Abstract
Background
Pediatric mechanical circulatory support (MCS) has evolved considerably over the past decade. Though marked improvements in waitlist survival have been realized, costs have not been reassessed. This project aimed to assess contemporary MCS costs in children bridged to heart transplant (HT).
Methods
All pediatric HT recipients (2002–2016) were identified from a unique, linked PHIS/SRTR dataset. Costs were calculated from hospital charges, inflated to 2016 Dollars and adjusted for patient-specific characteristics using generalized linear mixed-effects models. Costs and length of stay (LOS) were compared across support strategies at the time of HT (no MCS, VAD, or ECMO) with select subgroup analyses.
Results
A total of 2873 pediatric HT recipients were included; no MCS: 2268 (78.9%), VAD: 470 (16.4%), and ECMO: 135 (4.7%). Both VAD and ECMO were associated with greater total hospitalization costs compared to no MCS ($755,345 and $808,771 vs. $457,086; p<0.001). Total costs and LOS were similar between VAD and ECMO groups; however, costs and LOS were greatest for VAD-supported patients in the pre-HT period and greatest for ECMO-supported patients post-HT. Post-HT costs and LOS were similar between patients who did not require MCS and those supported with a VAD ($324,887 and 18 days vs. $329,198 and 18 days respectively, p=NS). Outpatients with VAD support at HT demonstrated significantly lower total costs compared to those who were inpatient with continuous flow devices ($552,222 vs. $663,071, p=0.003).
Conclusions
MCS as a bridge to HT in children is associated with greater total costs. While costs are similar between VAD and ECMO groups, the majority of costs associated with VAD support is incurred pre-HT while ECMO costs are incurred primarily post-HT. Discharging patients on VAD support awaiting HT may represent a strategy to reduce costs in this population.
Keywords: Pediatric, heart transplantation, cost, mechanical circulatory support
Introduction
Mechanical circulatory support (MCS) strategies to bridge children to heart transplant (HT) have evolved considerably over the past decade. Ventricular assist device (VAD) use in children has increased,1 and this shift has contributed to improvements in waitlist survival and post-HT outcomes. 2–4 However, VAD support is known to be very resource intensive. 5–8 Mahle and colleagues reported an average hospitalization cost of $758,199 (in 2007 US dollars) for children bridged to HT with a VAD between 2002 and 2007. 8 Interval improvements in candidate and device selection, patient management, detection of device complications, and an increased emphasis on outpatient management may have shifted these costs. In fact, since the prior report from Mahle et al. there has been a marked increase in the use of continuous flow VADs in pediatric patients and a greater emphasis on rehabilitation following VAD placement. 1, 9 In addition to this, improvements in waitlist mortality, in part due to the success of MCS, without a concurrent increase in the number of pediatric donors 10 has likely resulted in increased waitlist times. This may lead to longer durations of MCS prior to HT, further impacting costs.
The aim of this study was to utilize a novel linkage between the Scientific Registry of Transplant Recipients (SRTR) and the Pediatric Health Information System (PHIS) databases to report contemporary cost and length of stay (LOS) data for children bridged to HT with either VAD or extracorporeal membrane oxygenation (ECMO), compared to patients who did not require MCS.
Methods
This study utilized data from the SRTR and the PHIS databases. The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the U.S., submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Resources and Services Administration, U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. The SRTR database includes data from every organ transplant and waitlist addition within the U.S. since October 1987. The PHIS database is an administrative database that collects clinical and daily resource utilization data for hospital encounters from 49 tertiary care children’s hospitals. Hospital encounters captured by the PHIS database include inpatient hospitalizations, observation, ambulatory surgery, and emergency department visits. The SRTR and PHIS databases were linked at the patient level using indirect identifiers (hospital, date of birth, sex, and date of transplant). A total of 3188 unique transplants were identified as being present in both databases and amenable to linkage. Of these, 3057 (95.9%) were uniquely linked and 2896 (90.8%) had complete cost data. 11
All pediatric (<21 years) HT recipients with available hospital charge information in the linked database were included (2002 – 2016). Hospital charges were converted to costs using year-specific and hospital-specific cost-to-charge ratios. All costs were adjusted for inflation to 2016 U.S. dollars using the medical component of the Consumer Price Index. Costs were assessed for the entire transplant hospitalization and then separately for the pre- and post-HT periods. Costs were adjusted for clinical characteristics and severity of illness at the time of HT using generalized linear mixed effects models with a random hospital intercept. Variables included in the cost adjustment model were selected a priori to account for severity of illness and patient demographics thought to impact hospitalization costs. Variables included in the adjustment model were patient age, underlying cardiac diagnosis, race, blood type, hospital, ECMO support at HT, VAD support at HT, ventilator support at HT, inotropic support at HT, ICU length of stay post-HT, ECMO support post-HT, the need for dialysis post-HT, and transplant year. Since costs are not normally distributed, we modeled the natural log of actual costs. Adjusted model results were then transformed back to the original cost scale for interpretation.
HT recipients were divided into three groups based on MCS requirement (from SRTR data) at the time of HT: no MCS, VAD, or ECMO. Baseline demographics were compared across MCS groups using the chi square test or Kruskal-Wallis test, as appropriate. Unadjusted and adjusted total, pre-, and post-HT hospitalization costs were compared across MCS groups using the Kruskal-Wallis test or Wilcoxon rank sum test, as appropriate. Given the significant variation in cost based on underlying cardiac diagnosis,11 and the potential for differing MCS strategies in patients with cardiomyopathy and congenital heart disease (CHD), the analysis was repeated after stratifying by diagnosis. For patients with CHD, pre-HT cardiac surgery during the transplant hospitalization was documented using ICD procedure codes available in PHIS (Appendix A). To account for the impact of varying hospital LOS, average daily pre-HT costs were calculated and compared based on pre-HT MCS strategy. Costs were also assessed across MCS groups based on area of spending including pharmacy, laboratory, imaging, supply, clinical, and other (primarily room and nursing charges) costs. For those on VAD support, costs were also analyzed on the basis of pulsatile vs. continuous flow device type and left ventricular or biventricular support. Additionally, given potential variations in post-HT mortality based on pre-HT MCS, the analysis was repeated after stratifying by survival to hospital discharge. All statistical analyses were performed in SAS version 9.4 (SAS Institute; Cary, NC) or STATA version 13 (StataCorp LLC; College Station, TX) with p<0.05 considered statistically significant. This project was approved by the Vanderbilt University Institutional Review Board, SRTR, and PHIS.
Results
A total of 2896 pediatric HT recipients were identified in the linked database. Of these, 23 patients were excluded due to use of both ECMO and VAD support at the time of HT with an unclear temporal relationship between the modes of support. Demographics for the 2873 patients included in the analysis are shown in Table 1. A total of 2268 (78.9%) received HT with no MCS, 470 (16.4%) from VAD support, and 135 (4.7%) from ECMO support. The median time on ECMO prior to transplant was 8 days (IQR 4 – 14 days) with 50 days representing the longest duration of pre-HT ECMO support. Patients supported with ECMO at the time of HT were more likely to be younger, require ventilator support at HT, require post-HT iNO, and require dialysis after HT compared to both other groups. Patients supported with ECMO at HT also demonstrated an increased incidence of stroke compared to unsupported patients and were more likely to have prolonged mechanical ventilation post-HT and longer ICU LOS as compared to the no MCS and VAD groups. Patients supported with a VAD at HT were more likely to have a diagnosis of cardiomyopathy, a positive crossmatch, and less likely to require inotropic support at HT compared to other groups.
Table 1.
Demographics based on MCS
| Total N=2873 |
None N=2268 (78.9%) |
VAD N=470 (16.4%) |
ECMO N=135 (4.7%) |
p-valuea | |||||
|---|---|---|---|---|---|---|---|---|---|
| Age | |||||||||
| <1 year | 892 | (31.1%) | 729 | (32.1%) | 77 | (16.4%) | 86 | (63.7%) | <0.001 |
| 1–5 years | 681 | (23.7%) | 525 | (23.2%) | 130 | (27.7%) | 26 | (19.3%) | |
| 6–10 years | 401 | (14.0%) | 326 | (14.4%) | 67 | (14.3%) | 8 | (5.9%) | |
| 11–17 years | 823 | (28.7%) | 626 | (27.6%) | 184 | (39.2%) | 13 | (9.6%) | |
| 18–21 years | 76 | (2.7%) | 62 | (2.7%) | 12 | (2.6%) | 2 | (1.5%) | |
| Diagnosis | <0.001b | ||||||||
| Cardiomyopathy | 1318 | (46.4%) | 899 | (40.1%) | 373 | (80.2%) | 46 | (34.3%) | <0.001c |
| Dilated Cardiomyopathy | 1047 | (79.4%) | 659 | (73.3%) | 350 | (93.8%) | 38 | (82.6%) | |
| Restrictive Cardiomyopathy | 154 | (11.7%) | 142 | (15.8%) | 9 | (2.4%) | 3 | (6.5%) | |
| Hypertrophic Cardiomyopathy | 79 | (6.0%) | 71 | (7.9%) | 5 | (1.3%) | 3 | (6.5%) | |
| Other Cardiomyopathy | 38 | (2.9%) | 27 | (3.0%) | 9 | (2.4%) | 2 | (4.4%) | |
| Congenital Heart Disease | 1363 | (48.0%) | 1193 | (53.2%) | 85 | (18.3%) | 85 | (63.4%) | 0.001d |
| Single Ventricle Lesion | 572 | (41.9%) | 522 | (43.7%) | 20 | (23.5%) | 30 | (35.3%) | |
| Retransplant | 159 | (5.6%) | 149 | (6.7%) | 7 | (1.5%) | 3 | (2.2%) | |
| Race | |||||||||
| Caucasian | 1687 | (58.7%) | 1362 | (60.1%) | 237 | (50.4%) | 88 | (65.2%) | 0.002 |
| African-American | 531 | (18.5%) | 400 | (17.6%) | 112 | (23.8%) | 19 | (14.1%) | |
| Hispanic | 491 | (17.1%) | 379 | (16.7%) | 94 | (20.0%) | 18 | (13.3%) | |
| Other | 164 | (5.7%) | 127 | (5.6%) | 27 | (5.7%) | 10 | (7.4%) | |
| Male Sex | 1570 | (54.7%) | 1216 | (53.6%) | 270 | (57.5%) | 84 | (62.2%) | 0.061 |
| Blood Type | |||||||||
| O | 1298 | (45.2%) | 998 | (44.0%) | 240 | (51.1%) | 60 | (44.4%) | 0.109 |
| A | 1079 | (37.6%) | 877 | (38.7%) | 155 | (33.0%) | 47 | (34.8%) | |
| B | 373 | (13.0%) | 297 | (13.1%) | 57 | (12.1%) | 19 | (14.1%) | |
| AB | 123 | (4.3%) | 96 | (4.2%) | 18 | (3.8%) | 9 | (6.7%) | |
| Ventilator at Transplant | 478 | (16.6%) | 311 | (13.7%) | 77 | (16.4%) | 90 | (66.7%) | <0.001 |
| Inotropes at Transplant | 1426 | (49.6%) | 1206 | (53.2%) | 138 | (29.4%) | 82 | (60.7%) | <0.001 |
| Positive Crossmatch (any method) | 388 | (13.5%) | 287 | (12.7%) | 83 | (17.7%) | 18 | (13.3%) | 0.015 |
| Positive Crossmatch (CDC) | 143 | (5.0%) | 104 | (4.6%) | 34 | (7.2%) | 5 | (3.7%) | 0.044 |
| iNO Post-transplant | 1438 | (50.2%) | 1102 | (48.7%) | 251 | (53.4%) | 85 | (63.0%) | 0.002 |
| Total Length of Stay (Days) | 50 | (20–98) | 44 | (17–91) | 78 | (38–131) | 68 | (40–99) | <0.001 |
| Pre-transplant Length of Stay (Days) | 22 | (1–62) | 18 | (1–56) | 50 | (19–99) | 20 | (12–46) | <0.001 |
| Post-transplant Length of Stay (Days) | 18 | (12–32) | 18 | (11–31) | 18 | (13–32) | 37 | (22–57) | <0.001 |
| Outpatient Prior to Transplant | 939 | (32.7%) | 869 | (38.3%) | 70 | (14.9%) | 0 | - | <0.001 |
| Post-Transplant ICU Days | 9 | (4–20) | 9 | (4–20) | 8 | (4–16) | 24 | (12–46) | <0.001 |
| Post-Transplant Days on Ventilator | 2 | (1–8) | 2 | (1–7) | 2 | (1–5) | 17 | (6–37) | <0.001 |
| Post-Transplant Complications | |||||||||
| Dialysis | 158 | (5.5%) | 104 | (4.6%) | 24 | (5.1%) | 30 | (22.4%) | <0.001 |
| Rejection Prior to Discharge | 356 | (13.6%) | 272 | (13.3%) | 61 | (13.2%) | 23 | (20.2%) | 0.108 |
| Stroke | 96 | (3.4%) | 62 | (2.8%) | 24 | (5.2%) | 10 | (7.6%) | 0.001 |
| Chylothorax | 147 | (5.1%) | 117 | (5.2%) | 21 | (4.5%) | 9 | (6.7%) | 0.582 |
| Cardiac Reoperation | 183 | (8.1%) | 140 | (7.8%) | 27 | (7.9%) | 16 | (14.0%) | 0.061 |
Continuous variables reported as median (25% – 75%) and categorical variables reported as N(%)
p-values from the chi square test for categorical and Kruskal Wallis test for continuous variables
p-value comparing cardiomyopathy, congenital heart disease, and retransplant
p-value comparing forms of cardiomyopathy based on the presence of mechanical support
p-value comparing single ventricle lesions based on the presence of mechanical support
LOS varied significantly across MCS groups (Table 1). Total LOS was significantly shorter for the no MCS group compared to patients supported with either VAD or ECMO at the time of HT (median: 44 days vs. 78 and 68 days respectively, p<0.001 for both). However, there was no significant difference in total LOS between VAD and ECMO groups (p=0.129). VAD supported patients demonstrated the longest pre-HT LOS compared to the no MCS and ECMO groups (median: 50 days vs. 18 and 20 days respectively, p<0.001 for both). Conversely, patients supported with ECMO had the longest post-HT LOS (median: 37 days vs. 18 days, p<0.001 for both).
Costs based on MCS strategy are shown in Table 2. Median total adjusted costs were not significantly different between patients on VAD support at HT and those on ECMO support (p=0.292). However, both were significantly higher compared to the no MCS group (p<0.001 for both). From admission to HT, costs were highest for patients supported with a VAD, with median adjusted pre-HT costs of $420,636. This was significantly higher compared to the no MCS and ECMO groups with pre-HT costs of $124,019 and $252,497 respectively (p<0.001 for both). Costs incurred from the time of HT to discharge were greatest for patients supported with ECMO at the time of HT, with median adjusted post-HT costs of $540,540 (p<0.001 compared to both other groups). These results remained unchanged when ICU length of stay was excluded from the cost-adjustment model. Post-HT costs increased with more prolonged duration of pre-HT ECMO support, but this did not reach statistical significance (<1 week: $517,142, 1–2 weeks: $552,958, >2 weeks: $668,562; p=0.293). Post-HT costs were not significantly different between those supported with VAD at HT and those who did not require MCS ($329,198 vs. $324,887, p=0.588).
Table 2.
Adjusted total, pre-, and post-transplant hospitalization costs based on MCS strategy
| Total Hospitalization Costs | N (%) | Adjusted Costs ¥ | p-value § | |||
|---|---|---|---|---|---|---|
|
|
||||||
| All patients | 2873 | (100%) | $509,870 | ($374,529 - | $752,899) | - |
| Type of MCS | ||||||
| None | 2268 | (78.9%) | $457,086 | ($347,270 - | $644,527) | <0.001 |
| VAD | 470 | (16.4%) | $755,345 | ($580,149 - | $1,059,854) | Ref. |
| ECMO | 135 | (4.7%) | $808,771 | ($572,341 - | $1,157,392) | 0.292 |
|
| ||||||
| Pre-Transplant Costs | N (%) | Adjusted Costs ¥ | p-value | |||
|
|
||||||
| All patients | 2873 | (100%) | $163,718 | ($79,772 - | $307,702) | - |
| Type of MCS | ||||||
| None | 2268 | (78.9%) | $124,019 | ($65,758 - | $222,382) | <0.001 |
| VAD | 470 | (16.4%) | $420,636 | ($307,741 - | $628,794) | Ref. |
| ECMO | 135 | (4.7%) | $252,497 | ($161,216 - | $446,178) | <0.001 |
|
| ||||||
| Post-Transplant Costs | N (%) | Adjusted Costs ¥ | p-value | |||
|
|
||||||
| All patients | 2873 | (100%) | $330,631 | ($262,561 - | $448,521) | - |
| Type of MCS | ||||||
| None | 2268 | (78.9%) | $324,887 | ($258,402 - | $434,268) | 0.588 |
| VAD | 470 | (16.4%) | $329,198 | ($264,426 - | $442,113) | Ref. |
| ECMO | 135 | (4.7%) | $540,540 | ($391,916 - | $723,485) | <0.001 |
P-values from the Wilcoxon rank sum test comparing VAD vs. no support and VAD vs. ECMO
Cost model adjusted for the following variables: Patient age, diagnosis, race, blood type, hospital, ECMO (pre- or post-transplant), VAD, ventilator support, inotropic support, length of ICU stay post-transplant, dialysis post-transplant, and transplant year.
Costs based on MCS strategy stratified by underlying diagnosis are shown in Table 3. All costs were significantly higher for patients with a diagnosis of CHD compared to those with cardiomyopathy. Of patients with a diagnosis of CHD, 345 (25.3%) had pre-HT cardiac surgery during the transplant hospitalization. Total costs for CHD patients remained significantly higher than those with cardiomyopathy when excluding those who underwent prior cardiac surgery ($507,653 vs. $484,744, p<0.001). For both groups, either VAD or ECMO support at HT significantly increased total hospitalization costs compared to patients who were not on MCS at HT, but there was no significant difference in total hospital costs between patients supported with VAD or ECMO, regardless of diagnosis. Pre-HT costs were significantly higher for patients requiring VAD support at HT for both cardiomyopathy and CHD patients when compared to other forms of support. When stratifying the analysis based on diagnosis, VAD-supported patients demonstrated higher post-HT costs compared to patients who did not require MCS; however, post-HT costs for VAD patients remained significantly less compared to patients on ECMO at the time of HT.
Table 3.
Adjusted hospitalization costs based on MCS strategy by diagnosis
| Cardiomyopathy
| ||||||
|---|---|---|---|---|---|---|
| Total Hospitalization Costs | N (%) | Adjusted Costs ¥ | p-value § | |||
|
|
||||||
| All patients | 1318 | (100%) | $484,744 | ($340,184 - | $689,643) | - |
| Type of MCS | ||||||
| None | 899 | (68.2%) | $397,726 | ($304,486 - | $535,694) | <0.001 |
| VAD | 373 | (28.3%) | $699,220 | ($560,056 - | $977,340) | Ref. |
| ECMO | 46 | (3.5%) | $679,291 | ($526,415 - | $857,366) | 0.256 |
|
| ||||||
| Pre-Transplant Costs | N (%) | Adjusted Costs ¥ | p-value | |||
|
|
||||||
| All patients | 1318 | (100%) | $168,202 | ($80,675 - | $328,112) | - |
| Type of MCS | ||||||
| None | 899 | (68.2%) | $110,368 | ($60,051 - | $196,123) | <0.001 |
| VAD | 373 | (28.3%) | $401,943 | ($284,902 - | $575,938) | Ref. |
| ECMO | 46 | (3.5%) | $196,768 | ($125,764 - | $305,497) | <0.001 |
|
| ||||||
| Post-Transplant Costs | N (%) | Adjusted Costs ¥ | p-value | |||
|
|
||||||
| All patients | 1318 | (100%) | $293,246 | ($239,532 - | $385,874) | - |
| Type of MCS | ||||||
| None | 899 | (68.2%) | $282,340 | ($231,569 - | $364,733) | <0.001 |
| VAD | 373 | (28.3%) | $308,141 | ($256,566 - | $416,031) | Ref. |
| ECMO | 46 | (3.5%) | $476,258 | ($366,415 - | $610,768) | <0.001 |
|
| ||||||
| Congenital heart disease
| ||||||
| Total Hospitalization Costs | N (%) | Adjusted Costs ¥ | p-value § | |||
|
|
||||||
| All patients | 1363 | (100%) | $557,435 | ($415,292 - | $837,336) | - |
| Type of MCS | ||||||
| None | 1193 | (87.5%) | $519,921 | ($399,902 - | $757,307) | <0.001 |
| VAD | 85 | (6.2%) | $1,000,163 | ($693,263 - | $1,472,652) | Ref. |
| ECMO | 85 | (6.2%) | $898,993 | ($646,604 - | $1,273,851) | 0.111 |
|
| ||||||
| Pre-Transplant Costs | N (%) | Adjusted Costs ¥ | p-value | |||
|
|
||||||
| All patients | 1363 | (100%) | $175,097 | ($87,460 - | $307,907) | - |
| Type of MCS | ||||||
| None | 1193 | (87.5%) | $155,599 | ($80,176 - | $264,372) | <0.001 |
| VAD | 85 | (6.2%) | $561,807 | ($391,079 - | $903,231) | Ref. |
| ECMO | 85 | (6.2%) | $315,634 | ($195,343 - | $511,169) | <0.001 |
|
| ||||||
| Post-Transplant Costs | N (%) | Adjusted Costs ¥ | p-value | |||
|
|
||||||
| All patients | 1363 | (100%) | $373,063 | ($296,035 - | $517,102) | - |
| Type of MCS | ||||||
| None | 1193 | (87.5%) | $364,171 | ($291,514 - | $488,149) | 0.001 |
| VAD | 85 | (6.2%) | $404,650 | ($332,049 - | $591,514) | Ref. |
| ECMO | 85 | (6.2%) | $579,937 | ($466,061 - | $821,927) | <0.001 |
P-values from the Wilcoxon rank sum test comparing VAD vs. no support and VAD vs. ECMO
Cost model adjusted for the following variables: Patient age, diagnosis, race, blood type, hospital, ECMO (pre- or post-transplant), VAD, ventilator support, inotropic support, length of ICU stay post-transplant, dialysis post-transplant, and transplant year.
Average daily pre-HT costs are shown in Table 4. Both ECMO and VAD supported patients have increased daily pre-HT costs compared to unsupported patients and this remained consistent across diagnoses. There was no significant difference in daily pre-HT costs between patients supported with a VAD and those requiring ECMO support.
Table 4.
Adjusted pre-transplant cost per hospital day based on mechanical circulatory support and diagnosis
| N | % | Pre-transplant cost per hospital day | p-value* | |||
|---|---|---|---|---|---|---|
|
|
||||||
| Total (N=2873) | ||||||
| No MCS | 2268 | (78.9%) | $5,315 | ($2,182 - | $20,746) | <0.001 |
| VAD | 470 | (16.4%) | $8,897 | ($4,420 - | $21,508) | Ref. |
| ECMO | 135 | (4.7%) | $11,796 | ($5,450 - | $24,810) | 0.187 |
| Cardiomyopathy (N=1318) | ||||||
| No MCS | 899 | (68.2%) | $4,966 | ($2,032 - | $14,328) | <0.001 |
| VAD | 373 | (28.3%) | $8,855 | ($4,425 - | $22,060) | Ref. |
| ECMO | 46 | (3.5%) | $12,024 | ($5,975 - | $22,513) | 0.334 |
| Congenital heart disease (N=1363) | ||||||
| No MCS | 1193 | (87.5%) | $5,742 | ($2,485 - | $24,871) | 0.01 |
| VAD | 85 | (6.2%) | $9,451 | ($4,326 - | $22,763) | Ref. |
| ECMO | 85 | (6.2%) | $11,796 | ($4,841 - | $25,171) | 0.471 |
p-values from the Wilcoxon rank sum test
Cost expressed as median (interquartile range), inflated to 2016 dollars
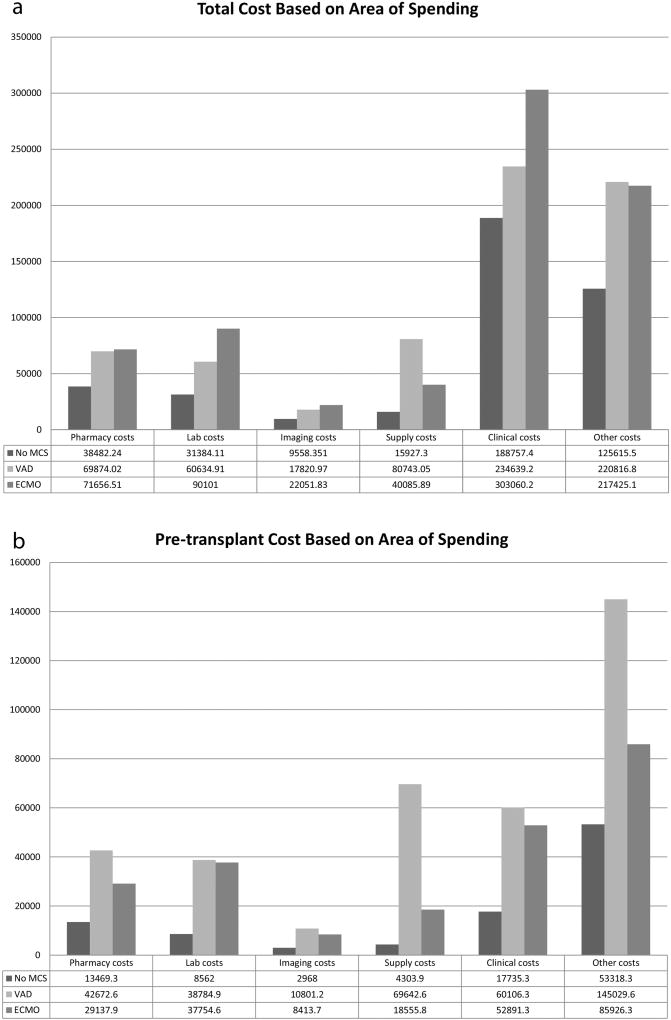
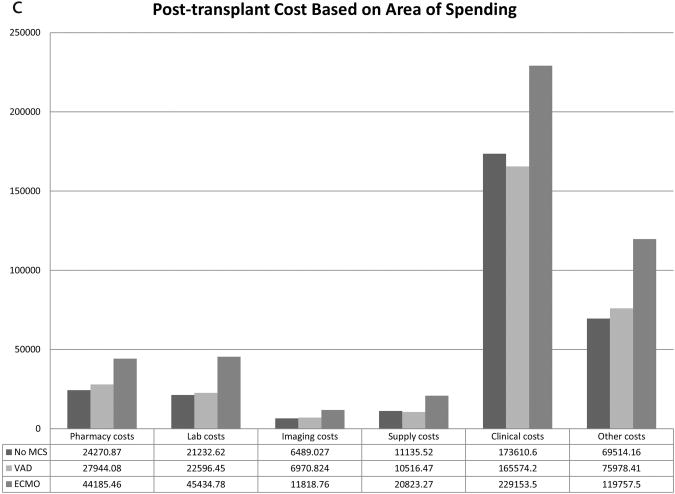
The breakdown of total, pre-HT, and post-HT costs based on area of spending are shown in Figures 1a, 1b, and 1c. Compared to patients not on MCS at HT, patients requiring VAD support have increased total and pre-HT costs across all areas of spending including pharmacy, laboratory, imaging, supply, clinical, and other costs. Post-HT costs for VAD supported patients were similar to patients who were not on MCS at HT for laboratory, imaging, supply, and clinical costs, but VAD supported patients demonstrated higher post-HT pharmacy ($27,944 vs. $24,271, p<0.001) and other costs ($75,978 vs. $69,514, p=0.002) compared to patients not requiring MCS. Patients requiring ECMO support have lower pre-HT pharmacy, imaging, supply, clinical, and other costs compared to VAD patients, but demonstrate increased post-HT costs across all spending areas.
Figure 1.
Cost based on area of spending for a) Total, b) Pre-transplant, and c) Post-transplant hospitalization. * Other costs consist primarily of room and nursing charges.
Costs stratified by VAD type (pulsatile vs. continuous flow and left ventricular support vs. biventricular support) are shown in Table 5. Pulsatile devices were present in 291 (61.9%) and continuous flow devices were used in 128 (27.2%) patients. Data was insufficient to classify VAD type in 51 (10.9%) patients. Berlin EXCOR (Berlin Heart; Berlin, Germany) was the most common pulsatile device used (N=241) and HeartWare HVAD (HeartWare; Framingham, MA) was the most common continuous flow device (N=83). Use of pulsatile VADs was associated with higher total and pre-HT costs compared to continuous flow devices ($802,081 and $471,315 vs. $645,452 and $333,745 respectively, p<0.001 for both). Post-HT costs were not significantly different between groups (pulsatile: $328,443 vs. continuous flow: $308,025, p=0.181). Median adjusted costs associated with the use of the Berlin EXCOR device were $839,027, $511,959, and $333,680 for the total, pre-HT, and post-HT hospitalization, respectively. Median adjusted costs associated with the use of the HeartWare HVAD device were $600,233, $297,372, and $293,497 for the total, pre-HT, and post-HT hospitalization, respectively. Median total cost associated with VAD equipment was $110,857 for pulsatile and $92,744 for continuous flow devices. There was no significant difference in total or pre-HT costs between patients receiving only left ventricular support and those who received biventricular support. However, post-HT costs were greater for patients receiving biventricular support ($378,736 vs. $317,481, p=0.047). Median total cost associated with VAD equipment was $83,844 for patients with left ventricular support and $158,600 for patients receiving biventricular support.
Table 5.
Adjusted total, pre-, and post-transplant hospitalization costs based on VAD type
| N | (%) | Median (IQR) | p-value* | |||
|---|---|---|---|---|---|---|
| Total cost | 470 | (100%) | $755,345 | ($580,149 - | $1,059,854) | - |
|
| ||||||
| Device Type | ||||||
| Pulsatile | 291 | (61.9%) | $802,081 | ($622,525 - | $1,134,111) | |
| Continuous Flow | 128 | (27.2%) | $645,452 | ($493,864 - | $914,146) | <0.001 |
| Other/Unspecified | 51 | (10.9%) | $788,114 | ($572,323 - | $1,174,256) | |
| Ventricular Support | ||||||
| LVAD Only | 326 | (69.4%) | $757,225 | ($580,018 - | $1,070,857) | |
| BiVAD | 102 | (21.7%) | $742,364 | ($581,739 - | $1,149,024) | 0.824 |
| Other/Unspecified | 42 | (8.9%) | $787,581 | ($571,696 - | $958,182) | |
|
| ||||||
| Pre-transplant costs | 470 | (100%) | $420,636 | ($307,009 - | $629,622) | - |
|
| ||||||
| Device Type | ||||||
| Pulsatile | 291 | (61.9%) | $471,315 | ($344,898 - | $708,881) | |
| Continuous Flow | 128 | (27.2%) | $333,745 | ($232,198 - | $463,225) | <0.001 |
| Other/Unspecified | 51 | (10.9%) | $421,844 | ($284,887 - | $665,486) | |
| Ventricular Support | ||||||
| LVAD Only | 326 | (69.4%) | $435,600 | ($317,010 - | $650,728) | |
| BiVAD | 102 | (21.7%) | $390,890 | ($282,342 - | $646,520) | 0.383 |
| Other/Unspecified | 42 | (8.9%) | $408,044 | ($298,648 - | $568,420) | |
|
| ||||||
| Post-transplant costs | 470 | (100%) | $329,198 | ($264,426 - | $442,113) | - |
|
| ||||||
| Device Type | ||||||
| Pulsatile | 291 | (61.9%) | $328,443 | ($269,194 - | $448,250) | |
| Continuous Flow | 128 | (27.2%) | $308,025 | ($260,492 - | $424,569) | 0.181 |
| Other/Unspecified | 51 | (10.9%) | $356,339 | ($274,761 - | $471,299) | |
| Ventricular Support | ||||||
| LVAD Only | 326 | (69.4%) | $317,481 | ($262,035 - | $436,904) | |
| BiVAD | 102 | (21.7%) | $378,736 | ($282,637 - | $472,461) | 0.047 |
| Other/Unspecified | 42 | (8.9%) | $350,867 | ($281,315 - | $402,451) | |
p-values from the Wilcoxon rank sum test comparing pulsatile to continuous flow VAD and LVAD to BiVAD
Cost expressed as median (interquartile range), inflated to 2016 dollars
- Age, diagnosis, race, blood type, hospital, ECMO (pre- or post-transplant), VAD, ventilator, inotropic support, ICU LOS, transplant year, need for dialysis post-transplant
A total of 70 (14.9%) VAD patients were outpatient prior to HT. Total LOS was not significantly different between outpatients with no MCS and those supported with continuous flow devices (median: 15 days vs. 14 days respectively, p=0.890). Patients supported with continuous flow VAD’s who were outpatient at the time of HT demonstrated significantly lower total costs compared to those who were inpatient ($552,222 vs. $663,071, p=0.003).
A total of 2716 (94.5%) patients survived to hospital discharge. Of the 157 patients who died prior to discharge, 109 (69.4%) did not require MCS at HT, 13 (8.3%) were supported with a VAD, and 35 (22.3%) were supported with ECMO. Patients supported with ECMO at HT were significantly more likely to have in-hospital mortality following HT (p<0.001). Total, pre-, and post-HT costs were significantly higher for patients who died compared to those who survived to hospital discharge, regardless of pre-HT MCS. When the analysis was limited to patients who survived to hospital discharge, the findings were unchanged from the primary analysis. In this subgroup analysis, post-HT costs for patients supported with a VAD were no different compared to patients not on MCS at HT ($325,414 vs. $317,559, p=0.359) and both were significantly less than patients supported with ECMO at HT ($517,142, p<0.001 compared to VAD and no MCS groups).
Discussion
We report contemporary cost data for children bridged to HT with VAD or ECMO from the largest U.S. cohort to date. VAD and ECMO both significantly increase total hospitalization costs in children undergoing HT compared to patients not on MCS. Though total costs and LOS were not significantly different between patients on VAD or ECMO at HT, the differences in the distribution of costs and LOS across the pre- vs. post-HT periods were significant. The costs associated with VAD support are incurred primarily in the pre-HT period, while patients supported with ECMO demonstrate increased post-HT costs. Patients supported with ECMO at HT demonstrated significantly shorter pre-HT LOS, suggesting that this was a select group of patients that was fortunate enough to receive a donor organ in a timely fashion. While VAD supported patients demonstrate significantly higher pre-HT costs, we believe this is most likely attributable the successful use of VADs to enable a longer duration of support, as the average daily cost pre-HT was similar between VAD and ECMO groups. Following HT, VAD supported patients had similar post-HT costs and LOS compared to patients who did not require MCS at HT. This is consistent with prior reports demonstrating that VAD support effectively mitigates the severity of illness in children bridged to HT.12
It is not surprising that patients requiring VAD support demonstrated increased pre-HT costs compared to the other groups as VAD support is known to be expensive. 5–8 In fact, our analysis demonstrates that patients who are bridged to HT with a VAD have increased pre-HT costs across all areas of spending including increased pharmacy, laboratory, imaging, supply, clinical, and other costs. So while device acquisition costs represent a large expenditure, the increased cost associated with VAD support is not solely attributable to the cost of the devices alone. The finding that average daily pre-HT costs were not significantly different between the VAD and ECMO groups, suggests that duration of inpatient support may be driving the pre-HT cost difference observed.
When the entire cohort was analyzed, patients supported with a VAD demonstrated similar post-HT costs compared to unsupported patients. However, when stratified by diagnosis, the VAD group had significantly higher post-HT costs. This finding likely reflects the overall increased costs in the CHD population in conjunction with decreased VAD utilization in this group, thereby increasing the costs of the unsupported group relative to the VAD population when the entire cohort was analyzed. It also highlights the need to consider separating patients with cardiomyopathy from those with CHD for analysis, as has been noted in prior studies. 13, 14 Importantly, despite a small increase in post-HT costs incurred by patients requiring VAD support compared to unsupported patients, post-HT costs for VAD patients remained significantly lower than those on ECMO at the time of HT.
Our analysis provides benchmark VAD costs based on device type. The Berlin EXCOR was the most common pulsatile device and costs were greater compared to continuous flow devices. While device acquisition costs may differ between the Berlin EXCOR compared to continuous flow devices, there are multiple reasons why overall costs may be greater. Pulsatile VADs are more likely to be used in younger patients, a group that has been demonstrated to have higher overall costs. 11 Pulsatile devices are also associated with more device complications,15–18 which may necessitate pump exchanges and lead to additional interventions. Lastly, continuous flow devices are more amenable to discharging patients from the hospital. Given that a large proportion of VAD costs are incurred during the pre-HT period, patient discharge while awaiting HT represents a cost-saving strategy. Adults are now routinely discharged following VAD placement 19 and there is increasing experience with this in the pediatric population. 20–22 In fact, 70 patients in our analysis on VAD support were outpatient prior to transplantation. Importantly, this group demonstrated significantly lower total costs compared to patients supported with continuous flow devices that were inpatient at the time of HT. This suggests that as the practice of discharging pediatric patients supported with a VAD expands, the costs associated with pediatric VAD support may decrease.
Prior reports on the costs associated with MCS as a bridge to HT in children are limited. Morales, et al. reported total hospitalization costs of $174,743 in 187 pediatric patients who underwent VAD placement in 2006. 23 However, only 26% of their cohort was bridged to HT and therefore the results cannot be directly compared with the findings of our analysis. A separate analysis by Mahle et al., reported a mean hospitalization cost of $758,199 (in 2007 U.S. dollars) for 94 children bridged to HT with a VAD between 2002 and 2007. 8 While this result is comparable to our findings, it describes an earlier cohort, where use of continuous flow devices was less common. 1 This report also describes mean costs, a likely biased estimate given that costs are not normally distributed. 24, 25 For this reason, we reported median costs associated with the use of MCS in the present analysis. With respect to ECMO support to HT, multiple prior reports have documented a relatively high cost, 26, 27 with one group showing that it does not meet the conventionally applied criteria for cost-effectiveness as a bridge to HT in children. 27
Our analysis has several limitations. A significant percentage of patients with CHD underwent cardiac surgery prior to HT, resulting in overestimation of costs that are not directly attributable to MCS. There is an inherent selection bias in our cohort. The decision regarding MCS is based on multiple factors and patients who received ECMO support may not have been suitable candidates for VAD placement. As evidence of this, in our analysis a higher portion of patients with hypertrophic cardiomyopathy, restrictive cardiomyopathy, and single ventricle anatomy received support with ECMO as opposed to a VAD. There are also likely differences across groups based on severity of illness. While our analysis attempted to account for this, there are likely factors not captured within the linked dataset that could not be adjusted for. The linked PHIS and SRTR database only includes transplanted patients and therefore excludes patients who were listed but not transplanted. The impact on our analysis of excluding patients requiring MCS who did not undergo transplantation is unknown, but represents an additional selection bias. Children bridged to transplant with a VAD have been shown to have higher costs relative to children with VADs who die on the waitlist and those who undergo successful device explant. 8 Therefore, exclusion of these patients likely biases our analysis toward higher costs and LOS estimates for VAD patients. In addition to this, patients supported with ECMO are less likely to survive to HT 28 and therefore our cohort of patients on ECMO at HT is a select group with short waitlist times, potentially underestimating costs of ECMO support by excluding patients who had longer waitlist durations but did not survive until HT. Patients supported with ECMO at HT also have a much higher incidence of post-HT mortality, potentially impacting our analysis. However, the results of our analysis did not change when patients who died prior to hospital discharge were excluded, suggesting that our findings are not attributable to differences in post-HT mortality between MCS groups. Also, the PHIS database does not include physician professional fees, thereby underestimating a small proportion of costs. As expected with any large dataset, missing and erroneous data can be problematic. While merger of these databases helps to improve data granularity, it is often not possible to reconcile discrepancies that may occur between datasets.
Conclusion
Our analysis provides contemporary cost and LOS data for the use of MCS as a bridge to HT in children from the largest U.S. sample to date. MCS as a bridge to HT in children is associated with greater costs during the entire HT hospitalization relative to children without MCS. VADs enable a longer duration of support which results in increased pre-HT costs, but effectively minimizes post-HT resource utilization. Discharging patients on VAD support awaiting HT represents a strategy to reduce costs in this population.
Acknowledgments
Funding sources
This project was supported through internal funding from the Katherine Dodd Faculty Scholar Program at Vanderbilt University (JG). Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K23HL123938 (Bethesda, MD) (JS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix A. ICD codes defining prior cardiac surgery
| ICD-10 Procedure Codes | ||||
|---|---|---|---|---|
| 021K0JP | 02HK0JZ | 02RW0KZ | 02UW0KZ | |
| 021V0ZQ | 02HL02Z | 02SP0ZZ | 02VP0CZ | |
| 021W0JQ | 02HL0JZ | 02SW0ZZ | 02VQ0CZ | |
| 021W0KP | 02LR0ZT | 02U50JZ | 02VR0CT | |
| 027R0DT | 02PA0JZ | 02UF0JZ | 02VR0CZ | |
| 02B50ZZ | 02PA0MZ | 02UG0JZ | 02WAXRZ | |
| 02BH0ZZ | 02PA0QZ | 02UJ0JZ | 02WY07Z | |
| 02BL0ZX | 02PA0RZ | 02UL0JZ | 03LH0ZZ | |
| 02BN0ZZ | 02PY0JZ | 02UM07Z | 03QH0ZZ | |
| 02BS0ZZ | 02Q50ZZ | 02UN0JZ | 05L00ZZ | |
| 02BT0ZZ | 02QF0ZZ | 02UP07Z | 06U007Z | |
| 02BW0ZZ | 02QM0ZZ | 02UQ07Z | 0JH606Z | |
| 02CK0ZZ | 02QP0ZZ | 02UQ0KZ | 0JH60XZ | |
| 02CL0ZZ | 02QQ0ZZ | 02UR0JZ | 0W3C0ZZ | |
| 02H60JZ | 02QR0ZZ | 02UR0KZ | 0WCD0ZZ | |
| 02H70MZ | 02QW0ZZ | 02UV07Z | 0WPD0YZ | |
| 02HA0QZ | 02RP0KZ | 02UV0KZ | 3E080GC | |
| 02HA0RZ | 02RQ08Z | 02UW07Z | ||
| ICD-9 Procedure Codes | ||||
| 35.10 | 35.39 | 35.99 | 37.49 | 38.45 |
| 35.11 | 35.51 | 36.03 | 37.52 | 38.64 |
| 35.12 | 35.53 | 36.11 | 37.53 | 38.65 |
| 35.13 | 35.61 | 36.15 | 37.54 | 38.7 |
| 35.14 | 35.62 | 36.19 | 37.55 | 38.85 |
| 35.21 | 35.63 | 36.2 | 37.60 | 39.0 |
| 35.22 | 35.71 | 36.31 | 37.63 | 39.21 |
| 35.23 | 35.72 | 36.91 | 37.64 | 39.23 |
| 35.24 | 35.73 | 36.99 | 37.65 | 39.29 |
| 35.25 | 35.81 | 37.10 | 37.66 | 39.54 |
| 35.26 | 35.82 | 37.11 | 37.74 | 39.61 |
| 35.27 | 35.83 | 37.12 | 37.91 | 39.62 |
| 35.28 | 35.84 | 37.24 | 37.99 | 39.64 |
| 35.31 | 35.91 | 37.31 | 38.04 | |
| 35.33 | 35.92 | 37.33 | 38.05 | |
| 35.34 | 35.94 | 37.36 | 38.34 | |
| 35.35 | 35.95 | 37.41 | 38.35 | |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
References
- 1.Villa CR, Khan MS, Zafar F, Morales DLS, Lorts A. United States Trends in Pediatric Ventricular Assist Implantation as Bridge to Transplantation. ASAIO J. 2017;63:470–475. doi: 10.1097/MAT.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 2.Jeewa A, Manlhiot C, McCrindle BW, Van Arsdell G, Humpl T, Dipchand AI. Outcomes with ventricular assist device versus extracorporeal membrane oxygenation as a bridge to pediatric heart transplantation. Artif Organs. 2010;34:1087–91. doi: 10.1111/j.1525-1594.2009.00969.x. [DOI] [PubMed] [Google Scholar]
- 3.Zafar F, Castleberry C, Khan MS, Mehta V, Bryant R, 3rd, Lorts A, Wilmot I, Jefferies JL, Chin C, Morales DL. Pediatric heart transplant waiting list mortality in the era of ventricular assist devices. J Heart Lung Transplant. 2015;34:82–8. doi: 10.1016/j.healun.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Singh TP, Almond CS, Piercey G, Gauvreau K. Trends in wait-list mortality in children listed for heart transplantation in the United States: era effect across racial/ethnic groups. Am J Transplant. 2011;11:2692–9. doi: 10.1111/j.1600-6143.2011.03723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulloy DP, Bhamidipati CM, Stone ML, Ailawadi G, Kron IL, Kern JA. Orthotopic heart transplant versus left ventricular assist device: a national comparison of cost and survival. J Thorac Cardiovasc Surg. 2013;145:566–73. doi: 10.1016/j.jtcvs.2012.10.034. discussion 573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marasco SF, Summerhayes R, Quayle M, McGiffin D, Luthe M. Cost comparison of heart transplant vs. left ventricular assist device therapy at one year. Clin Transplant. 2016;30:598–605. doi: 10.1111/ctr.12725. [DOI] [PubMed] [Google Scholar]
- 7.Digiorgi PL, Reel MS, Thornton B, Burton E, Naka Y, Oz MC. Heart transplant and left ventricular assist device costs. J Heart Lung Transplant. 2005;24:200–4. doi: 10.1016/j.healun.2003.11.397. [DOI] [PubMed] [Google Scholar]
- 8.Mahle WT, Ianucci G, Vincent RN, Kanter KR. Costs associated with ventricular assist device use in children. Ann Thorac Surg. 2008;86:1592–7. doi: 10.1016/j.athoracsur.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Hollander SA, Hollander AJ, Rizzuto S, Reinhartz O, Maeda K, Rosenthal DN. An inpatient rehabilitation program utilizing standardized care pathways after paracorporeal ventricular assist device placement in children. J Heart Lung Transplant. 2014;33:587–92. doi: 10.1016/j.healun.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Godown J, McKane M, Wujcik K, Mettler BA, Dodd DA. Expanding the donor pool: regional variation in pediatric organ donation rates. Pediatr Transplant. 2016;20:1093–1097. doi: 10.1111/petr.12779. [DOI] [PubMed] [Google Scholar]
- 11.Godown J, Thurm C, Dodd DA, Soslow JH, Feingold B, Smith AH, Mettler BA, Thompson B, Hall M. A Unique Linkage of Administrative and Clinical Registry Databases to Expand Analytic Possibilities in Pediatric Heart Transplantation Research. Am Heart J. 2017 doi: 10.1016/j.ahj.2017.08.014. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutcliffe DL, Pruitt E, Cantor RS, Godown J, Lane J, Turrentine MW, Law SP, Lantz JL, Kirklin JK, Bernstein D, Blume ED. Post-transplant outcomes in pediatric ventricular assist device patients: A PediMACS-Pediatric Heart Transplant Study linkage analysis. J Heart Lung Transplant. 2017 doi: 10.1016/j.healun.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Godown J, Donohue JE, Yu S, Friedland-Little JM, Gajarski RJ, Schumacher KR. Differential effect of body mass index on pediatric heart transplant outcomes based on diagnosis. Pediatr Transplant. 2014;18:771–6. doi: 10.1111/petr.12352. [DOI] [PubMed] [Google Scholar]
- 14.Schumacher KR, Almond C, Singh TP, Kirk R, Spicer R, Hoffman TM, Hsu D, Naftel DC, Pruitt E, Zamberlan M, Canter CE, Gajarski RJ Investigators PSG. Predicting graft loss by 1 year in pediatric heart transplantation candidates: an analysis of the Pediatric Heart Transplant Study database. Circulation. 2015;131:890–8. doi: 10.1161/CIRCULATIONAHA.114.009120. [DOI] [PubMed] [Google Scholar]
- 15.Almond CS, Morales DL, Blackstone EH, Turrentine MW, Imamura M, Massicotte MP, Jordan LC, Devaney EJ, Ravishankar C, Kanter KR, Holman W, Kroslowitz R, Tjossem C, Thuita L, Cohen GA, Buchholz H, St Louis JD, Nguyen K, Niebler RA, Walters HL, 3rd, Reemtsen B, Wearden PD, Reinhartz O, Guleserian KJ, Mitchell MB, Bleiweis MS, Canter CE, Humpl T. Berlin Heart EXCOR pediatric ventricular assist device for bridge to heart transplantation in US children. Circulation. 2013;127:1702–11. doi: 10.1161/CIRCULATIONAHA.112.000685. [DOI] [PubMed] [Google Scholar]
- 16.Schulman AR, Martens TP, Christos PJ, Russo MJ, Comas GM, Cheema FH, Naseem TM, Wang R, Idrissi KA, Bailey SH, Naka Y. Comparisons of infection complications between continuous flow and pulsatile flow left ventricular assist devices. J Thorac Cardiovasc Surg. 2007;133:841–2. doi: 10.1016/j.jtcvs.2006.09.083. [DOI] [PubMed] [Google Scholar]
- 17.Yuan N, Arnaoutakis GJ, George TJ, Allen JG, Ju DG, Schaffer JM, Russell SD, Shah AS, Conte JV. The spectrum of complications following left ventricular assist device placement. J Card Surg. 2012;27:630–8. doi: 10.1111/j.1540-8191.2012.01504.x. [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal DN, Almond CS, Jaquiss RD, Peyton CE, Auerbach SR, Morales DR, Epstein DJ, Cantor RS, Kormos RL, Naftel DC, Butts RJ, Ghanayem NS, Kirklin JK, Blume ED. Adverse events in children implanted with ventricular assist devices in the United States: Data from the Pediatric Interagency Registry for Mechanical Circulatory Support (PediMACS) J Heart Lung Transplant. 2016;35:569–77. doi: 10.1016/j.healun.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richenbacher WE, Seemuth SC. Hospital discharge for the ventricular assist device patient: historical perspective and description of a successful program. ASAIO J. 2001;47:590–5. doi: 10.1097/00002480-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Lin A, Liu E, Gowan M, May LJ, Doan LN, Almond CS, Maeda K, Reinhartz O, Hollander SA, Rosenthal DN. Outpatient Outcomes of Pediatric Patients with Left Ventricular Assist Devices. ASAIO J. 2016;62:163–8. doi: 10.1097/MAT.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins B, Fynn-Thompson F, Daly KP, Corf M, Blume E, Connor J, Porter C, Almond C, VanderPluym C. The Evolution of a Pediatric Ventricular Assist Device Program: The Boston Children's Hospital Experience. Pediatr Cardiol. 2017;38:1032–1041. doi: 10.1007/s00246-017-1615-8. [DOI] [PubMed] [Google Scholar]
- 22.Hollander SA, Chen S, Murray JM, Lin A, McBrearty E, Almond CS, Rosenthal DN. Rehospitalization Patterns in Pediatric Outpatients with Continuous-Flow VADs. ASAIO J. 2017;63:476–481. doi: 10.1097/MAT.0000000000000505. [DOI] [PubMed] [Google Scholar]
- 23.Morales DL, Zafar F, Rossano JW, Salazar JD, Jefferies JL, Graves DE, Heinle JS, Fraser CD., Jr Use of ventricular assist devices in children across the United States: analysis of 7.5 million pediatric hospitalizations. Ann Thorac Surg. 2010;90:1313–8. doi: 10.1016/j.athoracsur.2010.04.107. discussion 1318-9. [DOI] [PubMed] [Google Scholar]
- 24.Mihaylova B, Briggs A, O'Hagan A, Thompson SG. Review of statistical methods for analysing healthcare resources and costs. Health Econ. 2011;20:897–916. doi: 10.1002/hec.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nixon RM, Thompson SG. Parametric modelling of cost data in medical studies. Stat Med. 2004;23:1311–31. doi: 10.1002/sim.1744. [DOI] [PubMed] [Google Scholar]
- 26.Mahle WT, Forbess JM, Kirshbom PM, Cuadrado AR, Simsic JM, Kanter KR. Cost-utility analysis of salvage cardiac extracorporeal membrane oxygenation in children. J Thorac Cardiovasc Surg. 2005;129:1084–90. doi: 10.1016/j.jtcvs.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Brown KL, Wray J, Wood TL, Mc Mahon AM, Burch M, Cairns J. Cost utility evaluation of extracorporeal membrane oxygenation as a bridge to transplant for children with end-stage heart failure due to dilated cardiomyopathy. J Heart Lung Transplant. 2009;28:32–8. doi: 10.1016/j.healun.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Dipchand AI, Mahle WT, Tresler M, Naftel DC, Almond C, Kirklin JK, Pruitt E, Webber SA Pediatric Heart Transplant Study I. Extracorporeal Membrane Oxygenation as a Bridge to Pediatric Heart Transplantation: Effect on Post-Listing and Post-Transplantation Outcomes. Circ Heart Fail. 2015;8:960–9. doi: 10.1161/CIRCHEARTFAILURE.114.001553. [DOI] [PubMed] [Google Scholar]