Abstract
Astrocyte functions in white matter are less well understood than in gray matter. Our recent study of white matter in ventral prefrontal cortex (vPFC) revealed alterations in expression of myelin-related genes in major depressive disorder (MDD). Since white matter astrocytes maintain myelin, we hypothesized that morphometry of these cells will be altered in MDD in the same prefrontal white matter region in which myelin-related genes are altered. White matter adjacent to vPFC was examined in 25 MDD and 21 control subjects. Density and size of GFAP-immunoreactive (-ir) astrocyte cell bodies was measured. The area fraction of GFAP-ir astrocytes (cell bodies + processes) was also estimated. GFAP mRNA expression was determined using qRT-PCR. The density of GFAP-ir astrocytes was also measured in vPFC white matter of rats subjected to chronic unpredictable stress (CUS) and control animals. Fibrous and smooth GFAP-ir astrocytes were distinguished in human white matter. The density of both types of astrocytes was significantly decreased in MDD. Area fraction of GFAP immunoreactivity was significantly decreased in MDD, but mean soma size remained unchanged. Expression of GFAP mRNA was significantly decreased in MDD. In CUS rats there was a significant decrease in astrocyte density in prefrontal white matter. The decrease in density and area fraction of white matter astrocytes and GFAP mRNA in MDD may be linked to myelin pathology previously noted in these subjects. Astrocyte pathology may contribute to axon disturbances in axon integrity reported by neuroimaging studies in MDD and interfere with signal conduction in the white matter.
Keywords: White matter, astrocytes, glia, major depressive disorder, chronic unpredictable stress
INTRODUCTION
The pathology of astrocytes in the white matter in major depressive disorder (MDD) is less well understood than in gray matter. White matter astrocytes are morphologically and functionally differentiated from gray matter astrocytes, although both types are associated with the blood brain barrier and glucose metabolism (Sofroniew and Vinters, 2010). Functionally, astrocytes in white matter have specific roles in myelination and impulse conduction (Black and Waxman, 1988; Ishibashi et al., 2006; Liedtke et al., 1996). Thus, one of the main functions of white matter astrocytes is promotion of myelination and myelin maintenance (Lundgaard et al., 2014). These functions are supported by gap junction-based coupling of astrocytes to myelin-forming oligodendrocytes (Nualart-Marti et al., 2013). Defects in myelination or in saltatory impulse conduction could impair propagation of action potentials and neural connectivity. Altered connectivity in ventral prefrontal cortex (vPFC) and elsewhere is reported in patients with MDD, as determined with diffusion tensor imaging of white matter fiber tracts or with functional magnetic resonance imaging (Jalbrzikowski et al., 2017; Murphy and Frodl, 2011; Nobuhara et al., 2006; Rolls et al., 2017; Sexton et al., 2009; Shimony et al., 2009; Tham et al., 2011).
Our recent study in the white matter of postmortem vPFC revealed changes in the expression of myelin-related genes and a reduction in the size of oligodendrocyte cell bodies in MDD (Rajkowska et al., 2015), which may be related to white matter pathology detected using neuroimaging methods. Other molecular changes such as shortened telomere length or increased oxidation and DNA repair enzymes have been observed in white matter oligodendrocytes or astrocytes in Brodmann’s area 10 of the prefrontal cortex in MDD (Szebeni et al., 2014; 2017). Additionally, reductions in gray matter in immunohistochemically labeled astrocytic gap junctions and in the density and area fraction of astrocytes immunohistochemically labeled for glial fibrillary acidic protein (GFAP) have been observed in the vPFC in subjects with MDD (Miguel-Hidalgo et al., 2010; 2014). However, in white matter, 3-dimensional quantification of GFAP-immunoreactive (-ir) astrocytes has not been reported in vPFC from subjects with MDD.
Of the few studies that have examined pathology of white matter astrocytes in depression, only Torres-Platas et al. (2011) reported hypertrophy of fibrous astrocyte processes in the white matter that underlies the anterior cingulate cortex in subjects with a mood disorder who died by suicide. In studies using a two-dimensional sampling method, Williams et al. (2013; 2014) found no significant difference in the density of GFAP-ir astrocytes in the white matter of the subgenual cingulate cortex in subjects with MDD vs. controls. In another two-dimensional study, there was a reduction in immuno-autoradiographic labeling of GFAP in white matter of the anterior cingulate cortex of subjects with a mood disorder (Gittins and Harrison, 2011). In a qualitative study, Webster et al. (2001) reported that fewer subjects with MDD than controls had any astrocytes immunolabeled for phosphorylated GFAP in association with blood vessels in white matter lining the dorsolateral PFC.
Thus, the present study sought to determine whether there are alterations in the density of GFAP-ir astrocytes and the area fraction covered by GFAP-ir astrocytic cell bodies and processes in the white matter of vPFC in subjects diagnosed with MDD as compared to age-matched controls. The subjects with MDD used in the present study for GFAP-ir astrocyte morphometry were all used in our study of oligodendrocyte and myelin pathology in vPFC white matter (Rajkowska et al., 2015). We also compared the levels of expression of GFAP mRNA in white matter of the vPFC between MDD and control groups in a mostly different cohort of subjects from the same brain collection. Finally, using rats exposed to chronic unpredictable stress (CUS) as a model for the induction of depression-like behaviors (Willner, 2016), and control animals, we investigated the density of GFAP-ir astrocytes in a region of the rat white matter homologous to the human vPFC.
METHODS
Human subjects
Brain tissue was collected at autopsy at the Cuyahoga County Medical Examiner’s Office (Cleveland, OH). Legally-defined next-of-kin provided written informed consent for tissue collection, medical histories, and diagnostic interviews administered to knowledgeable informants. The institutional review boards of University Hospitals Case Medical Center, Cleveland, OH, and the University of Mississippi Medical Center, Jackson, MS, approved the protocol for recruitment, tissue collection, and interviews.
Tissue samples from the left ventral prefrontal white matter were collected from 25 subjects that met diagnostic criteria for MDD and 21 psychiatrically-normal control subjects that were matched for age and postmortem interval. The Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 1995) was administered by a trained interviewer to a knowledgeable informant for all subjects, as described (Cobb et al., 2013). Lifetime and recent psychopathology was determined by a board-certified psychiatrist and board-certified clinical psychologist according to the Diagnostic and Statistical Manual of Mental Disorders (4th ed.) (DSM-IV; American Psychiatric Association, 1994). Information was also collected from informants or medical records about psychoactive substance use and medication history. No subjects had evidence of head trauma, neurologic or neuropathological disease. Twenty four subjects met criteria for MDD in the last month of life and one other diagnosed with MDD was in remission for one year. Subjects with MDD were comorbid with delusional disorder (n=1), dysthymic disorder (n=1), generalized anxiety disorder (n=1), panic disorder with agoraphobia (n=1), and anxiety disorder (not otherwise specified, n=1). Only one subject with MDD had a psychoactive drug use disorder at the time of death (benzodiazepine abuse). None of the 21 control subjects met criteria for a DSM-IV diagnosis at the time of death, although three subjects met criteria for alcohol dependence at 6, 8, and 30 years prior to death. Eighteen of the 25 MDD subjects died by suicide (Tables 1 and 2). Postmortem samples of urine and blood were evaluated by the medical examiner for the presence of psychotropic medications or psychoactive substances. An antidepressant drug was identified postmortem in four of the 25 subjects with MDD (Tables 1 and 2). Control and MDD subjects were yoked for all procedures and matched for age and gender, and these values plus postmortem interval, tissue pH, and storage time in formalin are noted in Tables 1 and 2.
Table 1.
Characteristics of Control and MDD Subjects used for GFAP morphometry.
| Parameter | Controls (n=8) | MDD (n=8) |
|---|---|---|
| Age (years) (range) | 57±7 (27 – 80) | 58±7 (34 – 82) |
| Gender (F:M) | 3:5 | 4:4 |
| PMI (hrs) (range) | 22±2 (15 – 29) | 24±3 (12 – 44) |
| Tissue pH (range) | 6.7±0.1 (6.3 – 7.1) | 6.5±0.1 (6.1 – 6.9) |
| TF (months) (range) | 104±11 (38 – 131) | 102±16 (24– 141) |
| Cause of death | Cardiovascular disease n=7; hemorrhagic pancreatitis n=1 | Suicide n=6 (CO poisoning n=2; hanging n=2; drowning n=1; fall from height n=1) Natural causes n=2 (cardiovascular disease) |
| Duration of MDD (years) (range) | not applicable | 15±3 (0.2 – 62) |
| Antidepressant or psychotropic drugs present postmortem | none | none |
Data represent the mean ± S.E.M. AD – antidepressant; CO - carbon monoxide; MDD - major depressive disorder; PMI - Postmortem interval; TF - Time in formalin. The mean age (t=0.117, df=14, p=0.91), PMI (t=0.438, df=14, p=0.67), brain tissue pH (t=1.575, df=14, p=0.14), and TF (t=0.092, df=14, p=0.93) of subjects with MDD were not statistically different from the control subjects.
Table 2.
Characteristics of Control and MDD Subjects used for GFAP mRNA expression.
| Parameter | Controls (n=13) | MDD (n=17) |
|---|---|---|
| Age (years) (range) | 50±3 (32 – 74) | 52±4 (34 – 87) |
| Gender (F:M) | 4:9 | 6:11 |
| PMI (hrs) (range) | 23±2 (16 – 28) | 23±2 (11 – 27) |
| Tissue pH (range) | 6.6±0.1 (6.0 – 6.9) | 6.5±0.1 (6.1 – 7.0) |
| Cause of death | Cardiovascular disease n=10; acute pancreatitis n=1; blunt head trauma n=1; gastric ulcer hemorrhage n=1 | Suicide n=12 (CO poisoning n=4; SIGSW n=4; hanging n=1; drug overdose n=2; drowning n=1) Natural causes n=4 (cardiovascular disease) Undetermined n=1 |
| Duration of MDD (years) (range) | not applicable | 14±4 (0.1 – 61) |
| Psychotropic compounds present postmortem | none | ethanol n=4; alprazolam n=1; codeine n=1 |
| Antidepressant drug present postmortem | none | bupropion and citalopram n=1; fluoxetine n=1; sertraline n=2 |
Data represent the mean ± S.E.M. AD – antidepressant; CO - carbon monoxide; MDD - major depressive disorder; PMI - Postmortem interval; SIGSW – self-inflicted gunshot wound. The mean age (t=−0.492, df=28, p=0.63), PMI (t=0.054, df=28, p=0.96), and brain tissue pH (t=1.107, df=28, p=0.28) of subjects with MDD were not statistically different from the control subjects.
A total of 25 subjects with MDD and 21 control subjects were examined, with 8 MDD and 8 control subjects used for GFAP morphometry (Table 1) and 17 MDD and 13 control subjects used for mRNA expression (Table 2). One control subject and 4 subjects with MDD were used in both studies. Tissue frozen at autopsy was examined for mRNA expression, while other vPFC tissues were fixed in 10% phosphate-buffered formalin and used for GFAP immunohistochemistry and morphometry.
There were more subjects for expression of GFAP mRNA than for morphometry because there were more subjects in the brain collection with frozen than with fixed tissue. In addition, fixed tissue was chosen for morphometry of GFAP-ir astrocytes because there is minimal shrinkage of celloidin-embedded fixed tissue and it is ideally prepared for 3-D cell counting.
Rodents
To determine if any changes in the density of GFAP-ir astrocytes in white matter of the prefrontal cortex in MDD are a consequence of potential risk factors for depression such as stress, a rat model of depression-like behavior, namely CUS, was used. Body weight, sucrose preference, and novelty-suppressed feeding were examined in CUS-exposed and control rats. All procedures were approved by the Institutional Animal Care and Use Committee and conformed to the guidelines of the National Institutes of Health.
Twenty male Sprague–Dawley rats (Charles River, Wilmington, MA, USA) weighing 200–250 g were housed two per cage in a temperature and humidity-controlled colony room. Rats were assigned to one of two groups (n=10 per group) and were either exposed for 35 days to CUS or handled to serve as controls, and then behavioral tests were administered as described by investigators naïve to treatment group (Riaz et al., 2015). Body weight was measured on day 0, at 2 days after the beginning of CUS, and every 4 days thereafter. Sucrose preference was tested after 3 weeks of daily CUS. Novelty suppressed feeding was performed at the end of five weeks of CUS. Rats were sacrificed by decapitation and prefrontal cortical tissues were dissected, frozen on dry ice and stored at −80°C. Six rats were randomly selected from the CUS and from the control group for GFAP immunohistochemistry.
Tissue Preparation
Human and rodent tissues were coded and laboratory personnel were blinded to diagnoses or treatments. The fixed ventral portion of the left prefrontal cortex from 16 subjects (8 MDD and 8 controls, Table 1) was embedded in 12% celloidin (Rajkowska and Goldman-Rakic, 1995). Blocks were sectioned coronally (40 μm), and sections were stored in 70% ethanol. Three sections per subject located about 400 μm apart were selected for removal of celloidin and subsequent GFAP immunohistochemistry (Miguel-Hidalgo and Rajkowska, 1999). In addition, frozen sections (50 μm) were collected from the ventral half of the prefrontal cortex (17 MDD and 13 controls, Table 2). A total of about thirty punches (5 mm; Sklar Instruments, VWR, Radnor, PA) were collected from 5–6 frozen sections of deep white matter adjacent to orbitofrontal cortex (Rajkowska et al., 2015; Uylings et al., 2010) for measuring levels of GFAP mRNA. From rat brain, frozen sections (20 μm) containing the ventral part of the infralimbic prefrontal cortex (bregma level 2.7mm; Paxinos and Watson, 1986) were collected for GFAP immunohistochemistry.
Immunohistochemistry
Triplicate free-floating sections of human tissue were incubated overnight with the primary antibody (GFAP rabbit polyclonal, dilution 1:500, Millipore/Sigma AB5804, Billerica, MA). Triplicate frozen sections from rat brains were thaw-mounted on slides, dried for 20 min, washed 5 min in PBS, fixed 30 min in 4% paraformaldehyde, washed in Tris-HCl buffered saline solution (TBS, pH 7.6), and incubated overnight with the same GFAP antibody (dilution 1:500). Labelling with the primary antibody was detected with a secondary anti-rabbit antibody (dilution 1:200) using the ABC method (Vectastain Universal Elite ABC kit-PK6200; Vector Laboratories, Burlingame, CA) with 3′3′-diaminobenzidine as chromogen with color-enhancement by nickel ammonium sulfate. Sections were cover-slipped. To minimize inter-assay variability in the intensity of staining, each experiment included yoked sections from both cohorts of human and rat tissue. Omission of either primary or seconary antibodies yielded no discernable immunoreactivity. For human and rat tissues, three sections adjacent to those selected for immunohistochemistry were stained with cresyl violet acetate and used to delineate white matter of the vPFC.
Morphometric analyses
Density and soma size of GFAP-ir astrocytes
In human tissue, the cell packing density and soma size (cell volume) of GFAP-ir astrocytes (Fig. 1) was estimated with a Nikon Eclipse E600 microscope (Nikon Instruments, Melville, NY) using 3-dimensional cell counting (StereoInvestigator software, ver.10; MBF Bioscience, Williston, VT). For cell counting and soma size measurements of astrocytes, a contour was drawn enclosing the ventral part of the prefrontal white matter, but excluding white matter from adjacent gyri. The contour was adjacent to Brodmann areas 11, 12, 47, and the ventral part of area 24 (Rajkowska et al., 2015; Uylings et al., 2010). Counting boxes (75 × 75 × 5 μm) were randomly and systematically placed within contours. Astrocytes were counted and measured within each counting box with an oil objective (40X). The optical disector probe was used to estimate cell packing density and the nucleator probe was used to estimate the average soma size. In rat tissue, cell packing density was estimated as noted above using StereoInvestigator software (ver. 11). A contour enclosing white matter adjacent to the ventral part of the infralimbic prefrontal area was drawn in each section. Counting boxes (50 × 50 × 5 μm) were randomly and systematically placed within contours, and astrocytes were counted within each counting box using an oil objective (40X). The optical fractionator probe was used to estimate cell packing density.
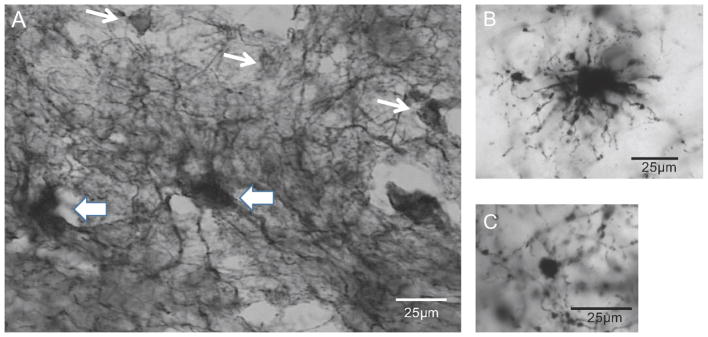
Figure 1.
Photomicrograph showing human astrocytes immunoreactive for glial fibrillary acidic protein (GFAP) in the deep ventral prefrontal white matter of human brain. Two types of GFAP-immunoreactive astrocytes were distinguished: fibrous, large astrocytes with numerous, long branches stemming from the cell body, A (thick arrows) and B; smooth, small astrocytes with very few processes, A (thin arrows) and C.
Area fraction of GFAP-ir structures
The area occupied by GFAP-ir structures (cell bodies and processes) was examined within the contours of three sections delineating white matter of the vPFC in human tissues, as described by Miguel-Hidalgo et al. (2000; 2010). A 500 × 500 μm frame was placed within each white matter contour in three GFAP-ir sections per brain. The area fraction occupied by GFAP immunoreactivity was calculated by dividing the immunoreactive area by the total area occupied by the white matter in the square frame. The extent of GFAP-ir was expressed as a percentage of immunoreactive area divided by the total area of sampled white matter.
Determination of GFAP mRNA levels by qRT-PCR
Total RNA was extracted with TRI-Reagent (Molecular Research Center) following manufacturer’s suggested protocols, re-suspended in nuclease free water and DNase treated with Turbo DNA-free kit (Invitrogen, Waltham, MA). Purified total RNA was quantified by UV absorbance using the Nanodrop spectrophotometer (Thermo Fisher, Waltham, MA). Total RNA (0.25 μg) was reverse transcribed with 330 ng T20VN oligo, 250 ng random hexamer primers and Superscript III (Invitrogen) following the manufacturer’s suggested protocol. Quantitative PCR (qPCR) was performed with TaqMan Fast Advanced Master Mix (Applied Biosystems, Carlsbad, CA) using 1 μl RT product and 1 μl of Taqman assay primer/probe mix. Taqman assays for human GFAP (Hs00909233_m1), GAPDH (Hs99999905_m1) and ACTB (Hs99999903_m1) were obtained from Applied Biosystems. Cycling conditions were 2 min at 50°C and 20 sec at 95°C, followed by 45 cycles of 3 sec at 95°C and 30 sec at 60°C. Fluorescent data were obtained during the extension phase and threshold cycle values were obtained at the log phase of each gene amplification. PCR product quantification was performed by the relative quantification method (Livak and Schmittgen, 2001) and expressed as arbitrary units standardized against the geometric mean of GAPDH and β-actin (Schmittgen and Livak, 2008).
Statistical analyses
The potential influence of age, postmortem interval (PMI), brain tissue pH, storage time in formalin (TF), duration of depression, and age of onset of depression on the packing density of GFAP-ir astrocytes, GFAP area fraction, and GFAP mRNA expression in human tissues was examined by Pearson correlational analysis (GraphPad Prism, ver. 5.0b; La Jolla, CA). There was no significant difference between cohorts in mean age, PMI, tissue pH, and TF (Tables 1 and 2). The density and soma size of GFAP-ir astrocytes, area fraction of GFAP immunoreactivity, and GFAP mRNA expression were compared between the MDD and control groups using analysis of covariance (ANCOVA) with age, PMI, tissue pH, and TF as covariates (IBM SPSS, ver. 22.0; Chicago, IL). An unpaired, two-tailed Student’s t-test was used to compare astrocyte density between CUS and control rats. Statistical significance for all tests was set at p ≤ 0.05. All values presented are mean ± S.E.M.
RESULTS
Morphology of GFAP-ir astrocytes in human tissue
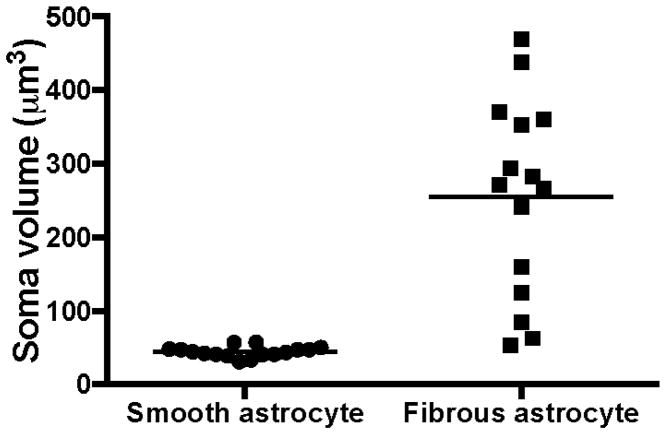
Two morphological types of GFAP-ir astrocytes were distinguished in the white matter of vPFC in human brain (Fig. 1). Fibrous astrocytes with multiple, long, ramified processes stemming from irregularly shaped, large cell bodies were identified (Fig. 1A-thick arrows, Fig. 1B). In contrast, astrocytes with rounded and much smaller cell bodies with only a few, truncated and thin immunoreactive processes, here termed smooth astrocytes, were also identified (Fig. 1A–thin arrows, Fig. 1C). Most cell bodies of fibrous astrocytes were large (soma volume = 255.4 ± 34.4 μm3) whereas smooth astrocytes had consistently smaller cell bodies (44.4 ± 1.8 μm3) (Fig. 2).
Figure 2.
Mean soma volume of smooth and fibrous GFAP-immunoreactive astrocytes in the ventral prefrontal white matter of human brain. The horizontal lines represent the mean value of volume for each cell type.
Density of GFAP-ir astrocytes in white matter of human vPFC
Fibrous astrocytes
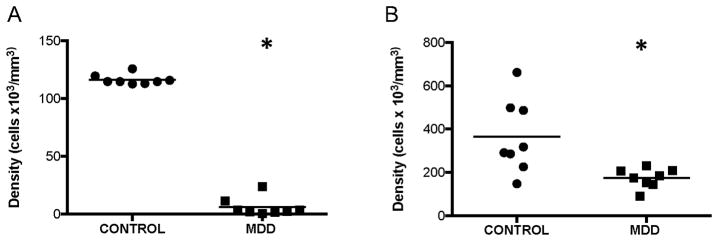
There was a marked, significant 95% decrease in the average density of fibrous astrocytes in subjects with MDD as compared to control subjects (ANCOVA, F(1, 10)=1701.375, p<0.0001; Fig. 3A). There was no significant correlation between cell packing density of fibrous astrocytes and age, PMI, tissue pH, or TF in subjects with MDD (age: r=−0.160, p=0.71; PMI: r=0.036, p=0.93; TF: r=−0.669, p=0.07) or control subjects (age: r=0.514, p=0.19; PMI: r=0.103, p=0.81; pH: r=−0.036, p=0.93). However, there was a significant negative correlation between the density of fibrous astrocytes and tissue pH in MDD subjects (r=−0.740, p=0.04) and a significant negative correlation between astrocyte density and TF in control subjects (r=−0.999, p<0.0001). Neither duration (r=−0.004, p=0.99) nor age at onset of depression (r=−0.144, p=0.73) was correlated with the density of fibrous astrocytes in MDD subjects.
Figure 3.
Density of GFAP-immunoreactive astrocytes in the ventral prefrontal white matter of human brain. There were significant decreases in the density of both types of astrocytes, fibrous (A) (ANCOVA, F(1, 10)=1701.375, *p<0.0001) and smooth (B) (ANCOVA, F(1, 10)=5.159, *p=0.046), in subjects with major depressive disorder (MDD) as compared to control subjects. The horizontal lines represent the mean value of density for each diagnostic group.
Smooth astrocytes
There was a less-pronounced, but also significant 52% decrease in the average density of smooth astrocytes in subjects with MDD as compared to control subjects (ANCOVA, F(1, 10)=5.159, p=0.046; Fig. 3B). There was no significant correlation between cell packing density of smooth astrocytes and age, PMI, tissue pH, or TF in subjects with MDD (age: r=0.534, p=0.17; PMI: r=−0.500, p=0.21; pH: r=−0.249, p=0.55; TF: r=−0.426, p=0.29) or control subjects (Age: r=0.261, p=0.53; pH: r=0.492, p=0.22; TF: r=−0.333, p=0.42). However, there was a significant negative correlation between the density of these astrocytes and PMI in control subjects (r=0.805, p=0.02). Neither duration (r=−0.130, p=0.76) nor age at onset of depression (r=−0.370, p=0.37) was correlated with the density of smooth astrocytes in MDD subjects.
Soma size of GFAP-ir astrocytes in white matter of human vPFC
There was no significant difference in the mean soma size of fibrous (ANCOVA, F(1, 10), p=0.346) or smooth (ANCOVA, F(1, 10)=0.086, p=0.776) astrocytes between control and MDD cohorts (fibrous: control: 281.1±41.33 vs. MDD: 233.0±54.87; smooth: control: 45.47±2.62 vs. MDD: 43.38±2.53).
Area fraction of GFAP-ir structures in white matter of human vPFC
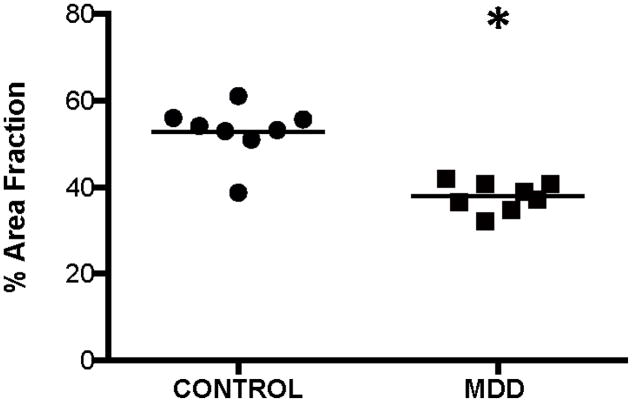
The mean area fraction of all GFAP-ir structures (cell bodies and processes) was significantly decreased by 28% in subjects with MDD as compared to control subjects (ANCOVA, F(1,10)=43.322, p<0.0001; Fig. 4). There was no significant correlation between area fraction and PMI, tissue pH, or TF in subjects with MDD (PMI: r=0.167, p=0.69; pH: r=−0.565, p=0.14; TF: r=−0.424, p=0.29) or control subjects (PMI: r=0.552, p=0.16; pH: r=−0.301, p=0.47; TF: r=−0.523, p=0.18). However, age was significantly correlated with area fraction in subjects with MDD (r=−786, p=0.02) and control subjects (r=0.731, p=0.04). Neither duration (r=−0.599, p=0.12) nor age at onset of depression (r=0.119, p=0.78) was significantly correlated with area fraction in subjects with MDD.
Figure 4.
Area fraction of all GFAP-immunoreactive structures (cell bodies + processes) in the ventral prefrontal white matter of human brain. Area fraction was significantly decreased (ANCOVA, F(1,10)=43.322, *p<0.0001) in subjects with major depressive disorder (MDD) as compared to control subjects. The horizontal lines represent the mean value of density for each diagnostic group.
Expression of GFAP mRNA in white matter of human vPFC
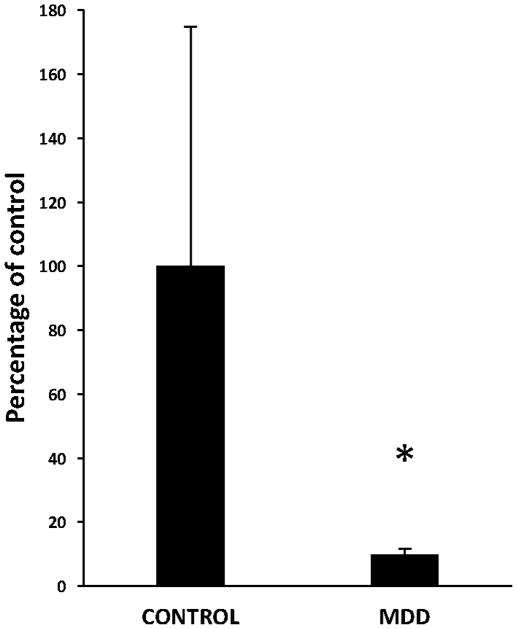
There was a significant 90% down-regulation in the expression of GFAP mRNA in subjects with MDD as compared to control subjects (ANCOVA, F(1, 25)=4.568, p=0.04; Fig. 5). There was no significant correlation between GFAP mRNA expression and age, PMI, or tissue pH in subjects with MDD (age: r=0.207, p=0.43; PMI: r=−0.343, p=0.18; pH: r=−0.069, p=0.79). However, in control subjects, GFAP mRNA expression was positively correlated with age (r=0.634, p=0.02) and negatively correlated with tissue pH (r=−810, p=0.001). There was no significant correlation between PMI and expression of GFAP mRNA in control subjects (r=0.494, p=0.09). Neither duration (r=0.114, p=0.66) nor age at onset of depression (r=0.108, p=0.68) was significantly correlated with expression of GFAP mRNA.
Figure 5.
Expression of GFAP mRNA in the human ventral prefrontal white matter as measured by qRT-PCR. Note decrease in the GFAP mRNA expression in subjects with major depressive disorder (MDD) as compared to control subjects (ANCOVA, F(1, 13)=5.551, *p=0.035).
Behavioral tests in CUS-treated rats
CUS attenuated the normal gain of body weight (t=1.909, df=18, p=0.05) and increased the mean latency to feed in a novelty environment, as compared to control rats (t=5.938, df=18, p<0.0001). However, CUS did not significantly alter sucrose preference in comparison to control rats (t=1.808, df=14, p=0.09).
Density of GFAP-ir astrocytes in white matter of CUS-treated rats
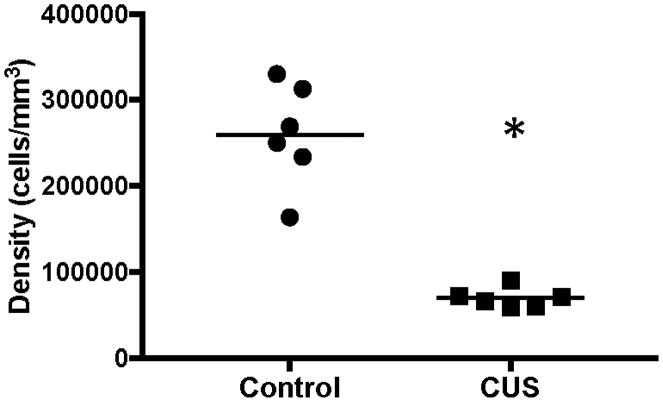
In the white matter of rat prefrontal cortex, nearly all GFAP-ir astrocytes were of the fibrous type with branches stemming from cell bodies. Although these branches were less numerous and shorter in rat brain than in the corresponding region of the human brain, rodent fibrous astrocytes still resembled human fibrous astrocytes. There were very few astrocytes in the rat that resembled the smooth astrocytes seen in humans. Due to their low number, smooth astrocytes in the rat were counted together with the larger fibrous astrocytes. There was a significant 73% decrease (t=7.688, df=10, p<0.0001) in the density of GFAP-ir astrocytes in white matter in CUS rats, as compared to control rats (Fig. 6).
Figure 6.
Density of GFAP-immunoreactive astrocytes in the white matter of ventral infralimbic cortex of rat brain. There was a significant decrease in the density of GFAP-immunoreactive astrocytes in rats exposed to chronic unpredictable stress (CUS) as compared to control rats (t=7.688, df=10, *p<0.0001). The horizontal lines represent the mean value of density for each experimental group.
DISCUSSION
Using 3-D quantification to measure cell density of GFAP-ir astrocytes and quantifying the tissue coverage by GFAP-ir structures (astrocytic cell bodies and processes), a significant decrease in the density of GFAP-ir astrocytes and GFAP-ir structures was detected in white matter of the vPFC in subjects with MDD as compared to control subjects. In addition, there was a significant decrease in the expression of mRNA for GFAP in white matter in MDD, as noted previously (Miguel-Hidalgo et al., 2017). Thus, the reductions in GFAP-ir astrocyte density, area fraction, and mRNA expression noted in prefrontal white matter in MDD in the present study parallel similar observations in prefrontal gray matter in MDD (Miguel-Hidalgo et al., 2000; 2010; Nagy et al., 2015; Si et al., 2004; Torres-Platas et al., 2016).
Three studies have quantified GFAP-ir astrocytes in white matter in depression (Gittins and Harrison, 2011; Williams et al., 2013; 2014). In subjects with MDD or bipolar disorder, Gittins and Harrison (2011) reported a reduction in GFAP detected immuno-autoradiographically in the white matter of the anterior cingulate cortex. In the current study in MDD, the reduction in the area fraction of immunohistochemically detected GFAP, a procedure potentially comparable to immuno-autoradiography, is in agreement with the observation in mood disorders by Gittins and Harrison (2011). While the density of GFAP-ir fibrous or smooth astrocytes in the present study was significantly decreased in MDD, in two other studies of white matter in the subgenual cingulate cortex, no significant difference was detected in the density of GFAP-ir astrocytes between subjects with MDD or bipolar disorder and control subjects (Williams et al., 2013; 2014). The apparent discrepancy between the studies by Williams et al. and the present study may be due to one or a combination of several factors: 1) differences in morphometric methodology (2-D cell counting in Williams et al. vs. 3-D in the present study), 2) targeting white matter in different cortical regions (cingulate cortex in Williams et al. vs. vPFC in the present study), 3) white matter region (gyral white matter in Williams et al. vs. deep white matter in the present study), and 4) the presence of antidepressant medication (undetermined in Williams et al. vs. no antidepressant medication detected in subjects at autopsy in the present study). The presence or absence of an antidepressant medication may be a critical determinant as shown for GFAP-ir astrocyte density in the hippocampal hilus, which is only decreased in subjects with MDD that were medication free at the time of death (Cobb et al, 2016).
Astrocyte pathology in cortical white matter was also described by Torres-Platas et al. (2011) using the Golgi method to stain the cell body and processes. In white matter adjacent to the anterior cingulate cortex, they found that the cell body area of fibrous astrocytes was larger, and the astrocyte processes were longer, more branched, and had more spines in suicide victims with MDD or bipolar disorder, as compared to controls. In contrast, in the present study, there was no significant difference between cohorts in soma volume of either fibrous or smooth GFAP-ir astrocytes. Furthermore, in the present study, the mean area fraction of GFAP-immunoreactivity, aggregating cell bodies and processes, was significantly decreased in MDD as compared to controls. In contrast to Torres-Platas et al. (2011), the reduction in GFAP-ir area fraction in MDD in the present study, combined with no change in volume of the cell body suggests a reduction in astrocytic processes. There are several potential reasons that may account, at least in part, for the difference in findings in the present study vs. Torres-Platas et al. (2011): 1) GFAP-ir labeling in the present study vs. Golgi silver-impregnation of astrocytes in Torres Platas et al., 2) the white matter brain region (left vPFC in the present study vs. right anterior cingulate cortex in Torres-Platas et al.), 3) all MDD subjects in the present study vs. a mix of subjects with MDD or bipolar disorder in Torres-Platas et al., 4) postmortem interval (22–24 hours in present study vs. 41–57 hours in Torres-Platas et al.), 5) age (mean of 57–58 in the present study vs. 48 in Torres-Platas et al.), 6) measurement of cell body volume in the present study vs. area in Torres-Platas et al., and 7) duration of depression (mean of 15 years in the present study vs. unreported by Torres-Platas et al.).
Studies in human postmortem tissue and animal models related to depression show significant changes in GFAP-ir astrocyte density and tissue coverage and GFAP expression in selective brain regions linked to depression. The expression of GFAP protein and mRNA was down-regulated in subcortical and prefrontal cortical areas implicated in mood disorders but not in other neocortical regions such as the primary motor and visual cortices (Nagy et al., 2015; Torres-Platas et al., 2016). In Wistar-Kyoto rats, a genetic model expressing depression-like behaviors, decreases in the density of GFAP-ir astrocytes and GFAP protein expression were specific to the fronto-limbic region but not to the corpus callosum (Gosselin et al., 2009). Finally, our study of GFAP-ir astrocyte density in CUS-treated rats revealed a significant reduction in the white matter adjacent to the vPFC (present study) but not in the more dorsally located regions of the prefrontal white matter (Rajkowska et al., data not shown), again suggesting that astrocyte pathology related to depression is brain region-specific.
The low density of GFAP-ir astrocytes in white matter in MDD noted in the present study suggests that MDD is accompanied by alterations in the physiology or metabolism of white matter astrocytes. Astrocytes promote myelination, myelin maintenance, and assist in the conduction of action potentials at nodes of Ranvier (Black and Waxman, 1988; Ishibashi et al., 2006; Liedtke et al., 1996). A role for astrocytes in myelin maintenance depends in part on gap junction-based astrocytic coupling to myelin-forming oligodendrocytes (Nualart-Marti et al., 2013). Interestingly, a significant decrease in immuno-labeling of connexin 43, the main protein of astrocytic gap junctions, was observed in MDD in our previous study in the orbitofrontal gray matter immediately adjacent to the white matter region examined in the present study (Miguel-Hidalgo et al., 2014). In addition, expression of mRNA for connexin 43 and connexin 30 (another gap junction protein expressed in astrocytes) was decreased in the dorsolateral prefrontal cortex of suicide victims, some of whom had MDD (Ernst et al., 2011). Interestingly, in vPFC white matter in the same controls and subjects with MDD as in the present study, we detected significant alterations in myelin-related gene expression (Rajkowska et al., 2015). Thus, alterations in the structure and function of astrocytes may be linked to myelin pathology in cortical white matter in MDD.
One consequence of reduced density of white matter astrocytes or their processes in depression could be altered propagation of action potentials. Astrocytes in white matter are important for the proper functioning of nodes of Ranvier located along myelinated axons, where the nodes are critical for regeneration of action potentials for saltatory impulse conduction (Black and Waxman, 1988; Waxman, 1986). Defects in myelination could impair the rapid propagation of action potentials and disturb neural connectivity. In fact, alterations in connectivity of white matter fiber tracts in the ventral PFC, among other regions, as measured with diffusion tensor imaging or functional neuroimaging, have been reported in patients with MDD (Jalbrzikowski et al., 2017; Murphy and Frodl, 2011; Nobuhara et al., 2006; Rolls et al., 2017; Sexton et al., 2009; Shimony et al., 2009; Tham et al., 2011).
Decreases in the density of GFAP-ir astrocytes, area fraction, and expression of GFAP mRNA observed in vPFC white matter in MDD, as noted in the present study, may represent a primary or early pathological event that alters maintenance of myelin. Mice with a null mutation of GFAP display abnormal myelination, poorly vascularized white matter, and an impaired blood-brain barrier (Liedtke et al., 1996). Thus, altered expression of GFAP in astrocytes may mediate abnormal white matter structure and connectivity in MDD.
There was a significant reduction in the expression of mRNA for GFAP in deep white matter of the vPFC in this study, as noted previously (Miguel-Hidalgo et al., 2017). However, Webster et al. (2005), using in situ hybridization in superficial white matter in the anterior cingulate cortex, reported no significant change in expression of mRNA for GFAP of subjects with MDD. Differences between Webster et al. and the present study that may account for varying results include: brain region examined (left or right anterior cingulate cortex in Webster et al. vs left vPFC in the present study), white matter region examined (superficial in Webster et al. vs. deep in present study), and method of assessing GFAP mRNA (film densitometry in Webster et al. vs. qRT-PCR in tissue in present study). In depressed suicide victims, reductions in the expression of mRNA for astrocyte-related genes, including GFAP, in prefrontal cortex may be related to differentially methylated genes regulating GFAP expression (Nagy et al., 2015; Pantazatos et al., 2017). While this regulatory mechanism is implicated in gray matter in depressed suicide victims, it remains to be determined if there is differential methylation of these genes in white matter. In addition, there is other evidence of astrocytic pathology in white matter in MDD that includes enhanced DNA oxidation and elevated expression of mRNA related to a DNA-repair enzyme (Szebeni et al., 2017), the exact connection of which to the present report remains to be determined.
In the present study there were several significant correlations between measured parameters of GFAP and potentially confounding variables such as tissue pH, TF, PMI, or age. Due to these significant correlations, these variables were included as covariates in the statistical analyses where significant differences were observed in GFAP-related parameters between our two cohorts. It is also important to mention that there were no significant differences between the two cohorts for tissue pH, TF, PMI, or age. In spite of this, the density of fibrous astrocytes and of expression of GFAP mRNA were negatively correlated with tissue pH only in subjects with MDD. Based on neuroimaging studies by Brody et al. (1999, 2001), Su et al. (2014), and cell culture work by Oh et al. (1995), metabolic disturbances reflecting changes in blood flow or tissue acidosis may be responsible for altered detection of GFAP mRNA and immunoreactivity of astrocytes in depression. Another significant negative correlation was found between astrocyte density and both TF and PMI in control subjects. Longer time in formalin and longer postmortem intervals may decrease the amount of detectable GFAP in control subjects in some unknown way, although statistical power for this observation was low. Such a hypothesis could be examined in future studies by comparing the processing of sections from tissues fixed in formalin over increasing periods of time and postmortem intervals. Finally, there was a significant positive correlation between GFAP mRNA and age of control subjects at death. This is not surprising as previous studies have shown that GFAP mRNA significantly increased with age in normal rat and human brain (e.g. Nichols et al., 1993).
It is of interest to establish whether astrocytes in white and gray matter are affected in animal models related to depression. To determine whether stress, a well-known risk factor for depression, produced changes in astrocytes in white matter comparable to those found in MDD, rats were exposed to CUS. We observed a significant decrease in the density of GFAP-ir astrocytes in white matter of the ventral prefrontal cortex of rats exposed to 35 days of unpredictable mild stress. In gray matter, CUS in rats was associated with decreased astrocyte cell density in prefrontal cortex, and chronic psychosocial stress in tree shrews was accompanied by decreased total numbers of astrocytes in the hippocampus (Banasr et al., 2008; Czeh et al., 2006). These effects of chronic stress in gray matter are consistent with our finding of decreased density of GFAP-ir astrocytes in the prefrontal white matter of rats treated with CUS. As noted here for CUS, Wistar-Kyoto rats, a strain considered to be a genetic model for depression and stress susceptibility, have reductions in GFAP-ir cell density and GFAP-ir protein content in the gray matter of various prefronto-limbic brain regions, as compared to Sprague-Dawley rats (Gosselin et al., 2009).
In the present study two distinct morphological types of GFAP-ir astrocytes were detected in white matter in the human vPFC. Fibrous astrocytes had numerous branched processes originating from cell bodies, while smooth astrocytes had no or only a few slender processes coming from cell bodies. Previous descriptions of astrocyte heterogeneity did not distinguish between types of astrocytes in white matter (Lundgaard et al., 2014; Oberheim et al., 2006; Torres-Platas; et al., 2011). However, morphology of GFAP-ir smooth astrocytes resembles that of dwarf cells described by Cajal (Garcia-Marin et al., 2007). Two types of GFAP-ir astrocytes were reported in the white matter of subgenual cingulate cortex and identified as fibrillary and gemistocytic astrocytes (Williams et al., 2014). Fibrillary astrocytes had multiple, visibly stained processes whereas larger gemistocytic astrocytes had either one or no stained processes. These fibrillary astrocytes are similar to our fibrous astrocytes and the gemistocytic astrocytes appear to resemble our smooth astrocytes. However, in the present study, cell bodies of smooth astrocytes were much smaller than fibrous astrocytes, while Williams et al. (2014) described gemistocytic astrocytes as larger than fibrillary astrocytes. However, in contrast to the present study, Williams et al. (2014) did not observe any significant decreases in either fibrillary or gemistocytic astrocyte density in white matter in MDD. Based on the morphology and abundance of fibrous astrocytes in the present study, we propose that these cells correspond to fibrous astrocytes described elsewhere in human white matter (Khakh and Sofroniew, 2015; Kimelberg, 2010; Miller and Raff, 1984). Smooth astrocytes may represent progenitors of mature fibrous astrocytes as GFAP is expressed in glial progenitor cells derived from radial glia (Khahn and Sofroniew, 2015). Alternatively, smooth astrocytes may represent fibrous astrocytes or their processes in the course of losing GFAP.
There are a number of potential limitations to the present study. While psychopathology was assessed using informant-based interviews, DeJong and Overholser (2009) determined for MDD that an informant-based interview is a reliable proxy for directly interviewing the patient. Despite the relatively small number of subjects examined for morphometry, the reduction in the density of GFAP-ir fibrous astrocytes and in GFAP mRNA in MDD was striking. While estimations of astrocyte density and mRNA were carried out in two differing cohorts of control and depressed subjects, the decrease in expression of mRNA for GFAP confirms the observation of Miguel-Hidalgo et al. (2017). Another limitation is that GFAP-ir astrocytes may only represent a subset of all astrocytes in white matter. Not all astrocytic processes contain GFAP, although the proportion of astrocytes that express GFAP is significantly greater in white matter than in gray matter (Bushong et al., 2002; Ludwin et al., 1976). Thus, our estimates of the area fraction of GFAP-ir processes may not have included all astrocytic processes and cell bodies. Nevertheless, significant reductions were noted in the area fraction of GFAP-ir structures in MDD as compared to non-psychiatric control subjects, which is consistent with the reduction in GFAP mRNA. Although the density of astrocytes was decreased both in white matter of rats exposed to 35 days of chronic unpredictable stress and in subjects with MDD, the psychopathology of MDD in humans may not be simply equated with chronic stress in the rat. There were no significant differences between the cohorts for time in formalin, PMI, tissue pH, or age. However, while there were several significant correlations between measured paramaters of GFAP and some of these potentially confounding variables in either control or MDD cohorts, these variables were included as covariates in the statistical comparisons of the two cohorts. More subjects in each cohort in future studies would reveal whether these potential relationships with confounding variables are real or spurious. No significant correlations were found between myelin genes and oligodendrocyte markers studied in Rajkowska et al. (2015) and the density of astrocytes or GFAP gene expression (present study), despite examining the same subjects and brain region in both studies. GFAP-related astrocyte parameters investigated in the present study may not be directly related to myelin maintenance. However, it is possible that other astrocyte-related components such as connexins, glutamate transporters, or components of the extracellular matrix are correlated with myelin-related markers. Other functions in which astrocytes participate, including saltatory impulse conduction, may be directly correlated with myelin and oligodendrocyte markers.
In summary, the present report identifies a reduction in the density and tissue coverage of GFAP-ir astrocytes and in expression of GFAP mRNA in deep white matter in the vPFC in MDD. Prolonged stress, a risk factor for human depression, may play an etiological role in MDD-related changes in astrocytes. Future studies are needed to determine whether the morphometric changes in white matter astrocytes in MDD are related to white matter pathology and alterations in connectivity detected in cortical and subcortical regions in MDD as determined with neuroimaging.
Acknowledgments
We thank the Cuyahoga County Medical Examiner’s Office, Cleveland, Ohio, and the next-of-kin of our subjects for their participation and support. We gratefully acknowledge the assistance of Drs. James C. Overholser, George Jurjus, Lisa Konick, and of Lesa Dieter in establishing the psychiatric diagnoses and in collecting the human tissues. This work was supported by the National Institutes of Health grants P30 GM103328 (Imaging Core and Postmortem Brain Core) (GR, CAS), R56 MH113828 (JJM-H, GR), R21 DK-113500 (DGR), and American Heart Association grant 12SDG8980032 (DGR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association (APA) Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; Washington: 1994. [Google Scholar]
- Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JA, Waxman SG. The perinodal astrocyte. Glia. 1988;1:169–183. doi: 10.1002/glia.440010302. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Mandelkern MA, Fairbanks LA, Ho ML, Baxter LR. Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biol Psychiatry. 2001;50:171–178. doi: 10.1016/s0006-3223(01)01117-9. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Silverman DH, Alborzian S, Fairbanks LA, Phelps ME, Huang SC, Wu HM, Maidment K, Baxter LR. Brain metabolic changes in major depressive disorder from pre- to post-treatment with paroxetine. Psychiatry Res. 1999;91:127–139. doi: 10.1016/s0925-4927(99)00034-7. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. The Journal of Neuroscience. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006;31:1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- Cobb JA, Simpson J, Mahajan G, Overholser JC, Jurjus GJ, Dieter L, Herbst N, May W, Rajkowska G, Stockmeier CA. Hippocampal volume and total cell numbers in major depressive disorder. J Psychiatr Res. 2013;47:299–306. doi: 10.1016/j.jpsychires.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb JA, O’Neill K, Milner J, Mahajan G, Laurence TJ, May WL, Rajkowska G, Stockmeier CA. Density of GFAP-immunoreactive astrocytes is decreased in left hippocampi in major depressive disorder. Neuroscience. 2016;316:209–220. doi: 10.1016/j.neuroscience.2015.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejong TM, Overholser JC. Assessment of depression and suicidal actions: agreement between suicide attempters and informant reports. Suicide Life Threat Behav. 2009;39:38–46. doi: 10.1521/suli.2009.39.1.38. [DOI] [PubMed] [Google Scholar]
- Ernst C, Nagy C, Kim S, Yang JP, Deng X, Hellstrom IC, Choi KH, Gershenfeld H, Meaney MJ, Turecki G. Dysfunction of astrocyte connexins 30 and 43 in dorsal lateral prefrontal cortex of suicide completers. Biol Psychiatry. 2011;70:312–319. doi: 10.1016/j.biopsych.2011.03.038. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for the DSM-IV Axis I disorders, SCID patient edition, version 2.0. New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- García-Marín V, García-López P, Freire M. Cajal’s contributions to glia research. Trends Neurosci. 2007;30:479–487. doi: 10.1016/j.tins.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Gittins RA, Harrison PJ. A morphometric study of glia and neurons in the anterior cingulate cortex in mood disorder. J Affect Disord. 2011;133:328–332. doi: 10.1016/j.jad.2011.03.042. [DOI] [PubMed] [Google Scholar]
- Gosselin RD, Gibney S, O’Malley D, Dinan TG, Cryan JF. Region specific decrease in glial fibrillary acidic protein immunoreactivity in the brain of a rat model of depression. Neuroscience. 2009;159:915–925. doi: 10.1016/j.neuroscience.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M, Larsen B, Hallquist MN, Foran W, Calabro F, Luna B. Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: associations with anxiety and depression. Biol Psychiatry. 2017;82:511–521. doi: 10.1016/j.biopsych.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015;18:942–952. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK. Functions of mature mammalian astrocytes: a current view. Neuroscientist. 2010;16:79–106. doi: 10.1177/1073858409342593. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Edelmann W, Bieri PL, Chiu FC, Cowan NJ, Kucherlapati R, Raine CS. GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron. 1996;17:607–615. doi: 10.1016/s0896-6273(00)80194-4. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ludwin SK, Kosek JC, Eng LF. The topographical distribution of S-100 and GFA proteins in the adult rat brain: an immunohistochemical study using horseradish peroxidase-labelled antibodies. The Journal of Comparative Neurology. 1976;165:197–207. doi: 10.1002/cne.901650206. [DOI] [PubMed] [Google Scholar]
- Lundgaard I, Osório MJ, Kress BT, Sanggaard S, Nedergaard M. White matter astrocytes in health and disease. Neuroscience. 2014;276:161–173. doi: 10.1016/j.neuroscience.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiatry. 2000;48:861–873. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Hall KO, Bonner H, Roller AM, Syed M, Park CJ, Ball JP, Rothenberg ME, Stockmeier CA, Romero DG. MicroRNA-21: Expression in oligodendrocytes and correlation with low myelin mRNAs in depression and alcoholism. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79:503–514. doi: 10.1016/j.pnpbp.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Rajkowska G. Immunohistochemistry of neural markers for the study of the laminar architecture in celloidin sections from the human cerebral cortex. J Neurosci Methods. 1999;93:69–79. doi: 10.1016/s0165-0270(99)00114-4. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Waltzer R, Whittom AA, Austin MC, Rajkowska G, Stockmeier CA. Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. J Affect Disord. 2010;127:230–240. doi: 10.1016/j.jad.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Wilson BA, Hussain S, Meshram A, Rajkowska G, Stockmeier CA. Reduced connexin 43 immunolabeling in the orbitofrontal cortex in alcohol dependence and depression. J Psychiatr Res. 2014;55:101–109. doi: 10.1016/j.jpsychires.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RH, Raff MC. Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J Neurosci. 1984;4:585–592. doi: 10.1523/JNEUROSCI.04-02-00585.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ML, Frodl T. Meta-analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biol Mood Anxiety Disord. 2011;1:3–12. doi: 10.1186/2045-5380-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy C, Suderman M, Yang J, Szyf M, Mechawar N, Ernst C, Turecki G. Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol Psychiatry. 2015;20:320–328. doi: 10.1038/mp.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NR, Day JR, Laping NJ, Johnson SA, Finch CE. GFAP mRNA increases with age in rat and human brain. Neurobiol Aging. 1993;14:421–429. doi: 10.1016/0197-4580(93)90100-p. [DOI] [PubMed] [Google Scholar]
- Nobuhara K, Okugawa G, Sugimoto T, Minami T, Tamagaki C, Takase K, Saito Y, Sawada S, Kinoshita T. Frontal white matter anisotropy and symptom severity of late-life depression: a magnetic resonance diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2006;77:120–122. doi: 10.1136/jnnp.2004.055129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nualart-Marti A, Solsona C, Fields RD. Gap junction communication in myelinating glia. Biochim Biophys Acta. 2013;1828:69–78. doi: 10.1016/j.bbamem.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Wang X, Goldman S, Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006;29:547–553. doi: 10.1016/j.tins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Oh TH, Markelonis GJ, Von Visger JR, Baik B, Shipley MT. Acidic pH rapidly increases immunoreactivity of glial fibrillary scidic protein in cultured astrocytes. Glia. 1995;13:319–322. doi: 10.1002/glia.440130408. [DOI] [PubMed] [Google Scholar]
- Pantazatos SP, Huang YY, Rosoklija GB, Dwork AJ, Arango V, Mann JJ. Whole-transcriptome brain expression and exon-usage profiling in major depression and suicide: evidence for altered glial, endothelial and ATPase activity. Mol Psychiatry. 2017;22:760–773. doi: 10.1038/mp.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2. Academic Press INC; San Diego: 1986. [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex. 1995;5:307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Mahajan G, Maciag D, Sathyanesan M, Iyo AH, Moulana M, Kyle PB, Woolverton WL, Miguel-Hidalgo JJ, Stockmeier CA, Newton SS. Oligodendrocyte morphometry and expression of myelin - related mRNA in ventral prefrontal white matter in major depressive disorder. J Psychiatr Res. 2015;65:53–62. doi: 10.1016/j.jpsychires.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz MS, Bohlen MO, Gunter BW, Henry Q, Stockmeier CA, Paul IA. Attenuation of social interaction-associated ultrasonic vocalizations and spatial working memory performance in rats exposed to chronic unpredictable stress. Physiol Behav. 2015;152:128–134. doi: 10.1016/j.physbeh.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Cheng W, Gilson M, Qiu J, Hu Z, Ruan H, Li Y, Huang CC, Yang AC, Tsai SJ, Zhang X, Zhuang K, Lin CP, Deco G, Xie P, Feng J. Effective connectivity in depression. Biol Psychiatry: Cognitive Neuroscience and Neuroimaging. 2017 doi: 10.1016/j.bpsc.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sexton CE, Mackay CE, Ebmeier KP. A systematic review of diffusion tensor imaging studies in affective disorders. Biol Psychiatry. 2009;66:814–23. doi: 10.1016/j.biopsych.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Shimony JS, Sheline YI, D’Angelo G, Epstein AA, Benzinger TL, Mintun MA, McKinstry RC, Snyder AZ. Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biol Psychiatry. 2009;66:245–252. doi: 10.1016/j.biopsych.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si X, Miguel-Hidalgo JJ, O’Dwyer G, Stockmeier CA, Rajkowska G. Age-dependent reductions in the level of glial fibrillary acidic protein in the prefrontal cortex in major depression. Neuropsychopharmacology. 2004;29:2088–2096. doi: 10.1038/sj.npp.1300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Cai Y, Xu Y, Dutt A, Shi S, Bramon E. Cerebral metabolism in major depressive disorder: a voxel-based meta-analysis of positron emission tomography studies. BMC Psychiatry. 2014;14:321–328. doi: 10.1186/s12888-014-0321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szebeni A, Szebeni K, DiPeri T, Chandley MJ, Crawford JD, Stockmeier CA, Ordway GA. Shortened telomere length in white matter oligodendrocytes in major depression: potential role of oxidative stress. Int J Neuropsychopharmacol. 2014;17:1579–1589. doi: 10.1017/S1461145714000698. [DOI] [PubMed] [Google Scholar]
- Szebeni A, Szebeni K, DiPeri TP, Johnson LA, Stockmeier CA, Crawford JD, Chandley MJ, Hernandez LJ, Burgess KC, Brown RW, Ordway GA. Elevated DNA Oxidation and DNA Repair Enzyme Expression in Brain White Matter in Major Depressive Disorder. Int J Neuropsychopharmacol. 2017;20:363–373. doi: 10.1093/ijnp/pyw114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham MW, Woon PS, Sum MY, Lee TS, Sim K. White matter abnormalities in major depression: evidence from post-mortem, neuroimaging and genetic studies. J Affect Disord. 2011;132:26–36. doi: 10.1016/j.jad.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Torres-Platas SG, Hercher C, Davoli MA, Maussion G, Labonté B, Turecki G, Mechawar N. Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology. 2011;36:2650–2658. doi: 10.1038/npp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Platas SG, Nagy C, Wakid M, Turecki G, Mechawar N. Glial fibrillary acidic protein is differentially expressed across cortical and subcortical regions in healthy brains and downregulated in the thalamus and caudate nucleus of depressed suicides. Mol Psychiatry. 2016;21:509–515. doi: 10.1038/mp.2015.65. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Sanz-Arigita EJ, de Vos K, Pool CW, Evers P, Rajkowska G. 3-D cytoarchitectonic parcellation of human orbitofrontal cortex correlation with postmortem MRI. Psychiatry Res. 2010;183:1–20. doi: 10.1016/j.pscychresns.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG. The astrocytes as a component of the node of Ranvier. Trends in Neuroscience. 1986;9:250–253. [Google Scholar]
- Webster MJ, Knable MB, Johnston-Wilson N, Nagata K, Inagaki M, Yolken RH. Immunohistochemical localization of phosphorylated glial fibrillary acidic protein in the prefrontal cortex and hippocampus from patients with schizophrenia, bipolar disorder, and depression. Brain Behav Immun. 2001;15:388–400. doi: 10.1006/brbi.2001.0646. [DOI] [PubMed] [Google Scholar]
- Webster MJ, O’Grady J, Kleinman JE, Weickert CS. Glial fibrillary acidic protein mRNA levels in the cingulate cortex of individuals with depression, bipolar disorder and schizophrenia. Neuroscience. 2005;133:453–461. doi: 10.1016/j.neuroscience.2005.02.037. [DOI] [PubMed] [Google Scholar]
- Williams MR, Hampton T, Pearce RK, Hirsch SR, Ansorge O, Thom M, Maier M. Astrocyte decrease in the subgenual cingulate and callosal genu in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2013;263:41–52. doi: 10.1007/s00406-012-0328-5. [DOI] [PubMed] [Google Scholar]
- Williams M, Pearce RK, Hirsch SR, Ansorge O, Thom M, Maier M. Fibrillary astrocytes are decreased in the subgenual cingulate in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2014;264:357–362. doi: 10.1007/s00406-013-0482-4. [DOI] [PubMed] [Google Scholar]
- Willner P. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol Stress. 2016;6:78–93. doi: 10.1016/j.ynstr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]