Abstract
Compared to their differentiated progeny, stem cells are often characterized by distinct metabolic landscapes that emphasize anaerobic glycolysis and a lower fraction of mitochondrial carbohydrate oxidation. Until recently, the metabolic program of stem cells had been thought to be a byproduct of the environment, rather than an intrinsic feature determined by the cell itself. However, new studies highlight the impact of metabolic behavior on the maintenance and function of intestinal stem cells and hair follicle stem cells. This review summarizes and discusses the evidence that metabolism is not a mere consequence of, but rather influential on stem cell fate.
Keywords: Glycolysis, Pyruvate Oxidation, Fatty Acid Oxidations, Intestinal Stem Cells, Hair follicle stem cells, Stemness
Stem Cells and Their Metabolism
Most fully developed organs, principally populated with terminally differentiated cells, can renew and recover from injury due to the function of tissue-resident adult stem cells. The demand for stem cell proliferation in response to injury is widely appreciated, but recent studies have demonstrated that adult tissue stem cells also take cues from environmental nutrients that impact their homeostatic fate [1, 2]. For example, caloric restriction increases the healthspan and lifespan of various organisms and one mechanism is likely to be the promotion of tissue stem cell function [3–7]. Recent studies have highlighted the impact of metabolic behavior on intestinal stem cells (ISCs) and hair follicle stem cells (HFSCs) and this phenomenon is the primary focus of this review [8–10].
Both quiescent and proliferative adult stem cells appear to have metabolic profiles that are distinct from their fully differentiated progeny [11–13]. One common metabolic feature is their preference to perform aerobic glycolysis, with a lower fractional mitochondrial oxidation of carbohydrate fuels [11–13]. Although this metabolic distinction has been known, it has not been widely considered to be a major driving factor in the management of stem cell homeostasis and tissue regeneration. Typically, the metabolic program of adult stem cells has been viewed to be a product of the environment, rather than being intrinsically determined by the cell itself [14]. This view was supported by the observation that hematopoietic stem cells (HSCs) homeostasis is augmented by hypoxia within their niche, perhaps through promotion of a glycolytic/non-oxidative metabolic program [14–17]. In contrast, tissue resident stem cells frequently have active mitochondria and oxidize other fuels, such as fatty acids and amino acids [18–20]. This is suggestive of a highly specific mode of metabolic control operating in tissue stem cells. Notably, metabolic profiles of pluripotent stem cells (PSC) vary with developmental stage and the metabolic regulation of PSC cell fate has been reviewed extensively elsewhere [21–23].
Evidence is accumulating that the metabolic program of stem cells is intrinsically determined and essential for the maintenance of those cells, perhaps through impacting the balance of self-renewal and differentiation [11, 21]. Here, we focus on how the consumption and metabolism of different substrates (see Text Box) differentially influences adult stem cell homeostasis and function.
Metabolic Signatures of Tissue Stem Cells
Nutrient availability could provide cues that impact stem cell fate decisions [1, 2] and the intestinal epithelium is particularly interesting because ISC progeny have intimate access to dietary nutrients. Caloric restriction, while maintaining adequate nutrition, leads to increased numbers of ISCs in mice and enhances their regeneration capacity [3]. Other studies show that different epithelial stem cells in various species prefer to utilize glycolysis and lactate production over mitochondrial carbohydrate oxidative [24, 25]. Now, multiple groups have set out to understand the regulation and impact of this stem cell metabolism signature across different systems and species.
The expression of Mpc1, one of the subunits of the Mitochondrial Pyruvate Carrier (MPC) complex, is low in ISCs and high in the differentiated progeny of those ISCs, the intestinal epithelium [10]. In both mouse and human proximal small intestine (jejunum), immunohistochemistry demonstrated that MPC is nearly absent from the base of the intestinal crypt, where ISCs are located, but is much more abundantly expressed as one moves up the crypts to the villi, which is populated with differentiated epithelial cells. Furthermore, using mice in which EGFP is expressed from the stem cell-specific LGR5 promoter, it was observed that MPC1 abundance inversely correlated with LGR5 gene expression [10]. Together, these data indicate that intestinal stem cells exhibit low MPC1 expression and presumably low MPC activity.
It is critical to note that the low MPC1 protein level in ISCs is not due to low abundance of mitochondria overall. Protein analysis and electron microscopy both show high mitochondrial content in mouse and human ISCs [10, 18]. This implies that the mitochondria in ISCs might be directed toward other functions, including biosynthetic programs as well as the oxidation of fatty acids or other non-carbohydrate fuels, rather than carbohydrate oxidation.
Unlike Lgr5-positive ISCs that appear to divide almost continuously, HFSCs undergo cycles of proliferation and quiescence corresponding to the beginning and end of the hair cycle, respectively [9]. Parallel to the discovery that pyruvate oxidation is low in ISCs, lactate dehydrogenase (LDHA), the cytosolic enzyme that converts pyruvate to lactate, is highly expressed and active in HFSCs [9]. Ldha expression is enriched in HFSCs relative to the total epidermis [9] and LDH activity assays on skin tissue sections and lysates from sorted cells also showed high LDH activity in HFSCs across the hair cycle [9]. Furthermore, Ldha expression and activity is induced at the beginning of the hair cycle, which is when HFSCs start their rapid proliferation phase[9]. These data are consistent with proliferative HFSCs also being highly glycolytic.
The observation of low MPC expression in ISCs and high LDH expression in HFSCs suggest that these tissue-resident stem cells might preferentially utilize glucose through glycolysis coupled with lactate production rather than with mitochondrial oxidation. Beyond these correlations, manipulation of pyruvate metabolism through MPC or LDH modulation also profoundly affects stem cell homeostasis and function, suggesting that metabolism is not a mere response to, but rather influential on stem cell fate.
Pyruvate oxidation limits stem cell maintenance and proliferation
Using multiple species and multiple stem cell populations, recent studies have demonstrated that stem cell number and proliferation is increased when mitochondrial pyruvate entry is blocked through MPC deletion or inhibition [10]. These discoveries support the notion that the metabolic program of stem cells is not a byproduct of their environments or a passive feature of their cell biology, but rather a driving force that influences their fate and function.
Loss of the Drosophila ortholog of MPC1, known as dMPC1 caused overgrowth of the intestinal epithelium [10]. ISCs from these mutant flies also exhibited increased proliferation, similar to what was observed with RNAi-mediated disruption of dMPC1 or dMPC2 in wild-type flies [10]. Drosophila ISC proliferation was also increased when pyruvate dehydrogenase (PDH), the mitochondrial enzyme that oxidizes MPC-imported pyruvate to acetyl-CoA, was disrupted by RNAi [10]. This collection of phenotypes indicates that MPC inhibition promotes stem cell proliferation, almost certainly through reducing pyruvate incorporation into the TCA cycle.
Mice that lack MPC1 selectively in Lgr5-positive ISCs also exhibited increased stem cell number in the intestinal crypt and these stem cells were hyperproliferative [10]. Moreover, in the proximal small intestine where deletion of MPC1 was incomplete, MPC1-deficient cells appeared to be positively selected to repopulate the intestinal epithelium over time [10]. In spite of these apparently stem cell-intrinsic phenotypes, the intestine was overtly normal, with no morphological changes observed in intestinal crypt length, villus height, or total intestinal length [10]. This suggests that although the homeostasis of the stem cell compartment is perturbed by MPC deletion, MPC-deficient stem cell progeny are still competent to differentiate.
Similar effects have also been observed in intestinal organoid cultures, which are an in vitro model that faithfully recapitulates many aspects of the architecture and function of the mammalian intestinal epithelium [10, 26]. MPC1 deletion enhanced organoid formation from both whole crypts and from sorted Lgr5-positive stem cells [10]. MPC inhibition by the well-established MPC inhibitor, UK-5099, had similar effects. UK-5099-treated or MPC1-deleted organoids displayed increased expression of stem cell markers (Lgr5, Ascl2, Cd44 and Myc) and decreased expression of differentiation markers (Krt20, Villin1, Chga and Fabp2) [10]. Metabolomics experiments also confirmed that MPC1-deleted organoids exhibited a steady-state increase in pyruvate and decrease in TCA cycle intermediates, such as citrate, malate and α-ketoglutarate. In addition, 13C-glucose flux tracing experiments in intestinal organoids also showed impaired glucose carbon entry into the mitochondrial TCA cycle in response to MPC1 deletion [10]. Together, these observations support the hypothesis that impaired mitochondrial pyruvate import enhances stem cell function and reinforces the stem cell molecular signature.
Like MPC abundance in ISCs, LDH abundance and activity also appears to affect stem cell function. Deletion of Ldha in HFSCs prevented activation and HFSCs failed to enter the start of the hair cycle and remained quiescent [9]. Similarly, enforcement of a glycolytic and non-oxidative metabolic program by deleting MPC1 accelerated HFSC activation [9], providing further evidence that such a program promotes stem cell maintenance and proliferation.
As might be expected from the observations described above, overexpression of MPC1 and MPC2 is sufficient to reduce stem cell proliferation, consistent with previous reports that expression of these two genes together establishes a function MPC complex and increases mitochondrial pyruvate uptake [10, 27]. Exogenous MPC expression in mouse ISCs decreased expression of the Lgr5 stemness marker [10]. Furthermore, intestines from flies overexpressing MPC1 and MPC2 displayed reduced size, consistent with impaired stem cell proliferation [10]. Therefore, it appears that MPC overexpression decreases ISC proliferation and probably eventually causes defects in tissue homeostasis.
MPC: an important effector of the APC/Wnt/β-catenin pathway?
The Wnt/β-catenin pathway is a highly conserved signaling pathway that regulates cell proliferation as well as the fate of many cell populations [28, 29]. In particular, this pathway plays a very important role in the development and maintenance of ISCs and HFSCs [30]. Due to its preferential expression and function in stem cells, it is not surprising that targets of the Wnt/β-catenin pathway are upregulated in MPC-depleted mouse organoids [10]. Additionally, HFSC activation, which is associated with high LDH activity, also induced expression of Myc, a known target of the Wnt/β-catenin pathway [9]. This suggests that these conditions of low pyruvate oxidation in stem cells may stimulate activity of the Wnt/β-catenin pathway.
Adenomatous polyposis coli (APC), which acts by suppressing Wnt/β-catenin signaling, is a well-known tumor suppressor in the context of colorectal cancer [31]. APC also controls the expression of MPC1 and MPC2 during intestinal development in zebrafish [32]. Indeed, MPC1 knockdown recapitulated the impaired intestinal differentiation phenotype caused by APC mutation. Moreover, the defective intestinal differentiation observed in APC mutant zebrafish was rescued by expression of human MPC1. This unexpected finding raises the possibility that maintaining low MPC1 expression could be one of the important effectors of the Wnt/β-catenin pathway during stem cell maintenance (see Outstanding Questions).
Outstanding Questions.
By which mechanisms does decreased pyruvate oxidation promote intestinal stem cell (ISC) and hair follicle stem cell (HFSC) function?
What is the role of mitochondrial pyruvate carrier (MPC) in the APC/Wnt/β-catenin pathway?
Do reduced cytosolic citrate levels (and with that acetyl-CoA) as a result of decreased mitochondrial pyruvate oxidation limit histone acetylation?
Lastly, while recent studies suggest that fatty acids regulate ISC function, it remains to be seen if this phenomenon is due to increase fatty acid oxidation or other mechanisms such as fatty acid-responsive transcription or signal transduction.
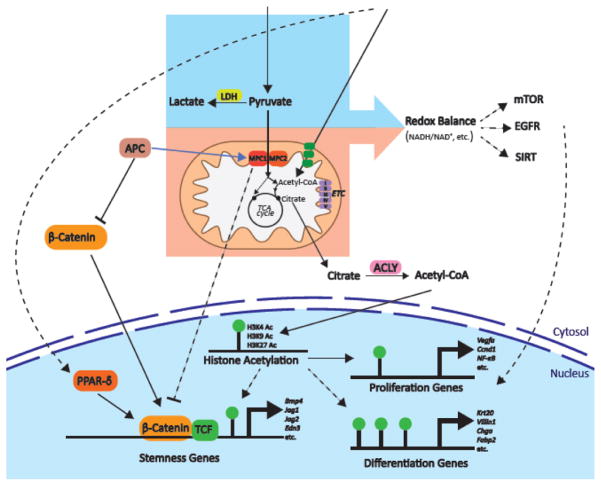
These disparate observations lead to a hypothetical model wherein APC activity induces expression and activity of the MPC complex to limit stemness. In contrast, impaired APC activity leads to decreased expression and activity of the MPC complex and this impairs differentiation. There appears to also be a feedback mechanism whereby low MPC activity also stimulates activation of the Wnt/β-catenin pathway (Figure 1).
Figure 1. Potential mechanisms by which glucose and fatty acid metabolism might affect stem cell homeostasis.
The Wnt/β-Catenin pathway is important in development and in the maintenance and proliferation of epithelial stem cells. In this review, we propose a model wherein Adenomatous polyposis coli (APC), most likely through the Wnt/β-catenin pathway, modulates the expression of MPC1 and MPC2 to alter the cellular metabolic program. This metabolic program then profoundly impacts cell fate, perhaps through feedback regulation on the Wnt/β-Catenin pathway itself. Targets of the Wnt/β-Catenin pathway are also up-regulated in response to fatty acids, possibly through PPARδ-driven gene expression.
Potential mechanisms underlying the effects of metabolic program on gene expression and stemness are depicted. Differential redox balance in the cytosol and mitochondria is influenced by the flux of glycolysis, lactate production, pyruvate oxidation and fatty acid oxidation. Redox balance could act through various pathways to modulate gene expression related to stem cell homeostasis. Finally, MPC inhibition also could impair differentiation through epigenetic modifications as MPC deletion decreased H3K4, H3K9 and H3K27 histone acetylation marks. This observation could be explained by a depleted cytosolic acetyl-CoA pool through the reduction of its precursor, citrate, as a result of limited mitochondrial pyruvate. Transporters and enzymes are shown in rounded boxes; transcriptional regulation is depicted by blue arrows; possible relationships are shown in dashed lines; metabolites and histone modifications are shown in regular type.
Fatty acids promote ISC proliferation and maintenance
As expected, MPC inhibition in mouse intestinal organoids decreased pyruvate flux into the mitochondrial TCA cycle and decreased carbohydrate oxidation [10]. Interestingly, in this MPC-deficient setting, maximal respiration became highly dependent on fatty acid oxidation [10]. This suggests that loss of MPC activity and pyruvate oxidation leads to a compensatory increased dependence on fatty acid oxidation. This is particularly intriguing because fatty acids have a profound effect on ISC stemness that is opposite that of glucose oxidation described above [8]. Indeed, a high-fat diet (HFD), wherein 60% of the calories come from fat, impacted ISCs in mice. Mice on a long-term HFD exhibited shorter intestinal villi with a decrease in crypt depth, but experienced no change in crypt-villus density [8]. This finding is consistent with a block in ISC differentiation. Staining for ISCs and Paneth cells further revealed that HFD increased ISC abundance, but decreased Paneth cell abundance [8]. Paneth cells intercalate with ISCs and support ISC function in the stem cell niche by secreting specific growth factors [33]. Moreover, a HFD not only decreased Paneth cell abundance, but also decreased the dependence of ISCs on Paneth cells for organoid formation [8]. In addition, HFD feeding increased ISC proliferation and regeneration in vivo as assessed by BrdU incorporation [8]. Recapitulating these in vivo findings, crypts isolated from HFD-fed mice showed enhanced primary and secondary organoid formation capacity in vitro [8], probably related to the increased frequency of LGR5-positive ISCs compared to control organoids. Single ISCs from HFD-fed mice also exhibited increased capacity to form new organoids. When mice were switched from a HFD to a standard normal chow diet, the enhanced organoid formation from crypts or single ISCs disappeared within four weeks [8]. These findings demonstrate that HFD feeding causes profound, reversible and acute effects on ISC homeostasis, maintenance and proliferation (see Outstanding Questions).
It appears that this effect of a HFD is likely related to direct effects of fatty acids. Treatment with the fatty acid palmitate in vitro was sufficient to increase ISC abundance and decrease the stem cell niche dependence of organoids isolated from normal chow fed mice [8]. Similar results were obtained when mouse and human intestinal crypts were treated with other fatty acids, such as oleic acid or a mixture of fatty acids. It is important to emphasize that this phenotype is diet-mediated and is not related to obesity. Leptin receptor deficient (db/db) mice, which develop profound obesity, show no increase in ISC proliferation when fed a normal chow diet [8]. These data suggest that ISC maintenance and function is affected by the diet directly and this is specifically related to fatty acid content. While other mechanisms might also be at play, it was demonstrated that fatty acids might stimulate PPARδ-driven expression of components of the Wnt/β-catenin pathway to increase stemness [8].
Concluding Remarks
While both carbohydrate and fatty acid oxidation generate mitochondrial acetyl-CoA and ultimately ATP in mitochondria, the simplest interpretation of these data is that pyruvate and fatty acid oxidation have opposing effects on stem cell homeostasis. Enhanced pyruvate oxidation decreases stem cell maintenance and proliferation, whereas increased fatty acid abundance promotes stem cell function. As noted above, these two metabolic programs are highly interlinked as decreased pyruvate oxidation leads to increased fatty acid oxidation, likely to compensate for the loss of pyruvate-derived mitochondrial acetyl-CoA. Therefore, it becomes important to determine whether elevated fatty acid availability and oxidation similarly reduces pyruvate oxidation as part of its effect on stem cell function.
Probably the most important question, however, and one of the most difficult to answer is how do these two metabolic pathways, with ostensibly similar outputs, have such different effects on cell fate. Is the critical distinction derived from the activity level of the different oxidative pathways per se and the generation of products like acetyl-CoA, or is it due to differential “side effects”, e.g. lactate production, redox changes, etc.? One thing seems clear; eventually this effect has to arrive at the nucleus to impact gene expression, and given the molecules involved, this seems likely to be related to epigenetic modification of histones and/or DNA. It is, therefore, noteworthy that histone acetylation was decreased in MPC-deficient organoids [10], which exhibit enhanced ISC proliferation and maintenance. It is tempting to speculate that decreased pyruvate-derived acetyl-CoA, due to MPC loss, might increase stem cell function through a secondary decrease in cytosolic acetyl-CoA. Cytosolic acetyl-CoA is thought to be primarily derived from either citrate or acetate. Mitochondrial citrate is a TCA cycle intermediate that can be exported to the cytosol and converted to acetyl-CoA by ATP citrate lyase [34]. However, this process depletes TCA cycle intermediates, known as cataplerosis, which need to be replenished through anaplerosis. Although pyruvate-derived mitochondrial acetyl-CoA drives the TCA cycle, it is not an anaplerotic substrate to restore TCA intermediates. However, besides being decarboxylated to acetyl-CoA, mitochondrial pyruvate can also be converted to the TCA cycle intermediate oxaloacetate via pyruvate carboxylase, which does replenish the TCA cycle [35]. Therefore, in the absence of MPC activity, pyruvate import into mitochondria is lost, which reduces production of mitochondrial acetyl-CoA as well as the production of TCA cycle anaplerotic substrates. As a result, citrate availability for cytosolic acetyl-CoA production would be decreased and this might be predicted to decrease the acetyl-CoA pool that is available for histone acetylation (Figure 1, see Outstanding Questions).
While fatty acid oxidation can generate mitochondria acetyl-CoA to compensate for loss of pyruvate-derived acetyl-CoA, it does not generate anaplerotic substrates to replenish TCA cycle intermediates. Therefore, the impact of these two oxidative pathways on the generation of cytosolic acetyl-CoA from mitochondria-derived citrate could be quite different. In the setting of elevated fatty acid oxidation, it is possible that mitochondrial pyruvate uptake and conversion to acetyl-CoA and oxaloacetate might be limited, which might further decrease the availability of cytosolic acetyl-CoA for histone acetylation.
It is also possible that altered redox state might be a key intermediate in the effects of metabolic program on stem cell function (Figure 1). Complete glucose oxidation produces NADH from NAD+ in the cytosol (via glycolysis) as well as in the mitochondrial matrix. In mitochondria, the electron transport chain recycles NADH to NAD+ by passing those electrons to oxygen, while LDH recycles NAD+ in the cytosol by converting pyruvate to lactate. Compared to differentiated cells, stem cells use a reduced fraction of their pyruvate for mitochondrial oxidation, which leads to predictable and differential effects on the cytosolic and mitochondrial NADH/NAD+ ratio. Different redox states might affect nuclear epigenetics and gene expression through altering the activity of NAD+ dependent deacetylases (sirtuins), or through any of a number of other redox-dependent mechanisms, including the mTOR or EGFR pathways [36]. This type of differential compartmentalization of reducing equivalents between glycolysis, glucose oxidation and fatty acid oxidation could constitute part of the mechanistic foundation underlying the differential effects on stemness [37]. At this point, it is critical to note that although high fatty acid abundance has been shown to promote ISC function, it remains to be determined whether this phenomenon depends primarily on elevated fatty acid oxidation or on other mechanisms like fatty acid-responsive transcription or signal transduction.
In summary, although the narrative still must be expanded to tell the full story, several recent studies have demonstrated that metabolism is not a mere signature of, but instead is highly influential on tissue-resident stem cell fate. Metabolic modulations that decrease the fractional oxidation of pyruvate or increase fatty acid abundance enhance epithelial stem cell maintenance and function, potentially through epigenetic modifications and/or changes in redox balance.
Metabolism Fundamentals.
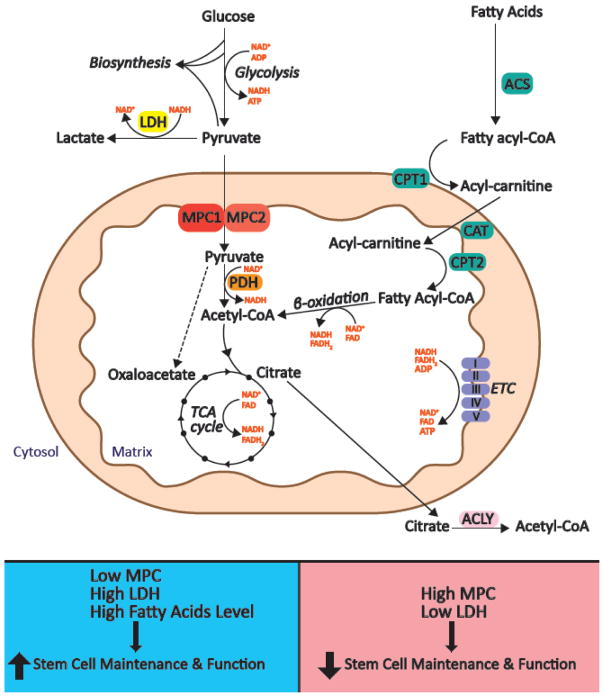
Glucose and fatty acids are two major fuels that drive ATP production (Figure I). Fatty acids are activated in the cytosol and then transported into mitochondria via the carnitine shuttle system. Once in the matrix, β-oxidation of fatty acids yields acetyl-CoA, which can then be further oxidized in the tricarboxylic acid (TCA) cycle to liberate reducing equivalents that fuel the electron transport chain and oxidative phosphorylation (OXPHOS) to eventually power the production of ATP. However, mitochondrial acetyl-CoA can also be derived from glucose. First, glucose is converted to pyruvate in the cytosol via glycolysis, which generates limited quantities of ATP and NADH. From there pyruvate has two major fates: Pyruvate imported into mitochondria can be decarboxylated to acetyl-CoA and enter the TCA cycle to ultimately produce NADH, which can fuel ATP synthesis through OXPHOS. Alternatively, pyruvate that remains in the cytosol can either be reduced to lactate or it (and/or its precursors) can be used in biosynthetic processes. Therefore, a major determinant of the fate of pyruvate is whether it enters the mitochondrial compartment or remains in the cytosol. The protein complex responsible for transporting pyruvate into mitochondria is known as the Mitochondria Pyruvate Carrier (MPC) [38, 39]. The yeast, fly and mammalian MPC is composed of two types of subunits, MPC1 and MPC2, and deletion of either one is sufficient to disrupt the formation of a functional MPC complex [27, 38, 39], thereby impairing the direct transport of pyruvate into mitochondria and reducing pyruvate oxidation. This places the MPC at an important position in determining the mode of carbohydrate metabolism and raises the possibility that its expression and activity could impact stem cell homeostasis.
Figure I. Overview of glucose and fatty acid metabolism pathways.
TOP. As the end product of glycolysis, pyruvate has two major fates. In the cytosol, it can either be reduced to lactate by lactate dehydrogenase (LDH) or it (and/or its precursors) can be used for biosynthesis. Alternatively, the mitochondrial pyruvate carrier (MPC) can import pyruvate into mitochondria where it can be converted to acetyl-CoA by pyruvate dehydrogenase (PDH). In the cytosol, fatty acyl-CoA synthases (ACS) activate fatty acids by converting them to fatty acyl-CoAs. Transport of fatty acyl-CoA across the mitochondrial membrane requires carnitine palmitoyltransferase 1 (CPT1), carnitine translocase (CAT) and carnitine palmitoyltransferase 2 (CPT2). In the mitochondrial matrix, fatty acyl-CoA is oxidized to acetyl-CoA through β-oxidation. Acetyl-CoA, whether derived from carbohydrates, fatty acids or other fuels, enters the tricarboxylic acid (TCA) cycle to generate NADH and FADH2, which ultimately fuel the electron transport chain (ETC) to produce ATP. The TCA cycle intermediate citrate can be transported to the cytosol, where it can be converted to cytosolic acetyl-CoA by ATP citrate lyase (ACLY). While this process depletes TCA cycle intermediates, they can be replenished through the conversion of pyruvate to oxaloacetate by pyruvate carboxylase (PC). BOTTOM. Decreased pyruvate oxidation caused by low MPC function and high LDH activity or potentially by high fatty acid oxidation can promote stem cell maintenance and function. Similarly, enhanced pyruvate oxidation by high MPC activity or low LDH activity reduces stem cell maintenance and function. Transporters/enzymes are shown in boxes; major metabolic pathways are shown in italics; metabolites are shown in regular type.
Highlights.
Reduced pyruvate oxidation enhances stem cell function and reinforces the stem cell molecular signature.
Expression of the mitochondrial pyruvate carrier (MPC) is low in intestinal stem cells (ISCs), but high in their differentiated progeny.
Lactate dehydrogenase (LDHA) is highly expressed and active in hair follicle stem cells (HFSCs).
Deletion of MPC leads to increased function of ISCs, while deletion of Ldha prevents activation of HFSCs and promotes their quiescence.
Altered levels of pyruvate oxidation might be transduced through the Wnt/β-catenin pathway.
High levels of fatty acids also promote ISC proliferation and maintenance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen J, et al. Hematopoietic senescence is postponed and hematopoietic stem cell function is enhanced by dietary restriction. Exp Hematol. 2003;31(11):1097–103. doi: 10.1016/s0301-472x(03)00238-8. [DOI] [PubMed] [Google Scholar]
- 2.Ertl RP, et al. Effects of dietary restriction on hematopoietic stem-cell aging are genetically regulated. Blood. 2008;111(3):1709–16. doi: 10.1182/blood-2007-01-069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yilmaz OH, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486(7404):490–5. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bondolfi L, et al. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol Aging. 2004;25(3):333–40. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 5.Colman RJ, et al. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattison JA, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489(7415):318–21. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Picca A, et al. Does eating less make you live longer and better? An update on calorie restriction. Clin Interv Aging. 2017;12:1887–1902. doi: 10.2147/CIA.S126458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beyaz S, et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature. 2016;531(7592):53–8. doi: 10.1038/nature17173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flores A, et al. Lactate dehydrogenase activity drives hair follicle stem cell activation. Nat Cell Biol. 2017;19(9):1017–1026. doi: 10.1038/ncb3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schell JC, et al. Control of intestinal stem cell function and proliferation by mitochondrial pyruvate metabolism. Nat Cell Biol. 2017;19(9):1027–1036. doi: 10.1038/ncb3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, et al. Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell. 2012;11(5):589–95. doi: 10.1016/j.stem.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stringari C, et al. Metabolic trajectory of cellular differentiation in small intestine by Phasor Fluorescence Lifetime Microscopy of NADH. Sci Rep. 2012;2:568. doi: 10.1038/srep00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varum S, et al. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One. 2011;6(6):e20914. doi: 10.1371/journal.pone.0020914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simsek T, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7(3):380–90. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takubo K, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7(3):391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol. 2014;15(4):243–56. doi: 10.1038/nrm3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takubo K, et al. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell. 2013;12(1):49–61. doi: 10.1016/j.stem.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez-Colman MJ, et al. Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature. 2017;543(7645):424–427. doi: 10.1038/nature21673. [DOI] [PubMed] [Google Scholar]
- 19.Knobloch M, et al. A Fatty Acid Oxidation-Dependent Metabolic Shift Regulates Adult Neural Stem Cell Activity. Cell Rep. 2017;20(9):2144–2155. doi: 10.1016/j.celrep.2017.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilberg MS, et al. Influence of Amino Acid Metabolism on Embryonic Stem Cell Function and Differentiation. Adv Nutr. 2016;7(4):780s–9s. doi: 10.3945/an.115.011031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shyh-Chang N, et al. Stem cell metabolism in tissue development and aging. Development. 2013;140(12):2535–47. doi: 10.1242/dev.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lees JG, et al. Pluripotent Stem Cell Metabolism and Mitochondria: Beyond ATP. Stem Cells Int. 2017;2017:2874283. doi: 10.1155/2017/2874283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teslaa T, Teitell MA. Pluripotent stem cell energy metabolism: an update. EMBO J. 2015;34(2):138–53. doi: 10.15252/embj.201490446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu W, et al. Glycolytic Metabolism Plays a Functional Role in Regulating Human Pluripotent Stem Cell State. Cell Stem Cell. 2016;19(4):476–490. doi: 10.1016/j.stem.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H, et al. Energy metabolism in intestinal epithelial cells during maturation along the crypt-villus axis. Sci Rep. 2016;6:31917. doi: 10.1038/srep31917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–5. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 27.Schell JC, et al. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol Cell. 2014;56(3):400–13. doi: 10.1016/j.molcel.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krausova M, Korinek V. Wnt signaling in adult intestinal stem cells and cancer. Cell Signal. 2014;26(3):570–9. doi: 10.1016/j.cellsig.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 29.Mah AT, et al. Wnt pathway regulation of intestinal stem cells. J Physiol. 2016;594(17):4837–47. doi: 10.1113/JP271754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuijers J, Clevers H. Adult mammalian stem cells: the role of Wnt, Lgr5 and R-spondins. EMBO J. 2012;31(12):2685–96. doi: 10.1038/emboj.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fearnhead NS, et al. The ABC of APC. Hum Mol Genet. 2001;10(7):721–33. doi: 10.1093/hmg/10.7.721. [DOI] [PubMed] [Google Scholar]
- 32.Sandoval IT, et al. A metabolic switch controls intestinal differentiation downstream of Adenomatous polyposis coli (APC) Elife. 2017:6. doi: 10.7554/eLife.22706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato T, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469(7330):415–8. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su X, et al. Metabolic control of methylation and acetylation. Curr Opin Chem Biol. 2016;30:52–60. doi: 10.1016/j.cbpa.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jitrapakdee S, et al. Structure, Mechanism and Regulation of Pyruvate Carboxylase. Biochem J. 2008;413(3):369–87. doi: 10.1042/BJ20080709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iqbal MA, Eftekharpour E. Regulatory Role of Redox Balance in Determination of Neural Precursor Cell Fate. Stem Cells Int. 2017;2017:9209127. doi: 10.1155/2017/9209127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Igarashi M, Guarente L. mTORC1 and SIRT1 Cooperate to Foster Expansion of Gut Adult Stem Cells during Calorie Restriction. Cell. 2016;166(2):436–450. doi: 10.1016/j.cell.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 38.Bricker DK, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337(6090):96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herzig S, et al. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337(6090):93–6. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]