Abstract
Objective
To determine the impact of physicians’ financial relationships with the pharmaceutical industry on prescribing marketed alpha-blockers and overactive bladder medications. We also aim to examine if the number or total value of transactions is influential.
Materials and Methods
We linked the Open Payments Program database of industry payments to prescribers with Medicare Part D prescription data. We used binomial logistic regression to identify the association between receipt of industry payment and prescribing of marketed alpha-blockers (silodosin) and overactive bladder (OAB) medications (fesoterodine, solifenacin, and mirabegron). We also evaluated the impact of increasing total value and number of payments on prescribing of marketed drugs.
Results
The receipt of industry payment was associated with increased odds of prescribing the marketed drug for all included drugs: silodosin (OR 34.1); fesoterodine (OR 5.9); solifenacin (OR 2.7); and mirabegron (OR 6.8) (all p<0.001). We also found that increasing value of total payment and increasing frequency of payments were both independently associated with increased odds of prescribing with a dose-response effect.
Conclusions
There is a consistent association between receipt of industry payment and prescribing marketed alpha-blockers and OAB medications. Both the total value and number of transactions is associated with prescribing.
Keywords: conflict of interest, physician prescribing patterns, overactive bladder, adrenergic alpha blockers
Introduction
Physicians’ financial relationships with the pharmaceutical industry are common and a source of concern for patients, policymakers, and the public1, 2. Almost half of physicians in the US received payments from industry in 2015, with a total value of $1.8 billion3. These payments include gifts, meals, and fees and can be seen as problematic sources of conflict of interest for physicians. To better examine these relationships, the Centers for Medicare & Medicaid Services (CMS) established the Open Payments Program (OPP) to systematically report the financial transactions between health product manufacturers and prescribers2, 4. Since its inception in 2014, the OPP has published over 40 million transactions representing almost $25 billion in total value4.
Despite the recent focus on physician-industry relationships, the impact of industry payments on prescribing habits is not well understood. Prior analyses using OPP data have suggested that the receipt of industry payments is associated with increased prescribing costs for some patients5 and increased prescribing of promoted oral anticoagulants, diabetes drugs, and intraocular anti-VEGF injections6, 7. Within urology, previous work has examined the association between payment and prescribing of drugs for prostate cancer8, 9. Contrary to studies in other fields, these studies found either a weak or no association between payments and prescribing. However, no previous study has evaluated more common drugs and those with several equivalent alternatives. Additionally, it is not clear how the effect of marketing may vary among different prescriber specialties.
To better characterize the association between the receipt of industry payments and the prescribing of promoted drugs, we conducted a study of two classes of medications for urinary dysfunction: alpha-blockers and overactive bladder (OAB) drugs. These classes of drugs are commonly prescribed by physicians of multiple specialties, vary with respect to intensity of marketing, and contribute significantly to excess spending10. By linking the OPP database with Medicare part D payment data, we examined a nationwide cohort of prescribers of all specialties who prescribed a drug in one of these two classes.
Methods
Data Sources
We identified prescribers from the 2014 Medicare Provider Utilization and Payment Data Part D Prescriber public use file. This file contains prescriber identifying information (name and practice address) and claim counts for all pharmaceuticals prescribed to beneficiaries with Medicare Part D coverage. After excluding prescribers whose prescriptions were redacted due to low claim count (<10 claims), we sorted prescribers into groups based on prescription claim counts for alpha blockers (silodosin) and OAB drugs (solifenacin, fesoterodine, and mirabegron). We selected these drugs because they had significant associated payments (each had more than $2 million dollars in non-research payments) while others in the class did not (Supplemental Table). We recorded the number of prescriptions for each study drug and the total number of prescriptions in each class (alpha-blockers and OAB drugs) for each prescribing provider.
We used the 2014 OPP database (https://www.cms.gov/openpayments/) to identify all payments from industry to prescribers for each drug within the two included classes. For each prescriber and each drug, we obtained the number of transactions and total dollar value of all transactions. Names, practice addresses, and specialties were also abstracted from this data. We then linked providers between the two databases using name and practice address. National Provider Identification (NPI) number is not available in the OPP data and therefore could not be used to link the files.
Statistical Analysis
Alpha-blockers
We recorded the financial payments each prescriber received for silodosin. Both the number of payments and the total value of payments were included. In order to remove prescribers who do not see patients with voiding dysfunction, we limited the cohort of prescribers to those who had at least one record for any alpha-blocker prescription to a Medicare Part D beneficiary in 2014. We used univariable logistic regression to model the association between the receipt of any payment and the probability of prescribing silodosin. No additional explanatory variables were included in this initial model. We then performed additional multivariable logistic regression analyses among prescribers who received at least one payment to evaluate the independent impact of increasing total value and increasing total number of payments as well as the effect on of urologist or non-urologist prescribers. In the multivariable models, we included a categorical variable for tertile of payment and a continuous variable for number of transactions as depicted in Table 2.
Table 2.
Impact of increasing value and frequency of payments on prescribing, among those who received at least one payment.
| Drug | Specialty | Total Payment (tertiles) | OR (95% CI) | p-value |
|---|---|---|---|---|
| Alpha-blockers | ||||
| Silodosin | All prescribers (n=4176) | Low (≤$20) | Ref | |
| Intermediate ($21–$120) | 1.22 (1.04–1.42) | p=0.013 | ||
| High (≥$123) | 1.39 (1.11–1.74) | p=0.004 | ||
| Each additional payment | 1.26 (1.20–1.32) | p<0.001 | ||
| Urologists (n=2700) | Low (≤$31) | Ref | ||
| Intermediate ($32–$171) | 1.14 (0.92–1.41) | p=0.226 | ||
| High (≥$172) | 1.41 (1.03–1.94) | p=0.030 | ||
| Each additional payment | 1.08 (1.04–1.12) | p<0.001 | ||
| Non-urologists (n=1476) | Low (≤$17) | Ref | ||
| Intermediate ($18–$70) | 1.1 (0.8–1.5) | p=0.571 | ||
| High (≥$71) | 1.07 (0.75–1.51) | p=0.715 | ||
| Each additional payment | 1.58 (1.42–1.76) | p<0.001 | ||
| OAB Drugs | ||||
| Fesoterodine | All prescribers (n=8666) | Low (≤$15) | Ref | |
| Intermediate ($16–$66) | 1.22 (1.09–1.36) | p=0.001 | ||
| High (≥$67) | 1.59 (1.38–1.82) | p<0.001 | ||
| Each additional payment | 1.07 (1.06–1.09) | p<0.001 | ||
| Urologists (n=2620) | Low (≤$26) | Ref | ||
| Intermediate ($27–$138) | 1.1 (0.9–1.34) | p=0.372 | ||
| High (≥$139) | 1.36 (1.00–1.84) | p=0.049 | ||
| Each additional payment | 1.04 (1.02–1.07) | p<0.001 | ||
| Non-urologists (n=6046) | Low (≤$13) | Ref | ||
| Intermediate ($14–$52) | 1.13 (0.98–1.31) | p=0.1 | ||
| High (≥$53) | 1.23 (1.03–1.47) | p=0.022 | ||
| Each additional payment | 1.08 (1.06–1.11) | p<0.001 | ||
| Solifenacin | All prescribers (n=23686) | Low (≤$20) | Ref | |
| Intermediate ($21–$119) | 1.12 (1.05–1.20) | p=0.001 | ||
| High (≥$120) | 1.25 (1.13–1.38) | p<0.001 | ||
| Each additional payment | 1.17 (1.15–1.19) | p<0.001 | ||
| Urologists (n=3857) | Low (≤$95) | Ref | ||
| Intermediate ($96–$597) | 1.22 (0.88–1.68) | p=0.225 | ||
| High (≥$598) | 1.22 (0.64–2.34) | p=0.544 | ||
| Each additional payment | 1.14 (1.07–1.21) | p<0.001 | ||
| Non-urologists (n=19829) | Low (≤$18) | Ref | ||
| Intermediate ($19–$80) | 1.10 (1.02–1.18) | p=0.010 | ||
| High (≥$81) | 1.26 (1.14–1.39) | p<0.001 | ||
| Each additional payment | 1.10 (1.09–1.12) | p<0.001 | ||
| Mirabegron | All prescribers (n=30144) | Low (≤$23) | Ref | |
| Intermediate ($24–$137) | 1.45 (1.33–1.58) | p<0.001 | ||
| High (≥$138) | 1.94 (1.75–2.15) | p<0.001 | ||
| Each additional payment | 1.19 (1.18–1.20) | p<0.001 | ||
| Urologists (n=4442) | Low (≤$154) | Ref | ||
| Intermediate ($155–$934) | 1.18 (0.96–1.43) | p=0.109 | ||
| High (≥$935) | 1.00 (0.71–1.41) | p=0.987 | ||
| Each additional payment | 1.13 (1.10–1.16) | p<0.001 | ||
| Non-urologists (n=25702) | Low (≤$21) | Ref | ||
| Intermediate ($22–$101) | 1.28 (1.15–1.43) | p<0.001 | ||
| High (≥$102) | 1.76 (1.56–1.98) | p<0.001 | ||
| Each additional payment | 1.14 (1.12–1.15) | p<0.001 | ||
OAB Drugs
We similarly recorded the number and total value of financial payments each prescriber received for fesoterodine, solifenacin, or mirabegron. For these analyses, we limited the cohort of prescribers to those who prescribed at least one OAB drug. We again used univariable logistic regression to model the association between the receipt of payment and the probability of prescribing the marketed drug, without any additional explanatory variables. Additional multivariable logistic regression analyses were then performed to calculate the independent effect of the total value and number of payments as well as differences by prescriber specialty among those who received at least one payment. These multivariable models are presented in Table 2.
For both groups of drugs, all analyses were performed using SAS 9.4 (Cary, NC) and STATA 15 (College Station, TX). Alpha was set at 0.05 for all analyses. This study uses publicly available data and was deemed exempt from institutional review board review.
Results
We identified 363,950 prescribers (6,602 urologists and 357,348 non-urologists) with linked data in the OPP and Medicare Part D databases. In 2014, 108,979 providers prescribed an alpha-blocker or OAB medication through Medicare Part D and 33,380 received at least one industry payment from the manufacturer of that drug. Each of the four pharmaceuticals included in this analysis was prescribed as a minority of total prescriptions in its class (Supplemental Table).
Alpha-blockers
Among alpha-blockers, silodosin prescriptions (n=292,526) accounted for 2.5% of total prescriptions, while tamsulosin (n=8,052,736) accounted for 69.2% of prescriptions (Supplemental Table). Prescribers who received any industry payment for silodosin were significantly more likely to prescribe silodosin than those who did not (OR 34.1, 95%CI 31.7–36.6, p<0.001) (Table 1). The effect of payment on prescribing was attenuated for urologists (OR 4.4) as opposed to non-urologist prescribers (OR 14.2).
Table 1.
Effect of receipt of any industry payment on prescribing of promoted drug by specialty
| OR | 95% CI | p-value | ||
|---|---|---|---|---|
| Alpha-blockers | ||||
| Silodosin | All physicians | 34.1 | 31.7–36.6 | p<0.001 |
| Urologists | 4.4 | 4.0–5.0 | p<0.001 | |
| Non-urologists | 14.2 | 12.5–16.1 | p<0.001 | |
| OAB Drugs | ||||
| Fesoterodine | All physicians | 5.9 | 5.6–6.2 | p<0.001 |
| Urologists | 3.3 | 2.9–3.6 | p<0.001 | |
| Non-urologists | 4 | 3.8–4.3 | p<0.001 | |
| Solifenacin | All physicians | 2.7 | 2.6–2.8 | p<0.001 |
| Urologists | 5.1 | 4.3–5.9 | p<0.001 | |
| Non-urologists | 2.1 | 2.0–2.2 | p<0.001 | |
| Mirabegron | All physicians | 6.8 | 6.5–7.1 | p<0.001 |
| Urologists | 4.5 | 4.0–5.1 | p<0.001 | |
| Non-urologists | 4.8 | 4.5–5.0 | p<0.001 | |
Among those who received at least one payment, increasing value of payments and increasing number of payments both increased the odds of prescribing silodosin (Table 2). Prescribers who received a total value of payments in the middle or highest tertile of payments had significantly increased odds of prescribing as compared to those who received payments in the lowest tertile of total value. Additionally, each additional payment received was associated with increased odds of prescribing silodosin.
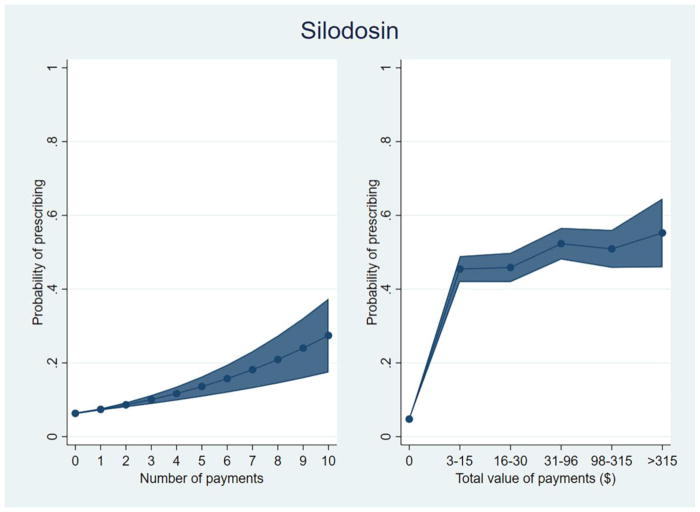
We plotted the predicted probability of prescribing silodosin among prescribers of at least one alpha-blocker, by increasing total value of payments (by quintile) and increasing number of payments (Figure 1). Prescribers who received no payments had a predicted probability of prescribing silodosin of 4.8% while those who received payments valued at ≤$15 had a probability of 45.4%. Among those who received payments in the highest tertile of value (>$315), the predicted probability of prescribing silodosin was 55.3%. Considering number of payments, the probability of prescribing increased from 6.3% for no payments to 13.6% for 5 payments and 27.4% for 10 payments, independent of total value received (Figure 1).
Figure 1.
Predicted probability of prescribing silodosin among prescribers of at least one alpha-blocker.
OAB Drugs
Solifenacin (n=1,338,104, 24.8%), mirabegron (355,950, 6.6%), and fesoterodine (n=298,692, 5.5%) were all prescribed less than the most commonly used drug in the class, oxybutynin (n=2,763,970, 51.2%).
Prescribers who received any industry payment for a one of the industry supported OAB drugs had significantly higher odds of prescribing that drug for all 3 medications (Table 1). These findings are consistent for urologists and non-urologists prescribers. Among prescribers who received at least one payment, those who received the highest total value were more likely to prescribe the marketed drug than those who received a lower total value of payments (Table 2). When considering urologists alone, increasing total value of payments did not have an additional impact on odds of prescribing for solifenacin or mirabegron. However, increasing number of payments was consistently associated with increased probability of prescribing for all drugs and all specialties (Table 2).
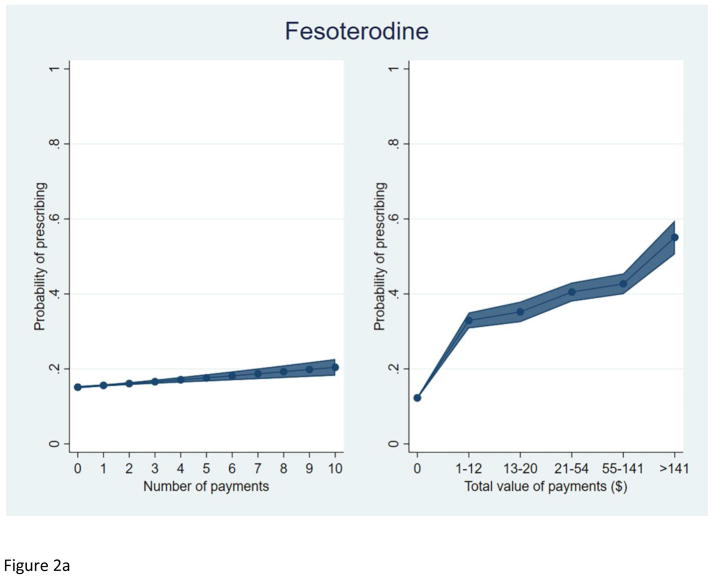
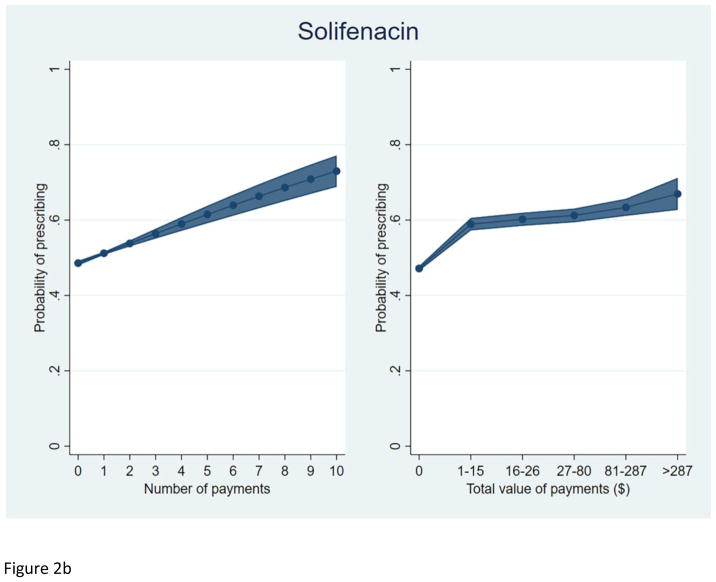
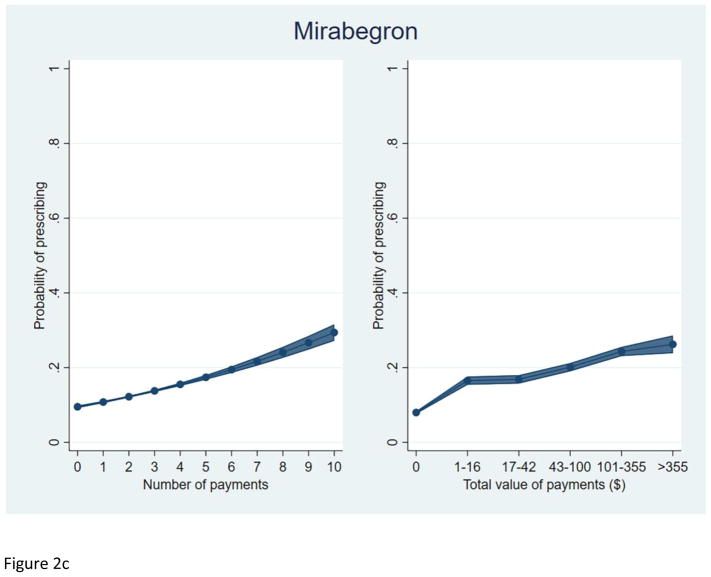
We plotted the predicted probability of prescribing each study drug, among prescribers of at least one OAB drug, by total number of payments and total value of payments received (Figure 2). As with silodosin, an increasing total value and number of payments were each independently associated with a higher predicted probability of prescribing each OAB drug.
Figure 2.
Predicted probability of prescribing among prescribers of at least one OAB drug.
a. Fesoterodine
b. Solifenacin
c. Mirabegron
Finally, we performed post-hoc sensitivity analyses including prescriber gender and geographic region in our models. These models demonstrated a similar association between receipt of payment and odds of prescribing (data not shown).
Discussion
We found that prescribers who received payments from pharmaceutical manufacturers were more likely to prescribe marketed alpha-blockers and OAB drugs. This finding was consistent across all four studied pharmaceuticals and for urologists and non-urologists. Furthermore, a higher total value of payments and a greater total number of payments were both associated with higher odds of prescribing the associated drug. These findings are important for urologists as recent studies have demonstrated that the marketed drugs we analyzed are not significantly better than generic alternatives11 and their use contributes in large proportion to the excess payments in Medicare Part D10.
An important detail of these results is our finding that increasing number of payments is associated with odds of prescribing the marketed drug, independent of total value of payments. This supports what has been described in the social psychology literature about the deeply ingrained social norm of reciprocity – a subconscious, but powerful, obligation to help those who have helped you12. This obligation can be engendered with small gifts, which are a mainstay of the pharmaceutical industry’s strategy to increase sales13.
The relationship between pharmaceutical industry payment and prescribing patterns has been investigated in other specialties and has been spurred by consistent and comprehensive payment data provided by CMS as part of the OPP5–7, 14, 15. Physicians who received industry-sponsored meals were more likely to prescribe name-brand statins, beta-blockers, ACE-inhibitors, angiotensin-receptor blockers, and selective serotonin and serotonin-norepinephrine reuptake inhibitors14, 15. An ecological study found that receipt of payments by industry was associated with greater regional prescribing of marketed oral anticoagulants and non-insulin diabetes drugs6. Another study that used OPP data demonstrated an association between payments and greater prescription costs per patient and more prescribing of brand-name medications5.
Not all studies of industry payments have shown a positive relationship between payments and prescriptions. Recent work evaluating the impacts of payments on urologist prescribers has not found strong associations. A study of denosumab and degarelix for prostate cancer demonstrated only a weak association between payments and prescribing habits9. Similarly, an analysis of enzalutamide and abiraterone prescribing among urologists and oncologists found no relationship between receipt of payments and prescription counts8. These results differ from ours for several possible reasons. First, the drugs studied in these previous analyses have defined benefits over less-marketed alternatives. We expect the impact of marketing to be greater in settings of uncertainty or small marginal benefits. In our study, all marketed drugs have generic and effective alternatives. Indeed, a recent meta-analysis found that these newer agents did not have evidence of superiority over older drugs11. It is in these discretionary settings that we would expect marketing payments to have the largest effect. Second, we limited the population of providers to those who prescribe drugs in the same class as the marketed drug. This eliminates a large number of physicians who may not be targeted for marketing as they do not treat the conditions for which these drugs are used. The consistency of our findings across different drugs and the effect of increasing value and frequency of payments lead us to conclude that industry payments do indeed have an impact on prescribing.
Our study has significant limitations. First, we cannot definitively infer a causal relationship from these cross-sectional findings. Due to the nature of these data sources, we were not able to include additional explanatory variables in our models. These variables, such as clinical information and cost-sharing requirements for different Medicare Part D plans would be expected to impact prescription decisions. However, we would not expect these variables to associate with receipt of industry payment by prescribers and therefore confound the analysis. Nevertheless, we found consistent results across study drugs. Additionally, a dose-response relationship exists between total payment value and frequency of payment with likelihood of prescribing. These findings are consistent with previous studies5, 7. Second, alternative explanations for our findings do exist: physicians who prescribe the promoted drugs in our study may also be more likely to accept meals or small gifts from industry sources. High prescribers of promoted drugs may be targeted by pharmaceutical companies to participate in speakers’ bureaus or other promotional events. Third, our study is limited to Medicare beneficiaries with Part D coverage and may not be representative of national prescribing practices. All Medicare beneficiaries are eligible for prescription drug coverage through Part D and approximately 70% enroll in one of the Part D plans. Medicare Part D beneficiaries can vary considerably in the specifics of their drug benefit (e.g., the out of pocket cost to the patient for any given drug) and this may influence physician prescribing. However, any variation in Part D prescription patterns would not be expected to correlate with industry payments to the prescribing physician and therefore should not bias the results of our analysis. Finally, our study is also limited by the nature of Medicare Part D data and the Open Payments Program database. Data from prescribers with a low claim count (<10 prescriptions) are redacted from the Part D file, which may skew our results. The reliability of payment data from the OPP has not been tested and errors are possible. Because the OPP is a relatively new initiative, physicians may not be aware of their option to review and challenge potentially incorrect reports. However, it is unlikely that errors in the payment data are systematic and bias our results.
These limitations notwithstanding, the association we found between pharmaceutical industry payment and prescribing should be of concern to patients, physicians, and policymakers. Urologists and other prescribers should carefully consider their relationships with industry and other potential conflicts of interest. Policymakers and clinical leaders should consider restricting the access of industry representatives to physicians and staff and continue to emphasize the importance of disclosure. Future research in this area should evaluate the possibility of a causal relationship and test the effect of physician-industry relationships on subjective and objective patient outcomes. Additional studies could examine the impacts of various types of payments and patterns of marketing interactions between industry and prescribers. Trust is central to the physician-patient relationship and the ability of physicians to provide high-quality care16. As a profession, physicians are responsible for self-regulation and should take steps to limit the influence of industry in medical decisions.
Supplementary Material
Acknowledgments
Funding: This work was supported by T32-CA180984 (PKM), P30CA072720 (EAS), JMD receives salary support from Blue Cross Blue Shield of Michigan for his role as the co-director of the Michigan Value Collaborative and his involvement in the Michigan Urological Surgery Improvement Collaborative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Campbell EG, Gruen RL, Mountford J, et al. A national survey of physician-industry relationships. N Engl J Med. 2007;356:1742. doi: 10.1056/NEJMsa064508. [DOI] [PubMed] [Google Scholar]
- 2.Santhakumar S, Adashi EY. The Physician Payment Sunshine Act: testing the value of transparency. JAMA. 2015;313:23. doi: 10.1001/jama.2014.15472. [DOI] [PubMed] [Google Scholar]
- 3.Tringale KR, Marshall D, Mackey TK, et al. Types and Distribution of Payments From Industry to Physicians in 2015. JAMA. 2017;317:1774. doi: 10.1001/jama.2017.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Open Payments - Centers for Medicare & Medicaid Services
- 5.Perlis RH, Perlis CS. Physician Payments from Industry Are Associated with Greater Medicare Part D Prescribing Costs. PLoS One. 2016;11:e0155474. doi: 10.1371/journal.pone.0155474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleischman W, Agrawal S, King M, et al. Association between payments from manufacturers of pharmaceuticals to physicians and regional prescribing: cross sectional ecological study. BMJ. 2016;354:i4189. doi: 10.1136/bmj.i4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor SC, Huecker JB, Gordon MO, et al. Physician-Industry Interactions and Anti-Vascular Endothelial Growth Factor Use Among US Ophthalmologists. JAMA Ophthalmol. 2016;134:897. doi: 10.1001/jamaophthalmol.2016.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandari J, Ayyash OM, Turner RM, 2nd, et al. The lack of a relationship between physician payments from drug manufacturers and Medicare claims for abiraterone and enzalutamide. Cancer. 2017;123:4356. doi: 10.1002/cncr.30914. [DOI] [PubMed] [Google Scholar]
- 9.Bandari J, Turner RM, 2nd, Jacobs BL, et al. The Relationship of Industry Payments to Prescribing Behavior: A Study of Degarelix and Denosumab. Urol Pract. 2017;4:14. doi: 10.1016/j.urpr.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirk PS, Borza T, Dupree JM, et al. Potential Savings in Medicare Part D for Common Urological Conditions. Urology Practice. doi: 10.1016/j.urpr.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahm P, Brasure M, MacDonald R, et al. Comparative Effectiveness of Newer Medications for Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: A Systematic Review and Meta-analysis. Eur Urol. 2017;71:570. doi: 10.1016/j.eururo.2016.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sah S, Fugh-Berman A. Physicians under the influence: social psychology and industry marketing strategies. J Law Med Ethics. 2013;41:665. doi: 10.1111/jlme.12076. [DOI] [PubMed] [Google Scholar]
- 13.Oldani MJ. Thick prescriptions: toward an interpretation of pharmaceutical sales practices. Med Anthropol Q. 2004;18:325. doi: 10.1525/maq.2004.18.3.325. [DOI] [PubMed] [Google Scholar]
- 14.Yeh JS, Franklin JM, Avorn J, et al. Association of Industry Payments to Physicians With the Prescribing of Brand-name Statins in Massachusetts. JAMA Intern Med. 2016;176:763. doi: 10.1001/jamainternmed.2016.1709. [DOI] [PubMed] [Google Scholar]
- 15.DeJong C, Aguilar T, Tseng CW, et al. Pharmaceutical Industry-Sponsored Meals and Physician Prescribing Patterns for Medicare Beneficiaries. JAMA Intern Med. 2016;176:1114. doi: 10.1001/jamainternmed.2016.2765. [DOI] [PubMed] [Google Scholar]
- 16.Pellegrino ED. Professionalism, profession and the virtues of the good physician. Mt Sinai J Med. 2002;69:378. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.