Abstract
Objective
Many survivors of acute respiratory distress syndrome (ARDS) have poor long term outcomes possibly due to supportive care practices during invasive mechanical ventilation. Helmet noninvasive ventilation (NIV) in ARDS may reduce intubation rates; however it is unknown if avoiding intubation with helmet NIV alters the consequences of surviving ARDS.
Design
Long-term follow up data from a previously published randomized controlled trial
Patients
Adults patients with ARDS enrolled in an previously published clinical trial
Setting
Adult ICU
Intervention
None
Measurements and Main Results
The primary outcome was functional independence at one year after hospital discharge defined as independence in activities of daily living and ambulation. At one year, patients were surveyed to assess for functional independence, survival,, and number of institution free days, defined as days alive spent living at home. The presence of ICU acquired weakness (ICU-AW) and functional independence was also assessed by a blinded therapist on hospital discharge. On hospital discharge, there was a greater incidence of ICU-AW (79.5% vs 38.6%; p=0.0002) and less functional independence (15.4% vs 50%; p=0.001) in the facemask group. One year follow-up data were collected for 81 of 83 patients (97.6%). One year mortality was higher in the facemask group (69.2% vs 43.2%; p=0.017). At one year, patients in the helmet group were more likely to be functionally independent (40.9% vs 15.4%; p=0.015) and had more institution free days (median 268.5 [0–354] vs 0 [0–323]; p=0.017).
Conclusions
Poor functional recovery after invasive mechanical ventilation for ARDS is common. Helmet NIV may be the first intervention that mitigates the long term complications that plague survivors of ARDS managed with noninvasive ventilation.
Clinical Trial Registration
clinicaltrials.gov Identifier: NCT01680783
Keywords: Acute Respiratory Distress Syndrome, Helmet, noninvasive ventilation, ICU-Acquired weakness, Early Mobilization
Introduction
Surviving acute respiratory distress syndrome (ARDS) is only the beginning of a prolonged recovery phase marked by neuromuscular weakness, functional impairment, and increased health care utilization.1 The complications that many ARDS survivors are set to inherit may be a consequence of supportive care practices that include deep sedation, neuromuscular blockade, and bed rest, which lay the foundation for persistent disability. Although some have shown that early mobilization during invasive mechanical ventilation improves functional impairment,2 data regarding its long term benefits are lacking.3 Furthermore, despite its short-term benefits, implementation of early mobilization in invasively mechanically ventilated may be challenging4,5 given that the mere presence of an endotracheal tube is often considered a major barrier to therapy.6 Therefore if the cost of surviving invasive mechanical ventilation for ARDS is not readily modified by early mobilization, we wondered if successful use of noninvasive ventilation in ARDS has the potential to alter short and long term outcomes. Our group has recently shown in a randomized clinical trial that noninvasive ventilation using a helmet interface can reduce intubation rates and improve mortality in patients with ARDS in comparison to facemask NIV. 7 However, it remains unknown if helmet NIV alters the long-term complications of surviving ARDS. The primary aim of this one-year follow-up study is to describe and compare the functional outcomes patients of ARDS patients enrolled in a randomized clinical trial of helmet versus facemask NIV.
Methods
The results from helmet versus facemask NIV, a single-center randomized clinical trial in patients with ARDS, have been previously reported. 7 Briefly, patients admitted to the medical ICU with ARDS requiring facemask NIV for at least 8 hours were eligible for enrollment. The institutional review board approved the study and informed written consent was obtained from participants or their authorized surrogate decision maker. Patients were randomly assigned to continue on NIV using the facemask (n=39) or switch to the helmet interface (n=44). The primary endpoint was the intubation rate, which was observed to be over 40% lower in the helmet NIV group. This result and other considerations led to discontinuation of the trial, as described in the primary report.7
Baseline functional independence was determined by Barthel Index score ≥70 obtained from a proxy describing patient function two weeks before admission.8,9 All enrolled patients received physical and occupational therapy during their ICU stay. The number of mobility sessions completed during the ICU stay and mobilization milestones such as completion of upper/lower extremity exercises, bed mobility, sitting on edge of bed, standing, sitting in a chair, and ambulating were also recorded for each ICU mobility session. If patients were not mobilized, the treating therapist was required to provide a reason for deferring the session.10 Given that ICU length of stay was different between groups, the total number of ICU days patients were mobilized was divided by the total number of ICU days to calculate the proportion of ICU days that patients were mobilized in each group. Delirium was assessed after interruption of sedation,11 using the Confusion Assessment Method (CAM-ICU).12 Patients with contraindication to sedative interruption (ie neuromuscular blockade) were assessed while on sedative. The proportion of days spent in delirium, coma, or CAM-ICU normal were calculated by dividing the total number of days in each cognitive category by the total ICU or hospital days for each group.
All patients who survived to hospital discharge had a functional and strength assessment using the Medical Research Council (MRC) scale by a therapist who was blinded to study allocation.13 A combined MRC score of <48 defined the presence of ICU acquired weakness (ICU-AW).14 Patients who died during their hospitalization without a strength assessment were recorded as having ICU-AW. Functional independence at hospital discharge was defined as independence in all activities of daily living (ADLs) and independent ambulation as determined by a blinded therapist.2
At one year after hospital discharge, patients were interviewed by phone to assess survival and functional independence. Functional independence at one year was defined as independence in all ADLs and independent ambulation as reported by the patient. We prospectively collected data on hospital, skilled nursing, long-term acute care, and rehabilitation facility admissions using patient interview corroborated by medical records to calculate the number of health care institution-free days, defined as days alive spent living at home. Deaths were also assessed using the social security death index.
Statistical analysis
All analyses were performed on the basis of an intention-to-treat approach. We used the χ2 test or Fischer’s exact test as appropriate to compare categorical outcomes between groups. Wilcoxon-Mann-Whitney two-sample rank-sum test (for medians) or t-tests (for means) were used to compare continuous outcomes. To evaluate the effect of the intervention on one year survival, we used the Kaplan-Meier procedure to estimate survival distributions in each group, with the effect of the intervention compared between groups using the log-rank test. Additional analyses were done with Cox-regression models that adjusted for baseline factors that predict outcomes (age and APACHE II) and the presence of the helmet intervention. Hazard ratios (HRs), together with 95% confidence intervals (CI) were estimated using this model. We used Stata 11.0 (StataCorp LP) software. This trial was registered with ClinicalTrials.gov, number NCT01680783.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Baseline characteristics
The majority of the patients who survived to hospital discharge resided at home (50 out of 52 survivors; 96%) and were functionally independent (43 out of 52 survivors; 87%) prior to their hospitalization (Table 1). There was no difference in severity of ICU illness between groups (APACHE II score 24 in the facemask group vs 25 in helmet group; p=0.97).
Table 1.
Baseline characteristics of survivors
| Baseline Characteristic | Facemask NIV (n=20) | Helmet NIV (n=32) | p-value | ||
|---|---|---|---|---|---|
|
| |||||
| Age (years) | 58.4 | [50.8–71.5] | 58.2 | [49.7–66.6] | 0.52 |
| Female | 10 | 50% | 16 | 50% | 1 |
| APACHE II | 24 | [22–27] | 25 | [20–28] | 0.87 |
| Pre admission Residence | |||||
| Home | 18 | 90% | 32 | 100% | 0.14* |
| Nursing Home | 2 | 10% | 0 | 0% | |
| Functionally independent** | 15 | 75% | 28 | 87.5% | 0.28 |
| Past Medical History | |||||
| Congestive Heart Failure | 8 | 40% | 8 | 25% | 0.25 |
| Hematologic malignancy | 2 | 10% | 4 | 13% | 1 |
| Cancer, solid tumor | 3 | 15% | 2 | 6% | 0.36 |
| Stem cell transplant | 1 | 5% | 3 | 9% | 1 |
| Solid organ transplant | 1 | 5% | 4 | 13% | 0.64 |
Short-term Functional and Neuromuscular outcomes
Patients in the helmet group had a greater proportion of ICU days with early mobilization as compared to patients in the facemask group (51.6% [111/215 ICU days] vs 38.4% [110/286 ICU days]; p=0.003). This difference in the number of mobilization sessions was primarily seen during the time patients were on noninvasive ventilation (Table 2). Patients in the helmet group participated in mobilization 63.6% of the days while on NIV as compared to 43.9% in the facemask group (p=0.02). There was no difference in completion of upper/lower extremity (90.9 vs 76.9%; p=0.13) or bed mobility exercises (90.9 vs 74.4%; p=0.08) between groups. However, more patients in the helmet group were able to sit at the edge of the bed (90.9 vs 69.2%; p=0.02), stand (84.1 vs 56.4%; p=0.006), transfer to a chair (77.3 vs 51.3%; p=0.01), and walk (70.5 vs 41%; p=0.007) as compared to the facemask group.
Table 2.
Ease of Early Mobilization and Short-term Functional/Neuromuscular Outcomes
| Mobilization Outcomes | Facemask NIV (N=39) | Helmet NIV (N=44) | p-value | ||
|---|---|---|---|---|---|
|
| |||||
| #ICU days mobilized* | 110/286 | 38.4% | 111/215 | 51.6% | 0.003 |
| # Mobility sessions during: | |||||
| Endotracheal intubation** | 34/125 | 21.7% | 16/56 | 28.6% | 0.76 |
| Noninvasive ventilation** | 29/66 | 43.9% | 50/77 | 63.6% | 0.02 |
| Supplemental oxygen** | 52/95 | 50.5% | 48/82 | 56.1% | 0.46 |
| Mobility Milestones while on NIV | |||||
| Upper and lower extremity exercises | 30 | 76.9% | 40 | 90.9% | 0.13 |
| Bed mobility | 29 | 74.4% | 40 | 90.9% | 0.08 |
| Sit at edge of bed | 27 | 69.2% | 40 | 90.9% | 0.02 |
| Stand | 22 | 56.4% | 37 | 84.1% | 0.006 |
| Chair | 20 | 51.3% | 34 | 77.3% | 0.01 |
| Ambulation | 16 | 41% | 31 | 70.5% | 0.007 |
| Reasons for missed mobility session | |||||
| Cardiovascular | 16 | 8.6% | 6 | 5.2% | 0.26 |
| Respiratory | 41 | 22.2% | 25 | 21.6% | 0.9 |
| Neurologic | 24 | 13.0% | 12 | 10.3% | 0.49 |
| Neuromuscular Blockade | 7 | 3.8% | 19 | 16.4% | 0.0002 |
| Procedure | 10 | 5.4% | 7 | 6.0% | 0.82 |
| Comfort Care | 28 | 15.1% | 12 | 10.3% | 0.23 |
| Cardiac Arrest | 4 | 2.2% | 3 | 2.6% | 1 |
| Intermittent HD | 4 | 2.2% | 4 | 3.4% | 0.49 |
| Patient Refusal | 18 | 9.7% | 1 | 0.9% | 0.001 |
| Discharged from therapy | 0 | 0% | 4 | 3.4% | 0.02 |
| Logistics | 25 | 13.51% | 18 | 15.5% | 0.63 |
| Unspecified | 8 | 4.3% | 5 | 4.3% | 1 |
| Outcomes on Hospital Discharge | |||||
| ICU- Acquired Weakness** | 31 | 79.5% | 17 | 38.6% | 0.0002 |
| Functional Independence** | 6 | 15.4% | 22 | 50% | 0.001 |
Data are expressed as # of days with early mobility/#ICU days
Data are expressed as # mobility sessions/# days on specified respiratory support and proportion of ICU days the patient was mobilized (%), where appropriate
Determined by therapist blinded to study allocation
Upon hospital discharge, there was a significant reduction in the proportion of patients with ICU-acquired weakness in the helmet group as compared to the facemask group (38.6%vs 79.5%; p=0.0002). In addition more patients in the helmet group were functionally independent on hospital discharge than the facemask group (50% vs 15.4%; p=0.001). After excluding patients who died during their hospitalization, these differences in the incidence of ICU-acquired weakness (helmet 18.8% vs facemask 60%; p=0.002) and functional independence (helmet 68.8% vs facemask 30%; p=0.006) remained statistically significant.
Neurocognitive outcomes
Both groups had similar sedative use (Table 3) with the exception of increased propofol use in the facemask group than in the helmet group due to greater intubation rates in the former group (helmet 15.9% vs facemask 41%; p=0.01). Overall, the incidence of delirium was lower in the helmet group in the ICU (15.8% [34/215 ICU days] vs 28.7% [82/286 ICU days]; p=0.0007) and entire hospitalization (15.7% [70/446 hospital days] vs 31.9% [132/414 hospital days]; p=0.0001); There were no differences in the proportion of days spent in coma. There was also a significant increase in the proportion of days spent as CAM-ICU normal in the ICU (62.7% [150/215 ICU days] vs 57.3% [164/286 ICU days]; p=0.004) and overall hospitalization (76.7% [342/446 hospital days] vs 58.7% [243/414 hospital days]; p=0.00001) in the helmet group.
Table 3.
Sedative Use and Neurocognitive outcomes
| Outcome | Facemask NIV (n=39) | Helmet NIV (n=44) | p-value | ||
|---|---|---|---|---|---|
|
| |||||
| No. ICU days spent in: | 286 ICU days | 215 ICU days | |||
| Delirium | 82/286 | 28.7% | 34/215 | 15.8% | 0.0007 |
| Coma | 40/286 | 14.0% | 31/215 | 14.2% | 0.89 |
| CAM-ICU Normal | 164/286 | 57.3% | 150/215 | 62.7% | 0.004 |
| No. Hospital days spent in: | 414 Hospital days | 446 Hospital days | |||
| Delirium | 132/414 | 31.9% | 70/446 | 15.7% | 0.00001 |
| Coma | 39/414 | 9.4% | 34/446 | 7.6% | 0.34 |
| CAM-ICU Normal | 243/414 | 58.7% | 342/446 | 76.7% | 0.00001 |
| Sedative Details | |||||
| No. receiving dexmedetomidine | 13 | 33.3% | 17 | 38.6% | 0.62 |
| No. receiving propofol | 16 | 41% | 7 | 15.9% | 0.01 |
| No. receiving midazolam | 16 | 41% | 15 | 34% | 0.52 |
| No. receiving IV opiate | 27 | 69% | 23 | 52.3% | 0.12 |
Data are expressed in n (%), where appropriate
ICU= Intensive Care Unit; CAM= Confusion Assessment Method
One year follow-up outcomes
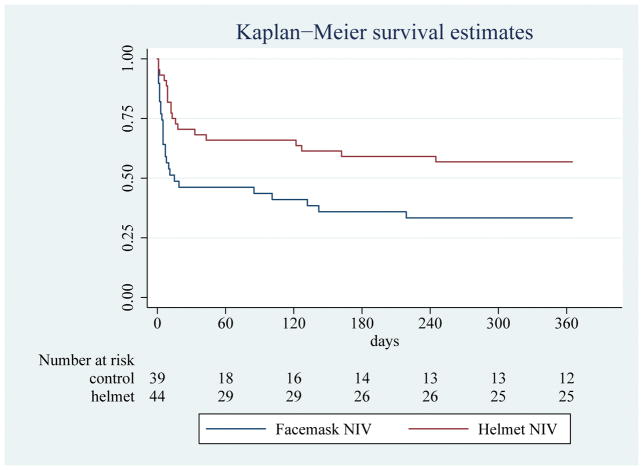
Eighty-one of the 83 enrolled patients underwent one-year follow-up (97.6%) (Supplemental Figure 1). Upon hospital discharge, 47.7% of the helmet group (Table 4) returned home in comparison to 20.5% of patients in the facemask group (p=0.009). A greater proportion of patients in the helmet group were alive and functionally independent at one year than in the facemask group (40.9 vs 15.4%; p=0.015). After excluding patients who died during their hospitalization, 30% of the patients in the facemask were functionally independent at one year as compared to 56.3% in the helmet group (p=0.06). In addition, patients in the helmet group had significantly more health care institution-free days than patients in the facemask group (268.5 vs 0 days; p=0.017). Finally, the helmet group had lower one year mortality than the facemask group (43.2 vs 69.2%; log-rank test for difference in survival distributions; p=0.007) (Fig 1). The unadjusted hazard ratio (HR) for death at one year was 0.46 (95% confidence interval (CI) [0.25–0.82]; p=0.009) in the helmet NIV group. APACHE II score was also independently associated with death at 90 days (HR 1.08 [1.02–1.14]; p=0.008). No association was detected with age (HR 1.01[0.99–1.04]; p=0. 34). The risk of death at one year remained significantly lower in the helmet NIV group after adjustment for APACHE II score (HR 0.48 [0.26–0.86], p=0.01).
Table 4.
Discharge Destination and One-Year Outcomes
| Outcome | Facemask NIV (n=39) | Helmet NIV (n=44) | p-value | ||
|---|---|---|---|---|---|
|
| |||||
| Discharge Destination | |||||
| Home | 8 | 20.5% | 21 | 47.7% | 0.009* |
| Long term acute care | 3 | 7.7% | 4 | 9.1% | |
| Acute rehab | 4 | 10.3% | 1 | 2.3% | |
| Subacute rehab | 0 | 0% | 4 | 9.1% | |
| Skilled nursing facility | 2 | 5.1% | 0 | 0% | |
| Hospice | 3 | 7.7% | 2 | 4.5% | |
| Died | 19 | 48.7% | 12 | 27.3% | |
| One Year Outcomes | |||||
| One-Year Mortality | 27 | 69.2% | 19 | 43.2% | 0.007 |
| Functional Independence | 6 | 15.4% | 18 | 40.9% | 0.015 |
| Institution Free Days (days)** | 0 | [0–323] | 268.5 | [0–354] | 0.017 |
Data are expressed in n (%) or median [Interquartile Range], where appropriate
Indicates comparison of proportion of patients who went home versus any other discharge destination
Equals one year (365 days) minus days in a health care institution, rather than home
Figure 1.
Kaplan Meier Analysis of One Year Survival
Discussion
In a long-term follow-up study of our trial of helmet noninvasive ventilation,7 we found that patients in the helmet group were more likely to be discharged home functionally independent and remain independent at one year after ICU admission in comparison with facemask NIV. The helmet group also had improved one year survival and less health care utilization. These findings are in stark contrast to prior work demonstrating persistent functional limitations, poor quality of life, and increased health care utilization in invasively mechanically ventilated ARDS survivors after critical illness.1 The complications described by Herridge et al 1,15 may be a direct result of supportive care practices in the management of ARDS which include bed rest, neuromuscular blockade, and deep sedation during invasive mechanical ventilation. One should be cautious directly comparing our work with the Herridge et al cohort,1,15 since all of those patients were intubated whereas many in our cohort did not require endotracheal intubation. However, interventions aimed at mitigating the complications of invasive mechanical ventilation such as mobilization, while possible in intubated patients,2 are more challenging to implement than in non-intubated patients.6 While noninvasive ventilation has the promise to avoid the complications of invasive ventilation,16 many have cautioned against its use in hypoxemic respiratory failure due to high failure rates17,18 and concerns for excess mortality19 possibly from delayed intubation and/or large tidal volumes.20,21 Our prior work suggests mortality and endotracheal intubation rates for noninvasive ventilation in ARDS may be significantly improved when the interface for the delivery of noninvasive ventilation is a helmet.7 Therefore the avoidance of complications of invasive mechanical ventilation could be possible with helmet ventilation without excess risk from high failure rates and mortality. Further work is needed to draw more definitive conclusions on this issue.
The prevention of functional and neuromuscular complications in the helmet group may be explained by the avoidance of immobility and deep sedation practices that may be associated with invasive mechanical ventilation. Indeed, the helmet group was mobilized more reliably in the ICU, had less delirium, and less neuromuscular weakness on hospital discharge. The ability to effectively implement early mobilization during the noninvasive ventilation was key to setting a path for functional recovery after ARDS.
Our study has several limitations. First, the original trial of helmet NIV was stopped early for efficacy and safety concerns in accord with pre-defined criteria by our Data Safety and Monitoring Board. This may alter the magnitude of the long term effects of this intervention. Second, functional independence at one year was determined by patient self-report, which can underestimate any impairment. However, there are data suggesting acceptable agreement in ADL proficiency by self-report and direct observation.22 Third, the study was not blinded which may introduce bias especially with the implementation of early mobilization. To investigate this possibility, therapists cited reasons for deferred mobility sessions. There was no difference in most reasons for missed mobility sessions, except for increased neuromuscular blockade on potential mobility days in the helmet group (16 vs 4%; p=0.0002). The reasons for missed mobility sessions were not expected to be all inclusive and thus secondary reasons omitted by the therapists for the control group may have included neuromuscular blockade. In addition therapists cited graduation from therapy due to achievement of functional independence more commonly in the helmet group (3% vs 0%; p= 0.02) and increased patient refusal of mobilization in the facemask group (10% vs 1%; p=0.001). The most common reason cited as to why patients refused was fatigue in the facemask group (15 out of 18 times; 83.3%). These data support our observation of patient intolerance of the facemask which limited the implementation of early mobilization during noninvasive ventilation in the control group.
Recent work has cautioned against the use of noninvasive ventilation in the management of ARDS in deference to the primacy of instituting lung protective strategy with invasive mechanical ventilation.21,23 This choice may reduce the risk of perpetuating lung injury but often necessitates deep sedation practices and bed rest which are at the cost of functional independence. Our work suggests a possible alternative approach in which helmet ventilation obviates the need for invasive mechanical ventilation and its associated detrimental supportive care practices. Patients have the opportunity to be awake and animated in spite of highly assisted spontaneous breathing during ARDS to set a foundation for functional recovery after ARDS. To date there have been no interventions shown to improve long term outcomes in ARDS survivors. Although these findings are preliminary, the potential of helmet noninvasive to alter the landscape of ARDS survivorship warrants further study.
Supplementary Material
Supplemental Figure 1: Consort diagram of patient flow through one year after randomization
Acknowledgments
Funding: Daniel Edelman grant, a gift of the Edelman family; T32 NIH/NHLBI; Parker B. Francis Foundation
Footnotes
Contributors:
BKP, ASPohlman, JBH, and JPK participated in the conception of the trial. BKP, ASP, JBH, JPK participated in study design. BKP, ASPohlman, and JPK recruited patients and collected data. KSW, EM, DS, CLE, ASPawlik, MS, CK, MT, EZ, JM collected data alone. BKP, KSW, EM, ASP, JBH, JPK analyzed the data. All authors participated in the interpretation of the results. BKP drafted the manuscript. All authors have seen and approved the final version of the manuscript.
Conflicts of interest
We declare that we have no relevant conflicts of interest.
Copyright form disclosure: Drs. Patel, Stulberg, and Kemple’s institution received funding from a Daniel Edelman grant. Dr. Patel’s institution received funding from a Parker B Francis Foundation Career Development Award (salary support). Drs. Patel and Wolfe’s institution received funding from the National Institutes of Health (NIH) T32 salary support, and they received support for article research from the NIH. Dr. Pohlman received funding from B. Braun (consultant). The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 2.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–82. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moss M, Nordon-Craft A, Malone D, et al. A Randomized Trial of an Intensive Physical Therapy Program for Patients with Acute Respiratory Failure. Am J Respir Crit Care Med. 2016;193:1101–10. doi: 10.1164/rccm.201505-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nydahl P, Ruhl AP, Bartoszek G, et al. Early mobilization of mechanically ventilated patients: a 1-day point-prevalence study in Germany. Crit Care Med. 2014;42:1178–86. doi: 10.1097/CCM.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 5.Berney SC, Harrold M, Webb SA, et al. Intensive care unit mobility practices in Australia and New Zealand: a point prevalence study. Crit Care Resusc. 2013;15:260–5. [PubMed] [Google Scholar]
- 6.Jolley SE, Moss M, Needham DM, et al. Point Prevalence Study of Mobilization Practices for Acute Respiratory Failure Patients in the United States. Crit Care Med. 2016 doi: 10.1097/CCM.0000000000002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of Noninvasive Ventilation Delivered by Helmet vs Face Mask on the Rate of Endotracheal Intubation in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA. 2016;315:2435–41. doi: 10.1001/jama.2016.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahoney FI, Barthel DW. Functional Evaluation: The Barthel Index. Md State Med J. 1965;14:61–5. [PubMed] [Google Scholar]
- 9.Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud. 1988;10:61–3. doi: 10.3109/09638288809164103. [DOI] [PubMed] [Google Scholar]
- 10.Pohlman MC, Schweickert WD, Pohlman AS, et al. Feasibility of physical and occupational therapy beginning from initiation of mechanical ventilation. Crit Care Med. 2010;38:2089–94. doi: 10.1097/CCM.0b013e3181f270c3. [DOI] [PubMed] [Google Scholar]
- 11.Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med. 2014;189:658–65. doi: 10.1164/rccm.201310-1815OC. [DOI] [PubMed] [Google Scholar]
- 12.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–10. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 13.Kleyweg RP, van der Meche FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barre syndrome. Muscle Nerve. 1991;14:1103–9. doi: 10.1002/mus.880141111. [DOI] [PubMed] [Google Scholar]
- 14.De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–67. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 15.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–93. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 16.Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–22. doi: 10.1056/NEJM199509283331301. [DOI] [PubMed] [Google Scholar]
- 17.Antonelli M, Conti G, Moro ML, et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi-center study. Intensive Care Med. 2001;27:1718–28. doi: 10.1007/s00134-001-1114-4. [DOI] [PubMed] [Google Scholar]
- 18.Thille AW, Contou D, Fragnoli C, Cordoba-Izquierdo A, Boissier F, Brun-Buisson C. Non-invasive ventilation for acute hypoxemic respiratory failure: intubation rate and risk factors. Crit Care. 2013;17:R269. doi: 10.1186/cc13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frat JP, Ragot S, Thille AW. High-Flow Nasal Cannula Oxygen in Respiratory Failure. N Engl J Med. 2015;373:1374–5. doi: 10.1056/NEJMc1508390. [DOI] [PubMed] [Google Scholar]
- 20.Carteaux G, Millan-Guilarte T, De Prost N, et al. Failure of Noninvasive Ventilation for De Novo Acute Hypoxemic Respiratory Failure: Role of Tidal Volume. Crit Care Med. 2016;44:282–90. doi: 10.1097/CCM.0000000000001379. [DOI] [PubMed] [Google Scholar]
- 21.Bellani G, Laffey JG, Pham T, et al. Noninvasive Ventilation of Patients with Acute Respiratory Distress Syndrome. Insights from the LUNG SAFE Study. Am J Respir Crit Care Med. 2017;195:67–77. doi: 10.1164/rccm.201606-1306OC. [DOI] [PubMed] [Google Scholar]
- 22.Dorevitch MI, Cossar RM, Bailey FJ, et al. The accuracy of self and informant ratings of physical functional capacity in the elderly. J Clin Epidemiol. 1992;45:791–8. doi: 10.1016/0895-4356(92)90057-t. [DOI] [PubMed] [Google Scholar]
- 23.Brochard L, Slutsky A, Pesenti A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am J Respir Crit Care Med. 2017;195:438–42. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Consort diagram of patient flow through one year after randomization