Abstract
Aging and aging-related declines in physical activity are associated with physical and metabolic impairments. Skeletal muscle capillarization is reduced in sedentary older adults, may contribute to impairments in skeletal muscle, and is modifiable by exercise training. This article examines the hypothesis that preservation of skeletal muscle capillarization is essential to maintain metabolism, fitness and function with aging.
Keywords: skeletal muscle, capillary, type 2 diabetes, sarcopenia, exercise training
INTRODUCTION
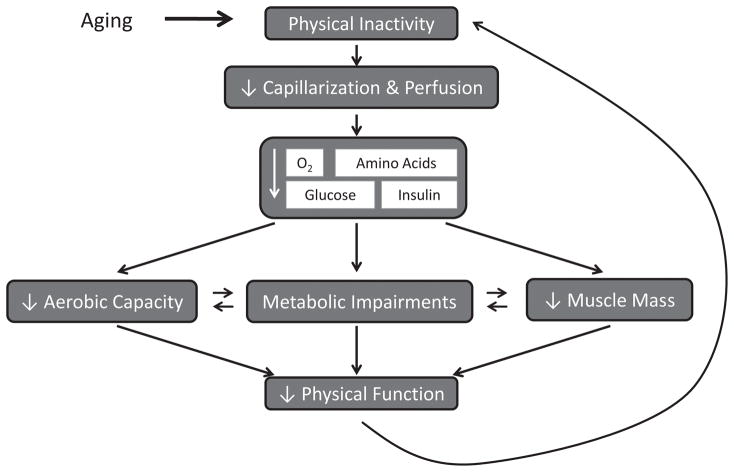
Aging is associated with reduced muscle mass, metabolic impairments, low cardiorespiratory fitness, and impaired physical function. Aging per se may account for some proportion of these declines; however, aging is associated with physical inactivity and lifestyle behaviors that may also contribute through vascular dysfunction and microvascular rarefaction. As the major interface between the circulation and skeletal muscle, the microvasculature can affect cardiometabolic and muscular health across the lifespan. For example, a reduction in skeletal muscle capillarization may contribute to aging-associated impairments in muscle mass, metabolism, fitness and function through limited delivery of oxygen, amino acids, nutrients and hormones (Figure 1). In this article, we explore the hypothesis that capillary rarefaction contributes to these aging-associated declines, and preservation of skeletal muscle capillarization is essential for maintaining fitness and function across the lifespan.
Figure 1.
Conceptual model of the consequences of reduced skeletal muscle capillarization on muscle mass, glucose metabolism, and aerobic capacity in aging with a sedentary lifestyle. In this model, aging-related decreases in physical activity contribute to reduced skeletal muscle capillarization, which can limit the delivery of oxygen, amino acids, glucose and insulin. This likely contributes to declines in muscle mass, aerobic capacity and muscle metabolism, all of which may interact with each other and ultimately reduce physical function and further contribute to physical inactivity in a cyclic manner.
A number of studies have compared gastrocnemius or vastus lateralis muscle capillarization between young (20–30 years) and older (>60 years) men and women, showing that older adults have 12–25% lower capillarization than young adults (1–4). These results typically hold true for overall skeletal muscle and type II muscle fibers (1, 3, 4), while at least one study has shown a similar trend in type I muscle fibers (1). Although some studies report lower capillary density [number of capillaries per mm2 of muscle cross-sectional area (CSA)] in older adults (2), the majority of studies show that capillarization is lower in older adults when expressed as the number of capillary contacts (the average number of capillaries in contact with each muscle fiber) or individual capillary-to-fiber ratio (the number of whole capillary equivalents in contact with each muscle fiber) (1, 3, 4), indicating that the number of capillaries is lower in muscle from older adults. It should be noted that not all studies have identified significant decreases in skeletal muscle capillarization in older adults. For example, Chilibeck et al. (5) found no statistically significant difference in capillary-to-fiber ratio between young and older adults who were recreationally active. Studies in animals have shown a decline (6), or no decline (7) in capillary-to-fiber ratio in older vs. younger rats, and one study using a murine model showed higher capillary-to-fiber ratio and capillary density in older compared with younger mice (8). The reason for these seemingly discrepant findings is somewhat unclear, but across studies there are varying age differences between groups of young and older subjects, differing levels of fitness of the subjects studied, and different muscles analyzed, all of which could contribute to different findings.
While most studies of sedentary, older humans appear to show some degree of capillary rarefaction, there is evidence that capillarization can be maintained later in life, or restored with regular exercise training. Coggan et al. (9) studied a group of master athletes compared with two groups of younger runners (competitive runners or runners matched to master athletes for performance and training volume). Interestingly, gastrocnemius capillary density in masters athletes was similar to that of matched young runners, while capillary contacts and capillary-to-fiber ratio was similar to the competitive young runners (9). Additionally, Hedman et al. (10) reported that in a large cohort of 70-year-old men that higher levels of physical activity were associated with higher vastus lateralis muscle capillarization. In sedentary older adults, exercise training has been shown to increase capillarization. Coggan et al. (11) reported that 9–12 months of aerobic exercise training increased gastrocnemius capillarization by ~20% in 60–70 year-old men and women. Charles et al. (12) found that 14 weeks of combined aerobic and resistance training increased capillary-to-fiber interface by~25% in older men, and we recently showed that 6 months of aerobic exercise training with (13) or without (14) weight loss increases vastus lateralis muscle capillary density by 15% in older men and women. Collectively, these findings indicate that exercise training, especially when training contains a strong aerobic component, can promote angiogenesis and restore skeletal muscle capillarization, even after aging-associated capillary rarefaction has occurred.
To date, the specific effects of skeletal muscle capillary rarefaction on aging-related skeletal muscle phenotypes, as well as the effects of exercise training on capillarization and downstream aging-associated phenotypes remain understudied. This review will summarize a) findings from cross-sectional and longitudinal studies in humans that demonstrate strong relationships between skeletal muscle capillarization, physical function, muscle mass, and metabolism; b) findings from studies of exercise interventions to increase capillarization in skeletal muscle with subsequent effects on these phenotypes; and c) findings from basic and translational animal studies that demonstrate a role of muscle capillarization in skeletal muscle function, mass and metabolism.
FITNESS AND PHYSICAL FUNCTION
Low levels of cardiorespiratory fitness are associated with increased risk for cardiovascular diseases and mortality (15), and reduced ambulatory capacity and physical function are associated with higher rates of disability, morbidity and mortality in older people (16). As the major interface for oxygen diffusion into muscle, capillary surface area contributes to oxygen uptake in working skeletal muscle and may serve as a limiting factor to fitness and physical function in older adults. Previous studies reported direct relationships between skeletal muscle capillarization and maximal oxygen consumption (VO2max) in young (r=0.636) and older (r=0.884) men (4, 17). In addition, our lab found that sarcopenic, older men and women had ~20% lower capillary-to-fiber ratio that was directly associated with ~15% lower VO2max compared to age-matched individuals without sarcopenia and capillary-to-fiber ratio explained ~30% of the variability in VO2max (18). While skeletal muscle capillarization is established as one determinant of VO2max, less is known about relationships between capillarization and physical function in aging. One cross-sectional study of older men and women reported that low skeletal muscle capillary density is associated with functional outcomes including low Short Physical Performance Battery score, slow gait speed, and greater disability |r| = 0.37–0.51, which persisted after adjustment for age, BMI and disease status (19). Additional research is needed to understand the relationships between capillarization, mobility function and ambulatory capacity in the elderly.
The effects of aerobic exercise training to improve VO2max and skeletal muscle capillarization are well-known; therefore, we will discuss specific studies that elucidate the role of skeletal muscle capillarization in determining responses to exercise training in older people. Coggan et al. (11) found that 9–12 months of vigorous aerobic exercise training significantly increased VO2maxin older men and women, and that the increase in VO2max occurred in tandem with increases in skeletal muscle capillarization and oxidative enzyme activities (11). These adaptations were similar in magnitude to those observed in younger adults, suggesting that aerobic exercise training can elicit fitness benefits in older adults that are, in part, mediated by skeletal muscle capillarization. Charles et al. (12) found that 14 weeks of combined resistance and aerobic interval exercise in older subjects increased VO2peak by 11%, with corresponding increases in microvascular filtration capacity and capillary-fiber interface. The increase in filtration capacity indicates a greater surface area for exchange with increased capillary-fiber interface area, suggesting that capillarization plays a role in increasing aerobic capacity (12). Another study evaluated 9 weeks of lower leg resistance training followed by 9 weeks of aerobic training compared to 18 weeks of only aerobic training in older men (20). Both groups increased vastus lateralis muscle capillarization which correlated with VO2max across time points, indicating a potential for increased O2 transport with both training interventions. As resistance training and aerobic exercise training both have distinct benefits for cardiometabolic and muscular health, this study provides support for a combination of both modes of exercise to improve capillarization and function in older adults.
Studies combining exercise training and detraining interventions have also been used to elucidate the role of capillarization in determining fitness responses. Vigelso et al. (21) compared the effects of leg immobilization followed by 6 weeks of aerobic re-training in younger and older men. Immobilization reduced capillary-to-fiber ratio in both age groups, while re-training restored capillarization, with corresponding changes in leg work capacity. In a study distinguishing the acute and chronic effects of aerobic exercise on metabolic outcomes in older men and women, we showed improvements in capillarization, intramyocellular measures (e.g., citrate synthase) and VO2max after 6-month aerobic exercise training (14). When this was followed by two weeks of detraining, intramyocellular changes reverted to baseline, while capillarization and ~2/3 of the improvement in VO2max was retained. Thus, part of the training-induced improvement in VO2max was attributable to metabolic improvements that reverted to baseline with detraining, while the sustained increase in VO2max was attributable to effects of chronic exercise training on variables such as skeletal muscle capillarization.
The current body of literature indicates a strong relationship between skeletal muscle capillarization and aerobic capacity in young and older adults. Furthermore, evidence suggests that skeletal muscle capillarization is subject to similar relative improvements with exercise training regardless of age. Thus, it appears that some proportion of the aging-related declines in fitness and function can be ameliorated by increasing skeletal muscle capillarization through regular exercise.
SKELETAL MUSCLE MASS
Advancing age is associated with a loss of muscle mass, termed sarcopenia, that reduces mobility and physical function and accelerates progression of other age-related disorders. The aging-associated decline in physical activity is a significant contributor to the development of sarcopenia, and this appears to be partially mediated by aging-associated reductions in capillarization of muscle that limit substrate delivery. Adequate perfusion of muscle is needed to maintain anabolic potential (22) and recent literature suggests that impairments of muscle fiber perfusion are involved in anabolic resistance (23) associated with aging. While muscle perfusion is regulated by a number of factors including cardiac output and macrovascular blood flow which also may be subject to aging-associated declines, the number of capillaries can serve as one limiting step in the perfusion of muscle and delivery of substrate. There is also evidence that capillaries may interact with satellite cells in the regulation of skeletal muscle morphology. Nederveen et al. (24) recently showed a lower number of satellite cells in type II muscle of older compared with younger adults, and a greater distance between satellite cells and capillaries in the older adults, which they propose may blunt overall satellite cell activation through reduced migration or exposure to circulating growth factors.
Studies investigating the relationship between skeletal muscle capillarization and skeletal muscle fiber size find a direct relationship between these variables in young adults (25, 26) and similar findings have been observed in older adults. Croley et al. (3) reported lower skeletal muscle type II fiber CSA that was associated with lower type II capillary-to-fiber ratio (r = 0.691) in older women compared with young women. In a subsequent study, Gavin et al. (27) reported similar results in groups of younger and older women, although when both groups were analyzed together, capillary-to-fiber ratio was associated with fiber CSA in both type I and II fibers (r = 0.575–0.693). A more recent study including both men and women found that older adults had lower capillary-to-fiber ratio in overall muscle compared with younger adults, and this was associated with lower fiber CSA (r = 0.644) (28). While relationships to muscle mass were not evaluated in these studies, the current body of literature provides evidence that capillary-to-fiber ratio and muscle fiber size are tightly linked across the spectrum of age and sex in humans, particularly in type II muscle fibers which may be more susceptible to aging-related declines.
Two recent studies have assessed skeletal muscle capillarization in older adults defined as having sarcopenia by current criteria. In a cohort of 68–76 year-old men with or without sarcopenia, defined by the European Working Group on Sarcopenia in Older People criteria, only a small proportion of the sample (6 of 99 subjects) met the criteria for sarcopenia; however, the sarcopenic subjects tended to have ~10% lower capillary-to-fiber ratio in overall skeletal muscle, as well as smaller type I and type II fibers (29). Another study from our laboratory (18) assessed muscle morphology in a group of 76 older (~61 years) men and women, 16 of whom were sarcopenic by appendicular lean mass divided by body mass index (ALMBMI) based on the Foundation for the National Institutes of Health Sarcopenia Project criteria (30). We found that older subjects with sarcopenia had 20% lower capillary-to-fiber ratio than non-sarcopenic older adults, as well as 21% lower muscle fiber CSA and 18% lower mid-thigh muscle CSA. In this cohort, both capillary-to-fiber ratio and capillary contacts per fiber directly correlated with fiber size (r = 0.69), mid-thigh muscle area (r = 0.35), and ALMBMI (r = 0.51, Figure 2). These studies show that the presence and degree of sarcopenia is associated with low skeletal muscle capillarization, and suggest that reduced capillarization may be one contributing mechanism to the development of sarcopenia.
Figure 2.
Scatterplot depicting the direct relationship between skeletal muscle capillary-to-fiber ratio and sarcopenic index (ALMBMI: appendicular lean mass divided by body mass index) in 76 sedentary, older men and women. (Reprinted from (18). Copyright © 2016 Oxford University Press. Used with permission.)
The aforementioned studies are cross-sectional, making it difficult to discern true cause and effect relationships. A common theme across these studies, however, is that capillary-to-fiber ratio is lower in sarcopenic subjects or those with smaller muscle fibers while capillary density (capillaries per mm2 of muscle fiber area) is not lower. This may provide some insight into the relationship between capillarization and muscle fiber size. If decreases in muscle fiber size preceded changes in capillarization, one would expect capillary density to be higher in sarcopenic subjects (i.e., a similar number of capillaries with lower overall fiber area). Given that research shows similar capillary density in sarcopenic and non-sarcopenic adults, it appears that either decreases muscle fiber size occur in tandem with capillary rarefaction, or that capillary rarefaction precedes changes in fiber size. At least one study in humans and additional studies in animal models may inform the temporal relationship between capillarization and muscle size. Frontera et al. (31) reported the results of a 12-year longitudinal study in older adults and found a significant decrease in capillary-to-fiber ratio. A decrease in thigh muscle CSA was observed, but there were no changes in fiber CSA. The authors attributed the overall reduction in muscle CSA to a reduction in the number of muscle fibers, and the findings suggested that capillary rarefaction may precede the decrease in fiber size with aging. This is supported by at least one animal study (32) finding that endothelial cell apoptosis preceded myofiber apoptosis in a rodent model of congestive heart failure. While not specifically a study of aging, this finding does provide evidence of a potential link between endothelial apoptosis and changes in muscle morphology. One additional study of muscle-specific vascular endothelial growth factor (VEGF) deficiency in a mouse model showed that VEGF deficiency resulted in a 48% lower capillary-to-fiber ratio and reduced mass of the gastrocnemius muscle, but no change in gastrocnemius muscle fiber CSA was observed (33). Despite these findings, it should be noted that some of the phenotypic differences could have been related to broad developmental effects of low VEGF because the study used a model of life-long VEGF deficiency.
In older humans, we propose that the ability of exercise training to promote muscle hypertrophy may, in part, be dependent on increases in capillarization. Several studies have assessed the effects of resistance training on skeletal muscle capillarization, showing that resistance training does induce angiogenesis and fiber hypertrophy in skeletal muscle (20, 34). For example, one study comparing young and older men found that 12 weeks of resistance training increased capillary contacts per fiber and capillary-to-fiber perimeter exchange index in both type I and II fibers of older men, nearly to levels observed in the young men (34). This occurred in tandem with an increase in the size of type II muscle fibers, which are typically more responsive to resistance training. That said, Hagerman et al. (35) found that sixteen weeks of resistance training did not induce a statistically significant increase in capillary-to-fiber ratio in older men. In another study of 24-week resistance training in older men, Snijders et al. observed no increase in capillarization, but a numerical increase in the size of type I and type II fibers, with only the change in type II fibers statistically significant (36). These subjects were then divided into two groups having either relatively low or high vastus lateralis capillarization at baseline. While capillarization increased in type II muscle fibers in low capillarization group, a significant increase in fiber size was only found in the high capillarization group, suggesting a critical level of capillarization is required to realize the anabolic response to resistance training in older men. At least one study in young adults has shown that a substantial increases in capillary-to-fiber ratio is not necessary for eight-week resistance training to increase thigh muscle cross sectional area (37), observing a 6% increase in muscle CSA, with only a trend for a modest increase in capillary-to-fiber ratio. That study did not report changes in muscle fiber cross sectional area, but this does show that that capillarization is not the only factor responsible for muscle hypertrophy in response to resistance training.
Some animal studies indicate that capillary proliferation may contribute to muscle fiber hypertrophy, which has implications for the prevention of sarcopenia with aging. Plyley et al. (38) assessed the time course of angiogenesis and muscle hypertrophy in compensatory overload of the plantaris muscle in rodents, finding increases in both capillary contacts per fiber and muscle fiber area, and that the t1/2 of capillary proliferation was slightly shorter (10.1 days) than for fiber hypertrophy (11.2 days). Conversely, Degens et al. (6) showed that a similar overload increased muscle fiber CSA and capillary-to-fiber ratio in rat plantaris muscle of young and old rats, but noted that the capillary proliferation lagged behind the increases in fiber area because of relatively lower capillary density in the overload animals compared with controls. While both of these studies do support a coupling of increases in capillary-to-fiber ratio and fiber CSA (regardless of which increase precedes the other), there is at least one study using a similar overload model that shows muscle fiber hypertrophy with no corresponding increase in capillary-to-fiber ratio (39).
While the effects of aerobic exercise training on the relationship between capillarization and muscle fiber size have received less attention, there is some evidence that aerobic exercise training may increase both capillarization and fiber size. In a long-term training study, Coggan et al. (11) reported that 9–12 months of high-intensity aerobic exercise was able to increase capillarization as well as muscle fiber CSA in both type I and type IIa fibers in older men and women. In a study shorter in duration, Gavin et al. (27) reported that 8 weeks of aerobic exercise training increased capillarization by 20–25% across fiber types in older women, and there was also a numerical increase in muscle fiber CSA in the older women, but this did not reach statistical significance. The authors further assessed this relationship by plotting individual capillary-to-fiber ratio against individual muscle fiber CSA before and after training in both young and older women, finding a greater slope of the relationship between capillarization and fiber area in older women (27). These findings in humans suggest that fiber size regulation is associated with capillarization and that the relationship may change with age such that capillarization and fiber size are more tightly coupled with aging. Studies of wheel running interventions in animals are more equivocal, however. One study demonstrated that increased skeletal muscle vascular density is associated with increases in muscle mass and fiber diameter in rodents when three weeks of wheel running exercise is supplemented with the nitric oxide donor isosorbide dinitrate, but not with exercise alone (40). Conversely, another study showed that three weeks of wheel running increased capillary-to-fiber ratio in mouse skeletal muscle, but there was no effect on muscle mass (41). When the mice were detrained for four weeks, capillary-to-fiber ratio regressed to a level similar to controls, and muscle mass again remained unchanged (41). In animals, and likely in humans, it seems that additional stimuli may be needed to increase muscle mass or fiber size along with increases in capillarization, or that long-term, high-intensity aerobic exercise training is necessary as shown by Coggan et al. (11).
Together, the current body of literature indicates the potential for capillary rarefaction to contribute to declines in muscle mass with aging, and also provides evidence that adaptations of both muscle mass and capillarization are possible in older adults with resistance training. In general, it appears that there is coupling of muscle capillarization and fiber size as it is likely that a greater number of capillaries are needed to support larger fibers and greater muscle mass. However, as some studies show muscle fiber hypertrophy without substantial increase in capillarization, it appears that capillarization is not the only factor responsible for aging-related declines in muscle mass and increases with exercise training. This is evidenced by aerobic exercise training, which is not well established to increase muscle fiber size, but has more robust effects on capillarization, indicating the likely need for an additional stimulus to increase muscle mass. That said, there is evidence that higher baseline levels of capillarization may contribute to larger increases in mass in response to resistance training, so there is some indication that an aerobic exercise component (either preceding or in tandem with resistance training) may enhance the effect of resistance training on capillarization, muscle morphology and mass.
GLUCOSE METABOLISM
Aging-associated changes in skeletal muscle contribute to insulin resistance, and the prevalence of both impaired glucose tolerance (IGT) or type 2 diabetes (T2DM) increase with age, such that more than half of adults over 65 years of age are affected (42). As the major interface between the circulation and skeletal muscle, capillaries play a critical role in muscle glucose uptake and metabolism. Transcapillary transport of insulin is one determinant of glucose uptake in metabolically active tissues (43) and there is lower capillary permeability surface area in people with T2DM compared with normal controls (44) that can limit the amount of insulin and glucose reaching skeletal muscle. Therefore, low skeletal muscle capillarization may be one mechanism contributing to the development of IGT and T2DM in aging.
To date, a number of cross-sectional studies have established a relationship between skeletal muscle capillarization and measures of glucose metabolism or insulin sensitivity across the spectrum of age. In one of the first such reports, Lithell et al. (45) found that lower gastrocnemius muscle capillarization correlated with higher fasting insulin levels (r = 0.87) and higher glucose levels (r = 0.51) during an oral glucose tolerance test in middle-aged men. This was followed by studies in obese women showing that higher capillarization in type I and type IIa muscle fibers correlated (|r| = 0.62–0.80) with lower plasma insulin levels (46) and studies in non-diabetic young men showing that capillarization directly correlated with insulin-stimulated glucose uptake (r = 0.63) during a euglycemic clamp (47). Subsequent studies by Hedman et al. in a cohort of 70-year old men showed that insulin sensitivity (48) and insulin-mediated changes in leg blood flow (49) directly correlated with vastus lateralis capillarization (r = 0.43–0.66). Our lab also reported lower skeletal muscle capillarization in sedentary, older men and women with IGT compared with normal glucose tolerant (NGT) subjects and found that the degree of glucose intolerance is associated with lower skeletal muscle capillarization (|r| = 0.55–0.70) in otherwise healthy older adults with a range of glucose tolerance (13, 14, 50), as well as in chronic stroke patients (50). Similarly, Solomon et al. (51) examined older adults across the spectrum of glucose tolerance and type 2 diabetes (NGT, IGT and T2DM) and found that skeletal muscle capillarization correlated with insulin sensitivity (r = 0.65) and was substantially reduced with greater glucose intolerance independent of age, body composition or energy expenditure. Snijders et al. (52) have also shown that capillarization directly correlates (r = 0.453–0.473) with insulin sensitivity indices derived from oral glucose tolerance tests in a homogeneous group of older men with NGT. Together, this body of research provides strong but associational evidence that low skeletal muscle capillarization may contribute to glucose intolerance and insulin resistance in humans.
These studies of older humans are supported by studies using animal models which demonstrate a direct role of skeletal muscle capillarization in determining insulin sensitivity. Vollus et al. (53) performed a study occluding capillaries in a rodent hindlimb model to experimentally demonstrate the role of capillarization in determining insulin sensitivity. This study used a graded occlusion of capillaries with increasing concentrations of microspheres, finding a dose-dependent reduction (−9% to −60%) in insulin stimulated glucose uptake with greater occlusion of capillaries. This reduction appeared, in part, attributable to reduced insulin action in skeletal muscle (measured as Akt phosphorylation), indicating that the experimental reduction in capillarization decreased delivery of insulin to skeletal muscle (53). In another study, muscle-specific deletion of the VEGF gene in a murine model resulted in 60% fewer capillaries in skeletal muscle: This had the effect of reducing insulin-stimulated glucose uptake by 56% with a similar worsening of glucose tolerance in mice with muscle VEGF deletion (54). While these experimental manipulations of capillarization may not mimic the magnitude of capillary rarefaction observed in aging and impaired glucose metabolism, they do provide more direct evidence of a role of capillarization in determining insulin-stimulated glucose uptake.
Conversely, increases in skeletal muscle capillarization may ameliorate age-related declines in insulin sensitivity and glucose tolerance by enhancing muscle perfusion to promote glucose uptake, but this has been difficult to directly assess. Experimental manipulations of capillarization are limited in humans; however, exercise training interventions have been used to elucidate the potential to restore skeletal muscle capillarization and glucose metabolism. One early study of combined aerobic and strength training in women with T2DM found an increase in the number of capillaries per fiber and in muscle fiber size, but no increase in capillary density after a 10-week intervention (55); however, the maintenance of capillary density along with increases in fiber size in that study indicate that angiogenesis did occur in the skeletal muscle of subjects with T2DM. Recently, it has become clearer that exercise training can attenuate rarefaction or restore capillarization in people with IGT or T2DM. In 2004, Kim et al. (56) reported that 12 weeks of moderate-vigorous intensity aerobic exercise training increased vastus lateralis muscle capillarization by ~10% in older men with IGT. In a rodent model, Frisbee et al. (57) studied the effects of chronic exercise in an obese Zucker rat model of metabolic syndrome, finding that regular exercise attenuated capillary rarefaction, preserving more than half of the rarefaction found in sedentary rats. These studies provided evidence for a role of exercise training in maintaining or increasing skeletal muscle capillarization in aging and impaired glucose metabolism, but it was unclear whether the effects on capillarization translated to improvements in glucose metabolism.
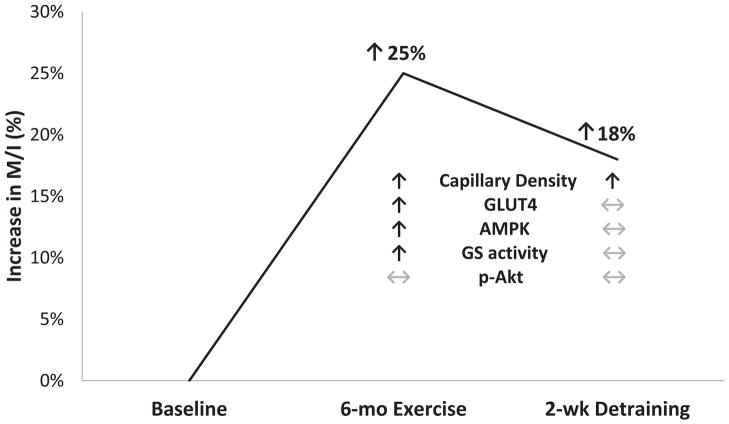
This led our group to conduct a study of 6-month aerobic exercise training with weight loss in overweight-obese older adults with IGT (13). We found that 6 months of moderate-vigorous intensity aerobic exercise training with ~8% weight loss increased vastus lateralis capillary density by 15% and that this increase was associated (|r| = 0.51–0.53) with 16% and 21% improvements in oral glucose tolerance and insulin-stimulated glucose uptake, respectively. We then conducted a second study to distinguish the effect of aerobic exercise training independent of weight loss, as well as the independent effect of increased capillarization on glucose metabolism. In this study, older adults with a range of glucose tolerance remained weight stable and underwent 2 weeks of detraining after the 6-month exercise intervention in order to eliminate the effects of intramyocellular mechanisms such as glycogen synthase activity, citrate synthase activity, glucose transporter-4 (GLUT4) and AMP-activated protein kinase-α1(AMPKα1) expression, and Akt phosphorylation (14). After exercise training, insulin sensitivity increased by 25%, which was associated with a 15% increase in skeletal muscle capillarization, as well as increases in GLUT4 and AMPKα1 expression, and an increase in insulin activation of glycogen synthase. After 2 weeks of detraining, the training-induced increase in capillarization was preserved and associated with an 18% increase in insulin sensitivity over baseline levels (r = 0.70), while all other training-induced improvements reverted to baseline levels (Figure 3). Regression analysis indicated that as much as one-half of the 18% increase in insulin sensitivity was attributable to increases in skeletal muscle capillarization, consistent with previous reports that 45–65% of limited insulin-stimulated glucose uptake is attributable to delivery of glucose (58). These findings are further supported by a recent study in rodents in which skeletal muscle-specific angiogenesis was induced by prazosin treatment (59). Three weeks of prazosin treatment increased skeletal muscle capillarization by ~20% and increased insulin sensitivity by 24% in the absence of other intramyocellular effects on AMPK, GLUT4, p-Akt, or glycogen synthase activation. Together, these two studies (14, 59) provide experimental evidence in animals and older humans that increases in skeletal muscle capillarization contribute to improvements insulin sensitivity in older adults.
Figure 3.
Contributors to increases in insulin sensitivity (M/I) in older men and women after 6 months of aerobic exercise training followed by 2 weeks of detraining. Sustained increases in capillarization accounted for approximately half of the exercise training-induced increase in insulin sensitivity after detraining when other intramyocellular mechanisms reverted to baseline levels. mo, month; wk, week; p-Akt, GLUT4, glucose transporter-4; AMPK, 5′ AMP-activated protein kinase; GS, glycogen synthase; phosphorylated AktSer473. (Reprinted from (14). Copyright © 2015 the American Diabetes Association®. Used with permission.)
Given the high prevalence of insulin resistance in older adults and the fact that skeletal muscle is responsible for the majority of insulin-stimulated glucose uptake, identifying microvascular mechanisms that mediate skeletal muscle glucose uptake has implications for the prevention and treatment of insulin resistance and T2DM in aging. Despite the capillary rarefaction observed in sedentary older people with insulin resistance and IGT, the ability to increase capillarization through exercise training is maintained and likely contributes to improvements in insulin sensitivity in older adults by increasing the delivery of insulin and the diffusible surface area to enhance glucose flux from blood to muscle. This may prevent declines in glucose metabolism in susceptible older adults and ultimately reduce progression to IGT and T2DM.
SUMMARY
There is now a substantial body of literature demonstrating reduced skeletal muscle capillarization in aging humans that is associated with aging-related declines in fitness, function, mass, and metabolism. Because sufficient capillary surface area is essential for delivery of hormones and substrates to skeletal muscle, microvascular rarefaction may represent a primary mechanism underlying these aging-related declines. Reduced capillarization may further impact the interrelated phenotypes of fitness, physical function and metabolism by contributing to reduced skeletal muscle mass. It is still somewhat unclear whether primary aging (i.e., the gradual, inevitable process of bodily deterioration taking place over the lifespan) or secondary aging (i.e., age-related structural and functional deficits attributable to disease and lifestyle factors) is responsible for reductions in skeletal muscle capillarization. The evidence presented herein suggests that a large proportion of microvascular rarefaction may be attributable to secondary aging in that masters athletes and older exercisers have higher capillarization than their sedentary or diseased counterparts. Not surprisingly, these individuals also have lower rates of physical and metabolic impairments associated with aging. This indicates that the development and promotion of therapies and interventions to maintain or increase capillarization in skeletal muscle may be critical to the maintenance of health, function, and metabolism with advancing age.
While links between skeletal muscle capillarization and aging-related declines have been established, there is still much that is not understood about the time course of microvascular rarefaction in aging and temporal relationships with adverse changes in muscle mass, function and metabolism. A comprehensive, longitudinal study will be needed to determine the time course and direct contribution of changes in capillarization to muscle adaptations in aging. Further studies should also focus on understanding the mechanism underlying capillary rarefaction in aging. This is a topic of investigation in our lab and others, with several current studies focusing on the contribution of growth factors that promote angiogenesis and inflammatory factors that adversely affect the endothelium, as well as endothelial progenitor cells and other circulating angiogenic cells that contribute to vascular homeostasis through paracrine mechanisms.
To date, exercise interventions have proven successful for promoting angiogenesis in skeletal muscle of older adults, even those who have already developed diseases associated with vascular complications such as T2DM. However, the optimal types and doses of exercise have not been established. We present evidence that both resistance and aerobic exercise training can stimulate angiogenesis in skeletal muscle. While aerobic exercise training may be a more robust angiogenic stimulus, resistance training represents a greater anabolic stimulus; therefore, a combination of aerobic and resistance exercise may have the most robust effects. Because there is some evidence that baseline capillarization may affect the anabolic response to resistance training (36), there may be a rationale for using aerobic exercise training before a resistance training intervention to maximize the angiogenic and anabolic responses to training in older adults.
Collectively, these findings support the notion that regular exercise and maintenance of skeletal muscle capillarization are essential for optimal health across the lifespan, and establishes the need for comprehensive studies to elucidate the mechanistic and temporal relationships between changes in skeletal muscle capillarization, muscle mass, metabolism, and function in aging humans.
Key Points.
Aging is associated with low fitness levels, reduced muscle mass, and metabolic impairments that may be mediated by a sedentary lifestyle and associated capillary rarefaction in skeletal muscle.
We present evidence that reduced skeletal muscle capillarization contributes to aging-related declines in cardiorespiratory fitness, skeletal muscle mass, and glucose metabolism, as well as reversal of those declines with exercise training.
We propose that maintaining skeletal muscle capillarization is essential for optimal health across the lifespan and justify the need for comprehensive studies to elucidate the mechanistic and temporal relationships between changes in skeletal muscle capillarization and adverse health outcomes in older age.
Acknowledgments
Dr. Landers-Ramos was supported by NIH T32-HL007698 and the Baltimore Veterans Affairs Medical Center Geriatric Research, Education and Clinical Center (GRECC). Dr. Prior was supported by a Paul B. Beeson Career Development Award in Aging (K23-AG040775 and the American Federation for Aging Research), Baltimore Veterans Affairs Medical Center GRECC, and the University of Maryland Claude D. Pepper Older Americans Independence Center (P30-AG028747). Conflicts of Interest: The authors have no conflict of interest to disclose.
Footnotes
Conflicts of Interest: The authors have no conflict of interest to disclose.
References
- 1.Groen BB, Hamer HM, Snijders T, et al. Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J Appl Physiol (1985) 2014;116(8):998–1005. doi: 10.1152/japplphysiol.00919.2013. [DOI] [PubMed] [Google Scholar]
- 2.Coggan AR, Spina RJ, King DS, et al. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol. 1992;47(3):B71–6. doi: 10.1093/geronj/47.3.b71. [DOI] [PubMed] [Google Scholar]
- 3.Croley AN, Zwetsloot KA, Westerkamp LM, et al. Lower capillarization, VEGF protein, and VEGF mRNA response to acute exercise in the vastus lateralis muscle of aged versus young women. JApplPhysiol. 2005;99(5):1872–9. doi: 10.1152/japplphysiol.00498.2005. [DOI] [PubMed] [Google Scholar]
- 4.Ryan NA, Zwetsloot KA, Westerkamp LM, et al. Lower skeletal muscle capillarization and VEGF expression in aged vs. young men. JApplPhysiol. 2006;100(1):178–85. doi: 10.1152/japplphysiol.00827.2005. [DOI] [PubMed] [Google Scholar]
- 5.Chilibeck PD, Paterson DH, Cunningham DA, Taylor AW, Noble EG. Muscle capillarization O2 diffusion distance, and VO2 kinetics in old and young individuals. J Appl Physiol (1985) 1997;82(1):63–9. doi: 10.1152/jappl.1997.82.1.63. [DOI] [PubMed] [Google Scholar]
- 6.Degens H, Turek Z, Hoofd L, van’t Hof MA, Binkhorst RA. Capillarisation and fibre types in hypertrophied m. plantaris in rats of various ages. Respiration physiology. 1993;94(2):217–26. doi: 10.1016/0034-5687(93)90049-g. [DOI] [PubMed] [Google Scholar]
- 7.Brown M. Change in fibre size, not number, in ageing skeletal muscle. Age and ageing. 1987;16(4):244–8. doi: 10.1093/ageing/16.4.244. [DOI] [PubMed] [Google Scholar]
- 8.Davidson YS, Clague JE, Horan MA, Pendleton N. The effect of aging on skeletal muscle capillarization in a murine model. J Gerontol A Biol Sci Med Sci. 1999;54(10):B448–51. doi: 10.1093/gerona/54.10.b448. [DOI] [PubMed] [Google Scholar]
- 9.Coggan AR, Spina RJ, Rogers MA, et al. Histochemical and enzymatic characteristics of skeletal muscle in master athletes. J Appl Physiol (1985) 1990;68(5):1896–901. doi: 10.1152/jappl.1990.68.5.1896. [DOI] [PubMed] [Google Scholar]
- 10.Hedman A, Byberg L, Reneland R, Lithell HO. Muscle morphology, self-reported physical activity and insulin resistance syndrome. Acta Physiol Scand. 2002;175(4):325–32. doi: 10.1046/j.1365-201X.2002.01000.x. [DOI] [PubMed] [Google Scholar]
- 11.Coggan AR, Spina RJ, King DS, et al. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol (1985) 1992;72(5):1780–6. doi: 10.1152/jappl.1992.72.5.1780. [DOI] [PubMed] [Google Scholar]
- 12.Charles M, Charifi N, Verney J, et al. Effect of endurance training on muscle microvascular filtration capacity and vascular bed morphometry in the elderly. Acta Physiol (Oxf) 2006;187(3):399–406. doi: 10.1111/j.1748-1716.2006.01585.x. [DOI] [PubMed] [Google Scholar]
- 13.Prior SJ, Blumenthal JB, Katzel LI, Goldberg AP, Ryan AS. Increased Skeletal Muscle Capillarization After Aerobic Exercise Training and Weight Loss Improves Insulin Sensitivity in Adults With IGT. Diabetes Care. 2014;37(5):1469–75. doi: 10.2337/dc13-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prior SJ, Goldberg AP, Ortmeyer HK, et al. Increased Skeletal Muscle Capillarization Independently Enhances Insulin Sensitivity in Older Adults After Exercise Training and Detraining. Diabetes. 2015;64(10):3386–95. doi: 10.2337/db14-1771. Epub 2015/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–35. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 16.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–61. doi: 10.1056/nejm199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gavin TP, Stallings HW, III, Zwetsloot KA, et al. Lower capillary density but no difference in VEGF expression in obese vs. lean young skeletal muscle in humans. JApplPhysiol. 2005;98(1):315–21. doi: 10.1152/japplphysiol.00353.2004. [DOI] [PubMed] [Google Scholar]
- 18.Prior SJ, Ryan AS, Blumenthal JB, et al. Sarcopenia Is Associated With Lower Skeletal Muscle Capillarization and Exercise Capacity in Older Adults. J Gerontol A Biol Sci Med Sci. 2016;71(8):1096–101. doi: 10.1093/gerona/glw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicklas BJ, Leng I, Delbono O, et al. Relationship of physical function to vastus lateralis capillary density and metabolic enzyme activity in elderly men and women. Aging clinical and experimental research. 2008;20(4):302–9. doi: 10.1007/bf03324860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hepple RT, Mackinnon SL, Goodman JM, Thomas SG, Plyley MJ. Resistance and aerobic training in older men: effects on VO2peak and the capillary supply to skeletal muscle. JApplPhysiol. 1997;82(4):1305–10. doi: 10.1152/jappl.1997.82.4.1305. [DOI] [PubMed] [Google Scholar]
- 21.Vigelsø A, Gram M, Wiuff C, et al. Six weeks’ aerobic retraining after two weeks’ immobilization restores leg lean mass and aerobic capacity but does not fully rehabilitate leg strength in young and older men. J Rehabil Med. 2015;47(6):552–60. doi: 10.2340/16501977-1961. [DOI] [PubMed] [Google Scholar]
- 22.Timmerman KL, Lee JL, Fujita S, et al. Pharmacological vasodilation improves insulin-stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes. 2010;59(11):2764–71. doi: 10.2337/db10-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips BE, Atherton PJ, Varadhan K, et al. The effects of resistance exercise training on macro- and micro-circulatory responses to feeding and skeletal muscle protein anabolism in older men. J Physiol. 2015;593(12):2721–34. doi: 10.1113/jp270343. Epub 2015/05/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nederveen JP, Joanisse S, Snijders T, et al. Skeletal muscle satellite cells are located at a closer proximity to capillaries in healthy young compared with older men. J Cachexia Sarcopenia Muscle. 2016;7(5):547–54. doi: 10.1002/jcsm.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed SK, Egginton S, Jakeman PM, Mannion AF, Ross HF. Is human skeletal muscle capillary supply modelled according to fibre size or fibre type? Exp Physiol. 1997;82(1):231–4. doi: 10.1113/expphysiol.1997.sp004012. [DOI] [PubMed] [Google Scholar]
- 26.Wüst RC, Gibbings SL, Degens H. Fiber capillary supply related to fiber size and oxidative capacity in human and rat skeletal muscle. Adv Exp Med Biol. 2009;645:75–80. doi: 10.1007/978-0-387-85998-9_12. [DOI] [PubMed] [Google Scholar]
- 27.Gavin TP, Kraus RM, Carrithers JA, Garry JP, Hickner RC. Aging and the Skeletal Muscle Angiogenic Response to Exercise in Women. J Gerontol A Biol Sci Med Sci. 2015;70(10):1189–97. doi: 10.1093/gerona/glu138. Epub 2014/09/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnouin Y, McPhee JS, Butler-Browne G, et al. Coupling between skeletal muscle fiber size and capillarization is maintained during healthy aging. J Cachexia Sarcopenia Muscle. 2017;8(4):647–59. doi: 10.1002/jcsm.12194. Epub 2017/04/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel HP, White MC, Westbury L, et al. Skeletal muscle morphology in sarcopenia defined using the EWGSOP criteria: findings from the Hertfordshire Sarcopenia Study (HSS) BMC Geriatr. 2015;15:171. doi: 10.1186/s12877-015-0171-4. Epub 2015/12/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cawthon PM, Peters KW, Shardell MD, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci. 2014;69(5):567–75. doi: 10.1093/gerona/glu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frontera WR, Hughes VA, Fielding RA, et al. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol (1985) 2000;88(4):1321–6. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 32.Vescovo G, Zennaro R, Sandri M, et al. Apoptosis of skeletal muscle myofibers and interstitial cells in experimental heart failure. Journal of molecular and cellular cardiology. 1998;30(11):2449–59. doi: 10.1006/jmcc.1998.0807. [DOI] [PubMed] [Google Scholar]
- 33.Olfert IM, Howlett RA, Tang K, et al. Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol. 2009;587(Pt 8):1755–67. doi: 10.1113/jphysiol.2008.164384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verdijk LB, Snijders T, Holloway TM, VAN Kranenburg J, VAN Loon LJ. Resistance Training Increases Skeletal Muscle Capillarization in Healthy Older Men. Med Sci Sports Exerc. 2016;48(11):2157–64. doi: 10.1249/mss.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 35.Hagerman FC, Walsh SJ, Staron RS, et al. Effects of high-intensity resistance training on untrained older men. I. Strength, cardiovascular, and metabolic responses. J Gerontol A Biol Sci Med Sci. 2000;55(7):B336–46. doi: 10.1093/gerona/55.7.b336. [DOI] [PubMed] [Google Scholar]
- 36.Snijders T, Nederveen JP, Joanisse S, et al. Muscle fibre capillarization is a critical factor in muscle fibre hypertrophy during resistance exercise training in older men. J Cachexia Sarcopenia Muscle. 2017;8(2):267–76. doi: 10.1002/jcsm.12137. Epub 2016/08/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kon M, Ohiwa N, Honda A, et al. Effects of systemic hypoxia on human muscular adaptations to resistance exercise training. Physiological reports. 2014;2(6) doi: 10.14814/phy2.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plyley MJ, Olmstead BJ, Noble EG. Time course of changes in capillarization in hypertrophied rat plantaris muscle. J Appl Physiol (1985) 1998;84(3):902–7. doi: 10.1152/jappl.1998.84.3.902. [DOI] [PubMed] [Google Scholar]
- 39.Huey KA, Smith SA, Sulaeman A, Breen EC. Skeletal myofiber VEGF is necessary for myogenic and contractile adaptations to functional overload of the plantaris in adult mice. J Appl Physiol (1985) 2016;120(2):188–95. doi: 10.1152/japplphysiol.00638.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leiter JR, Upadhaya R, Anderson JE. Nitric oxide and voluntary exercise together promote quadriceps hypertrophy and increase vascular density in female 18-mo-old mice. American journal of physiology Cell physiology. 2012;302(9):C1306–15. doi: 10.1152/ajpcell.00305.2011. [DOI] [PubMed] [Google Scholar]
- 41.Olenich SA, Audet GN, Roberts KA, Olfert IM. Effects of detraining on the temporal expression of positive and negative angioregulatory proteins in skeletal muscle of mice. J Physiol. 2014;592(15):3325–38. doi: 10.1113/jphysiol.2014.271213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–9. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 43.Yang YJ, Hope ID, Ader M, Bergman RN. Insulin transport across capillaries is rate limiting for insulin action in dogs. JClinInvest. 1989;84(5):1620–8. doi: 10.1172/JCI114339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gudbjornsdottir S, Sjostrand M, Strindberg L, Lonnroth P. Decreased muscle capillary permeability surface area in type 2 diabetic subjects. JClinEndocrinolMetab. 2005;90(2):1078–82. doi: 10.1210/jc.2004-0947. [DOI] [PubMed] [Google Scholar]
- 45.Lithell H, Lindgärde F, Hellsing K, et al. Body weight, skeletal muscle morphology, and enzyme activities in relation to fasting serum insulin concentration and glucose tolerance in 48-year-old men. Diabetes. 1981;30(1):19–25. doi: 10.2337/diab.30.1.19. [DOI] [PubMed] [Google Scholar]
- 46.Krotkiewski M, Bylund-Fallenius AC, Holm J, et al. Relationship between muscle morphology and metabolism in obese women: the effects of long-term physical training. Eur J Clin Invest. 1983;13(1):5–12. doi: 10.1111/j.1365-2362.1983.tb00057.x. [DOI] [PubMed] [Google Scholar]
- 47.Lillioja S, Young AA, Culter CL, et al. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. JClinInvest. 1987;80(2):415–24. doi: 10.1172/JCI113088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hedman A, Berglund L, Essen-Gustavsson B, Reneland R, Lithell H. Relationships between muscle morphology and insulin sensitivity are improved after adjustment for intra-individual variability in 70-year-old men. Acta Physiol Scand. 2000;169(2):125–32. doi: 10.1046/j.1365-201x.2000.00722.x. [DOI] [PubMed] [Google Scholar]
- 49.Hedman A, Andersson PE, Reneland R, Lithell HO. Insulin-mediated changes in leg blood flow are coupled to capillary density in skeletal muscle in healthy 70-year-old men. Metabolism. 2001;50(9):1078–82. doi: 10.1053/meta.2001.25604. [DOI] [PubMed] [Google Scholar]
- 50.Prior SJ, McKenzie MJ, Joseph LJ, et al. Reduced skeletal muscle capillarization and glucose intolerance. Microcirculation. 2009;16(3):203–12. doi: 10.1080/10739680802502423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solomon TP, Haus JM, Li Y, Kirwan JP. Progressive hyperglycemia across the glucose tolerance continuum in older obese adults is related to skeletal muscle capillarization and nitric oxide bioavailability. JClinEndocrinolMetab. 2011;96(5):1377–84. doi: 10.1210/jc.2010-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snijders T, Nederveen JP, Verdijk LB, et al. Muscle fiber capillarization as determining factor on indices of insulin sensitivity in humans. Physiological reports. 2017;5(10) doi: 10.14814/phy2.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vollus GC, Bradley EA, Roberts MK, et al. Graded occlusion of perfused rat muscle vasculature decreases insulin action. ClinSci(Lond) 2007;112(8):457–66. doi: 10.1042/CS20060311. [DOI] [PubMed] [Google Scholar]
- 54.Bonner JS, Lantier L, Hasenour CM, et al. Muscle-specific vascular endothelial growth factor deletion induces muscle capillary rarefaction creating muscle insulin resistance. Diabetes. 2013;62(2):572–80. doi: 10.2337/db12-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lithell H, Krotkiewski M, Kiens B, et al. Non-response of muscle capillary density and lipoprotein-lipase activity to regular training in diabetic patients. Diabetes Res. 1985;2(1):17–21. [PubMed] [Google Scholar]
- 56.Kim HJ, Lee JS, Kim CK. Effect of exercise training on muscle glucose transporter 4 protein and intramuscular lipid content in elderly men with impaired glucose tolerance. EurJApplPhysiol. 2004;93(3):353–8. doi: 10.1007/s00421-004-1214-2. [DOI] [PubMed] [Google Scholar]
- 57.Frisbee JC, Samora JB, Peterson J, Bryner R. Exercise training blunts microvascular rarefaction in the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2006;291(5):H2483–92. doi: 10.1152/ajpheart.00566.2006. Epub 2006/06/23. [DOI] [PubMed] [Google Scholar]
- 58.Wasserman DH. Four grams of glucose. American journal of physiology Endocrinology and metabolism. 2009;296(1):E11–21. doi: 10.1152/ajpendo.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akerstrom T, Laub L, Vedel K, et al. Increased skeletal muscle capillarization enhances insulin sensitivity. American journal of physiology Endocrinology and metabolism. 2014;307(12):E1105–16. doi: 10.1152/ajpendo.00020.2014. [DOI] [PubMed] [Google Scholar]