Abstract
As emergency physicians, we encounter patients suffering from either hypoxemic and/or hypercarbic respiratory problems on a daily basis. A stepwise approach to solving this problem seems logical from an emergency medicine perspective.
Current literature supports the notion that NIV decreases endotracheal intubation rates and, mortality in select patient populations. The key to the success of NIV is patient cooperation and support for the care givers.
In this narrative review, non-invasive ventilation (NIV) is discussed in terms of modes of delivery, interface and patient selection, as well as practical considerations.
1. Introduction
As emergency physicians, we encounter patients suffering from either hypoxemic and/or hypercarbic respiratory problems on a daily basis. A stepwise approach to solving this problem seems logical from an emergency medicine perspective. Nasal masks, non-rebreather masks with a reservoir, high flow nasal cannula (HFNC) and mechanical ventilation with either invasive or non-invasive methods are the procedures that can be performed during the ED course of a patient presenting with acute respiratory failure.
The history of the non-invasive ventilation (NIV) dates back to the 1940s. Although NIV was first used for patients with acute respiratory failure in the 1940s, it was not popular until the 1980s.1
Reversible disease processes, such as acute exacerbation of chronic obstructive pulmonary disease (AECOPD) or acute cardiogenic pulmonary edema (ACPE), are important for the success of NIV in an acute care setting. Current literature supports the notion that NIV decreases endotracheal intubation rates and, mortality in select patient populations. The key to the success of NIV is patient cooperation and support for the care givers.
In this narrative review, NIV is discussed in terms of modes of delivery, interface and patient selection, as well as practical considerations.
1.1. Modes
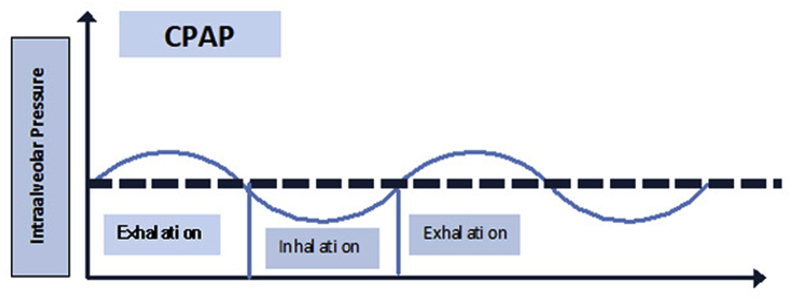
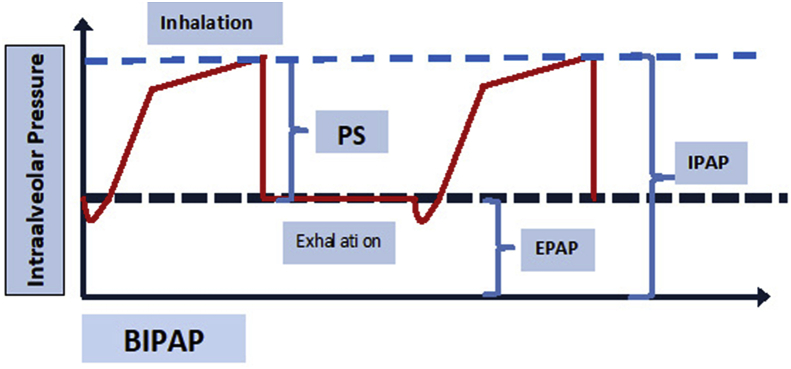
There are two types of NIV. The first mode is continuous positive airway pressure (CPAP), which applies a single pressure during inspiration and expiration (Fig. 1). During CPAP application, there are small variations in pressure during inspiration and expiration (Fig. 1). There is a pressure drop during inspiration and a rise in pressure during exhalation (Fig. 1). CPAP is most useful for those patients with hypoxemic respiratory failure (type 1). CPAP can be assumed to be analogous to positive end-expiratory pressure (PEEP) during mechanical ventilation with endotracheal intubation. BPAP is bi-level pressure applied during inspiration and expiration. Inspiratory positive airway pressure (IPAP) is the pressure support applied during inspiration, whereas expiratory positive airway pressure (EPAP) is the pressure applied during inspiration and expiration (Fig. 2). In this regard EPAP, CPAP and PEEP are analogous.
Fig. 1.
CPAP: continuous positive airway pressure. A single pressure is applied during inspiration and expiration.
Fig. 2.
BPAP IPAP: Inspiratory positive airway pressure, EPAP: Expiratory positive airway pressure, PS: Pressure Support. Here inspiration is triggered by patient effort and intra alveolar pressure increases; at the end of the inspiration a constant pressure (EPAP) is applied during expiration.
NIV reduces the work of breathing by counteracting with intrinsic PEEP, helps alveolar recruitment, decreases shunt and improves ventilation and perfusion. NIV also improves alveolar gas exchange and the removal of carbon dioxide, and decreases the oxygen consumption of the intercostal muscles. NIV increases the pressure inside the thorax. These pressure increases may impede venous return (preload) and, by lowering cardiac afterload, it acts as a left ventricular assist device. These physiological changes are the basis of NIV for the treatment of pulmonary edema, although for preload-dependent patients, the increase in intrathoracic pressure may cause hypotension.
2. Patient selection
The successful application of NIV depends on the cooperation of the patient with the provider. There are some certain features that are suggestive of NIV success. The patient's diagnosis, clinical characteristics (intact dentation, lower APACHE score, good initial response to NIV), less air leaking and fewer secretions are indicators of success.2 Strong, moderate and weak evidence for the application of NIV in certain diseases is indicated below (Table 1).
Table 1.
Types of evidences in certain diseases.
| Type of Evidence | Disease | Source |
|---|---|---|
| Strong | AECOPD ACPE Immunocompromised patients Facilitation of weaning in COPD patients |
Multiple randomized, controlled trials and meta-analyses |
| Intermediate | Preoxygenation in hypoxemic respiratory failure Postextubation respiratory failure |
Single controlled trial and cohort series or multiple randomized studies with conflicting findings |
| Weak | ALI/ARDS Neuromuscular disease Pneumonia Status asthmaticus |
Cohort studies, anecdotal reports and case series |
2.1. Acute exacerbation of chronic obstructive pulmonary disease
Chronic Obstructive Pulmonary Disease (COPD) is a disease characterized by expiratory airflow limitation and mucus hypersecretion. Exacerbation of COPD is known to have high mortality risk.4 Traditional treatment of a patient with exacerbation includes oxygen, bronchodilators, systemic steroids and antibiotics. When all these measures fail, patients are intubated. However, intubation and extubation of patients with COPD carry high mortality rates. COPD exacerbation is a reversible process, so if physicians are able to buy some time for their patients, they may negate the unenviable consequences of this disease. According to a Cochrane Review published in 2004, data from good quality randomized controlled trials showed the benefit of NIV as first line intervention to be an adjunct therapy in usual medical care in all suitable patients with AECOPD.5 When NIV plus usual medical care is compared with only usual medical care, treatment failure (RR 0.48, 95%CI:0.37–0.63), mortality (RR 0.52, 95%CI:0.35–0.76) and endotracheal intubation need (RR 0.42, 95%CI:0.33–0.53) were both less in the NIV group. The number needed to treat (NNT) for treatment failure, mortality and intubation was 5, 4 and 10 respectively.5 NIV is the suggested ventilation in COPD patients with acute respiratory failure according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 report.6 BPAP is the primary mode chosen. Early institution is crucial for the success of NIV. Indeed, a previous study indicated that the benefits of NIV were not seen after institution of usual treatment failure.7
In a recent Cochrane review, NIV was assessed for the management of hypercarbic respiratory failure due to exacerbation of COPD. The use of NIV decreased the risk of mortality (risk ratio (RR) 0.54, 95% confidence interval (CI) 0.38 to 0.76), and decreased the need for endotracheal intubation (RR 0.36, 95% CI 0.28 to 0.46).8
2.2. Acute cardiogenic pulmonary edema
ACPE is a leading cause of acute respiratory failure and it is an important medical issue with an increased in-hospital mortality rate.4
Increased end-diastolic pressure in the left ventricle decreases the preload in the left atrium and, according to communicating vessel law, increased pressure in the left atrium increases the pressure in the pulmonary vessel. Edema in the interstitial area and fluid in the alveoli disrupt gas exchange, and additionally cause a collapse in the alveoli and pathologic shunt formation, altogether causing hypoxemic respiratory failure.
The fundamental aim of the treatment of patients with ACPE is decreasing right ventricular preload and left ventricular afterload. The mainstay of treatment is oxygen, nitrates and diuretics. Here, the application of NIV not only decreases preload by increasing intrathoracic pressure, but acts also as a left ventricular assist device, thus decreasing afterload.
NIV is strongly suggested in the literature. In a Cochrane review, the addition of NIV to standard treatment was shown to be effective and safe intervention for patients with ACPE.9 NNT values for in-hospital mortality and endotracheal intubation with CPAP were 9 and 8 patients respectively.4 In a previous study, BPAP use was associated with increased risk of myocardial infarction in patients with acute pulmonary edema.10 However, subsequent studies found non-significant differences in the occurrence of acute myocardial infarction during treatment with BPAP.9 Therefore, either BPAP or CPAP can be applied to patients with ACPE.
2.3. Acute asthma
Asthma is a chronic inflammation of the lungs characterized by bronchoconstriction, airflow limitation and mucus hypersecretion. The mainstay of treatment is bronchodilators and steroids. A Cochrane review stated that, due to the paucity of existing data, the treatment of acute asthma patients with NIV remains controversial.11 A clinical practice guideline published in Canada in 2011 made no recommendation about the use of NIV in the exacerbation of asthma because of insufficient data.12 Consequently, it seems that, currently, the literature does not yet support the routine use of NIV for patients with asthma attacks. Therefore, a well-designed future RCT may provide conclusive answers to this debate.13
2.4. Chest trauma
Blunt thoracic injuries are the reason for up to a quarter of all injury-related deaths.14 The clinical course and outcome of a chest trauma is predicted by the mechanism of the injury and the tissue damage.15 Patients with blunt chest injury are prone to respiratory failure; endotracheal intubation and mechanical ventilation make these patients vulnerable to ventilator associated pneumonia and thus increased morbidity and mortality. In a systematic review CPAP or BIPAP delivered using any interface was compared to mechanical ventilation or supplemental oxygen therapy. It was found that NIV significantly reduced mortality (RR:0.26 (95% CI:0.09, 0.71), the need for endotracheal intubation, the length of intensive care unit stays and also improved oxygenation.16 In another systematic review, use of NIV was associated with a decrease in mortality regardless of the method chosen (continuous positive airway pressure, bi-level positive airway pressure and noninvasive intermittent-pressure support ventilation). This systematic review found no significant associated increase in the incidence of pneumothorax with NIV.17 NIV can be used for patients with blunt chest trauma, although there are currently no accepted standards for who NIV should be initiated with, so institutional protocols may be applied to this patient population.
2.5. Community acquired pneumonia
Pneumonia is the number one infectious cause of death and it is an acute infection of the alveoli. Either typical microorganisms (Streptococcus pneumoniae and Haemophilus influenza, with S. Pneumoniae) or atypical agents such as Legionella, Mycoplasma and Chlamydia may the cause of community acquired pneumonia (CAP).18 The use of NIV in patients with pneumonia is controversial. Failure rates of up to 50% have been reported in certain prospective studies.4 For instance, in a recent study NIV was associated with increased survival among the subgroup of patients hospitalized with pneumonia who had COPD or heart failure.19 Although current guidelines do not recommend NIV among patients with CAP, patients with COPD and heart failure may nonetheless obtain benefit from early and careful use of NIV in the acute care setting.12
2.6. Immunocompromised patients
The infectious complications related to endotracheal intubation and mechanical ventilation are particularly significant for immunocompromised patients. Endotracheal intubation by-passes the upper airway barrier and poses a great infectious risk for such a vulnerable patient population. Previous studies with solid organ transplant patients and studies with immunocompromised patients both indicated that the application of NIV decreased the need for endotracheal intubation and mortality.20 Although NIV failure is high in this population of patients, the use of NIV is nevertheless recommended for immunocompromised patients.
3. NIV and varying level of consciousness
Patient cooperation is an important factor for the success of NIV, so an altered level of consciousness is considered to be a possible contraindication to NIV in patients with acute respiratory failure. However, studies have thus far shown conflicting results.21 A study by Scala et al. suggested that NIV may be applied to patients with mild altered mental status and COPD exacerbation. In another prospective, open, noncontrolled study, hypercapnic patients were grouped according to their Glasgow coma scale (GCS) (≤8 or > 8). It was concluded that selected patients with hypercapnic coma secondary to acute respiratory failure can be treated successfully as patients with non-coma.22 Based on these findings, NIV may be applied to select patients with altered mental status due to hypercapnic coma secondary to acute respiratory failure.
4. Device and interface
4.1. Interfaces
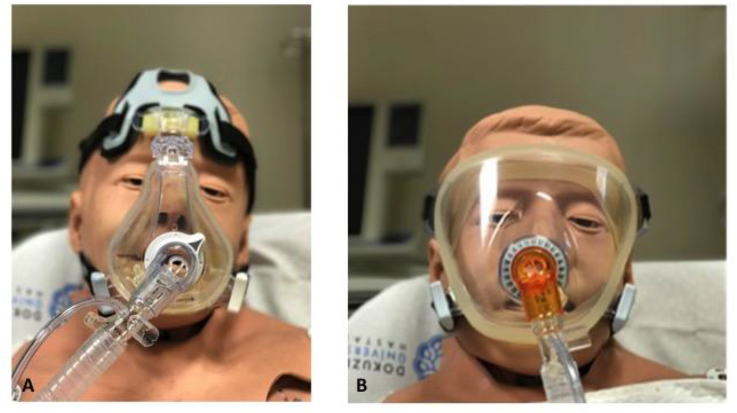
Interfaces are devices that connect the ventilator tubing to the patient's face. There are many different types of interface (nasal, oronasal, full face and helmets) (Fig. 3). Patients with acute respiratory failure in the ED are mainly mouth breathers, so nasal masks are not suitable for ED use. The helmet interface encompasses the entire head. As such, there is a risk of rebreathing carbon dioxide, especially if the respiratory rate of the patient is high. The helmet interface is also louder and this may impede communication with the patient, and it may also be claustrophobic and not practical for the ED patient. However, the helmet interface does not come into contact with the patient's face; therefore, it does not cause skin lesions. Nonetheless, full face masks may increase the dead space and may cause rebreathe of carbon dioxide. Oronasal masks cover the nasal bridge, go around the nose and forms a seal around the chin and the mouth of the patient. As such, oronasal masks are the most appropriate type for ED use, although there is risk of claustrophobia and skin deteriation.
Fig. 3.
A-Oronasal mask, B- Full face mask.
Another important point is whether single or double lumen circuits are preferable. If a classic intensive care mechanical ventilator is used for NIV, one end of the double lumen circuit will be connected to the machine and the other end to the interface. Mechanical ventilators use one lumen of this circuit for inspiration and the other for exhalation. Therefore, for double lumen circuits we should use an oronasal mask without an exhalation port. If use of a single lumen circuit is preferable, as machines specifically designed for NIV are typically used, the exhalation port must be either on the circuit or on the interface, but not both. Normally, the design of the interfaces and circuits does not allow for wrong connection, but it is also commonplace for practitioners to find an apparatus to connect these devices (Fig. 3).
Air leak is a typical feature of NIV.23 Many modern ventilators can compensate for a large amount of air leak, but the amount of air leak that a ventilator can compensated for is highly variable. EPs should be familiar with the device they use, and choosing the appropriate mask will increase the patient's comfort and decrease the need for unnecessary tightening of the straps of the mask. Leakage is important for patient-ventilator synchrony and the success of the procedure. Therefore, unintentional air leaks should be avoided.
5. Patient ventilator dyssynchrony
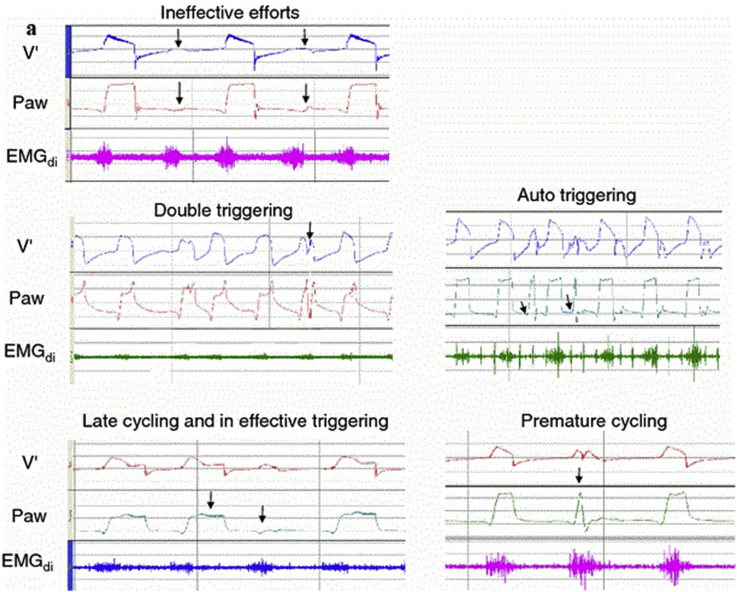
Patient and ventilator synchrony is one of the key factors for NIV success. There are numerous types of dyssynchrony (ineffective triggering, double-triggering, auto-triggering, premature cycling and delayed cycling) (see Fig. 4). In a multicenter prospective study, auto-triggering was present in 13% and double triggering in 15%, premature cycling in 12%, and ineffective breathing in 13% of the study population.24 A leak from the mask is one of the important factors associated with dyssynchrony. Ineffective triggering may occur when the sensitivity of the trigger is set too high, so the patient cannot trigger the ventilator. Ineffective triggering may also be secondary to high intrinsic PEEP, weakness of the patient, inappropriate ventilator sensitivity settings and ventilator dysfunction. Double triggering can be identified by two rapidly delivered breaths, and may be the result of too short inspiratory time, too low inspiratory flow and tidal volume and cough/hiccups. Auto triggering is seen when the ventilator is triggered in a way other than the patient's respiratory effort. Leaks are also the major factor leading to auto triggering.
Fig. 4.
Tracings of different types of asynchrony (with the permission of Springer-Verlag). V: Volume, Paw: Airway pressure, EMGdi: Electromyography of the diaphragm.
6. Practical considerations
Once the decision is given to apply NIV, EP should decide on the mode (either CPAP or BPAP), the interface, and the circuit that is suitable for the ventilator. CPAP is used mainly for patients with hypoxemic respiratory failure; therefore, patients with acute cardiogenic pulmonary edema may be suitable candidates. BPAP is the appropriate mode for the patients with either hypoxemic or hypercarbic respiratory failure. If a ventilator with a single lumen circuit is chosen, then the exhalation port must be at the end of the circuit or on the mask, whereas if a double lumen circuit is chosen, then there must be no exhalation port on the mask. Although the optimal interface and ventilator design is not known, oronasal masks are more appropriate for ED use. Application of the oronasal mask over the face of a patient creates the sensation of breathlessness, while application of the straps makes the scenario even worse, often causing claustrophobia and anxiety. As a preemptive measure, allowing the patient to hold the mask, while beginning with lower pressures may increase the cooperation of the patient. Even beginning with CPAP and then shifting to BIPAP may be an option for patient comfort. Although institutional preferences are important for the pressure preset, a common practice is to begin with 5 cmH2O of CPAP and to then increase the pressure 2–3 cmH2O every 10–15 min. A higher initial pressure setting such as 8–10 cmH2O may be applied to patients with acute cardiogenic pulmonary edema. For patients who are either hypoxemic or hypercarbic, performing either BPAP or EPAP will affect oxygenation and the pressure difference (pressure support) between IPAP and EPAP will affect the carbon dioxide level. The pressure difference between IPAP and EPAP should not be less than 3 cmH2O. A pressure difference of less than 3 cmH2O will actually be CPAP. A common initial setting for BPAP is IPAP of 10 and EPAP of 5 cmH2O.
In patients with high respiratory rates, sedatives may be used to enable the patients to tolerate the procedure23. Ketamine or remifentanil are the agents that can be used for this purpose.
The success of NIV can be monitored with both subjective and objective parameters (Table 2).25
Table 2.
Monitoring the efficacy of NIV.
| Subjective Parameters | Objective Parameters |
|---|---|
|
|
Once NIV is initiated, serial PaCO2 measurement is needed to assess the success of the therapy. In a study, authors compared naso-buccal ETCO2 sensor with ABG and found that naso-buccal sensor was inaccurate to predict both PaCO2 and PaCO2 variations over time.26 But there are some researches that measure transcutaneous partial pressure of carbon dioxide (PtcCO2) and results show that PtcCO2 measurement may provide reasonable estimate of PaCO2.27,28
7. Conclusion
Timely use of NIV in the ED may decrease the need for invasive ventilation and its associated complications. The appropriate device, interface and patient selection are, therefore, key components of NIV success.
Footnotes
Peer review under responsibility of The Emergency Medicine Association of Turkey.
References
- 1.Barach A.L., Eckman M. Studies on positive pressure respiration; general aspects and types of pressure breathing; effects on respiration and circulation at sea level. J Aviat Med. 1946;17 290–232. [PubMed] [Google Scholar]
- 2.Liesching T., Kwok H., Hill N.S. Acute applications of noninvasive positive pressure ventilation. Chest. 2003 Aug;124(2):699–713. doi: 10.1378/chest.124.2.699. [DOI] [PubMed] [Google Scholar]
- 3.Felix Yu, Erik Garpestad, Nicholas S. Hill. What is the role of noninvasive ventilation in the intensive care unit? Evidence Based Pract Critical Care, 7, 36–42.
- 4.Allison M.G., Winters M.E. Noninvasive ventilation for the emergency physician. Emerg Med Clin North Am. 2016 Feb;34(1):51–62. doi: 10.1016/j.emc.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Ram F.S., Picot J., Lightowler J. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2004;3 doi: 10.1002/14651858.CD004104.pub3. [DOI] [PubMed] [Google Scholar]
- 6.http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/).
- 7.Conti G., Antonelli M., Navalesi P. Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med. 2002;28(12):1701–1707. doi: 10.1007/s00134-002-1478-0. [DOI] [PubMed] [Google Scholar]
- 8.Osadnik C.R., Tee V.S., Carson-Chahhoud K.V. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017 Jul 13;7 doi: 10.1002/14651858.CD004104.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vital F.M., Ladeira M.T., Atallah A.N. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev. 2013 May 31;5 doi: 10.1002/14651858.CD005351.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Mehta S., Jay G.D., Woolard R.H. Randomized, prospective trial of bilevel versus continuous positive airway pressure in acute pulmonary edema. Crit Care Med. 1997;25(4):620–628. doi: 10.1097/00003246-199704000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Lim W.J., Mohammed Akram R., Carson K.V. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2012 Dec 12;12 doi: 10.1002/14651858.CD004360.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keenan S.P., Sinuff T., Burns K.E. Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ. 2011;183:E195–E214. doi: 10.1503/cmaj.100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutton L. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. BET 3. non-invasive positive pressure ventilation for patients with acute exacerbations of asthma. Emerg Med J. 2009 Jan;26(1):59–60. doi: 10.1136/emj.2008.069609. [DOI] [PubMed] [Google Scholar]
- 14.http://www.cdc.gov/nchs/FASTATS/acc-inj.htm (Centers for Disease Control and Prevention: Accidents or unintentional injuries.) Accessed March 4, 2014.
- 15.Richter M., Krettek C., Otte D. Correlation between crash severity, injury severity and clinical course in car occupants with thoracic trauma: a technical and medical study. J Trauma. 2001;50:10. doi: 10.1097/00005373-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Chiumello D., Coppola S., Froio S. Noninvasive ventilation in chest trauma: systematic review and meta-analysis. Intensive Care Med. 2013 Jul;39(7):1171–1180. doi: 10.1007/s00134-013-2901-4. [DOI] [PubMed] [Google Scholar]
- 17.Hua A., Shah K.H. Does noninvasive ventilation have a role in chest trauma patients? Ann Emerg Med. 2014 Jul;64(1):82–83. doi: 10.1016/j.annemergmed.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 18.Long B., Long D., Koyfman A. Emergency medicine evaluation of community acquired pneumonia: history, examination, imaging and laboratory assessment, and risk scores. J Emerg Med. 2017 Sep 20;17:S0736–S4679. doi: 10.1016/j.jemermed.2017.05.035. 30481-X. [DOI] [PubMed] [Google Scholar]
- 19.Stefan M.S., Priya A., Pekow P.S. The comparative effectiveness of noninvasive and invasive ventilation in patients with pneumonia. J Crit Care. 2017 May 23;43:190–196. doi: 10.1016/j.jcrc.2017.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antonelli M., Conti G., Bufi M. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation. JAMA. 2000;283:235–241. doi: 10.1001/jama.283.2.235. [DOI] [PubMed] [Google Scholar]
- 21.Scala R., Naldi M., Archinucci I. Noninvasive positive pressure ventilation in patients with acute exacerbations of COPD and varying levels of consciousness. Chest. 2005 Sep;128(3):1657–1666. doi: 10.1378/chest.128.3.1657. [DOI] [PubMed] [Google Scholar]
- 22.Díaz G.G., Alcaraz A.C., Talavera J.C. Noninvasive positive-pressure ventilation to treat hypercapnic coma secondary to respiratory failure. Chest. 2005 Mar;127(3):952–960. doi: 10.1378/chest.127.3.952. [DOI] [PubMed] [Google Scholar]
- 23.Nava S. Behind a mask: tricks, pitfalls, and prejudices for noninvasive ventilation. Respir Care. 2013 Aug;58(8):1367–1376. doi: 10.4187/respcare.02457. [DOI] [PubMed] [Google Scholar]
- 24.Vignaux L., Vargas F., Roeseler J. Patient-ventilator asynchrony during non-invasive ventilation for acute respiratory failure: a multicenter study. Intensive Care Med. 2009 May;35(5):840–846. doi: 10.1007/s00134-009-1416-5. [DOI] [PubMed] [Google Scholar]
- 25.Wright BJ, Slesinger TL. Noninvasive positive pressure ventilation. In: Farcy DA, Chiu WC, Marshall JP, Osborn TM. eds. Critical Care Emergency Medicine, 2e New York, NY: McGraw-Hill; Web site. Available at: http://accessemergencymedicine.mhmedical.com/content.aspx?bookid=1934§ionid=142834224. Accessed July 11, 2017.
- 26.Piquilloud L., Thevoz D., Jolliet P. End-tidal carbon dioxide monitoring using a naso-buccal sensor is not appropriate to monitor capnia during non-invasive ventilation. Ann Intensive Care. 2015 Feb 12;5:2. doi: 10.1186/s13613-014-0042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lermuzeaux M., Meric H., Sauneuf B. Superiority of transcutaneous CO2 over end-tidal CO2 measurement for monitoring respiratory failure in nonintubated patients: a pilot study. J Crit Care. 2016 Feb;31(1):150–156. doi: 10.1016/j.jcrc.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Aarrestad S., Tollefsen E., Kleiven A.L. Validity of transcutaneous PCO2 in monitoring chronic hypoventilation treated with non-invasive ventilation. Respir Med. 2016 Mar;112:112–118. doi: 10.1016/j.rmed.2016.01.017. [DOI] [PubMed] [Google Scholar]