Abstract
BACKGROUND
Temporal trends in prostate cancer incidence and death rates have been attributed to changing patterns of screening and improved treatment (mortality only), among other factors. This study evaluated contemporary national‐level trends and their relations with prostate‐specific antigen (PSA) testing prevalence and explored trends in incidence according to disease characteristics with stage‐specific, delay‐adjusted rates.
METHODS
Joinpoint regression was used to examine changes in delay‐adjusted prostate cancer incidence rates from population‐based US cancer registries from 2000 to 2014 by age categories, race, and disease characteristics, including stage, PSA, Gleason score, and clinical extension. In addition, the analysis included trends for prostate cancer mortality between 1975 and 2015 by race and the estimation of PSA testing prevalence between 1987 and 2005. The annual percent change was calculated for periods defined by significant trend change points.
RESULTS
For all age groups, overall prostate cancer incidence rates declined approximately 6.5% per year from 2007. However, the incidence of distant‐stage disease increased from 2010 to 2014. The incidence of disease according to higher PSA levels or Gleason scores at diagnosis did not increase. After years of significant decline (from 1993 to 2013), the overall prostate cancer mortality trend stabilized from 2013 to 2015.
CONCLUSIONS
After a decline in PSA test usage, there has been an increased burden of late‐stage disease, and the decline in prostate cancer mortality has leveled off. Cancer 2018;124:2801‐2814. © 2018 American Cancer Society
Keywords: Gleason score, incidence, mortality, prostate cancer, prostate‐specific antigen, trends
Short abstract
For the first time, the US cancer surveillance community has performed an analysis of long‐term trends in the incidence of prostate cancer by stage with delay‐adjusted rates. According to nationwide cancer registry and vital registration data, prostate cancer incidence rates for distant‐stage disease have increased and mortality rates for all stages combined have leveled off in the United States since the US Preventive Services Task Force recommendations against prostate‐specific antigen–based screening.See also pages 2785‐800 and 2690‐2.
INTRODUCTION
The introduction of prostate‐specific antigen (PSA) screening into the population has been linked with the dramatic increase in prostate cancer incidence between 1988 and 1992.1, 2 Since the peak in 1992, prostate cancer incidence has been decreasing, with an acceleration in the rate of decrease in more recent years. Several previous studies have suggested that the decline in incidence is associated with decreased screening since the 2012 US Preventive Services Task Force (USPSTF) recommendations against routine PSA testing.3, 4, 5, 6 In addition, the decline may also reflect a reduction in the pool of indolent cancers detectable by screening each year; such a reduction is an expected effect of the gradual stabilization of screening and biopsy practices in the 1990s and early 2000s. Some of these previous studies also reported recent increases in distant‐stage disease based on changes in the proportion of cases.4, 5 However, the interpretation of incidence trends based on the proportion of cases can be misleading; proportions of late‐stage disease could increase without a corresponding increase in the overall incidence rate simply because of a decline in the incidence of early‐stage disease. A recent study reported increases in the rate of distant‐stage prostate cancer in men aged 50 to 74 years.7 However, this recent study and none of the aforementioned studies simultaneously examined prostate cancer incidence and death rates.
In the United States, information on incident prostate cancer cases is collected and curated by central cancer registries with support from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program and the Centers for Disease Control and Prevention's National Program of Cancer Registries. Sometimes, case information is transmitted to central cancer registries with a delay because of the need to observe the staging workup and the initiation of treatment. Patients with local and regional disease are more likely to undergo radical prostatectomy.8, 9 Because the surgery is performed in a hospital setting whereas other treatment modalities are administered in outpatient centers, there might be a differential delay in reporting by stage based on the location of treatment. Statistical modeling of reporting delays adjusts the current case count to correct for the undercount of cases in the most recent diagnosis years.10 These adjusted counts and associated rates are valuable for more precisely determining changes in cancer trends.11
Using population‐based incidence and mortality data and nationally representative screening data, this study was aimed at examining trends in prostate cancer incidence and death rates with respect to changes in PSA screening rates. Temporal trends in incidence rates and proportions of cases were examined by stage and other disease characteristics, including the PSA level, Gleason score, and clinical extension. Notably, the analysis by stage was adjusted for delays in reporting; this is the first study to do so.
MATERIALS AND METHODS
Data Sources
Several data sources have been used to estimate trends for annual PSA testing prevalence, prostate cancer incidence rates, and prostate cancer mortality. Annual PSA testing rates were derived from self‐reported screening tests captured as part of the National Health Interview Survey (NHIS) conducted in 2000, 2003, 2005, 2008, 2010, 2013, and 2015. From 2005 forward, we used coding consistent with Jemal et al6 to determine whether a survey respondent received a PSA test in the prior year. Questionnaires before 2005 were not consistent with those from 2005 onward; for respondents who could not recall precisely the timing of their last test, there were more alternative options for ascertaining whether the test was received within the past year, and response options varied for the question asking the main reason for having received a PSA test (eg, a routine physical examination/screening test vs a routine examination). For these reasons, the data are displayed as a disconnected series.
Because questions about PSA testing were not included in the NHIS until the 2000 survey, longer term trends back to the late 1980s (when PSA screening rapidly disseminated in the US population) must be reconstructed through modeling. Long‐term trends in the proportion of men having a PSA test in the previous year and in the proportion of men having a first PSA test between 1987 and 2005 are an update of previous modeled estimates.12 The NHIS survey conducted in 2005 asked respondents to specify the year in which they had received their last PSA test, which implicitly was the year of the first test for respondents with just 1 test. For the corresponding NHIS survey years, intervals between PSA testing were estimated from claims available for a 5% random sample of Medicare beneficiaries who were residents of SEER areas and had not been diagnosed with cancer.13 Age at first test and intervals between tests were first combined in 1987 to simulate PSA testing patterns.
We used 3 different prostate cancer incidence series, including different geographic areas depending on the purpose of our analysis. To study the impact of long‐term PSA testing use on prostate cancer incidence trends, we used cases diagnosed between 1975 and 2014 by site recode definition14 and reported by 9 SEER registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco–Oakland, Seattle–Puget Sound, and Utah). Distant‐stage incidence for this series was calculated with SEER historic stage A. For a shorter term series that included more geographic areas within the United States, we used a set of registries that satisfied criteria required by the North American Association of Central Cancer Registries (NAACCR) for inclusion in the Cancer in North America annual reports.15 This set included 42 state central cancer registries covering 89% of the US population,16 and it was consistent with the data set used in an accompanying article.17 Because consistent staging for these registries was available only back to 2001, we used this series only for cases from 2001 forward. Consistent staging was achieved with SEER Summary Stage 200018, 19 for 2001 to 2003 and with Derived Summary Stage 200020 for 2004 to 2014. When the Derived Summary Stage 2000 was missing, the abstracted stage was used if the reporting source was deemed reliable. Finally, to compute prostate cancer incidence trends by the PSA test value at diagnosis, Gleason score, and clinical extension of the tumor, we used data from the SEER 18 registries21 for the years 2004‐2014, which represented 28% of the US male population. The data set excluded patients whose only reporting source was a death certificate, an autopsy report, or a nursing home record. For analyses involving disease characteristics, only SEER data were used because they had been recently reviewed for PSA accuracy and any errors corrected back to 2004.22
The highest value of serum PSA within the 3 months preceding the diagnostic biopsy has been collected by cancer registries for cases diagnosed in 2004 and after. For each calendar year between 2004 and 2014, we categorized patients into 3 categories of tumor aggressiveness based on the PSA value at diagnosis (0.1‐9.9, 10.0‐19.9, and ≥20 ng/mL), following clinical management guidelines issued by the American Joint Committee on Cancer (AJCC)23 and the National Comprehensive Cancer Network.8
In addition, disease aggressiveness at the time of diagnosis was assessed on the basis of the Gleason score and clinical extension of the primary tumor. All cases were classified according to the most recent clinical guideline recommendations into Gleason grade groups: 2 to 6, 7, 8, 9 to 10, and unknown.23, 24, 25 Per cancer registration rules, if the Gleason score was available from multiple pathology examinations, including core biopsy, transurethral resection, and prostatectomy specimens, the highest known Gleason score was used to assign the grade group.26 The incidence of cases with unknown Gleason scores decreased over time, but the decline was not statistically significant.
Using the Collaborative Stage–derived AJCC‐6 T variable,27 we categorized patients diagnosed from 2004 to 2014 by extraprostatic extension into the following groups: no extraprostatic extension (corresponding to clinical T1‐T2), extraprostatic extension (corresponding to clinical T3‐T4), and unknown extraprostatic extension status. The extraprostatic extension definitions used were those recommended for the assignment of the TNM clinical T variable for the prostate by the AJCC28 and included physical examinations, biopsies (including biopsies of the seminal glands), and imaging examinations.
The mortality files were obtained from the National Center for Health Statistics. We calculated prostate cancer mortality for the years 1975‐2015 by using all cases with an underlying cause of death coded as a malignant neoplasm of the prostate (International Classification of Diseases, Adapted, 8th Revision and International Classification of Diseases, Ninth Revision code 185 in 1975‐1998 and International Classification of Diseases, Tenth Revision code C61 in 1999‐2015). Race information available on the death certificate was used to calculate race‐specific prostate cancer death rates for black men and white men.
The 2000 US standard population was used for the age adjustment of all incidence and mortality estimates. A more in‐depth description of incidence and death rate estimation is available in an accompanying article.17 All incidence rates were adjusted for reporting delays according to the methodology described in the Statistical Methods section. When used, age and race variables were collected and categorized according to the NAACCR data standards.29
Statistical Methods
Historically, delay‐adjustment modeling was done only for registries in the SEER areas, but in the last several years, it has been expanded to include registries throughout the United States and Canada that participate in the NAACCR Call for Data.30 Delay‐adjustment factors are typically developed by registry, cancer site, age, and race, but for this project, they were also developed for prostate cancer by stage of disease for cases diagnosed from 2001 to 2014; SEER Summary Stage 200018, 19 was used for 2001 to 2003, and Derived Summary Stage20 was used for 2004 to 2014. For example, for US cases from 2014 for all races combined, the count needed to be adjusted upward of approximately 7% for localized and regional disease cases and 15% for distant and unstaged cases. Both incidence rates and proportions of cancer cases were delay‐adjusted. For rates, only the numerator (ie, case counts) was delay‐adjusted, whereas for the proportions of cases (eg, the proportion of cases with extraprostatic extension), both the numerator and the denominator were delay‐adjusted. Delay‐adjusted rates and proportions involving disease characteristics were not stage‐specific.
SEER*Stat software, version 8.3.4,31 was used to calculate the age‐adjusted incidence with the delay‐adjusted counts for each of the risk categories. In addition, the counts were used to calculate the proportion of patients in each risk group by diagnosis year. A trend analysis was conducted to identify the joinpoints (ie, trend change years) and estimate the annual percent change (APC) of the incidence rate. Separately, the same analysis was used to study the changes in the proportion of cases diagnosed with certain disease characteristics (eg, low, intermediate, and high PSA levels). We calculated the incidence and trends for all cases by age category (50‐74 vs ≥ 75 years) and race (black men vs white men). Age categories were selected to replicate those relevant in the context of the USPSTF recommendations for PSA‐based screening.3, 4
Temporal trends in age‐standardized, delay‐adjusted cancer incidence and death rates were estimated with joinpoint regression,32 which uses statistical criteria to determine both the number and location of trend changes. The joinpoint model fits linear trends to the log‐transformed incidence or death rates, with the slope of the trend changing at the joinpoints. The maximum possible number of joinpoints is determined by the number of observation points. The slope of the model represents the APC in rates. A 2‐sided statistical‐significance (P < .05) t test is used to determine if the APC differs significantly from zero.
RESULTS
Trends in PSA Testing and Prostate Cancer Incidence
Figure 1 displays the long‐term trends in PSA testing and the incidence of prostate cancer (all stages combined and distant stage). The use of PSA testing increased very rapidly in the initial years after the test was first approved for the surveillance of prostate cancer patients by the US Food and Drug Administration in 1986, with the incidence of newly tested men peaking in 1992. The dissemination of PSA testing among men was practically zero in 1987, but by 1992, 24% of men aged 50 years or older had undergone at least 1 test. The rapid increase in PSA testing between 1987 and 1992 coincides with the dramatic increase in prostate cancer incidence during 1988 through 1992 and a slightly delayed sharp decline in distant‐stage prostate cancer incidence between 1991 and 1994. The rise and fall of first PSA testing mimicked that of prostate cancer incidence. The incidence of PSA testing in previously unscreened men peaked between 1991 and 1992 when the prevalence of men undergoing their first PSA test was close to 10%.
Figure 1.
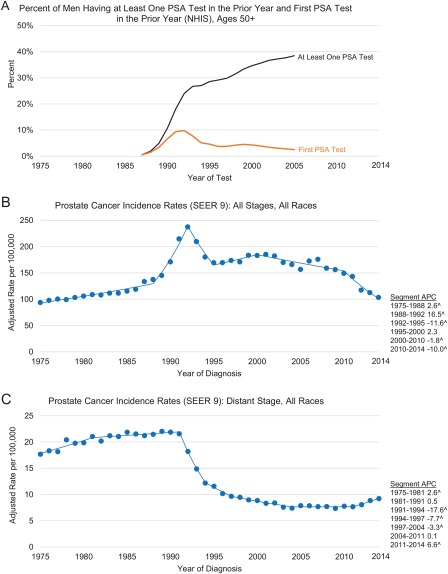
Trends in the proportion of men aged ≥50 years who received PSA testing in the prior year in the United States in 1987‐2005 (reconstructed from data from Medicare claims and the NHIS and based on methodology in Mariotto et al12) and trends in age‐ and delay‐adjusted prostate cancer incidence rates among men of all races combined by stage at diagnosis in SEER 9 in 1975‐2014. (A) Percentage of men having at least 1 PSA test in the prior year and their first PSA test in the prior year (NHIS): ages ≥ 50 years. (B) Prostate cancer incidence rates (SEER 9): all stages and all races. (C) Prostate cancer incidence rates (SEER 9): distant stage and all races. Rates are per 100,000 persons and have been age‐adjusted to the 2000 US standard population and delay‐adjusted for age and stage at diagnosis. Note that the y‐axis ratio for panel B to panel C is 10:1. The SEER 9 registries are Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco–Oakland, Seattle–Puget Sound, and Utah. ^The trend was statistically significant from 0 (P < .05). APC indicates annual percent change; NHIS, National Health Interview Survey; PSA, prostate‐specific antigen; SEER, Surveillance, Epidemiology, and End Results.
While Figure 1A shows the trends of PSA testing, Figure 2A‐C presents the most recent trends in PSA screening by race and age category. In addition, Figure 2D‐I presents prostate cancer incidence for all stages combined and distant‐stage disease. Screening rates declined after 2008 for all age and race categories shown, except for black men aged 75 years or older. As measured by the NHIS, PSA testing rates for all races and white men declined for men aged 50 to 74 years in 2013 in comparison with the prior survey in 2010. For all age groups shown, prostate cancer incidence rates consistently declined 6.5% per year from 2007 for all races combined, 6.8% per year from 2007 for white men, and 5.9% per year from 2009 for black men. For distant‐stage disease, there was an inflection point in the trends across all ages and racial groups. In all cases, except for black men aged 75 years or older, the rise in rates was significant after the joinpoint. The joinpoint varied from 2010 to 2011 for all ages combined and for men aged 75 years or older and from 2008 to 2009 for men aged 50 to 74 years. However, the confidence intervals constructed around the starting point of the final segment of distant‐stage disease overlapped (95% confidence interval for the joinpoint, 2006‐2011 for the 50‐ to 74‐year age category vs 2009‐2012 for the ≥ 75‐year age category [data not shown in figures]). The rise in the incidence rate of distant‐stage prostate cancer occurred at a time when fewer incident cases were reported as unstaged (2004‐2014 APCs for the unstaged incidence rate, –4.0 for all races, –5.3 for whites, and –3.6 for blacks [data not shown in the figures]). Thus, the increase in distant‐stage incidence rates can be partially explained by better staging workup and/or better stage documentation.
Figure 2.
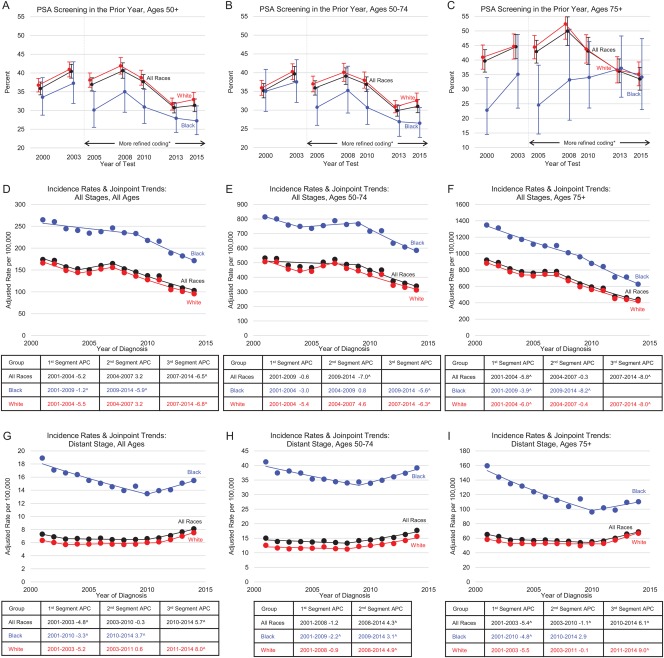
Trends in the proportion of men aged ≥50 years who received a PSA screening test in the prior year by age group in 2000‐2015 (NHIS) and trends in age‐ and delay‐adjusted prostate cancer incidence rates by race, age group, and stage at diagnosis from 42 registries representing 89% of the US population in 2001‐2014 (NAACCR). (A) PSA screening in the prior year: ages ≥ 50 years. (B) PSA screening in the prior year: ages of 50 to 74 years. (C) PSA screening in the prior year: ages ≥ 75 years. (D) Incidence rates and joinpoint trends: all stages and all ages. (E) Incidence rates and joinpoint trends: all stages and ages of 50 to 74 years. (F) Incidence rates and joinpoint trends: all stages and ages ≥ 75 years. (G) Incidence rates and joinpoint trends: distant stage and all ages. (H) Incidence rates and joinpoint trends: distant stage and ages of 50 to 74 years. (I) Incidence rates and joinpoint trends: distant stage and ages ≥ 75 years. Error bars represent 95% confidence intervals. Rates are per 100,000 persons and have been delay‐adjusted for age and stage at diagnosis. Note that panels D to I have different y‐axes. ^The trend was statistically significant from 0 (P < .05). *Better ascertainment of the year in which the test was received. APC indicates annual percent change; NAACCR, North American Association for Central Cancer Registries; NHIS, National Health Interview Survey; PSA, prostate‐specific antigen.
Although Figures 1 to 5 show incidence rate estimates from 3 different data sets representing varying levels of population coverage and time intervals, the average annual prostate cancer incidence trend for the common time period (years 2004‐2014) and the data set covering the largest population (89% of the United States) was −5.1% (data not shown in figures).
Figure 5.

Trends in age‐ and delay‐adjusted prostate cancer incidence rates and proportions of cases by clinical extension and age category in SEER 18 in 2004‐2014. (A) Incidence rates by cT: all ages. (B) Proportions by cT: all ages. (C) Incidence rates by cT: ages of 50 to 74 years. (D) Proportions by cT: ages of 50 to 74 years. (E) Incidence rates by cT: ages ≥ 75 years. (F) Proportions by cT: ages ≥ 75 years. Rates are per 100,000 persons and have been delay‐adjusted for age and stage at diagnosis. The SEER 18 registries are Connecticut, Georgia, the Greater California, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico, Utah, the Alaska Native Tumor Registry, Arizona Indians, the Cherokee Nation Cancer Registry, metropolitan Atlanta and rural Georgia, San Francisco–Oakland and San Jose–Monterey, Los Angeles, Detroit, and Seattle–Puget Sound. ^The trend was statistically significant from 0 (P < .05). APC indicates annual percent change; SEER, Surveillance, Epidemiology, and End Results.
Trends in Prostate Cancer Incidence and Proportions by Disease Characteristics
As shown in Figure 3, most patients were diagnosed at PSA levels below 10 ng/mL (a low recurrence risk); this is true for all age categories. Proportionally, fewer men aged 75 years or older were diagnosed at PSA levels below 10 ng/mL in comparison with men aged 50 to 74 years. The highest incidence rate of cases diagnosed at PSA levels below 10 ng/mL among men aged 75 years or older was observed in 2007, and a statistically significant downtrend occurred after 2007. However, the incidence rate of diseases diagnosed at low risk peaked earlier among patients diagnosed at the age of 75 years or older (2007) in comparison with patients aged 50 to 74 years (2009). Meanwhile, the incidence rate of prostate cancer diagnosed at PSA levels higher than 20 ng/mL (a high recurrence risk) for men of all ages declined steadily through 2012 and has plateaued in more recent years, except for men aged 50 to 74 years, whose incidence rate of diseases diagnosed at high risk has continued to decline through 2014.
Figure 3.
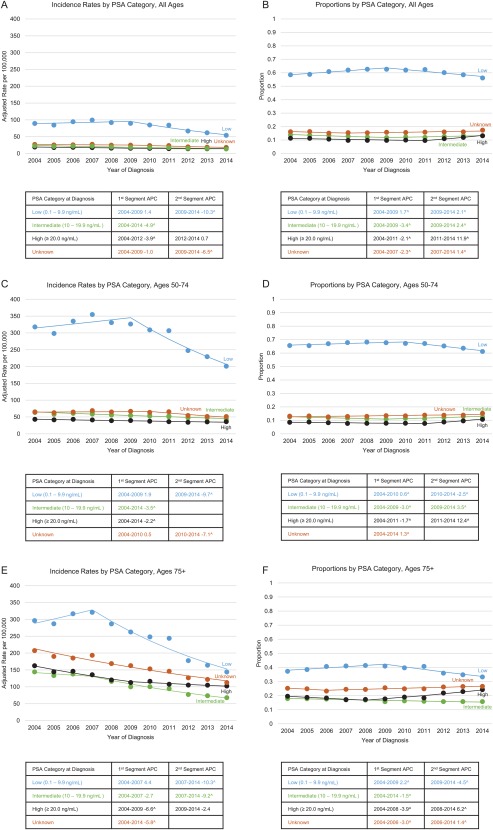
Trends in age‐ and delay‐adjusted prostate cancer incidence rates and proportions of cases by PSA value and age category in SEER 18 in 2004‐2014. (A) Incidence rates by PSA category: all ages. (B) Proportions by PSA category: all ages. (C) Incidence rates by PSA category: ages of 50 to 74 years. (D) Proportions by PSA category: ages of 50 to 74 years. (E) Incidence rates by PSA category: ages ≥ 75 years. (F) Proportions by PSA category: ages ≥ 75 years. Rates are per 100,000 persons and have been delay‐adjusted for age and stage at diagnosis. The SEER 18 registries are Connecticut, Georgia, the Greater California, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico, Utah, the Alaska Native Tumor Registry, Arizona Indians, the Cherokee Nation Cancer Registry, metropolitan Atlanta and rural Georgia, San Francisco–Oakland and San Jose–Monterey, Los Angeles, Detroit, and Seattle–Puget Sound. ^The trend was statistically significant from 0 (P < .05). APC indicates annual percent change; PSA, prostate‐specific antigen; SEER, Surveillance, Epidemiology, and End Results.
Figure 4 shows that the incidence rate of tumors with an aggressive histologic grade (ie, Gleason score of 9‐10) for all ages combined remained steady between 2004 and 2014 (APC, 0.0). However, for men aged 75 years or older, there was a small but persistent decline (APC, –1.1) in the incidence rate of patients who presented with a Gleason score of 9 to 10 at diagnosis between 2004 and 2014. For the same age category, the downtrend in cases diagnosed at a Gleason score of 2 to 6 was sharp, as shown by an almost 3‐fold decrease in incidence between 2004 and 2014 (293 per 100,000 vs 89 per 100,000). Gleason score 7 disease became the most prevalent presentation of prostate tumors at diagnosis in 2010 (40%), and it has been increasing slightly ever since (41% in 2014). After 2010, the proportion of new tumors diagnosed at a Gleason score of 2 to 6 decreased, whereas the proportion of tumors that presented with a more aggressive Gleason score increased. This trend (APC, 12.4) was particularly apparent among men aged 50 to 74 years, with nearly 9% of patients newly diagnosed in 2014 presenting with the most aggressive Gleason score category.
Figure 4.

Trends in age‐ and delay‐adjusted prostate cancer incidence rates and proportions of cases by GS and age category in SEER 18 in 2004‐2014. (A) Incidence rates by GS category: all ages. (B) Proportions by GS category: all ages. (C) Incidence rates by GS category: ages of 50 to 74 years. (D) Proportions by GS category: ages of 50 to 74 years. (E) Incidence rates by GS category: ages ≥ 75 years. (F) Proportions by GS category: ages ≥ 75 years. Rates are per 100,000 persons and have been delay‐adjusted for age and stage at diagnosis. The SEER 18 registries are Connecticut, Georgia, the Greater California, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico, Utah, the Alaska Native Tumor Registry, Arizona Indians, the Cherokee Nation Cancer Registry, metropolitan Atlanta and rural Georgia, San Francisco–Oakland and San Jose–Monterey, Los Angeles, Detroit, and Seattle–Puget Sound. ^The trend was statistically significant from 0 (P < .05). APC indicates annual percent change; GS, Gleason score; SEER, Surveillance, Epidemiology, and End Results.
As presented in Figure 5, the clear majority of tumors were assessed as confined to the prostate gland at the time of diagnosis (corresponding to clinical T1‐T2). Overall, the incidence of unknown T classifications increased between 2004 and 2009 and decreased significantly with the introduction of the seventh edition of the AJCC system in 2010. The incidence of prostate‐confined tumors (clinical T1‐T2) has been declining steadily since it peaked in 2007 (144 per 100,000) at approximately the same slope for all cases combined (APC, –7.4), men aged 50 to 74 years (APC, –8.9), and men aged 75 years or older (APC, –9.2). The incidence of tumors with clinical extraprostatic extension (corresponding to cT3‐cT4) has also been decreasing in all age categories (APC, –3.8, –4.3, and –1.4, respectively), albeit at a slower pace than the cT1 to cT2 decrease.
Trends in Prostate Cancer Mortality Rates
Prostate cancer mortality increased slowly before 1987 (APC, 0.9), but the trend moved upward at a steeper rate after 1987 for all races (APC, 3.0) and white men (APC, 3.1) and after 1988 for black men (APC, 3.2), as shown in Figure 6. The highest mortality during the observation period (1975‐2015) for all races combined was observed in 1993 (39.3 per 100,000). Mortality for black men peaked in 1993 (81.9 per 100,000), 2 years after mortality peaked for white men (36.5 per 100,000). After the peak, a greater decline in mortality was observed in black men (APC, –2.5) in comparison with white men (APC, –0.7). Between 2001 and 2015, the rate of decline among black men increased to an APC of –4.2. However, after a more sustained fall between 1994 and 1999 (APC, –4.3), the mortality decline slowed among white men (APC, –3.3) and then leveled off after 2013 (APC, –0.4 [statistically nonsignificant]).
Figure 6.
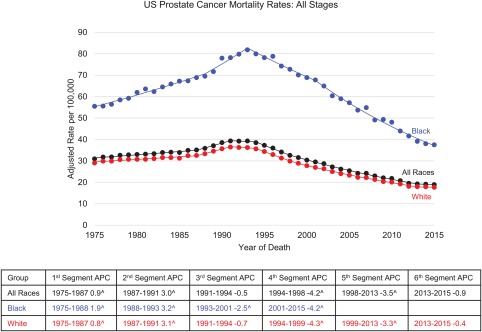
Trends in prostate cancer death rates by race in the United States in 1975‐2015 (National Center for Health Statistics). Rates are per 100,000 persons and have been age‐adjusted to the 2000 US standard population. ^The trend was statistically significant from 0 (P < .05). APC indicates annual percent change.
DISCUSSION
Original Contribution
This article uses national surveillance data to examine in detail the recent trends in prostate cancer incidence rates (based on data representing 89% of the US population) and prostate cancer death rates (based on mortality data covering the entire United States). The incidence estimates are presented by race and stage concurrent with PSA testing. To improve the accuracy of incidence estimation, the analysis used stage‐specific delay‐adjustment coefficients, which were found to be twice as large for distant and unstaged disease in comparison with local and regional disease. These stage‐specific delay‐adjustment coefficients allowed for a better correction of the reporting delay, a bias that potentially masks a trend change in the most recent years of a time series. Trends in the distribution of tumor characteristics, including the PSA test value, Gleason score, and clinical extension of the tumor, at the time of diagnosis allowed the examination of differences associated with more aggressive disease. The analysis has been conducted with the only population‐based data set for which PSA values have been fully audited and corrected.22 Presenting prostate cancer statistics alongside PSA testing estimates gives us insight into the interpretation of prostate cancer trends and how PSA test usage may have influenced national statistics.
An observation not fully explained is the dissonance of generally downward or stable incidence trends for prostate cancer diagnosed with the most aggressive disease characteristics (ie, high PSA levels, Gleason score of 9‐10, and extraprostatic extension) and the upward trend of distant disease incidence after 2010. Specifically, in most recent years (2010‐2014), the incidence of cases with high PSA levels and cases with Gleason scores of 9 to 10 was relatively flat, and the incidence of extraprostatic extension cases decreased significantly at the same time that the incidence of distant disease increased significantly.
This analysis included all incident prostate cancer diagnosed in the covered areas and observation periods, regardless of the diagnosing facility type (eg, hospital vs independent urology practice) and disease management plan (eg, active surveillance vs curative‐intent treatment). Underreporting of outpatients under active surveillance is a general concern in cancer surveillance; however, this type of bias is less likely to affect patients diagnosed at a late stage because these patients typically receive treatment in inpatient settings.
Difference Between Incidence and Proportion
When we look at the characteristics of cancer at diagnosis, data are shown as incidence rates and proportions of cases in each PSA test value, Gleason score, and clinical extraprostatic extension category. Incidence rates reflect the population risk of being diagnosed with a cancer having certain characteristics, whereas the proportions of cases reflect the probability that a man diagnosed with prostate cancer will present with specific characteristics. For example, if we assume a steady rate of cases with high PSA levels, as the incidence rate decreases for cases with low PSA levels (the cases most likely to be detected by testing), the proportion of cases presenting with high PSA levels will increase. However, this increase in the proportion of cases with high PSA levels does not necessarily imply either an increase in the incidence rate of cases with high PSA levels or an increase in the number of men diagnosed with high PSA levels. This report shows that as the proportions of cases with high PSA levels, high Gleason scores, and clinical extraprostatic extension increased, the actual incidence rates of those cases decreased or remained the same. When testing is introduced into the population or when testing usage declines, changes in the proportion of cases diagnosed with aggressive disease can be incorrectly interpreted as a change in risk. Moreover, changes in early detection procedures and in staging workup sensitivity make the interpretation of proportions even more difficult. For example, waiting for serial PSA measurements to calculate the PSA velocity before the first biopsy is performed can increase the counts of patients diagnosed with high‐risk PSA. Similarly, more sensitive imaging techniques may result in better detection of cases with extraprostatic extension. Thus, the distribution of disease characteristics at diagnosis and the trend based on proportions alone are difficult to interpret. Looking at incidence rates and proportion distributions side by side for prostate cancer demonstrates the importance of considering both measures when one is interpreting trends to understand the cancer burden.
Changes in Screening Recommendations and the Expected Impact and Timing on Incidence
In this report, trends in PSA testing seem to reflect the timing of changes in the USPSTF recommendations. In August 2008, the USPSTF recommended an I rating (insufficient evidence) for PSA‐based prostate cancer screening for men younger than 75 years but a D rating (a recommendation against screening) for men aged 75 years or older.3 PSA testing use declined after 2008 for all races and for white men in particular. In May 2012, the USPSTF revised its recommendations to a D rating for PSA‐based prostate cancer screening, regardless of age.4 PSA screening rates for all races and for white men in particular declined for men aged 50 to 74 years in 2013 in comparison with 2010.
We show that when PSA testing was initially introduced in the late 1980s, there was a very rapid decline in the incidence of distant‐stage prostate cancer. From this, it might be anticipated that reduced testing usage may trigger a similarly rapid increase in distant‐stage disease. Starting with the 2008 NHIS, we report a modest fall in PSA testing that is consistent with other reports.33 Concomitantly, we observed an increase in distant‐stage disease incidence of 4.4 per 100,000 (between 2008 and 2014). There is a continuum of potential for progression in the cancers poised to be diagnosed at any point in time. When medical providers increase the intensity of screening, certain would‐be distant cases are likely to be detected at an earlier stage. The rapid decline in distant‐stage prostate cancer incidence in the early 1990s, shortly after PSA‐based prostate cancer screening started, corroborates this explanation, as do simulations using well‐calibrated models of the natural history of prostate cancer.34
In May 2017, the USPSTF issued a draft recommendation statement changing a D rating to a C rating for men aged 55 to 69 years; this indicated that the decision about whether to be screened for prostate cancer should be an individual one.35 This could again result in changes in PSA testing and, consequently, the incidence of distant‐stage prostate cancer.
In contrast to overall prostate cancer incidence rates, trend inflection points for cases diagnosed at low PSA levels by age category followed the chronology of the USPSTF recommendations (the decrease occurred first and was more significant among those aged 75 years or older). The incidence rate of prostate cancer with high PSA levels decreased slightly until 2012 and appears to have flattened from 2012 to 2014. High PSA levels at prostate cancer diagnosis are associated with a higher risk of recurrent disease.36 The incidence rate of high‐grade disease was relatively stable, and the risk of extraprostatic extension decreased both before and after the USPSTF recommendations.
Change in Assigning the Gleason Score
The 2005 International Society of Urological Pathology consensus conference recommended that a high‐grade tumor of any quantity should be included in the calculations of the Gleason score.25 These recommendations have been reflected in the distribution of cases diagnosed in 2006 and later.37 As other authors reported, we found a migration of cases from the group with Gleason scores of 2 to 6 to the group with Gleason scores of 7. This migration has been seen in younger patients and had been observed before the 2012 USPSTF recommendations. In addition to the changes in the International Society of Urological Pathology guidelines, Gleason score abstracting instructions for cancer registrars changed in 2010. The score of the specimen obtained at prostatectomy was collected separately from the biopsy score and used for this analysis whenever the information was available. Thus, 2010 and later results may reflect better data collection practices. Nevertheless, the underlying population risk for aggressive tumors was unchanged during the 11 years of observation (2004‐2014). Among men aged 75 years or older, the risk of an aggressive histologic grade decreased slightly. However, as with the PSA, the proportion of new cases diagnosed at higher Gleason scores increased significantly in later years. This is expected to have an impact on the distributions of treatment because having a Gleason score of 9 to 10 is a contraindication for active surveillance or short‐term androgen deprivation therapy.8
Plausible Explanations for Changes in Mortality Trends
A decline in prostate cancer mortality rate for men of all races combined started in 1994 but stabilized between 2013 and 2015. The decline in the prostate cancer–specific mortality rate among white men slowed after 1999, well before the 2012 USPSTF D recommendation for PSA‐based prostate cancer screening. Gulati et al38 showed an expected flattening of mortality even in the absence of the recent decline in PSA testing. Interpreting trends in mortality is somewhat complex. Although this flattening of the mortality trend is temporally associated with the fall in PSA testing and the rise of distant‐stage disease, there are other possible contributing factors. For example, the timing and duration of the flattening could have been affected by improvements in treatment.39, 40 Since 1994, multiple factors might have contributed to a continuing decline in prostate cancer mortality, such as recent trends toward earlier detection and improved treatment of metastatic and castration‐resistant disease.41, 42 In conjunction with incidence data, death rate trends over the next few years can be used to track the role of PSA screening in declining prostate cancer mortality, although these trends may be partially confounded by steady improvements in prostate cancer treatment and by earlier detection of recurrent disease.
Limitations
Joinpoint regression is a powerful method for detecting changes in trends when the magnitude of change is small. However, there is uncertainty about the exact location of a joinpoint (ie, year of rate change), and this uncertainty is greater when the APC change between 2 successive segments is small but still statistically significant. This makes it particularly difficult to determine the exact temporal relation between interventions (ie, screening and therapeutics) and population‐based effects.
A comprehensive evaluation of any preventive intervention involves weighing both benefits and harms. This analysis did not attempt to cover patterns of adverse effects at the population or individual level and did not focus on indicators that may point to changes in trends in the frequency of adverse effects. Better information on the trends of adverse effects is necessary to understand whether the changes in incidence and mortality trends reported by this article translated into a change in the risk‐benefit ratio at the population level. In addition to understanding how the risk‐benefit ratio has changed, cancer control efforts for prostate cancer may be informed by a more in‐depth analysis of how measures of occurrence based on disease characteristics are related to the incidence of late‐stage disease and disease‐specific mortality.
In conclusion, this analysis of prostate cancer trends adjusted for delays in reporting by stage of disease showed an increase in the incidence of late‐stage disease from 2010 to 2014. This change chronologically followed new recommendations in the USPSTF guidelines for PSA‐based prostate cancer screening.3, 4 In addition, the incidence of disease characteristics has changed because newly diagnosed patients are less likely to present with low‐risk localized disease and, consequently, are less likely to be eligible for active surveillance. However, there was no increase in the incidence of patients with other high‐risk characteristics (ie, high PSA level, high Gleason score, and extraprostatic extension) to date. These findings, together with the flattening of previously declining mortality trends, illustrate a trend of increasing late‐stage disease after decreasing PSA screening at the population level.
FUNDING SUPPORT
This work was supported by the National Cancer Institute, the Centers for Disease Control and Prevention, the American Cancer Society, and the North American Association of Central Cancer Registries.
CONFLICT OF INTEREST DISCLOSURES
Stacey Fedewa, Jiemin Ma, and Ahmedin Jemal are employed by the American Cancer Society, which received a grant from Merck, Inc, for intramural research outside the submitted work; however, their salaries are solely funded through American Cancer Society funds.
AUTHOR CONTRIBUTIONS
Serban Negoita: Conceptualization, formal analysis, investigation, methodology, project administration, resources, supervision, validation, visualization, writing–original draft, and writing–review and editing. Eric J. Feuer: Conceptualization, formal analysis, investigation, methodology, software, validation, visualization, writing–original draft, and writing–review and editing. Angela Mariotto: Conceptualization, formal analysis, investigation, methodology, visualization, writing–original draft, and writing–review and editing. Kathleen A. Cronin: Conceptualization, formal analysis, investigation, methodology, visualization, writing–original draft, and writing–review and editing. Valentina I. Petkov: Conceptualization, methodology, visualization, and writing–review and editing. Sarah K. Hussey: Conceptualization, investigation, project administration, validation, visualization, writing–original draft, and writing–review and editing. Vicki Benard: Conceptualization, funding acquisition, supervision, and writing–review and editing. S. Jane Henley: Formal analysis, methodology, visualization, and writing–review and editing. Robert N. Anderson: Conceptualization, investigation, methodology, and writing–review and editing. Stacey Fedewa: Investigation and writing–review and editing. Recinda L. Sherman: Data curation and investigation. Betsy A. Kohler: Conceptualization and investigation. Barbara J. Dearmon: Conceptualization and writing–review and editing. Andrew J. Lake: Data curation, formal analysis, investigation, resources, software, validation, visualization, and writing–review and editing. Jiemin Ma: Investigation and writing–review and editing. Lisa C. Richardson: Conceptualization, supervision, and writing–review and editing. Ahmedin Jemal: Conceptualization, formal analysis, investigation, methodology, supervision, validation, visualization, and writing–review and editing. Lynne Penberthy: Conceptualization, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing–original draft, and writing–review and editing.
See companion article and editorial on pages 2785‐800 and 2690‐2, this issue.
This article has been contributed to by US Government employees, and their work is in the public domain in the United States.
The last 2 authors are co‐senior authors.
We gratefully acknowledge the contributions of the state and regional cancer registry staff for their work in collecting the data used in this study. In addition, we thank Danny Miller, Joe Zou, Steve Scoppa, and Rick Firth of Information Management Services, Inc, for their assistance in creating the data and generating the results used in this report.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official positions of the author's agencies (the Centers for Disease Control and Prevention, the National Cancer Institute, the American Cancer Society, the North American Association of Central Cancer Registries, and the National Cancer Registrars Association).
REFERENCES
- 1. Potosky AL, Miller BA, Albertsen PC, Kramer BS. The role of increasing detection in the rising incidence of prostate cancer. JAMA. 1995;273:548‐552. [PubMed] [Google Scholar]
- 2. Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;2012:152‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. US Preventive Services Task Force . Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:185‐191. [DOI] [PubMed] [Google Scholar]
- 4. US Preventive Services Task Force . Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120‐134. [DOI] [PubMed] [Google Scholar]
- 5. Hu JC, Nguyen P, Mao J, et al. Increase in prostate cancer distant metastases at diagnosis in the United States. JAMA Oncol. 2017;3:705‐707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jemal A, Fedewa SA, Ma J, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314:2054‐2061. [DOI] [PubMed] [Google Scholar]
- 7. Houston KA, King J, Li J, Jemal A. Trends in prostate cancer incidence rates and prevalence of prostate‐specific antigen screening by socioeconomic status and regions in the US, 2004‐2013. J Urol. 2018;199:676‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Comprehensive Cancer Network . NCCN clinical practice guidelines in oncology: prostate cancer. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed May 7, 2018.
- 9. Pettersson A, Robinson D, Garmo H, Holmberg L, Stattin P. Age at diagnosis and prostate cancer treatment and prognosis: a population‐based cohort study. Ann Oncol. 2018;29:377‐385. [DOI] [PubMed] [Google Scholar]
- 10. Clegg LX, Feuer EJ, Midthune DN, Fay MP, Hankey BF. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94:1537‐1545. [DOI] [PubMed] [Google Scholar]
- 11. Huang L, Midthune D, Krapcho M, Zou Z, Horner MJ, Feuer EJ. Adjusting for reporting delay in cancer incidence when combining different sets of cancer registries. Biom J. 2013;55:755‐770. [DOI] [PubMed] [Google Scholar]
- 12. Mariotto AB, Etzioni R, Krapcho M, Feuer EJ. Reconstructing PSA testing patterns between black and white men in the US from Medicare claims and the National Health Interview Survey. Cancer. 2007;109:1877‐1886. [DOI] [PubMed] [Google Scholar]
- 13. National Cancer Institute . SEER‐Medicare: about the data files. https://healthcaredelivery.cancer.gov/seermedicare/aboutdata/. Accessed October 12, 2017.
- 14. National Cancer Institute . Site recode. https://seer.cancer.gov/siterecode/. Accessed October 12, 2017.
- 15. NAACCR Research Application Review Workgroup . Information for CINA Deluxe Data Investigators. https://20tqtx36s1la18rvn82wcmpn-wpengine.netdna-ssl.com/wp-content/uploads/2017/02/Information-for-CINA-Deluxe-Investigators-2014.pdf. Accessed October 12, 2017.
- 16. North American Association of Central Cancer Registries . Registry data fitness for use by data year (1995‐2014). https://www.naaccr.org/cina-deluxe-for-researchers/. Accessed October 12, 2017.
- 17. Cronin KA, Lake AJ, Scott S, et al. The annual report to the nation on the status of cancer part I: national cancer statistics. Cancer. 2018;124:2785‐2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Surveillance, Epidemiology, and End Results Program . Summary Staging Guide for the Cancer Surveillance, Epidemiology, and End Results (SEER) Program. Bethesda, MD: National Institutes of Health; 1977. [Google Scholar]
- 19. Surveillance, Epidemiology, and End Results Program . Summary Staging Guide for the Cancer Surveillance, Epidemiology, and End Results (SEER) Program. Bethesda, MD: National Institutes of Health; 2000. [Google Scholar]
- 20. North American Association of Central Cancer Registries . Derived SS2000. http://datadictionary.naaccr.org/default.aspx?c=10#3020. Accessed October 12, 2017.
- 21. Surveillance, Epidemiology, and End Results Program . Research data (1973‐2014), National Cancer Institute, DCCPS, Surveillance Research Program, released April 2017, based on the November 2016 submission. https://seer.cancer.gov/data/. Accessed October 12, 2017.
- 22. Adamo MP, Boten JA, Coyle LM, et al. Validation of prostate‐specific antigen laboratory values recorded in Surveillance, Epidemiology, and End Results registries. Cancer. 2016;123:697‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amin MB, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. New York: Springer Nature; 2016. [Google Scholar]
- 24. Srigley JR, Humphrey PA, Amin MB, et al. Protocol for the examination of specimens from patients with carcinoma of the prostate gland. Version: prostate 4.0.0.0. http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebContent/pdf/prostate-12protocol-3200.pdf. Accessed October 12, 2017.
- 25. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL; ISUP Grading Committee . The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228‐1242. [DOI] [PubMed] [Google Scholar]
- 26. Adamo M, Dickie, L , Ruhl J. SEER Program Coding and Staging Manual 2016. Bethesda, MD: National Cancer Institute; 2016:100‐101. [Google Scholar]
- 27. North American Association of Central Cancer Registries . Derived AJCC‐6 T. http://datadictionary.naaccr.org/default.aspx?c=10#2940. Accessed October 12, 2017.
- 28. Greene FL, Page DL, Fleming ID, et al, eds. AJCC Cancer Staging Manual. 6th ed. Philadelphia, PA: J B Lippincott; 2002. [Google Scholar]
- 29. Thornton ML, ed. Standards for Cancer Registries Volume II: Data Standards and Data Dictionary, Record Layout Version 16. 20th ed. Springfield, IL: North American Association of Central Cancer Registries; 2015. [Google Scholar]
- 30. National Cancer Institute . Cancer incidence rates adjusted for reporting delay. https://surveillance.cancer.gov/delay/. Accessed April 11, 2017.
- 31. Surveillance Research Program . SEER*Stat Software. Version 8.3.4. Bethesda, MD: National Cancer Institute; 2017. [Google Scholar]
- 32. Surveillance Research Program . Joinpoint Regression Program. Version 4.5.0.1. Bethesda, MD: National Cancer Institute; 2017. [Google Scholar]
- 33. Berkowitz Z, Li J, Richards TB, Marcus PM. Patterns of prostate‐specific antigen test use in the U.S., 2005‐2015. Am J Prev Med. 2017;53:909‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stout NK, Knudsen AB, Kong CY, McMahon PM, Gazelle GS. Calibration methods used in cancer simulation models and suggested reporting guidelines. Pharmacoeconomics. 2009;27:533‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. US Preventive Services Task Force . Prostate cancer screening draft recommendation. http://www.screeningforprostatecancer.org. Accessed October 12, 2017.
- 36. Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169:517‐523. [DOI] [PubMed] [Google Scholar]
- 37. Epstein JI, Amin MB, Reuter VE, Humphrey PA. Contemporary Gleason grading of prostatic carcinoma: an update with discussion on practical issues to implement the 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2017;41:e1‐e7. [DOI] [PubMed] [Google Scholar]
- 38. Gulati R, Tsodikov A, Etzioni R, et al. Expected population impacts of discontinued prostate‐specific antigen screening. Cancer. 2014;120:3519‐3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. James ND, de Bono JS, Spears MR, et al.; STAMPEDE Investigators . Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Poorthuis MHF, Vernooij RWM, van Moorselaar RJA, de Reijke TM. First‐line non‐cytotoxic therapy in chemotherapy‐naive patients with metastatic castration‐resistant prostate cancer: a systematic review of 10 randomised clinical trials. BJU Int. 2017;119:831‐845. [DOI] [PubMed] [Google Scholar]
- 41. Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control. 2008;19:175‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shore N, Heidenreich A, Saad F. Predicting response and recognizing resistance: improving outcomes in patients with castration‐resistant prostate cancer. Urology. 2017;109:6‐18. [DOI] [PubMed] [Google Scholar]


