Abstract
Until the late 1990s class A G protein-coupled receptors (GPCRs) were believed to function as monomers. Then indirect evidence that they might internalize or even signal as dimers has emerged, along with proof that class C GPCRs are obligatory dimers. Crystal structures of GPCRs and their much larger binding partners were consistent with the idea that two receptors might engage a single G protein, GRK, or arrestin. However, recent biophysical, biochemical, and structural evidence invariably suggested that a single GPCR binds G proteins, GRKs, and arrestins. Here we review existing evidence of the stoichiometry of GPCR interactions with signal transducers and discuss potential biological roles of class A GPCR oligomers, including proposed homo- and heterodimers.
Keywords: GPCRs, oligomerization, G proteins, GRKs, arrestins
A Bit of History
Ever since single photon sensitivity of mammalian rod photoreceptors was demonstrated [1], the visual field was convinced that the activation of just one rhodopsin molecule was necessary and sufficient for signaling. The similarity to rhodopsin in sequence and membrane topology of the first hormone receptor cloned, the β2-adrenergic receptor (β2AR) [2], revealed the existence of a family of rhodopsin-like receptors, which was termed G protein-coupled receptors (GPCRs, see Glossary). This homology suggested functional similarity, so the field believed that all class A (rhodopsin-like) GPCRs signal as monomers (reviewed in [3]). However, indirect evidence that the process of internalization [4] and signaling [5] of some class A receptors can be explained by their dimerization accumulated. Unraveling the mechanisms of function of class C GPCRs, which are obligatory homo- or heterodimers (recently reviewed in [6]), inspired many to believe that class A receptors can also function as dimers (see [7,8] for review). The first GPCR crystal structure, that of rhodopsin [9], revealed a relatively small cytoplasmic tip with a diameter of no more than 44Å (Figure 1A). The structure of another prototypical GPCR, β2AR [10], appeared remarkably similar (Figure 1C). The cytoplasmic surfaces of activated rhodopsin [11] (Figure 1B) and β2AR [12] (Figure 1D) were also relatively small. In contrast, the structures of potential signal transducers, such as heterotrimeric G proteins [13,14] (Figure 1E), GRKs [15–19] (Figure 1F), and arrestins [20–23] (Figure 1G), showed a much greater potential footprint of these proteins (90–92Å), which specifically bind active GPCRs. Thus, it was fairly easy to structurally fit a G protein [8] or an arrestin [24] to a GPCR dimer. The debate regarding what constitutes the functional unit of class A GPCRs continues unabated to this day [25–28].
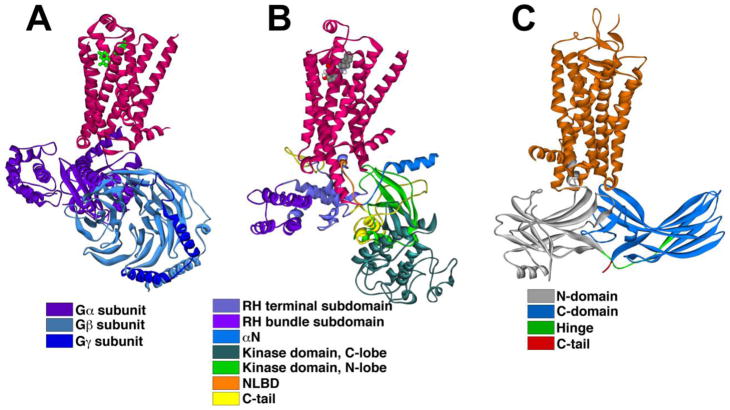
Figure 1. Crystal structures of GPCRs and signal transducers.
A–D. Crystal structures of inactive (A)(PDB ID: 1F88)[9] and active (B)(PDB ID: 5W0P)[77] rhodopsin, as well as inactive (C)(PDB ID: 2RH1) [10] and active (D)(PDB ID: 3SN6) [52] β2-adrenergic receptor. Note that the diameter of the cytoplasmic tip does not exceed 49A. E. Crystal structure of arrestin-2 (PDB ID: 1G4M)[21] with the elements colored, as follows: the N-domain, gray; the C-domain, blue; the inter-domain hinge, green; the part of the C-terminus anchored to the N-domain and visible in the structure, violet. F. Crystal structure of heterotrimeric G protein transducin (PDB ID: 1GOT) [14], with the subunits colored, as follows: guanyl nucleotide binding α-subunit, violet; β-subunit, teal; γ-subunit, dark blue. G. Crystal structure of GRK2 (PDB ID: 1OMW) [17], with the elements colored, as follows: RGS homology domain, light violet; kinase domain, dark green; C-terminus containing plekstrin homology domain, brown.
GPCR Superfamily
G protein-coupled receptors are the most numerous signaling proteins in animals, with 800–900 subtypes in primates (including humans) and bats, and a significantly greater variety in most mammalian species (http://sevens.cbrc.jp/). GPCRs are membrane proteins responding to a wide variety of stimuli: hormones, neurotransmitters, peptides, proteins, small molecule odorants, pheromones, light, extracellular calcium, protease activity, etc [29]. A large number of GPCRs are odorant/pheromone receptors, but every mammalian species expresses ~400 non-odorant GPCRs. Structurally, the only common part in all these receptors is an arrangement of seven trans-membrane helices (Figure 1A–D) (often termed the heptahelical domain, or HD), whereas the extracellular N-terminus and loops, as well as intracellular loops and the C-terminus widely vary in size and function, so that GPCRs range from fewer than 350 to more than 5,900 residues [30]. It appears that in all cases receptor activation requires the movement of the trans-membrane helices relative to each other, leading to the emergence of the cavity between them on the cytoplasmic side of the receptor [31,12].
The majority of the GPCRs belong to the three classes. Class A, or rhodopsin-like receptors, is by far the largest (more than 700 in humans) [32]. These GPCRs bind orthosteric ligands (those that bind to the same site as the native ligands)(or light-sensitive 11-cis-retinal in case of photopigments) in the HD between the helices closer to the extracellular surface. The binding of activating ligands (agonists) or light-induced isomerization of retinal directly causes the shift of the α-helices, which is the hallmark of receptor activation. Humans have 15 class B (secretin receptor-like) GPCRs [32]. These receptors have a large extracellular N-terminal domain, which contains the high-affinity binding site for their peptide ligands, with the lower affinity site localized between the HD helices, pretty much where the orthosteric ligands of class A receptors bind [30]. We have 15 class C GPCRs, which are obligatory dimers (reviewed in [6]). These receptors also have a large N-terminal domain, but it is different from class B: all class C GPCRs have a bilobal Venus flytrap domain (VFT), homologous to the periplasmic bacterial proteins that bind amino acids and ions, and most have a cysteine-rich domain between the VFT and HD. Class C dimers in most cases are stabilized by covalent sulfhydryl bonds, although separated VFTs form dimers even without S-S bonds, just like their bacterial ancestors [6]. Orthosteric ligands of class C receptors bind between the lobes of their VFT domains, inducing closed conformation of the VFT. Many of their allosteric modulators bind within the HD, pretty much where the orthosteric ligands of class A receptors bind [30]. In fact, when the HD of class C GPCR is expressed without its extracellular N-terminal elements, it can be activated by positive allosteric modulators binding within the HD just like class A GPCR are activated by their orthosteric agonists [33,34]. Some class C receptors are homodimers (e.g., calcium-sensing receptor and metabotropic glutamate receptors (mGluRs)), whereas others are heterodimers consisting of two different protomers (GABAB receptors [35,36], sweet and umami taste receptors [37]; mGluRs can also apparently form hetetodimers within subfamilies [38,39]). Interestingly, it has been established that in the GABAB receptor, the best studied heterodimer, only one VFT binds the ligand, whereas the HD of the other protomer couples to a G protein [35,36], suggesting that allosteric interactions between VFT and HD domains are necessary for receptor activation [6]. Inter-domain allosteric interactions and functional asymmetry, where only one protomer couples to the G protein, appear to apply to all class C receptors, although there are nuances. In homodimeric mGluRs, agonist binding to one VFT activates the receptor, but its binding to both VFTs further enhances the activity [6]. To the best of our knowledge, dimeric class C receptors can engage only one G protein molecule at a time.
Class A GPCRs in the Plasma Membrane
When the monomer-dimer equilibrium of class A GPCRs was studied in near-native conditions of the cellular plasma membrane, both states were shown to be transient [40]. The dimers of M1 muscarinic [40] and N-formylpeptide receptor [41] had very short lifetimes, of 0.5 s and 91 ms, respectively. However, the lifetime of the N-formylpeptide receptor monomer was also short, ~150 ms. Thus, at near-physiological expression level of ~6,000 receptors of this subtype per cell, at any given moment ~2,500 receptors existed as dimers and ~3,500 receptors as monomers [41]. Two recent studies addressed the dimerization of D2 dopamine receptors [42,43]. One found the half-life of a dimer being ~0.5 s at 24°C, and showed that the agonist increases the fraction of the D2 receptors present as dimers [42]. The other measured a much shorter dimer half-life, ~68 ms, at the physiological temperature of 37 °C [43]. The addition of an antagonist UH-232 did not change it, whereas the addition of agonists, dopamine or quinpirole, significantly increased the half-life of D2 receptor dimers to ~99 and ~104 ms, respectively [43]. Thus, the dimers of class A GPCRs do exist, even though they are fairly transient. At near-physiological receptor expression levels, GPCR monomers and dimers co-exist in equilibrium. These data suggest that dimerization of class A GPCRs has functional significance.
One group reported specific orthosteric ligand binding properties of the M2 muscarinic receptor in liposomes, where it can form oligomers, that were not reproduced in high density lipoprotein particles (nanodiscs) containing monomeric M2 receptor [44]. Similarly, a unique interplay between the binding of orthosteric and allosteric ligands was found in the M2 receptors in liposomes, that was absent in the case of nanodisc-reconstituted monomers [45]. Interpretation of ligand binding studies is far from straightforward, and is sometimes complicated by the fact that orthosteric ligands also bind to the allosteric site, as was shown by careful studies of the M2 muscarinic receptors [46]. In general, the ligand binding data essentially confirm that class A GPCR oligomers exist, as shown by single-molecule studies [40,43,41,42], but does not shed light on their functional role. Below we review potential functional roles of GPCR homodimers in signal transduction, focusing on biochemical and structural evidence that has emerged in the last 10 years, and then discuss the data used to suggest class A GPCR heterodimerization.
G proteins
The first question asked experimentally regarding GPCR-G protein interaction was whether a single monomeric GPCR could activate a heterotrimeric G protein. Both rhodopsin [47,48] and β2AR [49] reconstituted as monomers into the lipid bilayer in nanodiscs were shown to effectively couple to their cognate G proteins, transducin and Gs, respectively. Monomeric receptors facilitated G protein activation catalyzing the exchange of GDP bound to the inactive G protein for GTP. In the case of β2AR, high agonist affinity receptor-G protein complex was shown to be formed [49], just like in cell membranes or liposomes [50], where the receptor could have potentially oligomerized. Moreover, this complex demonstrated the same high sensitivity to GTP analog as a similar high agonist affinity complex formed by the β2AR in native membranes [49]. Interestingly, rhodopsin reconstituted at two molecules per nanodisc was only half as active per rhodopsin molecule, suggesting that only one of the two was activating G proteins [47], and is in agreement with a previous report that dimerization reduces G protein coupling of another GPCR, the neurotensin NTS1 receptor [51]. The crystal structure of the β2AR complex with Gs revealed a single receptor coupled to the heterotrimeric G protein, with the interaction largely mediated by the Gα subunit [52] (Figure 2A). The structure of another class A receptor, the adenosine A2A, with an engineered single-subunit G protein revealed the same 1:1 complex [53], although in this case one could argue that this artificial G protein was engineered to fit a single GPCR. Crystallization of GPCR complexes with interacting proteins remains tricky, so that other methods that yield structural information, such as cryo-electron microscopy (cryo-EM), are becoming popular. Recently cryo-EM structures with near-atomic resolution of two different class B receptors in complex with G protein also revealed the same 1:1 stoichiometry [54,55]. In contrast, there were no follow-up structures after reports of the isolation of pentameric complexes containing two GPCR molecules and a single heterotrimeric G protein [56,57]. Of course, one can argue that in all available crystal and cryo-EM structures only one type of G protein, Gs, was used. Yet transducin that couples to rhodopsin belongs to the Gi subfamily. Generally speaking, protein structures in crystals, which are usually obtained under highly non-physiological conditions, could be misleading. However, when many structures show the same thing, and it fully agrees with existing biochemical evidence, it has to be taken seriously. Undoubtedly, the structure of full-length truly dimeric class C GPCR with coupled G protein would be very illuminating. Yet the evidence available today, biochemical as well as structural, suggests that a single GPCR is necessary and sufficient to activate its cognate G protein. If anything, clustering of two GPCRs appears to impede G protein coupling [47,51], suggesting that in a dimer likely only one protomer interacts with the G protein, like in dimeric class C GPCRs [6]. Interestingly, oligomerization of the naturally dimeric GABAB receptor that belongs to class C also reduces its signaling via G proteins [58]. In fact, this phenomenon might reveal an underappreciated regulatory mechanism that needs to be further investigated.
Figure 2. Structures of GPCR complexes with signal transducers.
A. Crystal structure of the complex of β2-adrenergic receptor with cognate G protein Gs (PDB ID: 3SN6) [52]. B. Deduced structure of the complex of β2-adrenergic receptor with GRK5 [124]. C. Crystal structure of the complex of constitutively active rhodopsin with “pre-activated” arrestin-1 mutant (PDB ID: 5W0P) [77].
GRKs
GRKs were shown to be directly activated by physical interaction with an active GPCR [59]. Incorporation of monomeric GPCRs into nanodiscs was used to test whether a monomer is sufficient to activate GRK and serve as its substrate at the same time. First, these experiments were performed with wild type (WT) native rhodopsin purified from bovine retinas. Monomeric light-activated rhodopsin was found to successfully activate GRK1 (historic name - rhodopsin kinase) and become phosphorylated by it to the extent slightly exceeding that of the rhodopsin in the native disc membrane, where it was free to form oligomers [60]. Later the same was found to be true for the constitutively active rhodopsin mutants, also purified and reconstituted into nanodiscs as monomers, which were effectively phosphorylated by purified GRK1 [61,62]. Non-visual GRKs 2 and 5 were shown to phosphorylate the monomeric agonist-activated NTS1 receptor [63], as well as the monomeric β2AR [64], both purified and reconstituted into nanodiscs. Thus, biochemical experiments suggested that GPCR monomers effectively activate GRKs of all three subfamilies represented in vertebrates [65] and become phosphorylated by these GRKs. Structural studies later confirmed this 1:1 arrangement in case of β2AR complex with GRK5 [19] (Figure 2B) and rhodopsin complex with both GRK1 and GRK5 [66]. It should be noted that these are not crystal structures, but a generalized view based on several lines of experimental evidence supported by modeling. Thus, all available evidence suggests that a single active class A GPCR binds a GRK molecule and is phosphorylated by it. In view of these data, earlier reports that upon light activation of a small fraction of rhodopsin GRK1 can phosphorylate multiple inactive rhodopsin molecules [67,68], as well as being co-expressed in the same membrane inactive cone opsin [69], suggests that the complex of activated GRK1 with active rhodopsin lives long enough to allow other molecules of visual pigment to diffuse into its vicinity and become phosphorylated. In fact, these findings are in excellent agreement with the observation that GRK1 bound to light-activated rhodopsin becomes active and can phosphorylate any available substrate, including exogenously added peptides [59].
Arrestins
The first attempt to gauge the stoichiometry of the arrestin-receptor interaction was made in vivo, using established translocation of visual arrestin-1 (please note that we use systematic names of arrestin proteins, where the number after the dash indicates the order of cloning: arrestin-1 [historic names S-antigen, 48 kDa protein, visual or rod arrestin], arrestin-2 [β-arrestin or β-arrestin1], arrestin-3 [β-arrestin2 or hTHY-ARRX], and arrestin-4 [cone or X-arrestin]) to the outer segment of rod photoreceptors in the light, where it remains due to rhodopsin binding [70]. WT mice express eight molecules of arrestin-1 per ten rhodopsins [71,72]. The arrestin-1 translocation in genetically modified mouse lines expressing arrestin-1 and rhodopsin at different ratios, from 0.4 to 2.4, was measured [73]. In bright light ensuring virtually 100% light activation of rhodopsin, up to 8 molecules of arrestin-1 per 10 rhodopsins present translocated to the outer segment, suggesting that two rhodopsins cannot be needed to bind a single arrestin-1 [73]. Of course, one could argue that there are many proteins in the rod outer segment, and we cannot be sure that every molecule of translocated arrestin-1 actually bound rhodopsin in this compartment. To exclude participation of other proteins, the titration of fixed amount of purified phosphorylated rhodopsin with increasing amounts of purified arrestin-1 was performed. It revealed that the saturation is achieved at 0.99 + 0.08 mol/mol, i.e., at about 1:1 ratio [73]. However, visual arrestin-1 self-associates, forming dimers and tetramers [74,75]. So, these data did not exclude the role of rhodopsin oligomerization, as the binding of an arrestin dimer to a rhodopsin dimer could have yielded the same 1:1 ratio. A biophysical study of arrestin-1 oligomerization showed that only arrestin-1 monomer binds rhodopsin, or, rather, that when bound to rhodopsin, arrestin-1 becomes monomeric [74], thereby excluding dimer-to-dimer binding mode. Naturally, the next step was reconstitution of single molecules of phosphorylated rhodopsin into nanodiscs and testing whether it still binds arrestin-1. These experiments, performed independently by two different labs, showed that it does [76,60]. Monomeric rhodopsin in nanodiscs also bound non-visual arrestin-2 [76]. Moreover, it was shown that arrestin-1 binds monomeric active phosphorylated rhodopsin with the KD of 3–4 nM, i.e., with physiological high affinity [60]. Subsequent studies showed that arrestin-1 effectively binds monomeric constitutively active rhodopsin mutants as well [61,62]. Finally, a recent crystal structure of the arrestin-1-rhodopsin complex (Figure 2C) also revealed the 1:1 arrangement predicted by these studies [77,78]. The central arrestin “finger loop”, earlier implicated in direct receptor binding of arrestin-1 [79] and non-visual arrestin-2 [80], was inserted into the cavity between the trans-membrane helices that appears upon rhodopsin activation due to activation-induced movement of helices V and VI [31]. Importantly, the engagement of the inter-helical cavity by arrestin, which is an important part of the receptor-G protein interface [52], is consistent with earlier data that arrestin blocks G protein binding to the receptor via direct competition [81,82], and that arrestin-2 competes with GRK2 for the β2-adrenergic receptor [83]. The structure of the arrestin-rhodopsin complex was rigorously tested by H/D exchange, disulfide cross-linking between the two proteins, and distance measurements between selected points in rhodopsin and arrestin-1 using pulse EPR technique double electron-electron resonance [77,78]. It is also consistent with numerous lines of accumulated biological, biochemical and biophysical evidence (reviewed in [84]). Thus, similar to G proteins and GRKs, a single molecule of active phosphorylated GPCR is necessary and sufficient to bind arrestins.
New Twist: One Receptor, Two Signal Transducers
In full agreement with an old model positing that arrestin has two distinct receptor-binding elements, one engaging receptor-attached phosphates, and the other specifically recognizing active receptor conformation [85], two “flavors” of GPCR complexes with arrestin were visualized by cryo-EM [86]. One was “hanging”, with arrestin interacting solely with the phosphorylated receptor C-terminus, whereas the other appeared fully engaged, where this interaction was accompanied by the binding of the center of the arrestin molecule to the center of the cytoplasmic side of the receptor [86], closely resembling the crystal structure of the arrestin-1 complex with rhodopsin [77,78]. Clearly, in the hanging conformation arrestin does not block the inter-helical cavity necessary for the G protein binding [52]. Indeed, further studies revealed that a single GPCR molecule can bind G protein and arrestin simultaneously, which might explain sustained G protein signaling by internalized receptors [87]. Using arrestin lacking the finger loop, which can only bind GPCRs in hanging configuration, it was recently shown that this partially engaged arrestin mediates receptor internalization and ERK activation, but does not preclude G protein coupling, and therefore does not mediate receptor desensitization [88]. Similar results were obtained with mutant vasopressin receptor that did not bind the finger loop of arrestin, which is considered a hallmark of core engagement [89,90]. Collectively, these data suggest something that even the most devout monomer believers did not envision: simultaneous interaction of a single GPCR molecule with two potential signal transducers. One obvious caveat of these studies is that in all of them engineered receptors, β2AR with the C-terminus of V2 vasopressin receptor, β2AR with the deletion of the 3rd cytoplasmic loop to preclude the engagement of arrestin finger loop, or vasopressin receptor with the deletion in the 3rd cytoplasmic loop, were used, rather than native WT GPCRs. The other important caveat is that in neither of these studies the activation of ERK1/2 was shown to be strictly arrestin-mediated, independent of G proteins. This is particularly important in view of a recent study with the WT forms of these receptors, and many other GPCRs, showing that in the absence of G protein activation no arrestin-mediated signaling can be detected [91]. This issue needs to be resolved experimentally before any general conclusions are made. These results also appear to contradict a large body of evidence that the key function of arrestins is to preclude GPCR coupling to G proteins, thereby mediating desensitization (reviewed in [92]). However, these recent data are rather suggestive, although it remains to be elucidated whether this partial arrestin engagement by any naturally occurring GPCR actually happens and yields stable complexes.
GPCR Heterodimers
Numerous studies claimed the existence of class A GPCR heterodimers with properties distinct from those of their protomer components (Figure 3). Several studies suggested the existence of heterodimers based on the ligand binding properties. For example, co-expression of κ- and δ-opioid receptors was found to generate ligand-binding sites with properties not found in cells expressing these receptor subtypes individually [5]. Many studies have reported positive or negative cooperativity of agonist binding upon activation of the other protomer [93–95]. Then there is a more rare phenomenon of antagonist cross-inhibition, when an antagonist of one protomer blocks signaling via the other [96]. Ligand binding data extended the observations of the existence of class A GPCR homodimers in the plasma membrane to heterodimers. The key question is, does GPCR heterodimerization affect signaling?
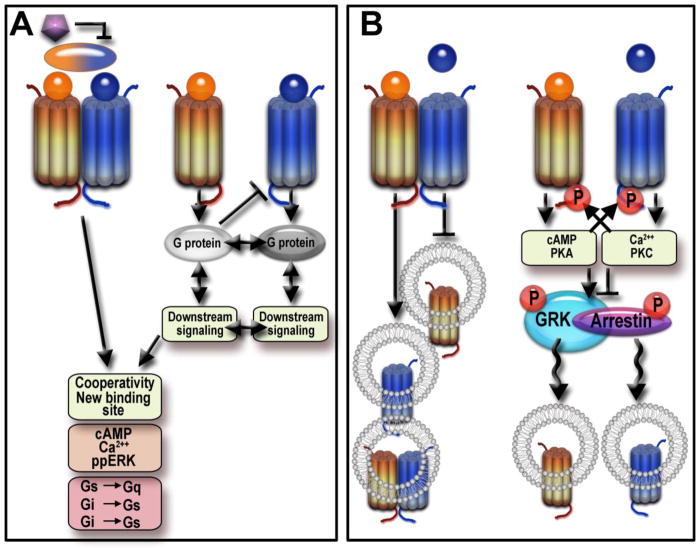
Figure 3. GPCR heterodimerization and possible alternative explanations.
A. Unique ligand binding characteristics and/or signaling, in cells co-expressing two different GPCRs was often explained by the formation of heterodimers (left panel). The ligand binding and signaling could be affected by simultaneous activation of both protomers or by ligand selective for the heterodimer (bi-color oval). Dimers could also display cross-antagonism when the binding of an antagonist (purple pentagon) to one protomer inhibits signaling via another. However, the observed phenomena can be explained equally well by signaling crosstalk (right panel). It can be at the level of G proteins as well as crosstalk of the signaling pathways downstream of G proteins. These processes can result in changes in ligand binding, second messenger (cAMP, Ca2+) concentrations, or signaling outcomes, such as ERK phosphorylation. Both Gs and Gi/o regulate the same pool of cAMP; the activity of various adenylyl cyclases can be modulated by intracellular Ca2+ and other factors. The level of Ca2+ can be regulated indirectly via downstream signaling affecting various Ca2+ channels. These phenomena are sometimes interpreted as a switch in GPCR coupling to a different G protein type. B. A change in trafficking pattern of a GPCR subtype co-expressed with another GPCR was also interpreted as evidence for the formation of heterodimers. Stimulation of one protomer could promote internalization of the other or both, or change the fate of the internalized receptor (left panel). However, alternative mechanisms that could mediate the effect of one GPCR on the trafficking of another without heterodimerization have been documented (right panel): for example, competition for the same pool of arrestins or arrestin binding due to receptor phosphorylation by second messenger-activated kinases. Moreover, both key actors in GPCR trafficking, GRKs and arrestins, are also regulated via phosphorylation by various kinases that can be affected by the signaling of another receptor. Thus, as it is virtually impossible to exclude all alternative explanations of the observations in cell-based experiments, the experiments performed so far do not definitively prove GPCR heterodimerization.
In some cases, co-expression of two different GPCRs was reported to result in a unique pattern of signaling or trafficking (Figure 3). The formation of the κ- and δ-opioid receptor heterodimers has been shown to potentiate the G protein-mediated signaling and MAP kinase activation [5]. The heterodimers of D1 and D2 dopamine receptors [97,98], angiotensin AT1 and α2C-adrenergic receptors [99], and melatonin MT1–MT2 receptors [100] coupled to different G protein species as compared to the protomers: Gq instead of Gs or Gi in case of the D1–D2 dimer, Gq instead of Gi in case of melatonin receptors, and Gs instead of Gi or Gq in case of α2c-AT1 dimer (Figure 3A). Altered signaling properties attributable to the heterodimer formation have been reported for numerous other GPCRs (for a comprehensive review, see [101]). The surface expression of the α1D adrenergic receptor in heterologous HEK293 cells requires its co-expression with the α1B- or β2-adrenoreceptor, whereas it is retained in the endoplasmic reticulum when expressed alone [102,103] - a situation clearly reminiscent of that of the stably heterodimeric class C GABAB receptor [104]. The removal of a GPCR from the cell membrane was also reported to be modified by heteromerization. For example, the presence of one protomer could suppress agonist-dependent internalization of the other [5] (Figure 3B). Alternatively, an agonist to one protomer could promote internalization of the partner protomer or both [105–108], or alter the fate of the internalized receptors [108,109] (Figure 3B). Unfortunately, studies of GPCR trafficking altered by coexpression of a different subtype usually do not take into account alternative ways one GPCR can affect the trafficking of another. These include documented competition of two GPCRs for a common pool of arrestins [110], the effects of GPCR phosphorylation by second messenger-activated kinases on arrestin binding [111], as well as the regulation of critical players in GPCR trafficking, GRKs and arrestins, by various protein kinases [65,112], the activity of which can be affected by the second GPCR.
It is important to keep in mind that many of these studies have been performed with GPCRs heterologously expressed in cultured cells. The field recognizes that GPCR over-expression in heterologous cultured cells can lead to artifacts due to crowding potentially exacerbated by the localization of certain GPCRs in membrane micro-domains that constitute only a small fraction of plasma membrane, as has been shown for neurotensin NTS1 receptors [113]. Therefore, lately the attention shifted to the demonstration of the existence of heteromers and the exploration of their potential functional role in native tissues. Three criteria were proposed for the proof of the existence of GPCR heterodimers: 1) heteromer components should colocalize and physically interact; 2) heteromers should exhibit properties distinct from those of the protomers; 3) heteromer disruption should lead to a loss of heteromer-specific properties [101].
To detect co-expression and, particularly, interaction of different GPCRs, numerous sophisticated methods have been used [114]. Although these techniques can demonstrate co-existence of the protomers within a limited space in cells, they generally do not have the ability to prove that the receptors are close enough to enable allosteric modulation, presumably via interaction of their HDs. Energy transfer-based methods show that the fluorescent or luminescent tags are within 40–45Å of each other, which, depending of the structure of particular GPCRs and the positions of these moieties in engineered receptors, can reflect the formation of a true dimer with interacting HDs, or report that the HDs of the two receptors are up to 500Å apart (discussed in [115]). Recently developed proximity ligation assay suffers from the same drawback: it only shows that the two receptors are within ~400Å of each other [114], which is many times greater than the diameter of the HD of any GPCR (Figure 1). Complementation-based methods, where the two receptors are tagged with two parts of the same protein (e.g., GFP) that becomes functional only after the receptors come together were also used. A major flaw of these methods is that complementation is irreversible. Random encounter of the two diffusing proteins can yield complementation, forcing them to stay together regardless of the specificity of their interaction. Thus, all available methods can demonstrate that the two receptors are fairly close to each other or at least were close at some point in time, but do not prove that they specifically interact and form a dimer.
A review of GPCR heterodimers reported to exist in native tissues concluded that in very few cases all three criteria have been met [101]. Furthermore, even when they apparently were, the evidence can still be insufficient or misleading (Figure 3). Putative dopamine D1 and D2 receptor heterodimer offers an instructive example. D1 receptors couple to Gs, whereas D2 couple to Gi/o. An interesting report suggested that the D1–D2 heterodimer has a unique ability to couple to Gq that neither individual subtype had [97,98]. The claim was supported by a significant amount of the in vitro and in vivo data obtained with the use of a wide array of experimental tools apparently demonstrating the existence, unique signaling properties, and the functional role of the heterodimer [98,97,116–119]. Although it is very hard to prove a negative, a recent comprehensive study using experiments in cell culture, brain slices, and genetically modified mice presented compelling evidence that while D1–D2 heterodimers can be formed in over-expressing cultured cells, they do not exist in the mouse brain, where the two receptor subtypes are not colocalized even in neurons co-expressing them [120]. That study showed that SKF83959-induced motor responses in mice, which were ascribed to the coupling of D1–D2 dimers to Gq, require the D1 receptor but not the D2, the dimer, or Gq-mediated signaling, since these responses were preserved in both D2 and Gq knockout animals [120]. This example shows how careful one has to be claiming that certain class A GPCR heterodimers detectable in over-expressing cells exist and possess unique signaling properties in vivo [121].
There are other ambiguities in methods used to demonstrate the existence of GPCR heterodimers. Often the data interpreted as the evidence of heterodimerization could be equally satisfactorily explained by signaling crosstalk (Figure 3). Unique ligand binding, in addition to the existence of transient heterodimers similar to the transient homodimers [40,43,41,42], can reflect that one receptor depletes the G protein necessary for the other. While this mechanism was discussed regarding agonist binding [114], G proteins were recently shown to affect the binding of antagonists, as well [122]. Unique trafficking properties of a GPCR co-expressed with another subtype can be the result of the activity of GRK bound to one receptor towards another brought close to it by lateral diffusion, as was demonstrated in case of photopigments [69,67,68], or even of phosphorylation of one GPCR by second messenger-activated protein kinases activated by another receptor [111]. A GPCR can also deplete proteins, such as arrestins, in the cell, thereby causing changes in the trafficking of another subtype [110]. In fact, the unique signaling pattern attributed to heterodimers has never been unequivocally proven to be the result of oligomerization of different GPCRs, rather than the interplay of signaling pathways in the cell [3]. Generally speaking, there is only one type of experiment that would prove beyond reasonable doubt the existence and unique functional characteristics of putative GPCR heterodimers. Two different native GPCRs must be purified and reconstituted into phospholipids (liposomes, nanodiscs, or bicelles) separately and together. It is important to mimic the lipid composition of the native membrane: dimerization of serotonin 5HT2A receptor was found to require cholesterol, whereas upon cholesterol depletion this receptor exists exclusively as a monomer [123]. If joint reconstitution leads to an activation of purified G protein type that individual receptors do not activate, or the binding of purified arrestin that individual receptors do not bind, or the interactions with a particular G protein or arrestin in response to a ligand of one GPCR that does induce this interaction when this receptor is reconstituted alone, one can be certain that heterodimers with unique functional properties are actually formed. So far, this type of experiment was never performed, so the definitive proof of the existence of GPCR heterodimers is lacking.
Finally, it is important to keep in mind that specific antibodies, bivalent ligands, or any other means that can simply force the dimerization of two different GPCRs might have therapeutic value even if the heterodimer in question is never formed naturally. Thus, in and of itself therapeutic usefulness of the tools of this kind does not prove (or disprove) the existence of GPCR homo- or heterodimers.
Concluding Remarks
So far all available data with class A and class B GPCRs indicate that a single receptor is necessary and sufficient to bind all three classes of proteins that specifically recognize active receptors: G proteins, GRKs, and arrestins. This statement is based on a huge body of biological, biochemical, and biophysical evidence. It is illustrated by structures of the complexes in Figure 2 not because crystallography is considered to be the ultimate proof, but simply because the structures provide a very clear picture. Despite the profusion of indirect evidence, not a single case was reported where a dimer or higher order oligomer of class A or B GPCR is necessary for signaling. However, as single-molecule experiments invariably show the existence of transient GPCR homodimers in equilibrium with transient monomers, and these data are supported by complex ligand binding kinetics in some cases, the dimers likely have biological functions. Numerous cell culture experiments with heterologously expressed GPCRs, as well as studies in native tissues and living animals, have demonstrated that some class A GPCRs might heterodimerize producing signaling units with properties different from those of the protomers. However, heterodimerization of class A GPCRs has not been proved to the exclusion of alternative mechanisms that do not imply receptor dimerization. The lifecycle of a GPCR is complex: it includes maturation and trafficking to the plasma membrane from the endoplasmic reticulum through Golgi, signaling, as well as endocytosis and subsequent sorting of internalized receptors. Thus, as homo- or heterodimers and higher order oligomers do not appear to be required for signaling, they might serve as inactive “storage” forms in the plasma membrane, or play a role at other stages of the GPCR lifecycle. Elucidation of the biological role(s) of class A and B GPCR oligomers, which are formed but do not apparently participate in GPCR signaling, is the next challenge (see Outstanding questions).
Outstanding questions.
Is reduced signaling capability of class A GPCR dimers a general rule?
Can GPCRs simultaneously engage more than one signal transducer?
Do oligomeric forms of class A GPCRs play a role in receptor maturation?
Do oligomeric forms of class A GPCRs play a role in receptor trafficking from ER via Golgi to the plasma membrane?
Do oligomers of class A GPCRs play a role in receptor internalization?
Do oligomers of class A GPCRs play a role in post-internalization sorting of receptors?
Do oligomers of class A GPCRs play a role in receptor ubiquitination and/or deubiquitination?
Highlights.
Class A GPCRS exist in monomer-dimer equilibrium
Monomeric class A GPCRs effectively activate G proteins, are phosphorylated by GRKs, and bind arrestins
GPCR oligomers may play a role at other stages of the receptor lifecycle that do not involve signaling
Acknowledgments
Supported in part by NIH RO1 grants EY011500, GM077561 and GM109955 (the latter two RO1s were replaced by R35 GM122491) (VVG), NS065868 and DA030103 (EVG).
Glossary
- GPCRs
G protein-coupled receptors. Common core of all GPCRs consists of seven trans-membrane helices (hence the synonym of GPCRs, seven trans-membrane domain receptors, or 7TMRs), whereas extracellular N-terminus and loops, as well as intracellular C-terminus and loops widely vary in size. Three classes of proteins preferentially bind active GPCRs: heterotrimeric G proteins, which were the first discovered signal transducers, GPCR kinases (GRKs), and arrestins.
- Heterotrimeric G proteins
Guanyl nucleotide binding proteins that consist of three subunits, α, β, and γ. The α-subunit is ~42–44 kDa. It consists of a~ 20 kDa Ras domain (homologous to small G proteins) and helical domain, with the nucleotide bound in the cleft between these two domains. G proteins selectively bind to active GPCRs. GPCRs serve as guanyl nucleotide exchange factors (GEFs) for heterotrimeric G proteins. Receptor binding facilitates the release of GDP bound to an inactive G protein, whereupon GTP, which is much more abundant in the cytoplasm binds. GTP binding induces G protein dissociation from GPCR, as well as the dissociation of Gα-GTP and Gβγ dimer, both of which regulate various effectors. Mammals express ~20 different α-subunits and smaller numbers of β- and γ-subunits.
- GRKs
G protein-coupled receptor kinases that preferentially phosphorylate active GPCRs. The reason for this specificity (in contrast to other protein kinases that recognize certain sequences around targeted residues) is believed to be that GRKs are activated by physical binding to active GPCRs. GRKs belong to the AGC kinase family (to which PKA, PKG, and PKC also belong). Most mammals express seven GRK subtypes (nocturnal rodents express six, lacking GRK7, which is specifically expressed in cone photoreceptors that function in relatively bright light).
- Arrestins
44–48 kDa proteins that specifically bind active phosphorylated GPCRs. Vertebrates have four subtypes: arrestin-1 and -4 are specialized visual, and are predominantly expressed in photoreceptor cells in the retina, whereas arrestin-2 and -3 (a.k.a. β-arrestins 1 and 2) are non-visual, and are expressed in virtually every cell. These two arrestin subtypes bind hundreds of different GPCRs and dozens of non-receptor protein partners, mediating signaling in numerous pathways.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baylor DA, Lamb TD, Yau KW. Responses of retinal rods to single photons. J Physiol. 1979;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon RA, Kobilka BK, Strader DJ, Benovic JL, Dohlman HG, Frielle T, Bolanowski MA, Bennett CD, Rands E, Diehl RE, Mumford RA, Slater EE, Sigal IS, Caron MG, Lefkowitz RJ, Strader CD. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986;321:75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- 3.Chabre M, le Maire M. Monomeric G-protein-coupled receptor as a functional unit. Biochemistry. 2005;44:9395–9403. doi: 10.1021/bi050720o. [DOI] [PubMed] [Google Scholar]
- 4.Cvejic S, Devi LA. Dimerization of the delta opioid receptor: implication for a role in receptor internalization. J Biol Chem. 1997;272(43):26959–26964. doi: 10.1074/jbc.272.43.26959. [DOI] [PubMed] [Google Scholar]
- 5.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399(6737):697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pin JP, Bettler B. Organization and functions of mGlu and GABAB receptor complexes. Nature. 2016;540(7631):60–66. doi: 10.1038/nature20566. [DOI] [PubMed] [Google Scholar]
- 7.Milligan G. G protein-coupled receptor dimerisation: molecular basis and relevance to function. Biochim Biophys Acta. 2007;1768:825–835. doi: 10.1016/j.bbamem.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Fotiadis D, Jastrzebska B, Philippsen A, Muller DJ, Palczewski K, Engel A. Structure of the rhodopsin dimer: a working model for G-protein-coupled receptors. Curr Opin Struct Biol. 2006;16:252–259. doi: 10.1016/j.sbi.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, LeTrong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, WIW, Kobilka BK. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- 11.Deupi X, Edwards P, Singhal A, Nickle B, Oprian D, Schertler G, Standfuss J. Stabilized G protein binding site in the structure of constitutively active metarhodopsin-II. Proc Natl Acad Sci U S A. 2012;109(1):119–124. doi: 10.1073/pnas.1114089108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, Devree BT, Rosenbaum DM, Thian FS, Kobilka TS, Schnapp A, Konetzki I, Sunahara RK, Gellman SH, Pautsch A, Steyaert J, Weis WI, Kobilka BK. Structure of a nanobody-stabilized active state of the β(2) adrenoceptor. Nature. 2011;469(7329):175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wall MA, Coleman DE, Lee E, Iñiguez-Lluhi JA, Posner BA, Gilman AG, Sprang SR. The structure of the G protein heterotrimer Giα1 β1γ2. Cell. 1995;83(6):1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 14.Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, Sigler PB. The 2.0 Å crystal structure of a heterotrimeric G protein. Nature. 1996;379(6563):311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- 15.Lodowski DT, Tesmer VM, Benovic JL, Tesmer JJ. The structure of G protein-coupled receptor kinase (GRK)-6 defines a second lineage of GRKs. J Biol Chem. 2006;281(24):16785–16793. doi: 10.1074/jbc.M601327200. [DOI] [PubMed] [Google Scholar]
- 16.Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJ. Snapshot of activated G proteins at the membrane: the Galphaq-GRK2-Gbetagamma complex. Science. 2005;310(5754):1686–1690. doi: 10.1126/science.1118890. [DOI] [PubMed] [Google Scholar]
- 17.Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJ. Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gbetagamma. Science. 2003;300(5623):1256–1262. doi: 10.1126/science.1082348. [DOI] [PubMed] [Google Scholar]
- 18.Homan KT, Waldschmidt HV, Glukhova A, Cannavo A, Song J, Cheung JY, Koch WJ, Larsen SD, Tesmer JJ. Crystal Structure of G Protein-coupled Receptor Kinase 5 in Complex with a Rationally Designed Inhibitor. J Biol Chem. 2015;290(34):20649–20659. doi: 10.1074/jbc.M115.647370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komolov KE, Bhardwaj A, Benovic JL. Atomic Structure of GRK5 Reveals Distinct Structural Features Novel for G Protein-coupled Receptor Kinases. J Biol Chem. 2015;290(34):20629–20647. doi: 10.1074/jbc.M115.647297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirsch JA, Schubert C, Gurevich VV, Sigler PB. The 2.8 A crystal structure of visual arrestin: a model for arrestin’s regulation. Cell. 1999;97(2):257–269. doi: 10.1016/s0092-8674(00)80735-7. [DOI] [PubMed] [Google Scholar]
- 21.Han M, Gurevich VV, Vishnivetskiy SA, Sigler PB, Schubert C. Crystal structure of beta-arrestin at 1.9 A: possible mechanism of receptor binding and membrane translocation. Structure. 2001;9(9):869–880. doi: 10.1016/s0969-2126(01)00644-x. [DOI] [PubMed] [Google Scholar]
- 22.Sutton RB, Vishnivetskiy SA, Robert J, Hanson SM, Raman D, Knox BE, Kono M, Navarro J, Gurevich VV. Crystal Structure of Cone Arrestin at 2.3 Å: Evolution of Receptor Specificity. J Mol Biol. 2005;354:1069–1080. doi: 10.1016/j.jmb.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 23.Zhan X, Gimenez LE, Gurevich VV, Spiller BW. Crystal structure of arrestin-3 reveals the basis of the difference in receptor binding between two non-visual arrestins. J Mol Biol. 2011;406:467–478. doi: 10.1016/j.jmb.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modzelewska A, Filipek S, Palczewski K, Park PS. Arrestin interaction with rhodopsin: conceptual models. Cell Biochem Biophys. 2006;46(1):1–15. doi: 10.1385/CBB:46:1:1. [DOI] [PubMed] [Google Scholar]
- 25.Lambert NA, Javitch JA. CrossTalk opposing view: Weighing the evidence for class A GPCR dimers, the jury is still out. J Physiol. 2014;592(12):2443–2445. doi: 10.1113/jphysiol.2014.272997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambert NA, Javitch JA. Rebuttal from Nevin A. Lambert and Jonathan A. Javitch. J Physiol. 2014;592(12):2449. doi: 10.1113/jphysiol.2014.274241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouvier M, Hébert TE. CrossTalk proposal: Weighing the evidence for Class A GPCR dimers, the evidence favours dimers. J Physiol. 2014;592(12):2439–2441. doi: 10.1113/jphysiol.2014.272252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouvier M, Hébert TE. Rebuttal from Michel Bouvier and Terence E. Hébert. J Physiol. 2014;592(12):2447. doi: 10.1113/jphysiol.2014.274233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bockaert J, Pin JP. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J. 1999;18(7):1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 31.Farrens DL, Altenbach C, Yang K, Hubbell WL, Khorana HG. Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science. 1996;274(5288):768–770. doi: 10.1126/science.274.5288.768. [DOI] [PubMed] [Google Scholar]
- 32.Stevens RC, Cherezov V, Katritch V, Abagyan R, Kuhn P, Rosen H, Wüthrich K. The GPCR Network: a large-scale collaboration to determine human GPCR structure and function. Nat Rev Drug Discov. 2013;12(1):25–34. doi: 10.1038/nrd3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Binet V, Brajon C, Le Corre L, Acher F, Pin JP, Prezeau L. The heptahelical domain of GABA(B2) is activated directly by CGP7930, a positive allosteric modulator of the GABA(B) receptor. J Biol Chem. 2004;279:29085–29091. doi: 10.1074/jbc.M400930200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Binet V, Duthey B, Lecaillon J, Vol C, Quoyer J, Labesse G, Pin JP, Prezeau L. Common structural requirements for heptahelical domain function in class A and class C G protein-coupled receptors. J Biol Chem. 2007;282:12154–12163. doi: 10.1074/jbc.M611071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galvez T, Duthey B, Kniazeff J, Blahos J, Rovelli G, Bettler B, Prezeau L, Pin JP. Allosteric interactions between GB1 and GB2 subunits are required for optimal GABA(B) receptor function. EMBO J. 2001;20:2152–2159. doi: 10.1093/emboj/20.9.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galvez T, Prezeau L, Milioti G, Franek M, Joly C, Froestl W, Bettler B, Bertrand HO, Blahos J, Pin JP. Mapping the agonist-binding site of GABAB type 1 subunit sheds light on the activation process of GABAB receptors. J Biol Chem. 2000;275:41166–41174. doi: 10.1074/jbc.M007848200. [DOI] [PubMed] [Google Scholar]
- 37.Xu H, Staszewski L, Tang H, Adler E, Zoller M, Li X. Different functional roles of T1R subunits in the heteromeric taste receptors. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(39):14258–14263. doi: 10.1073/pnas.0404384101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levitz J, Habrian C, Bharill S, Fu Z, Vafabakhsh R, Isacoff EY. Mechanism of Assembly and Cooperativity of Homomeric and Heteromeric Metabotropic Glutamate Receptors. Neuron. 2016;92(1):143–159. doi: 10.1016/j.neuron.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreno Delgado D, Müller TC, Ster J, Giraldo J, Maurel D, Rovira X, Scholler P, Zwier JM, Perroy J, Durroux T, Trinquet E, Prezeau L, Rondard P, Pin J-P. Pharmacological evidence for a metabotropic glutamate receptor heterodimer in neuronal cells. eLife. 2017;6:e25233. doi: 10.7554/eLife.25233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hern JA, Baig AH, Mashanov GI, Birdsall B, Corrie JE, Lazareno S, Molloy JE, Birdsall NJ. Formation and dissociation of M1 muscarinic receptor dimers seen by total internal reflection fluorescence imaging of single molecules. Proc Natl Acad Sci U S A. 2010;107(6):2693–2698. doi: 10.1073/pnas.0907915107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasai RS, Suzuki KG, Prossnitz ER, Koyama-Honda I, Nakada C, Fujiwara TK, Kusumi A. Full characterization of GPCR monomer-dimer dynamic equilibrium by single molecule imaging. J Cell Biol. 2011;192(3):463–480. doi: 10.1083/jcb.201009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tabor A, Weisenburger S, Banerjee A, Purkayastha N, Kaindl JM, Hubner H, Wei L, Gromer TW, Kornhuber J, Tschammer N, Birdsall NJ, Mashanov GI, Sandoghdar V, Gmeiner P. Visualization and ligand-induced modulation of dopamine receptor dimerization at the single molecule level. Sci Rep. 2016;6:33233. doi: 10.1038/srep33233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kasai RS, Ito SV, Awane RM, Fujiwara TK, Kusumi A. The Class-A GPCR Dopamine D2 Receptor Forms Transient Dimers Stabilized by Agonists: Detection by Single-Molecule Tracking. Cell Biochem Biophys. 2017 doi: 10.1007/s12013-017-0829-y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Redka DS, Morizumi T, Elmslie G, Paranthaman P, Shivnaraine RV, Ellis J, Ernst OP, Wells JW. Coupling of g proteins to reconstituted monomers and tetramers of the M2 muscarinic receptor. J Biol Chem. 2014;289(35):24347–24365. doi: 10.1074/jbc.M114.559294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shivnaraine RV, Kelly B, Sankar KS, Redka DS, Han YR, Huang F, Elmslie G, Pinto D, Li Y, Rocheleau JV, Gradinaru CC, Ellis J, Wells JW. Allosteric modulation in monomers and oligomers of a G protein-coupled receptor. Elife. 2016;5:e11685. doi: 10.7554/eLife.11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Redka DS, Pisterzi LF, Wells JW. Binding of orthosteric ligands to the allosteric site of the M(2) muscarinic cholinergic receptor. Mol Pharmacol. 2008;74(3):834–843. doi: 10.1124/mol.108.048074. [DOI] [PubMed] [Google Scholar]
- 47.Bayburt TH, Leitz AJ, Xie G, Oprian DD, Sligar SG. Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J Biol Chem. 2007;282:14875–14881. doi: 10.1074/jbc.M701433200. [DOI] [PubMed] [Google Scholar]
- 48.Whorton MR, Jastrzebska B, Park PS, Fotiadis D, Engel A, Palczewski K, Sunahara RK. Efficient coupling of transducin to monomeric rhodopsin in a phospholipid bilayer. J Biol Chem. 2008;283(7):4387–4394. doi: 10.1074/jbc.M703346200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whorton MR, Bokoch MP, Rasmussen SG, Huang B, Zare RN, Kobilka B, Sunahara RK. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci U S A. 2007;104:7682–7687. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samama P, Cotecchia S, Costa T, Lefkowitz RJ. A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J Biol Chem. 1993;268(7):4625–4636. [PubMed] [Google Scholar]
- 51.White JF, Grodnitzky J, Louis JM, Trinh LB, Shiloach J, Gutierrez J, Northup JK, Grisshammer R. Dimerization of the class A G protein-coupled neurotensin receptor NTS1 alters G protein interaction. Proc Natl Acad Sci U S A. 2007;104:12199–12204. doi: 10.1073/pnas.0705312104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah ST, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carpenter B, Nehmé R, Warne T, Leslie AG, Tate CG. Structure of the adenosine A(2A) receptor bound to an engineered G protein. Nature. 2016;536(7614):104–107. doi: 10.1038/nature18966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang YL, Khoshouei M, Radjainia M, Zhang Y, Glukhova A, Tarrasch J, Thal DM, Furness SGB, Christopoulos G, Coudrat T, Danev R, Baumeister W, Miller LJ, Christopoulos A, Kobilka BK, Wootten D, Skiniotis G, Sexton PM. Phase-plate cryo-EM structure of a class B GPCR-G-protein complex. Nature. 2017;546(7656):118–123. doi: 10.1038/nature22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Sun B, Feng D, Hu H, Chu M, Qu Q, Tarrasch JT, Li S, Kobilka TS, Kobilka BK, Skiniotis G. Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature. 2017;546(7657):248–253. doi: 10.1038/nature22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baneres JL, Parello J. Structure-based analysis of GPCR function: evidence for a novel pentameric assembly between the dimeric leukotriene B4 receptor BLT1 and the G-protein. J Mol Biol. 2003;329:815–829. doi: 10.1016/s0022-2836(03)00439-x. [DOI] [PubMed] [Google Scholar]
- 57.Jastrzebska B, Fotiadis D, Jang GF, Stenkamp RE, Engel A, Palczewski K. Functional and structural characterization of rhodopsin oligomers. J Biol Chem. 2006;281:11917–11922. doi: 10.1074/jbc.M600422200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Comps-Agrar L, Kniazeff J, Nørskov-Lauritsen L, Maurel D, Gassmann M, Gregor N, Prézeau L, Bettler B, Durroux T, Trinquet E, Pin JP. The oligomeric state sets GABA(B) receptor signalling efficacy. EMBO J. 2011;30(12):2336–2349. doi: 10.1038/emboj.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palczewski K, Buczylko J, Kaplan MW, Polans AS, Crabb JW. Mechanism of rhodopsin kinase activation. J Biol Chem. 1991;266:12949–12955. [PubMed] [Google Scholar]
- 60.Bayburt TH, Vishnivetskiy SA, McLean M, Morizumi T, Huang C-c, Tesmer JJ, Ernst OP, Sligar SG, Gurevich VV. Rhodopsin monomer is sufficient for normal rhodopsin kinase (GRK1) phosphorylation and arrestin-1 binding. J Biol Chem. 2011;286:1420–1428. doi: 10.1074/jbc.M110.151043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singhal A, Ostermaier MK, Vishnivetskiy SA, Panneels V, Homan KT, Tesmer JJ, Veprintsev D, Deupi X, Gurevich VV, Schertler GF, Standfuss J. Insights into congenital night blindness based on the structure of G90D rhodopsin. EMBO Rep. 2013;14(6):520–526. doi: 10.1038/embor.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vishnivetskiy SA, Ostermaier MK, Singhal A, Panneels V, Homan KT, Glukhova A, Sligar SG, Tesmer JJ, Schertler GF, Standfuss J, Gurevich VV. Constitutively active rhodopsin mutants causing night blindness are effectively phosphorylated by GRKs but differ in arrestin-1 binding. Cell Signal. 2013;25(11):2155–2162. doi: 10.1016/j.cellsig.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inagaki S, Ghirlando R, Vishnivetskiy SA, Homan KT, White JF, Tesmer JJ, Gurevich VV, Grisshammer R. G Protein-Coupled Receptor Kinase 2 (GRK2) and 5 (GRK5) Exhibit Selective Phosphorylation of the Neurotensin Receptor in Vitro. Biochemistry. 2015;54(28):4320–4329. doi: 10.1021/acs.biochem.5b00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li L, Homan KT, Vishnivetskiy SA, Manglik A, Tesmer JJ, Gurevich VV, Gurevich EV. G Protein-coupled Receptor Kinases of the GRK4 Protein Subfamily Phosphorylate Inactive G Protein-coupled Receptors (GPCRs) J Biol Chem. 2015;290(17):10775–10790. doi: 10.1074/jbc.M115.644773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gurevich EV, Tesmer JJ, Mushegian A, Gurevich VV. G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol Ther. 2012;133(1):40–46. doi: 10.1016/j.pharmthera.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He Y, Gao X, Goswami D, Hou L, Pal K, Yin Y, Zhao G, Ernst OP, Griffin P, Melcher K, Xu HE. Molecular assembly of rhodopsin with G protein-coupled receptor kinases. Cell Res. 2017;27(6):728–747. doi: 10.1038/cr.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Binder BM, Biernbaum MS, Bownds MD. Light activation of one rhodopsin molecule causes the phosphorylation of hundreds of others. A reaction observed in electropermeabilized frog rod outer segments exposed to dim illumination. J Biol Chem. 1990;265:15333–15340. [PubMed] [Google Scholar]
- 68.Binder BM, O’Connor TM, Bownds MD, Arshavsky VY. Phosphorylation of non-bleached rhodopsin in intact retinas and living frogs. J Biol Chem. 1996;271:19826–19830. doi: 10.1074/jbc.271.33.19826. [DOI] [PubMed] [Google Scholar]
- 69.Shi GW, Chen J, Concepcion F, Motamedchaboki K, Marjoram P, Langen R, Chen J. Light causes phosphorylation of nonactivated visual pigments in intact mouse rod photoreceptor cells. J Biol Chem. 2005;280:41184–41191. doi: 10.1074/jbc.M506935200. [DOI] [PubMed] [Google Scholar]
- 70.Nair KS, Hanson SM, Mendez A, Gurevich EV, Kennedy MJ, Shestopalov VI, Vishnivetskiy SA, Chen J, Hurley JB, Gurevich VV, Slepak VZ. Light-dependent redistribution of arrestin in vertebrate rods is an energy-independent process governed by protein-protein interactions. Neuron. 2005:46. doi: 10.1016/j.neuron.2005.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Strissel KJ, Sokolov M, Trieu LH, Arshavsky VY. Arrestin translocation is induced at a critical threshold of visual signaling and is superstoichiometric to bleached rhodopsin. J Neurosci. 2006;26:1146–1153. doi: 10.1523/JNEUROSCI.4289-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song X, Vishnivetskiy SA, Seo J, Chen J, Gurevich EV, Gurevich VV. Arrestin-1 expression in rods: balancing functional performance and photoreceptor health. Neuroscience. 2011;174:37–49. doi: 10.1016/j.neuroscience.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hanson SM, Gurevich EV, Vishnivetskiy SA, Ahmed MR, Song X, Gurevich VV. Each rhodopsin molecule binds its own arrestin. Proc Nat Acad Sci USA. 2007;104:3125–3128. doi: 10.1073/pnas.0610886104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hanson SM, Van Eps N, Francis DJ, Altenbach C, Vishnivetskiy SA, Klug CS, Hubbell WL, Gurevich VV. Structure and function of the visual arrestin oligomer. EMBO J. 2007;26:1726–1736. doi: 10.1038/sj.emboj.7601614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim M, Hanson SM, Vishnivetskiy SA, Song X, Cleghorn WM, Hubbell WL, Gurevich VV. Robust self-association is a common feature of mammalian visual arrestin-1. Biochemistry. 2011;50:2235–2242. doi: 10.1021/bi1018607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsukamoto H, Sinha A, Dewitt M, Farrens DL. Monomeric Rhodopsin Is the Minimal Functional Unit Required for Arrestin Binding. J Mol Biol. 2010;399:501–511. doi: 10.1016/j.jmb.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kang Y, Zhou XE, Gao X, He Y, Liu W, Ishchenko A, Barty A, White TA, Yefanov O, Han GW, Xu Q, de Waal PW, Ke J, Tan MHE, Zhang C, Moeller A, West GM, Van Eps N, Caro LN, Vishnivetskiy SA, Lee RJ, Suino-Powell KM, Gu X, Pal K, Ma J, Zhi X, Boutet S, Williams GJ, Messerschmidt M, Gati C, Zatsepin NA, Wang D, James D, Basu S, Roy-Chowdhuty S, Conrad S, Coe J, Liu H, Lisova S, Kupitz C, Grotjohann I, Fromme R, Jiang Y, Tan M, Yang H, Li J, Wang M, Zheng Z, Li D, Zhao Y, Standfuss J, Diederichs K, Dong Y, Potter CS, Carragher B, Caffrey M, Jiang H, Chapman HN, Spence JCH, Fromme P, Weierstall U, Ernst OP, Katritch V, Gurevich VV, Griffin PR, Hubbell WL, Stevens RC, Cherezov V, Melcher K, Xu HE. Crystal structure of rhodopsin bound to arrestin determined by femtosecond X-ray laser. Nature. 2015;523(7562):561–567. doi: 10.1038/nature14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou XE, He Y, de Waal PW, Gao X, Kang Y, Van Eps N, Yin Y, Pal K, Goswami D, White TA, Barty A, Latorraca NR, Chapman HN, Hubbell WL, Dror RO, Stevens RC, Cherezov V, Gurevich VV, Griffin PR, Ernst OP, Melcher K, Xu HE. Structural Identification of Phosphorylation Codes for Arrestin Recruitment by G protein-Coupled Receptors. Cell. 2017;170(3):457–469. doi: 10.1016/j.cell.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanson SM, Francis DJ, Vishnivetskiy SA, Kolobova EA, Hubbell WL, Klug CS, Gurevich VV. Differential interaction of spin-labeled arrestin with inactive and active phosphorhodopsin. Proc Natl Acad Sci U S A. 2006;103:4900–4905. doi: 10.1073/pnas.0600733103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hanson SM, Cleghorn WM, Francis DJ, Vishnivetskiy SA, Raman D, Song X, Nair KS, Slepak VZ, Klug CS, Gurevich VV. Arrestin mobilizes signaling proteins to the cytoskeleton and redirects their activity. J Mol Biol. 2007;368(2):375–387. doi: 10.1016/j.jmb.2007.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilden U. Duration and amplitude of the light-induced cGMP hydrolysis in vertebrate photoreceptors are regulated by multiple phosphorylation of rhodopsin and by arrestin binding. Biochemistry. 1995;34:1446–1454. doi: 10.1021/bi00004a040. [DOI] [PubMed] [Google Scholar]
- 82.Krupnick JG, Gurevich VV, Benovic JL. Mechanism of quenching of phototransduction. Binding competition between arrestin and transducin for phosphorhodopsin. J Biol Chem. 1997;272:18125–18131. doi: 10.1074/jbc.272.29.18125. [DOI] [PubMed] [Google Scholar]
- 83.Pan L, Gurevich EV, Gurevich VV. The nature of the arrestin x receptor complex determines the ultimate fate of the internalized receptor. J Biol Chem. 2003;278:11623–11632. doi: 10.1074/jbc.M209532200. [DOI] [PubMed] [Google Scholar]
- 84.Gurevich VV, Hanson SM, Song X, Vishnivetskiy SA, Gurevich EV. The functional cycle of visual arrestins in photoreceptor cells. Prog Retin Eye Res. 2011;30:405–430. doi: 10.1016/j.preteyeres.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gurevich VV, Benovic JL. Visual arrestin interaction with rhodopsin: Sequential multisite binding ensures strict selectivity towards light-activated phosphorylated rhodopsin. J Biol Chem. 1993;268:11628–11638. [PubMed] [Google Scholar]
- 86.Shukla AK, Westfield GH, Xiao K, Reis RI, Huang LY, Tripathi-Shukla P, Qian J, Li S, Blanc A, Oleskie AN, Dosey AM, Su M, Liang CR, Gu LL, Shan JM, Chen X, Hanna R, Choi M, Yao XJ, Klink BU, Kahsai AW, Sidhu SS, Koide S, Penczek PA, Kossiakoff AA, Wods VL, Jr, Kobilka BK, Skiniotis G, Lefkowitz RJ. Visualization of arrestin recruitment by a G-protein-coupled receptor. Nature. 2014;512(7513):218–222. doi: 10.1038/nature13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thomsen ARB, Plouffe B, Cahill TJ, III, Shukla AK, Tarrasch JT, Dosey AM, Kahsai AW, Strachan RT, Pani B, Mahoney JP, Huang L, Breton B, Heydenreich FM, Sunahara RK, Skiniotis G, Bouvier M, Lefkowitz RJ. GPCR-G Protein-β-Arrestin Super-Complex Mediates Sustained G Protein Signaling. Cell. 2016;166(4):907–919. doi: 10.1016/j.cell.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cahill TJ, 3rd, Thomsen AR, Tarrasch JT, Plouffe B, Nguyen AH, Yang F, Huang LY, Kahsai AW, Bassoni DL, Gavino BJ, Lamerdin JE, Triest S, Shukla AK, Berger B, Little J, 4th, Antar A, Blanc A, Qu CX, Chen X, Kawakami K, Inoue A, Aoki J, Steyaert J, Sun JP, Bouvier M, Skiniotis G, Lefkowitz RJ. Distinct conformations of GPCR-β-arrestin complexes mediate desensitization, signaling, and endocytosis. Proc Natl Acad Sci U S A. 2017;114(10):2562–2567. doi: 10.1073/pnas.1701529114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kumari P, Srivastava A, Ghosh E, Ranjan R, Dogra S, Yadav PN, Shukla AK. Core engagement with β-arrestin is dispensable for agonist-induced vasopressin receptor endocytosis and ERK activation. Mol Biol Cell. 2017;28(8):1003–1010. doi: 10.1091/mbc.E16-12-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kumari P, Srivastava A, Banerjee R, Ghosh E, Gupta P, Ranjan R, Chen X, Gupta B, Gupta C, Jaiman D, Shukla AK. Functional competence of a partially engaged GPCR-β-arrestin complex. Nat Commun. 2016;7:13416. doi: 10.1038/ncomms13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grundmann M, Merten N, Malfacini D, Inoue A, Preis P, Simon K, Rüttiger N, Ziegler N, Benkel T, Schmitt NK, Ishida S, Müller I, Reher R, Kawakami K, Inoue A, Rick U, Kühl T, Imhof D, Aoki J, König GM, Hoffmann C, Gomeza J, Wess J, Kostenis E. Lack of beta-arrestin signaling in the absence of active G proteins. Nat Commun. 2018;9(1):341. doi: 10.1038/s41467-017-02661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carman CV, Benovic JL. G-protein-coupled receptors: turn-ons and turn-offs. Curr Opin Neurobiol. 1998;8:335–344. doi: 10.1016/s0959-4388(98)80058-5. [DOI] [PubMed] [Google Scholar]
- 93.Albizu L, Balestre M-N, Breton C, Pin J-P, Manning M, Mouillac B, Barberis C, Durroux T. Probing the Existence of G Protein-Coupled Receptor Dimers by Positive and Negative Ligand-Dependent Cooperative Binding. Molecular Pharmacology. 2006;70(5):1783–1791. doi: 10.1124/mol.106.025684. [DOI] [PubMed] [Google Scholar]
- 94.El-Asmar L, Springael J-Y, Ballet S, Andrieu EU, Vassart G, Parmentier M. Evidence for Negative Binding Cooperativity within CCR5-CCR2b Heterodimers. Molecular Pharmacology. 2005;67(2):460–469. doi: 10.1124/mol.104.003624. [DOI] [PubMed] [Google Scholar]
- 95.Romero-Fernandez W, Borroto-Escuela DO, Agnati LF, Fuxe K. Evidence for the existence of dopamine d2-oxytocin receptor heteromers in the ventral and dorsal striatum with facilitatory receptor–receptor interactions. Molecular Psychiatry. 2012;18:849. doi: 10.1038/mp.2012.103. [DOI] [PubMed] [Google Scholar]
- 96.Callén L, Moreno E, Barroso-Chinea P, Moreno-Delgado D, Cortés A, Mallol J, Casadó V, Lanciego JL, Franco R, Lluis C, Canela EI, McCormick PJ. Cannabinoid Receptors CB1 and CB2 Form Functional Heteromers in Brain. J Biol Chem. 2012;287(25):20851–20865. doi: 10.1074/jbc.M111.335273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Verma V, Hasbi A, O’Dowd BF, George SR. Dopamine D1–D2 receptor Heteromer-mediated calcium release is desensitized by D1 receptor occupancy with or without signal activation: dual functional regulation by G protein-coupled receptor kinase 2. J Biol Chem. 2010;285(45):35092–35103. doi: 10.1074/jbc.M109.088625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O’Dowd BF, George SR. D1–D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci USA. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bellot M, Galandrin S, Boularan C, Matthies HJ, Despas F, Denis C, Javitch J, Mazères S, Sanni SJ, Pons V, Seguelas MH, Hansen JL, Pathak A, Galli A, Sénard JM, Galés C. Dual agonist occupancy of AT1-R-α2C-AR heterodimers results in atypical Gs-PKA signaling. Nat Chem Biol. 2015;11(4):271–279. doi: 10.1038/nchembio.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baba K, Benleulmi-Chaachoua A, Journé AS, Kamal M, Guillaume JL, Dussaud S, Gbahou F, Yettou K, Liu C, Contreras-Alcantara S, Jockers R, Tosini G. Heteromeric MT1/MT2 melatonin receptors modulate photoreceptor function. Sci Signal. 2013;6(296):ra89. doi: 10.1126/scisignal.2004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gomes I, Ayoub MA, Fujita W, Jaeger WC, Pfleger KD, Devi LA. G Protein-Coupled Receptor Heteromers. Annu Rev Pharmacol Toxicol. 2016;56:403–425. doi: 10.1146/annurev-pharmtox-011613-135952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hague C, Uberti MA, Chen Z, Hall RA, Minneman KP. Cell Surface Expression of α1D-Adrenergic Receptors Is Controlled by Heterodimerization with α1B-Adrenergic Receptors. J Biol Chem. 2004;279(15):15541–15549. doi: 10.1074/jbc.M314014200. [DOI] [PubMed] [Google Scholar]
- 103.Uberti MA, Hague C, Oller H, Minneman KP, Hall RA. Heterodimerization with β2-Adrenergic Receptors Promotes Surface Expression and Functional Activity of α1D-Adrenergic Receptors. Journal of Pharmacology and Experimental Therapeutics. 2005;313(1):16–23. doi: 10.1124/jpet.104.079541. [DOI] [PubMed] [Google Scholar]
- 104.Prosser HM, Gill CH, Hirst WD, Grau E, Robbins M, Calver A, Soffin EM, Farmer CE, Lanneau C, Gray J, Schenck E, Warmerdam BS, Clapham C, Reavill C, Rogers DC, Stean T, Upton N, Humphreys K, Randall A, Geppert M, Davies CH, Pangalos MN. Epileptogenesis and Enhanced Prepulse Inhibition in GABAB1-Deficient Mice. Molecular and Cellular Neuroscience. 2001;17(6):1059–1070. doi: 10.1006/mcne.2001.0995. [DOI] [PubMed] [Google Scholar]
- 105.Liu X-Y, Liu Z-C, Sun Y-G, Ross M, Kim S, Tsai F-F, Li Q-F, Jeffry J, Kim J-Y, Loh Horace H, Chen Z-F. Unidirectional Cross-Activation of GRPR by MOR1D Uncouples Itch and Analgesia Induced by Opioids. Cell. 2011;147(2):447–458. doi: 10.1016/j.cell.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hillion J, Canals M, Torvinen M, Casadó V, Scott R, Terasmaa A, Hansson A, Watson S, Olah ME, Mallol J, Canela EI, Zoli M, Agnati LF, Ibáñez CF, Lluis C, Franco R, Ferré S, Fuxe K. Coaggregation, Cointernalization, and Codesensitization of Adenosine A2A Receptors and Dopamine D2Receptors. J Biol Chem. 2002;277(20):18091–18097. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- 107.Torvinen M, Torri C, Tombesi A, Marcellino D, Watson S, Lluis C, Franco R, Fuxe K, Agnati LF. Trafficking of adenosine A2A and dopamine D2 receptors. Journal of Molecular Neuroscience. 2005;25(2):191–200. doi: 10.1385/JMN:25:2:191. [DOI] [PubMed] [Google Scholar]
- 108.He S-Q, Zhang Z-N, Guan J-S, Liu H-R, Zhao B, Wang H-B, Li Q, Yang H, Luo J, Li Z-Y, Wang Q, Lu Y-J, Bao L, Zhang X. Facilitation of μ-Opioid Receptor Activity by Preventing δ-Opioid Receptor-Mediated Codegradation. Neuron. 2011;69(1):120–131. doi: 10.1016/j.neuron.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 109.Terrillon S, Barberis C, Bouvier M. Heterodimerization of V1a and V2 vasopressin receptors determines the interaction with β-arrestin and their trafficking patterns. Proc Natl Acad Sci USA. 2004;101(6):1548–1553. doi: 10.1073/pnas.0305322101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schmidlin F, Déry O, Bunnett NW, Grady EF. Heterologous regulation of trafficking and signaling of G protein-coupled receptors: beta-arrestin-dependent interactions between neurokinin receptors. Proc Natl Acad Sci U S A. 2002;99(5):3324–3329. doi: 10.1073/pnas.052161299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tóth AD, Prokop S, Gyombolai P, Várnai P, Balla A, Gurevich VV, Hunyady L, Turu G. Heterologous phosphorylation-induced formation of a stability lock permits regulation of inactive receptors by β-arrestins. J Biol Chem. 2018;293(3):876–892. doi: 10.1074/jbc.M117.813139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gurevich VV, Gurevich EV. Arrestins: Critical Players in Trafficking of Many GPCRs. Prog Mol Biol Transl Sci. 2015;132:1–14. doi: 10.1016/bs.pmbts.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Meyer BH, Segura JM, Martinez KL, Hovius R, George N, Johnsson K, Vogel H. FRET imaging reveals that functional neurokinin-1 receptors are monomeric and reside in membrane microdomains of live cells. Proc Natl Acad Sci U S A. 2006;103:2138–2143. doi: 10.1073/pnas.0507686103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vischer HF, Castro M, Pin JP. G Protein-Coupled Receptor Multimers: A Question Still Open Despite the Use of Novel Approaches. Mol Pharmacol. 2015;88(3):561–571. doi: 10.1124/mol.115.099440. [DOI] [PubMed] [Google Scholar]
- 115.Gurevich VV, Gurevich EV. GPCR monomers and oligomers: it takes all kinds. Trends Neurosci. 2007;31:74–81. doi: 10.1016/j.tins.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ng J, Rashid AJ, So CH, O’Dowd BF, George SR. Activation of calcium/calmodulin-dependent protein kinase IIalpha in the striatum by the heteromeric D1–D2 dopamine receptor complex. Neuroscience. 2010;165:535–541. doi: 10.1016/j.neuroscience.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Perreault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, Seeman P, O’Dowd BF, George SR. The dopamine D1–D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J Biol Chem. 2010;285:36625–36634. doi: 10.1074/jbc.M110.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Perreault ML, Hasbi A, O’Dowd BF, George SR. Heteromeric dopamine receptor signaling complexes: emerging neurobiology and disease relevance. Neuropsychopharmacology. 2014;39:156–168. doi: 10.1038/npp.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.So CH, Varghese G, Curley KJ, Kong MM, Alijaniaram M, Ji X, Nguyen T, O’dowd BF, George SR. D1 and D2 dopamine receptors form heterooligomers and cointernalize after selective activation of either receptor. Mol Pharmacol. 2005:568–578. doi: 10.1124/mol.105.012229. [DOI] [PubMed] [Google Scholar]
- 120.Frederick AL, Yano H, Trifilieff P, Vishwasrao HD, Biezonski D, Mészáros J, Urizar E, Sibley DR, Kellendonk C, Sonntag KC, Graham DL, Colbran RJ, Stanwood GD, Javitch JA. Evidence against dopamine D1/D2 receptor heteromers. Mol Psychiatry. 2015;20(11):1373–1385. doi: 10.1038/mp.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hiranita T. In Vivo Significance of In Vitro Studies on G-Protein-Coupled Receptor Heteromers. J Alcohol Drug Depend. 2015;3(2):e120. doi: 10.4172/2329-6488.1000e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.DeVree BT, Mahoney JP, Vélez-Ruiz GA, Rasmussen SG, Kuszak AJ, Edwald E, Fung JJ, Manglik A, Masureel M, Du Y, Matt RA, Pardon E, Steyaert J, Kobilka BK, Sunahara RK. Allosteric coupling from G protein to the agonist-binding pocket in GPCRs. Nature. 2016;535(7610):182–186. doi: 10.1038/nature18324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mahesh G, Jaiswal P, Dey S, Sengupta J, Mukherjee S. Cloning, expression, purification and characterization of oligomeric states of the native 5HT2A G-Protein-Coupled Receptor. Protein Pept Lett. 2018 doi: 10.2174/0929866525666180207110137. in press. [DOI] [PubMed] [Google Scholar]
- 124.Komolov KE, Du Y, Duc NM, Betz RM, Rodrigues JPGLM, Leib RD, Patra D, Skiniotis G, Adams CM, Dror RO, Chung KY, Kobilka BK, Benovic JL. Structural and Functional Analysis of a β2-Adrenergic Receptor Complex with GRK5. Cell. 2017;169:407–421. doi: 10.1016/j.cell.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]