Abstract
Introduction
Essential tremor (ET) is increasingly recognized as a multi-dimensional disorder with both motor and non-motor features. For this reason, imaging studies are more broadly examining regions outside the cerebellar motor loop. Reliable detection of cerebral gray matter (GM) atrophy requires optimized processing, adapted to high-resolution magnetic resonance imaging (MRI). We investigated cerebral GM volume loss in ET cases using automated segmentation of MRI T1-weighted images.
Methods
MRI was acquired on 47 ET cases and 36 controls. Automated segmentation and voxel-wise comparisons of volume were performed using Statistical Parametric Mapping (SPM) software. To improve upon standard protocols, the high-resolution International Consortium for Brain Mapping (ICBM) 2009a atlas and tissue probability maps were used to process each subject image. Group comparisons were performed: all ET vs. Controls, ET with head tremor (ETH) vs. Controls, and severe ET vs. Controls. An analysis of variance (ANOVA) was performed between ET with and without head tremor and controls. Age, sex, and Montreal Cognitive Assessment (MoCA) score were regressed out from each comparison.
Results
We were able to consistently identify regions of cerebral GM volume loss in ET and in ET subgroups in the posterior insula, superior temporal gyri, cingulate cortex, inferior frontal gyri and other occipital and parietal regions. There were no significant increases in GM volume in ET in any comparisons with controls.
Conclusion
This study, which uses improved methodologies, provides evidence that GM volume loss in ET is present beyond the cerebellum, and in fact, is widespread throughout the cerebrum as well.
Keywords: Essential Tremor, Gray Matter Volume, VBM, High-Resolution MRI, Cerebrum
Introduction
Essential tremor (ET) is among the most common movement disorders [1]. Tremor in the head and neck may occur in addition to arm tremor. The precise localization of the problem that results in tremor in ET is not known, but is thought to involve motor loops passing through the cerebellum, and there is even some evidence that the cerebellum itself may be the primary seat of the problem [2–4]. Hence, imaging studies in ET have focused particular attention on the cerebellum [2]. While on the one hand investigators have been honing in on the cerebellum, there is greater and greater appreciation of the fact that ET seems to be a multi-dimensional disorder, with both motor and non-motor (e.g., cognitive) features [5], and studies of the cerebral cortex have become the subject of greater interest. Interestingly, imaging studies of subjects with spinocerebellar ataxia (SCA) similarly show that while there is an expected involvement of the cerebellum, the cerebral cortex is involved, pointing to a more diffuse form of degeneration [6,7]. On this basis, we hypothesized that (1) volume loss in ET would not be restricted to the cerebellum and (2) volume loss would involve areas in the cerebral cortex that are involved in movement.
One of the most important factors that introduces inconsistency between studies assessing brain volume loss is the multitude of anatomical segmentation algorithms used to classify the brains tissues. While many studies use the same software, SPM (http://www.fil.ion.ucl.ac.uk/spm/), the preprocessing steps have many variables that greatly affect the overall measure of tissues in the brain. Standard procedures are outlined by Ashburner and Friston [8], but there are more recent papers [9,10] that update preprocessing procedures to more accurately reflect improvements in the algorithms and image quality. Segmentation in SPM is performed using Bayesian probability and tissue probability maps (TPMs). The TPMs are morphed to the subject brain and used to create tissue maps for each subject. Thus, the quality of the TPMs will greatly affect the quality of the subject’s tissue maps. The brain is segmented into three tissue types: gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF). After segmentation, the subjects’ brain images must be normalized to a common space, typically Montreal Neurologic Institute (MNI) space, using an atlas. This involves both linear and nonlinear warping of the subject brain. An important step in normalization is the “modulation” of the image, which preserves the amount of a specific tissue type in each voxel [10]. Finally, smoothing of the normalized tissue maps is necessary to ensure parametric statistics can be performed [8]. In order to use parametric statistics to test for volume changes, a minimum of 30 degrees of freedom is recommended [11]. Therefore, when working with a population of fewer than 30 subjects, using non-parametric statistics is recommended. The current study greatly exceeds that threshold, having 47 and 36 subjects in the case/control groups respectively, for a total of 83 subjects. This threshold is maintained in subgroup tests as well.
To date, seven studies (Table 1) [12–18] have investigated brain volume loss in ET, both in the cerebrum and cerebellum, although they are inconsistent across methods and results. The inconsistencies in results are likely due to the underutilization of the high-resolution 3D images that are required for segmentation. Using a set of TPMs with a lower resolution than the original image essentially down-samples the resulting tissue maps to the resolution of the TPMs. The same can be said about the smoothing kernels; too large of a smoothing kernel will effectively lower the resolution and waste the high resolution benefits. By sampling to the same resolution as the original image, segmenting with TPMs of the same resolution as your 3D image, and smoothing by a smaller kernel, the high resolution of the original 3D image can improve the accuracy of the end result. Finally, correcting for multiple comparisons is essential when performing a voxel-wise analysis; however, identifying a correction procedure that minimizes both type I and type II errors is challenging.
Table 1.
Chronological summary of VBM studies in essential tremor. A check mark indicates the attribute was included in the study.
| Authors | Main Result | 3.0T MRI |
1×1×1 mm3 GM maps |
≥ 30 subjects in every test |
Multiple comparison correction |
4 mm isotropic smoothing |
High resolution atlas |
|---|---|---|---|---|---|---|---|
| Daniels et al. 2006 [12] | No GM changes were reported when comparing cases to controls. ET with intention tremor showed an increase in GM volume in the bilateral superior temporal gyrus compared to ET with postural tremor only. This test was performed with both ET groups compared to their respective controls (i.e. ET-I vs. C-I > ET-P vs C-P). | ||||||
| Benito-Leon et al. 2009 [13] | Comparing cases and controls, GM change (direction not specified) was found in the bilateral parietal lobe, right frontal lobe, and right insula. Comparing ET with head tremor and controls, GM change was reported in the right parietal and temporal lobes. | ✓ | ✓ | ||||
| Bagepally et al. 2012 [14] | Comparing cases and controls, GM atrophy was found in the bilateral frontal and occipital lobes, left middle temporal gyrus, and right superior parietal lobe. Comparing ET with and without head tremor, GM atrophy was found in the bilateral temporal and frontal lobes, right parietal lobe, and insula. | ✓ | ✓ | ||||
| Lin et al. 2013 [15] | Comparing cases and controls, GM atrophy was reported in the caudate, left mid temporal pole, insula, left precuneus, and superior temporal gyrus. Also, an increase in GM volume was reported in the mid temporal pole and precentral gyrus. | ✓ | |||||
| Bhalsing et al. 2014 [16] | This study separated the cases into those with and without cognitive impairment. An ANOVA comparing these two groups with controls found GM atrophy in the left anterior cingulate, right precentral gyrus, right occipital lobe, left superior temporal gyrus, and right insula. | ✓ | ✓ | ✓ | ✓ | ||
| Buijink et al. 2015 [17] | No GM changes were reported when comparing cases to controls. Comparing ET with and without head tremor, an increase in GM volume was reported in ET with head tremor in the bilateral pre-/post-central gyri, and left superior medial gyrus. | ✓ | ✓ | ✓ | |||
| Nicoletti et al. 2015 [18] | No GM changes were reported when comparing cases to controls or when comparing subgroups of ET with and without resting tremor. | ✓ | ✓ | ✓ | |||
| Cameron et al. 2017 | When comparing cases to controls, GM volume loss is found in the posterior insula, superior temporal gyri, cingulate cortex, inferior frontal gyri and other occipital and parietal regions. Multiple subgroups including ET with head tremor and ET with severe tremor were compared against controls. An ANOVA between ET with and without head tremor and controls was performed as well. | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
Abbreviations: Gray Matter (GM), ET with intention tremor (ET-I), ET with postural tremor (ET-P), control for ET-I (C-I), control for ET-P (C-P).
This study aims to address these issues by utilizing the International Consortium for Brain Mapping (ICBM) 2009a high-resolution (1×1×1 mm3) atlas and TPMs [19,20] for segmentation and normalization. Additionally, the parameters for preprocessing are carefully selected to optimize the detection of GM atrophy in the cerebrum. Cerebellar volume loss in this ET cohort has been reported previously [21].
Methods
Clinical Assessment
The study protocol was approved by the Yale, Cornell, and Purdue Human Subjects Institutional Review Boards. Written informed consent was obtained from each subject upon enrollment.
ET cases were recruited from a clinical-epidemiological case-control study of ET, from the neurological practice of one of the authors (E.D.L.), and via study advertisements [21]. Normal control subjects were recruited during the same time period and from the same sources as the ET cases, with some being spouses of the ET cases [21]. They were matched to ET cases on age. As cases were more readily available, their recruitment occurred more easily than those of controls, and this contributed to an unequal number of cases and controls [21]. Controls were excluded if they had history or family history of ET (a first- or second-degree relative with ET). Inclusion criteria for ET was a diagnosis of ET from the treating neurologist and a willingness to undergo MRI. General exclusion criteria included heavy ethanol exposure [22], a history of neurodegenerative disease (dementia, Parkinson’s disease), prior deep brain stimulation or other neurosurgery, or contraindication for MRI.
Upon enrollment, each ET case had an in-person assessment by a trained research assistant to collect demographic and clinical data, including the Montreal Cognitive Assessment (MoCA) (score range 0 – 30) to briefly assess cognitive function and screen for mild cognitive impairment (MCI) [23]. Questionnaires collected data on a broad range of aging-related comorbidities (e.g., hearing loss, osteoarthritis, stroke) as well as years since most recent hospitalization and number of prescription medications, both of which also reflect burden of comorbidity. Additionally, a video-taped neurological examination was performed on all subjects, which included 12 tests to assess postural and kinetic tremor. The video-tapes were reviewed by a neurologist specialized in tremors (E.D.L.) who scored each of 12 tests using the 0–3 Washington Heights-Inwood Genetic Study of Essential Tremor (WHIGET) rating scale (range of total tremor score [TTS] = 0 – 36). Head (i.e., neck) and jaw tremor were each scored as absent (0) or present (1). Diagnoses of ET were re-confirmed by the neurologist (E.D.L.) based on the history and videotaped neurological examination - WHIGET diagnostic criteria were applied (moderate or greater amplitude kinetic tremor [tremor rating ≥ 2]) during three or more tests or the presence of a head tremor, in the absence of Parkinson’s disease, dystonia or another cause.
MRI Acquisition
All MRI data was acquired at Weill Cornell Medicine at the Citigroup Biomedical Imaging Center on a 3 Tesla Siemens Tim Trio scanner (Siemens Healthcare, Erlangen, Germany) with a 32-channel head coil. For brain tissue segmentation, high resolution MPRAGE images were acquired (TR/TE/TI=2300/2.91/900ms, flip angle=9°, bandwidth:240 Hz/pixel, voxel size: 1.0mm×1.0mm×1.2mm, GRAPPA=2).
Data Processing
This study utilizes the “Old Segment” and “Old Normalize” programs available in SPM12 for their ability to select different atlases and TPMs compared to the standard version. Although the newer versions have updated algorithms to accomplish the segmentation and normalization, the ability to select more accurate reference images is essential. To improve the segmentation and normalization, the updated ICBM 2009a atlas was used for normalization, and the included TPMs were used for segmentation. The atlas and the TPMs have a resolution of 1×1×1 mm3, matching the resolution of the subject images.
Each subject image was first manually aligned to the atlas using the Check Reg function in SPM12. Manual adjustment ensures a proper registration of the subject to the atlas during segmentation and greatly improves the likelihood of proper segmentation.
The batch “Old Segment” was run to segment the brain into three separate tissue classes; GM, WM, and CSF, with the TPMs included in the ICBM 2009a atlas. Each image was checked for proper anatomical segmentation by overlaying the resulting tissue maps onto the original T1-weighted image. Those that did not pass a visual inspection were manually realigned to the atlas and segmented a second time. Often segmentation will fail if the image to be segmented is too far out of alignment with the atlas. This can be remedied by simply translating and rotating the image, contoured over the atlas, until it visually matches the atlas.
Normalization was performed using the “Old Normalize” function where the original T1-weighted image was normalized to the ICBM 2009a atlas and the subsequent transformation applied to the T1-weighted image and the three tissue maps from segmentation. Additionally, the tissue maps were modulated to preserve the amount of tissue in each voxel. This changes the intensity (which corresponds to percent tissue) in each voxel proportional to how much the volume of that voxel changes during the applied transformation. The tissue maps were resampled to 1×1×1 mm3 resolution after transformation to retain the original resolution of the image and atlas.
Finally, the tissue maps were smoothed with a 4×4×4 mm3 FWHM kernel. This smoothing kernel is smaller than that used in previous studies (which mostly used an 8×8×8 mm3 FWHM kernel), but follows the guidelines that the smoothing kernel should be at least twice the voxel size [24]. There is a trade-off between detection efficiency and noise when considering the size of smoothing kernel. By increasing the size of the smoothing kernel, the signal-to-noise ratio (SNR) of the image is increased at the expense of image resolution, which in turn lowers detection efficiency [24]. Therefore, due to overall better SNR from MRI images compared to earlier studies, and the improved segmentation with a high-resolution atlas, the images were smoothed by a reduced kernel.
Statistical Analysis
All analyses were performed using the factorial design specification function in SPM12, which performs statistical comparisons on a voxel-wise level. Demographics, clinical features, and associated comorbidities of ET cases and controls were compared using Student’s t and χ2 tests. A two-sample t-test was used to test group differences with nuisance variables age, sex, and MoCA score. Intracranial volume was calculated as the sum of the three tissue maps (GM, WM, and CSF) and included as a global calculation factor. Comparisons were performed for ET vs. Controls, ET with head or jaw tremor (ETH) vs. Controls, and ET with severe tremor (ET-ST) (i.e., total tremor score (TTS)≥23, which required a rating of 3 [severe] on at least one item) vs. Controls. ETH and ET-ST were not mutually exclusive categories, as it was possible for an ET case to present both with head tremor and a TTS≥23. Additionally, an ANOVA was performed to compare ET with and without head or jaw tremor and controls. A peak (voxel-wise) threshold was set at p<0.001 with a correction for multiple comparison using a cluster corrected p-value<0.05. The cluster correction requires a certain number of significant voxels be connected (clustered) in order to be deemed statistically significant. The size threshold for a cluster to be deemed significant (cluster p-value set to p<0.05) is dependent on the voxel size, smoothing kernel, and other variables [11,25].
Results
ET and controls differed with respect to sex (p=0.033) and years since most recent hospitalization (p=0.002). Differences in age, MoCA score, and hearing loss (p=0.076, p=0.135, and p=0.068 respectively) were marginally significant (Table 2). Age, sex, and MoCA score were used as covariates in all statistical tests to factor out any possible confounding effects.
Table 2.
Demographic and clinical characteristics.
| ET Cases | ETH | ET-ST | Controls | |
|---|---|---|---|---|
|
| ||||
| Sex | 24M/23F (51% M) | 13M/14F (48% M) | 14M/7F (67% M) | 10M/26F (28% M) |
| p = 0.033 | p = 0.099 | p = 0.004 | ||
|
| ||||
| Age (years) | 76.0±6.8 | 77.4±6.9 | 77.8±6.6 | 73.3±6.5 |
| p = 0.075 | p = 0.023 | p = 0.019 | ||
|
| ||||
| Total Tremor Score | 20.4±6.1 | 20.4±6.3 | 24.8±1.8 | 5.3±2.5 |
| p < 0.001 | p < 0.001 | p < 0.001 | ||
|
| ||||
| MoCA Score | 27.4±2.5 | 27.8±1.9 | 26.8±3.0 | 28.1±1.7 |
| p = 0.135 | p = 0.426 | p = 0.040 | ||
|
| ||||
| Age of Onset (years) | 41.0±20.5 | 41.9±20.8 | 36.0±21.6 | N/A |
|
| ||||
| #Years Hospit | 8.4±13.3 | 8.0±10.2 | 6.9±6.9 | 22.8±23.9 |
| p = 0.002 | p = 0.002 | p < 0.001 | ||
|
| ||||
| #Prescrip | 3.56±2.48 | 3.93±2.29 | 3.70±3.00 | 3.19±3.21 |
| p=0.575 | p=0.372 | p=0.641 | ||
|
| ||||
| Hearing loss | 12/47 (26%) | 8/27 (30%) | 5/21 (24%) | 3/36 (8%) |
| p=0.068 | p=0.045 | p=0.132 | ||
|
| ||||
| Stroke | 2/47 (4%) | 1/27 (4%) | 2/21 (10%) | 0/36 (0%) |
| p=0.216 | p=0.248 | p=0.064 | ||
|
| ||||
| TIA | 3/47 (6%) | 0/27 (0%) | 1/21 (5%) | 1/36 (3%) |
| p=0.458 | p=0.386 | p=0.700 | ||
|
| ||||
| CHF | 5/47 (11%) | 2/27 (7%) | 2/21 (10%) | 1/36 (3%) |
| p=0.187 | p=0.405 | p=0.284 | ||
|
| ||||
| MI | 1/47 (2%) | 0/27 (0%) | 1/21 (5%) | 0/36 (0%) |
| p=0.381 | p=N/A | p=0.190 | ||
|
| ||||
| OA | 13/47 (28%) | 7/27 (26%) | 5/21 (24%) | 8/36 (22%) |
| p=0.626 | p=0.766 | p=0.904 | ||
P-values are with respect to controls for each group. 9M/4F ET cases were included both in the ETH and ET-ST subgroups.
Abbreviations: ET with head tremor (ETH), ET with severe tremor [TTS≥23] (ET-ST), Montreal Cognitive Assessment (MoCA), Not Applicable (N/A), Years since hospitalization (#Years Hospit), Number of prescription medications (# Prescrip), Transient Ischemic Attack (TIA), Congestive Heart Failure (CHF), Myocardial Infarction (MI), Osteoarthritis (OA)
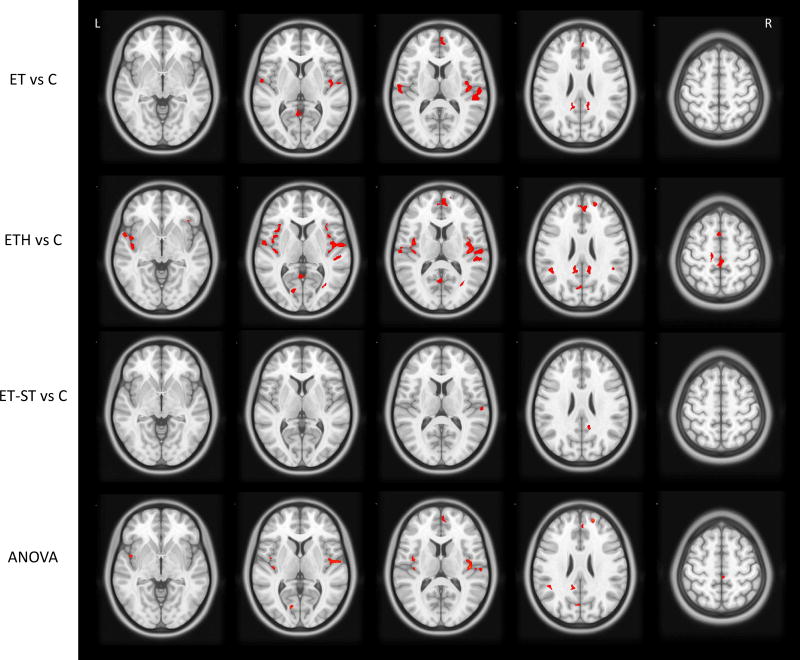
Statistically significant cerebral GM volume loss (cluster corrected p-value<0.05) was found widespread throughout the temporal, parietal, frontal and occipital lobes in ET vs C, ETH vs C and in the ANOVA (Table 3). The regions of highest significance were the Heschl, inferior insula, and superior temporal gyri, which had cluster corrected p-values≤0.001. ETH vs C comparison and ANOVA showed the most widespread cerebral GM volume loss involving additional regions such as the anterior, middle, and posterior cingulate. Fewer regions were found to show statistically significant GM volume loss in the ET-ST vs C group comparisons, such as the right superior temporal gyrus, right rolandic operculum, right supramarginal gyrus, right precuneus, and right superior occipital lobe. Importantly, no regions of cerebral GM volume increase in ET cases were detected in any comparisons. Figure 1 presents the statistically significant clusters overlaid on an axial slice of the 3D T1 atlas used for normalization. In additional analyses, we included hearing loss and years since most recent hospitalization as additional covariates in our adjusted models; results did not differ (data not shown).
Table 3.
Summary of statistically significant regions of GM volume loss in each test.
| Brain Region | ET vs C | ETH vs C | ET-ST vs C | ANOVA |
|---|---|---|---|---|
|
| ||||
| Temporal Lobe | ||||
|
| ||||
| R Heschl | <0.001 | <0.001 | <0.001 | |
| L Heschl | 0.003 | |||
|
| ||||
| R Insula | <0.001 | <0.001 | <0.001 | |
| L Insula | 0.027 | 0.008 | ||
|
| ||||
| R Sup Temporal | <0.001 | <0.001 | ||
| L Sup Temporal | 0.003 | <0.001 | 0.014 | |
|
| ||||
| R Rolandic Oper | 0.014 | |||
| L Rolandic Oper | <0.001 | 0.008 | ||
|
| ||||
| Parietal Lobe | ||||
|
| ||||
| R Supramarginal | 0.033 | <0.001 | <0.001 | |
| L Supramarginal | 0.002 | 0.028 | ||
|
| ||||
| R Post Cingulate | 0.006 | 0.001 | ||
| L Post Cingulate | 0.037 | 0.003 | 0.042 | |
|
| ||||
| R Paracentral lobule | 0.019 | |||
| L Paracentral lobule | <0.001 | 0.019 | ||
|
| ||||
| R Mid Cingulate | 0.016 | |||
|
| ||||
| Frontal Lobe | ||||
|
| ||||
| R Supp Motor area | 0.016 | |||
| L Supp Motor area | <0.001 | |||
|
| ||||
| R Frontal Sup | 0.007 | 0.016 | ||
|
| ||||
| R Frontal Sup Medial | 0.001 | <0.001 | 0.001 | |
|
| ||||
| R Ant Cingulate | 0.001 | <0.001 | 0.001 | |
|
| ||||
| Occipital Lobe | ||||
|
| ||||
| R Calcarine | 0.018 | |||
| L Calcarine | 0.026 | <0.001 | 0.022 | |
|
| ||||
| R Precuneus | 0.006 | 0.015 | ||
| L Precuneus | 0.037 | 0.011 | 0.032 | |
|
| ||||
| R Sup Occipital | 0.047 | |||
|
| ||||
| L Mid Occipital | 0.011 | |||
|
| ||||
| L Cuneus | <0.001 | 0.022 | ||
Presented p-values are on the cluster level and are deemed statistically significant using a peak-voxel p-value < 0.001 and a cluster corrected threshold at the cluster level of p < 0.05. The regional specificity of the cluster is defined by one or more “peak” voxels in the cluster. Therefore, it is possible for a single cluster to belong to multiple brain regions in close proximity. Peak voxels are presented in SPM with a set of MNI coordinates that can be checked on the Automated Anatomical Labeling (AAL) atlas.
ANOVA compared three groups: ET with head tremor, ET without head tremor and controls.
Abbreviations: “vs” refers to a group comparison between two subject groups. Essential tremor (ET), Control (C), ET with head tremor (ETH), ET with severe tremor [TTS ≥ 23] (ET-ST). Right side (R), left side (L). Analysis of Variance (ANOVA).
Figure 1.
Axial slice view of statistically significant clusters (in red). Statistical significance is determined using a peak-voxel p-value < 0.001 and a cluster corrected threshold at the cluster level of p < 0.05. ANOVA was run with three groups: ET with head tremor, ET without head tremor and controls.
Abbreviations: “vs” refers to a group comparison between two subject groups. Essential Tremor (ET), Control (C), ET with head tremor (ETH), ET with total tremor score ≥ 23 (ET-ST), Analysis of Variance (ANOVA).
Discussion
We investigated cerebral GM volume loss in phenotypic subgroups of ET and in the ET group as a whole compared to controls. Previous studies have used SPM to investigate cerebral GM volume changes in ET populations and many have conducted subgroup analyses as well [12–17]. To our knowledge, however, this is the first study to investigate cerebral GM volume loss in ET using the high-resolution ICBM 2009a atlas and TPMs for segmentation and normalization.
Although GM volume loss can be a feature of aging, it is important to emphasize that our study accounted for the effects of age and aging. First, we enrolled an age-matched control group, thereby allowing us to demonstrate changes in ET that were above and beyond those seen in normal aging. Second, we included age as a covariate in all statistical analyses; thus differences between ET groups and controls were completely independent of the effects of age. We also considered a broad range of aging-associated comorbidities, the vast majority of which did not differ between ET and controls, and for those that did (years since most recent hospitalization and hearing loss, marginally), adjustment in our models revealed that they did not influence our results.
By utilizing the high-resolution atlas and TPMs, this study was able to consistently identify regions of GM volume loss in ET and in ET subgroups. Regions such as the Heschl region, posterior insula, and superior temporal lobe were previously reported [12,15,16], however, we now report more widespread differences throughout the cerebrum as well. In addition to the regions noted above, significant GM volume loss was noted in the anterior, middle, and posterior cingulate, right frontal superior medial lobule, and occipital regions such as the calcarine, precuneus and cuneus. This study points clearly to the fact that volume loss is not restricted solely to the cerebellum in ET. Of additional note is that we found no statistically significant increases in GM volume in any of our case groups compared to controls, as have been previously reported [12,15,17]. The consistency of results between the ET vs C, ETH vs C, and ANOVA comparisons speaks to the improved detectability of GM volume loss using the high-resolution atlas.
Regions of significant difference between ET-ST and controls were less widespread than between ETH and controls. It is possible that sample size played a role (n = 21 for ET-ST vs. n = 27 for ETH). It is also possible that ETH represents a bone fide disease subtype (i.e., a “trait” difference) whereas ET-ST, merely a reflection of duration of tremor (i.e., a “state” difference). Indeed, other studies have consistently pointed to head tremor as a separable group of ET cases.
As noted above, this study suggests that volume loss is not restricted solely to the cerebellum in ET. Similarly, studies of patients with diseases characterized by more marked cerebellar involvement (e.g., SCA) indicate the presence of volume loss in the cerebral cortex as well [6,7], indicating a more diffuse degeneration.
One limitation of some studies is population size. It is recommended to have a minimum of 30 degrees of freedom (i.e. 30 subjects) when performing parametric statistics to test for GM volume differences between groups [11]. Three prior studies (Table 1) [12,14,15] performed group or subgroup tests that violated this threshold. This study was well above the threshold with a total of 83 subjects, with more than 30 in the control group, therefore the use of parametric statistics was validated for each test.
Functional and diffusion-based connectivity studies on healthy volunteers [26,27] have shown connections between the posterior insula and many of the regions this study reports as having statistically significant GM volume loss. The posterior insula is the most consistent finding among our subgroup comparisons, indicating that this could be one of the first regions in the cerebrum to be affected by GM volume loss in ET. Diffusion tractography showed structural connectivity between the posterior insula and multiple regions in the cingulate cortex and somatosensory regions such as the supplementary motor cortex, which showed GM volume loss in our ETH comparison [27]. Similar results are presented in a functional connectivity study on healthy volunteers [26], where the posterior insula is connected to the cerebellum as well as the posterior cingulate, sensorimotor, premotor, supplementary motor, and temporal cortices. Many, but not all, of these regions were found to have statistically significant GM volume loss in this study. This promotes the hypothesis that GM volume loss follows the motor paths from the cerebellum (see [21]) up into the insula and continues along the associated motor pathways.
Several prior studies [12,18,28–30] have reported no GM volume change in the cerebrum when comparing ET cases and controls. We have shown that GM volume loss can be observed in the ET population as a whole, and that it is even more prevalent in ET with head tremor. The null results in the prior studies are likely a byproduct of lower resolution tissue maps.
We did not investigate regional correlations of GM volume and cognitive variables as this would have required a lobule approach to segmentation. Furthermore, the use of the MoCA in place of a full neuropsychological test battery was a limitation. While MoCA score was factored into each statistical test, it is not a fully reliable score that could be used to compare GM volume loss between groups of differing cognitive function. Another limitation of this study was that all subjects remained on prescribed medication for the exam and MRI. Tremor medication is well-known to affect cognitive function, but it is not associated with cortical atrophy, so it was not considered necessary to withhold medication in the current study.
Conclusions
This study provides evidence that GM volume loss in ET is present beyond the cerebellum, and in fact, is widespread throughout the cerebrum as well. Further, head tremor in ET has been shown to potentially be a subtype of the disease, with a propensity towards greater GM volume loss in the cerebrum, specifically in the posterior insula and its functionally connected regions. The consistency between comparisons validates the use of an updated and improved atlas for use in VBM. The depth and complexity of such analysis requires careful consideration of each step in the processing. Comparison of regional cerebral GM volume loss in ET may serve to provide information on disease progression and further identify subtypes of disease.
Highlights.
-
-
ET is increasingly recognized as a multi-dimensional disorder
-
-
Imaging studies are broadly examining regions outside the cerebellar motor loop
-
-
We studied cerebral gray matter (GM) volume loss in ET using improved methods
-
-
GM volume loss in ET was present beyond the cerebellum
-
-
GM volume loss in ET appears to be widespread throughout the cerebrum
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke to E.D.L. [Grant # 5R01NS085136].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: E.D.L. has received research grants from the NINDS. U.D. has received research grants from the National Institute of Environmental Health Sciences. J.D., E.C and N.H. have no conflicts to report.
References
- 1.Louis ED, Ottman R. How many people in the USA have essential tremor? Deriving a population estimate based on epidemiological data. Tremor Other Hyperkinet. Mov. (N. Y) 2014;4:259. doi: 10.7916/D8TT4P4B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerasa A, Quattrone A. Linking Essential Tremor to the Cerebellum: Neuroimaging Evidence. Cerebellum. 2016;15:263–275. doi: 10.1007/s12311-015-0739-8. [DOI] [PubMed] [Google Scholar]
- 3.Filip P, Lungu OV, Manto M, Bares M. Linking Essential Tremor to the Cerebellum : Physiological Evidence. Cerebellum. 2016;15:774–780. doi: 10.1007/s12311-015-0740-2. [DOI] [PubMed] [Google Scholar]
- 4.Benito-León J, Labiano-Fontcuberta A. Linking Essential Tremor to the Cerebellum: Clinical Evidence. Cerebellum. 2016;15:253–262. doi: 10.1007/s12311-015-0741-1. [DOI] [PubMed] [Google Scholar]
- 5.Louis ED. Non-motor symptoms in essential tremor: A review of the current data and state of the field. Park. Relat. Disord. 2016;22:S115–S118. doi: 10.1016/j.parkreldis.2015.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez-Castillo CR, Galvez V, Diaz R, Fernandez-Ruiz J. Specific cerebellar and cortical degeneration correlates with ataxia severity in spinocerebellar ataxia type 7. Brain Imaging Behav. 2016;10:252–257. doi: 10.1007/s11682-015-9389-1. [DOI] [PubMed] [Google Scholar]
- 7.Dohlinger S, Hauser T-K, Borkert J, Luft AR, Schulz J. Magnetic Resonance Imaging in Spinocerebellar Ataxias. The Cerebellum. 2008;7:204–214. doi: 10.1007/s12311-008-00. [DOI] [PubMed] [Google Scholar]
- 8.Ashburner J, Friston KJ. Voxel-Based Morphometry—The Methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 9.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Ashburner J. Computational anatomy with the SPM software. Magn. Reson. Imaging. 2009;27:1163–1174. doi: 10.1016/j.mri.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear model approach. Hum. Brain Mapp. 1995;2:189–210. doi: 10.1002/hbm.460020402. [DOI] [Google Scholar]
- 12.Daniels C, Peller M, Wolff S, Alfke K, Witt K, Gaser C, Jansen O, Siebner HR, Deuschl G. Voxel-based morphometry shows no decreases in cerebellar gray matter volume in essential tremor. Neurology. 2006;67:1452–1456. doi: 10.1212/01.wnl.0000240130.94408.99. [DOI] [PubMed] [Google Scholar]
- 13.Benito-León J, Alvarez-Linera J, Hernández-Tamames JA, Alonso-Navarro H, Jiménez-Jiménez FJ, Louis ED. Brain structural changes in essential tremor: Voxel-based morphometry at 3-Tesla. J. Neurol. Sci. 2009;287:138–142. doi: 10.1016/j.jns.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 14.Bagepally BS, Bhatt MD, Chandran V, Saini J, Bharath RD, Vasudev MK, Prasad C, Yadav R, Pal PK. Decrease in cerebral and cerebellar gray matter in essential tremor: A voxel-based morphometric analysis under 3T MRI. J. Neuroimaging. 2012;22:275–278. doi: 10.1111/j.1552-6569.2011.00598.x. [DOI] [PubMed] [Google Scholar]
- 15.Lin C-H, Chen C-M, Lu M-K, Tsai C-H, Chiou J-C, Liao J-R, Duann J-R. VBM Reveals Brain Volume Differences between Parkinson’s Disease and Essential Tremor Patients. Front. Hum. Neurosci. 2013;7:247. doi: 10.3389/fnhum.2013.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhalsing KS, Upadhyay N, Kumar KJ, Saini J, Yadav R, Gupta AK, Pal PK. Association between cortical volume loss and cognitive impairments in essential tremor. Eur. J. Neurol. 2014;21:874–883. doi: 10.1111/ene.12399. [DOI] [PubMed] [Google Scholar]
- 17.Buijink AWG, Broersma M, van der Stouwe AMM, Sharifi S, Tijssen MAJ, Speelman JD, Maurits NM, van Rootselaar AF. Cerebellar Atrophy in Cortical Myoclonic Tremor and Not in Hereditary Essential Tremor-a Voxel-Based Morphometry Study. Cerebellum. 2015 doi: 10.1007/s12311-015-0734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicoletti V, Cecchi P, Frosini D, Pesaresi I, Fabbri S, Diciotti S, Bonuccelli U, Cosottini M, Ceravolo R. Morphometric and functional MRI changes in essential tremor with and without resting tremor. J. Neurol. 2015;262:719–728. doi: 10.1007/s00415-014-7626-y. [DOI] [PubMed] [Google Scholar]
- 19.Fonov V, Evans A, McKinstry R, Almli C, Collins D. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Neuroimage. 2009;47:S102. doi: 10.1016/S1053-8119(09)70884-5. [DOI] [Google Scholar]
- 20.Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyke JP, Cameron E, Hernandez N, Dydak U, Louis ED. Gray matter density loss in essential tremor : a lobule by lobule analysis of the cerebellum. Cerebellum & Ataxias. 2017;4:1–7. doi: 10.1186/s40673-017-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harasymiw JW, Bean P. Identification of heavy drinkers by using the early Detection of Alcohol Consumption Score. Alcohol. Clin. Exp. Res. 2001;25:228–236. [PubMed] [Google Scholar]
- 23.Nasreddine Z, Phillips N, Bédirian V, Charbonneau S, Whitehead V, Colllin I, Cummings J, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 24.Friston KJ, Holmes a, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4:223–35. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- 25.Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum. Brain Mapp. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- 26.Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- 27.Jakab A, Molnár PP, Bogner P, Béres M, Berényi EL. Connectivity-based parcellation reveals interhemispheric differences in the insula. Brain Topogr. 2012;25:264–271. doi: 10.1007/s10548-011-0205-y. [DOI] [PubMed] [Google Scholar]
- 28.Choi S-M, Kim BC, Chang J, Choi K-H, Nam T-S, Kim J-T, Lee S-H, Park M-S, Yoon W, de Leon MJ. Comparison of the Brain Volume in Essential Tremor and Parkinson’s Disease Tremor Using an Automated Segmentation Method. Eur. Neurol. 2015;73:303–9. doi: 10.1159/000381708. [DOI] [PubMed] [Google Scholar]
- 29.Quattrone A, Cerasa a, Messina D, Nicoletti G, Hagberg GE, Lemieux L, Novellino F, Lanza P, Arabia G, Salsone M. Essential head tremor is associated with cerebellar vermis atrophy: A volumetric and voxel-based morphometry MR imaging study. Am. J. Neuroradiol. 2008;29:1692–1697. doi: 10.3174/ajnr.A1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cerasa A, Messina D, Nicoletti G, Novellino F, Lanza P, Condino F, Arabia G, Salsone M, Quattrone A. Cerebellar atrophy in essential tremor using an automated segmentation method. Am. J. Neuroradiol. 2009;30:1240–1243. doi: 10.3174/ajnr.A1544. [DOI] [PMC free article] [PubMed] [Google Scholar]