Abstract
Objective
To evaluate the accuracy of emphysema volume (EV) and airway measurements (AMs) produced by various iterative reconstruction (IR) algorithms and virtual monoenergetic images (VME) at both low- and standard-dose settings.
Materials and Methods
Computed tomography (CT) images were obtained on phantom at both low- (30 mAs at 120 kVp) and standard-doses (100 mAs at 120 kVp). Each CT scan was reconstructed using filtered back projection, hybrid IR (iDose4; Philips Healthcare), model-based IR (IMR-R1, IMR-ST1, IMR-SP1; Philips Healthcare), and VME at 70 keV (VME70). The EV of each air column and wall area percentage (WA%) of each airway tube were measured in all algorithms. Absolute percentage measurement errors of EV (APEvol) and AM (APEWA%) were then calculated.
Results
Emphysema volume was most accurately measured in IMR-R1 (APEvol in low-dose, 0.053 ± 0.002; APEvol in standard-dose, 0.047 ± 0.003; all p < 0.001) and AM was the most accurate in IMR-SP1 on both low- and standard-doses CT (APEWA% in low-dose, 0.067 ± 0.002; APEWA% in standard-dose, 0.06 ± 0.003; all p < 0.001). There were no significant differences in the APEvol of IMR-R1 between low- and standard-doses (all p > 0.05). VME70 showed a significantly higher APEvol than iDose4, IMR-R1, and IMR-ST1 (all p < 0.004). VME70 also showed a significantly higher APEWA% compared with the other algorithms (all p < 0.001).
Conclusion
IMR was the most accurate technique for measurement of both EV and airway wall thickness. However, VME70 did not show a significantly better accuracy compared with other algorithms.
Keywords: Computed tomography, Model-based iterative reconstruction, Virtual monoenergetic image, Emphysema volume, Airway wall thickness
INTRODUCTION
Quantification of pulmonary emphysema and airway wall thickness using computed tomography (CT) is correlated with lung function and pathologic findings in chronic obstructive pulmonary disease (COPD) patients and even in normal subjects, and is also increasingly being used to quantify the features of COPD (1,2). Because radiation exposure is an obvious disadvantage of CT-based COPD quantification, various methods including iterative reconstruction (IR) have been developed to reduce radiation dose and maintain image quality.
Filtered back projection (FBP) reconstruction assumes that each pixel accurately indicates attenuation (3), however, FBP reconstruction impairs image quality due to large variations in these pixel values caused by noise. Among various IR, the use of hybrid IR (HIR) reduced image noise or artifacts, and improved image quality over FBP in low-dose chest CT (4,5,6). In recent studies, model-based IR (MIR), which is the latest development among reconstruction algorithms, also showed diagnostically acceptable images in low-dose chest CT which were better than HIR (6,7,8). Few studies have quantitatively examined emphysema volume (EV) or airway measurements (AMs) using IR in low-dose CT. The use of MIR seemed to provide the most accurate AMs (9). Meanwhile, emphysema measure was significantly different at HIR when compared to FBP (10), and low-dose CT images reconstructed with HIR dose reduction using three-dimensional processing could yield reliable emphysema quantification (11). However, AMs were not affected by HIR (10).
Meanwhile, introduction of spectral CT offers virtual monoenergetic images (VME), which allows materials in the scanned body to be rendered at photon energies as though the body was scanned with a monoenergetic X-ray beam of a desired kiloelectron volt (keV) level (12,13). This post-processing technique in spectral CT improves image quality and has been increasingly used for quantitative imaging (14,15). However, no studies have compared the effects of different IR and VME algorithms on quantitative measurements of emphysema and airway thickness.
Therefore, the aim of our study was to evaluate the accuracy of EV and AMs on the CT phantom produced by various IR methods and in VME images created at a fixed keV, at different radiation dose levels.
MATERIALS AND METHODS
Phantom
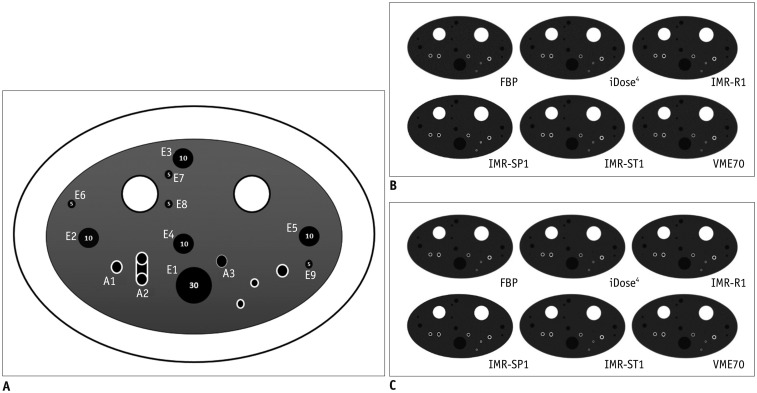
We used a commercially available phantom (CTP674 Lung phantom; The Phantom Laboratory, Salem, NY, USA) to simulate the human thorax. Characteristics of the phantom are described and shown in Table 1 and Figure 1. The physical phantom consisted of a central oval insert which simulates lung attenuation (250 × 150-mm) and an outer ring that simulates the surrounding soft tissue (350 × 250-mm). In the central oval insert, there are air columns of varying dimensions which simulates emphysema, six polycarbonate tubes which simulates airways of different dimensions, and angles and holes for the sterile water bottle. Among these, 9 air columns with three different diameters and volumes were chosen for the EV measurements (E1–E9), and three polycarbonate tubes (airway tubes) with different wall thicknesses and angles were analyzed for the airway wall measurements (A1–A3). Two airway tubes (A1 and A2) were positioned at a different angle of 30° from the z-axis of the CT couch.
Table 1. Characteristics of Each Air Column and Each Airway Tube Inserted into CTP674 Lung Phantom (The Phantom Laboratory).
| Diameter (mm) | Air Columns for EV | Reference Volume (mm3) |
|---|---|---|
| 30 | E1 | 28.416 |
| 10 | E2, E3, E4, E5 | 3.157 |
| 5 | E6, E7, E8, E9 | 0.789 |
EV = emphysema volume, WA% = wall area percentage
Fig. 1. Illustration and CT images of CTP674 lung phantom (The Phantom Laboratory).
A. Illustration of CTP674 lung phantom. Nine air columns with three different diameters and volumes were chosen for EV measurements (E1–E9), and three polycarbonate tubes (airway tubes) with different wall thicknesses and angles were analyzed for airway wall measurements (A1–A3). Two airway tubes (A1 and A2) were placed at different angle of 30° from z-axis of CT couch. B. CT scans with various algorithms at low-dose setting. C. CT scans with various algorithms at standard-dose setting. CT = computed tomography, EV = emphysema volume, FBP = filtered back projection, IMR-R1 = MIR with image definition of body routine level 1, IMR-SP1 = MIR with image definition of sharp plus level, IMR-ST1 = MIR with image definition of body soft tissue level 1, MIR = model-based iterative reconstruction, VME70 = standard virtual monoenergetic dataset at 70 keV
CT Image Acquisition
All CT images, from the phantom, were obtained on a Philips IQon 128-slice spectral CT (Philips Healthcare, Cleveland, OH, USA). The acquisitions were carried out using two different radiation dose levels: low dose (30 mAs at 120 kVp) and standard-dose (100 mAs at 120 kVp). The dose on low-dose CT was 69.5% less than that on standard dose CT. Radiation dose of low-dose CT was as follows: volume CT dose index (CTDIvol), 2.7 mGy; dose-length product (DLP), 25.4 mGy; effective dose (k: 0.014), 0.36 mSv. Radiation dose of standard-dose CT was as follows: CTDIvol, 9 mGy; DLP, 84.3 mGy; effective dose (k: 0.014), 1.18 mSv.
Static scan parameters were 64 × 0.625-mm collimation, 40-mm beam width, 0.67-mm slice thickness, 1.08 pitch, and a rotation time of 0.75 seconds.
Image Reconstruction Techniques
A series of CT scans with two radiation dose settings were reconstructed with FBP, HIR (iDose4; Philips Healthcare), MIR (IMR; Philips Healthcare), and a VME created at a fixed keV (Fig. 1). For iDose4, level 4 was used with a reconstruction filter B. With IMR image, sharpness is controlled by the “Image Definition” setting instead of filter kernel. For IMR, level 1 was used with three different image definitions: body routine (IMR-R1), body soft tissue (IMR-ST1), and sharp plus (IMR-SP1). For VME, a standard virtual monoenergetic dataset at 70 keV (VME70) was calculated and level 4 was used with reconstruction filter B. The 70 keV was chosen to be equivalent to 120 kVp, which is standard algorithm for CT angiography examinations at our institution. Also, it is known that monochromatic images from 70 keV produce similar CT numbers of the subcutaneous fat which are comparable to 120 kVp polychromatic results.
Among these six reconstructions, FBP, iDose4, IMR-R1, IMR-ST1, and VME70 were used for EV analysis, and FBP, iDose4, IMR-R1, IMR-SP1, and VME70 were used for the airway wall measurement. Therefore, five datasets for each radiation dose and emphysema/airway measurement were reconstructed.
Emphysema Volume Measurements and Airway Wall Measurements
All measurements were performed by a technician using a commercial software (Aview, Coreline Soft, Seoul, Korea). To measure the EV, automatic segmentation and volume quantification were performed by clicking on each air column. A threshold of −950 HU was used to define emphysema in the air columns. For correctly selected and segmented air columns, the volume of each air column was recorded. For AM, the airway wall area (WA) and the luminal area (LA) were measured. Then, the WA percentage (WA%) was calculated as follows: WA / (WA + LA). Finally, five calculated WA% were obtained using five LA and WA measurements at randomly selected five locations. Correctness of the lesion segmentation and selection was inspected visually and there was no manual correction. For the analysis of the difference between measurements in each algorithm and reference measurements, the absolute percentage measurement error of EV (APEvol) and WA% (APEWA%) was calculated. The APEvol or APEWA% was calculated as follows: |measurement in each algorithm - reference measurement| × 100 / reference measurement. The APEvol or APEWA% was expressed as mean ± standard deviation.
Statistical Analysis
Repeated measures analysis of variance (ANOVA) was performed on the repeated measured data. The Bonferroni method for confidence interval adjustment was used to compare the main effects. If the sphericity assumption for ANOVA was not met, and then the p value from the Greenhouse-Geisser correction was used instead. A p value of less than 0.05 indicated statistical significance. All statistical analyses were performed using SPSS (version 20; IBM Corp., Armonk, NY, USA).
RESULTS
Accuracy of Emphysema Volume
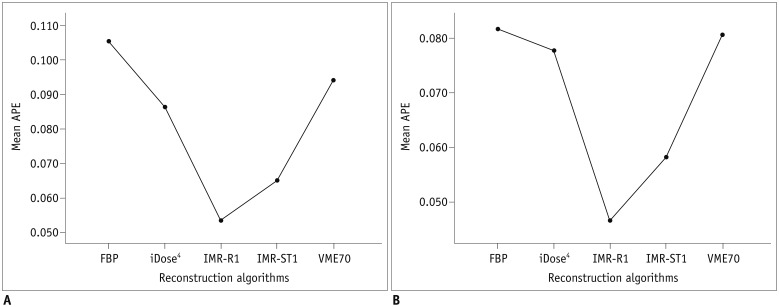
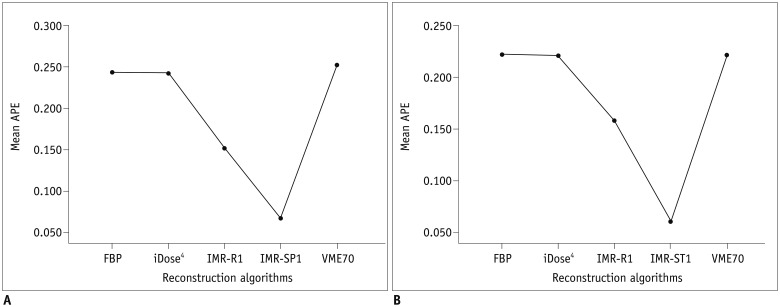
The APEvol of across all air columns (all of 5-, 10-, and 30-mm air columns) was significantly lower when IMR-R1 was used for reconstruction in both low-dose (p < 0.001) and standard-dose CT (p < 0.001) (Fig. 2). Also, the APEvol of each column (each 5-, 10-, and 30-mm air column) was significantly lower when IMR-R1 was used in both low-dose and standard-dose (all p < 0.05) (Table 2, Fig. 3). The mean APEvol decreased as the volume of air columns increased in all reconstruction algorithms and in both low-dose and standard-dose CT (all p < 0.01). VME70 showed significantly lower APEvol than FBP (p = 0.003), but showed significantly higher APEvol than iDose4, IMR-R1, and IMR-ST1 (all p < 0.004).
Fig. 2. APEvol of across all air columns (all of 5-, 10-, and 30-mm air columns) on (A) low-dose CT and (B) standard-dose CT.
Mean APEvol in all air columns was lowest in IMR-R1 in both low-dose and standard-dose settings. APE = absolute percentage measurement error, APEvol = APE of EV
Table 2. APEvol according to Different Air Columns, CT Tube Current, and Reconstruction Algorithms.
| Air Column Diameter | FBP | iDose4 | IMR-R1 | IMR-ST1 | VME70 | P† | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low Dose | Standard Dose | Low Dose | Standard Dose | Low Dose | Standard Dose | Low Dose | Standard Dose | Low Dose | Standard Dose | Low Dose | Standard Dose | |
| Across all air columns | 0.105 ± 0.003 | 0.082 ± 0.002* | 0.086 ± 0.002 | 0.078 ± 0.003 | 0.053 ± 0.002 | 0.047 ± 0.003 | 0.065 ± 0.002 | 0.058 ± 0.003 | 0.094 ± 0.003 | 0.081 ± 0.002 | < 0.001 | < 0.001 |
| 5 mm | 0.207 ± 0.004 | 0.165 ± 0.004* | 0.176 ± 0.004 | 0.159 ± 0.004* | 0.107 ± 0.008 | 0.097 ± 0.004 | 0.128 ± 0.004 | 0.118 ± 0.005 | 0.187 ± 0.006 | 0.160 ± 0.004* | 0.015 | 0.019 |
| 10 mm | 0.090 ± 0.004 | 0.069 ± 0.004* | 0.071 ± 0.004 | 0.064 ± 0.004 | 0.047 ± 0.003 | 0.039 ± 0.004 | 0.058 ± 0.004 | 0.050 ± 0.005 | 0.080 ± 0.006 | 0.069 ± 0.004 | 0.042 | 0.021 |
| 30 mm | 0.019 ± 0.004 | 0.011 ± 0.004 | 0.012 ± 0.004 | 0.011 ± 0.004 | 0.006 ± 0.003 | 0.004 ± 0.004 | 0.009 ± 0.004 | 0.007 ± 0.005 | 0.015 ± 0.006 | 0.013 ± 0.004 | < 0.001 | < 0.001 |
APEvol are expressed as mean ± standard deviation. *Showing significant difference between APE between low dose and standard dose (p < 0.05), †Showing significant difference between reconstruction algorithms. APE = absolute percentage measurement error, APEvol = APE of EV, CT = computed tomography, FBP = filtered back projection, IMR-R1 = body routine level 1, IMR-ST1 = body soft tissue level 1, VME70 = standard virtual monoenergetic dataset at 70 keV
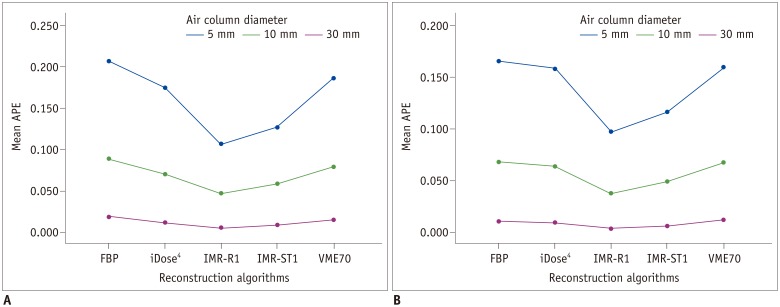
Fig. 3. APEvol of each column (each 5-, 10-, and 30-mm) on (A) low-dose CT and (B) standard-dose CT.
Mean APEvol in all air columns was lowest in IMR-R1 in both low-dose and standard-dose settings. Mean APEvol decreased as volume of air columns increased.
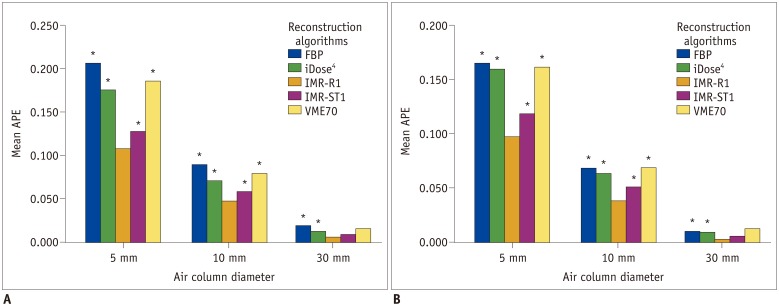
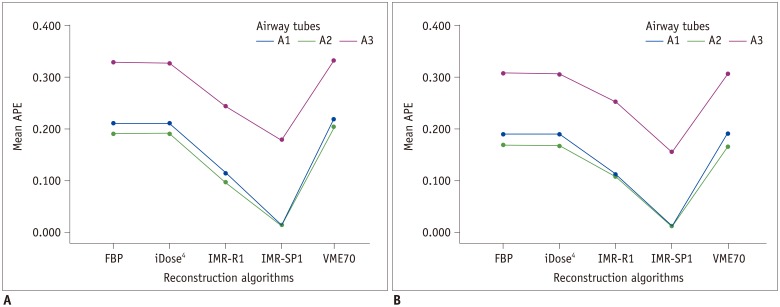
There were also significant differences in APEvol between IMR-R1 and each algorithm in 5-mm and 10-mm air columns on both low-dose and standard-dose CT (Fig. 4). However, the APEvol of the largest air column volume (30-mm air column) showed significant differences only between IMR-R1 and FBP and between IMR-R1 and iDose4, whereas there were no significant differences between IMR-R1, IMR-ST1, and VME70 on low-dose and standard-dose CT.
Fig. 4. Mean APEvol in each air column on (A) low-dose CT and (B) standard-dose CT.
Significant differences in APEvol from IMR-R1 are marked with an asterisk (*).
The lowest mean APEvol is shown in IMR-R1 at a standard dose, follow by IMR-R1 at a low dose, IMR-ST1 at a standard dose, and IMR-ST1 at a low dose. There were no significant differences in mean APEvol of all the air columns between IMR-R1 at a low dose and a standard dose (all p > 0.05).
Accuracy of Airways Measurement
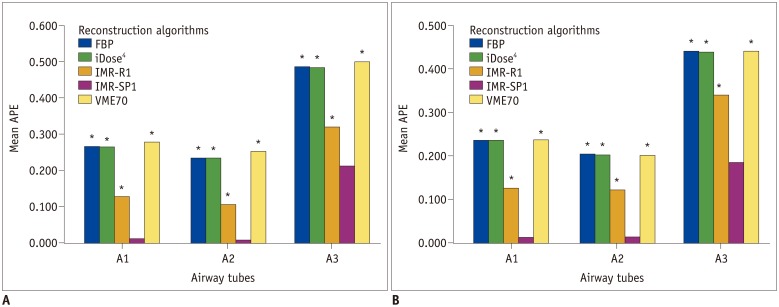
The APEWA% of across all airway tubes (all of A1, A2, and A3 airway tubes) was significantly lower when IMR-SP1 was used for reconstruction in both low-dose (p < 0.001) and standard-dose CT (p < 0.001) (Fig. 5). The APEWA% of each airway tube (each A1, A2, and A3 airway tube) was also significantly lower when IMR-SP1 was used for reconstruction on both low-dose and standard-dose CT (all p < 0.05) (Table 3, Fig. 6). The mean APEWA% increased as the WA% of airway tubes decreased in all reconstruction algorithms and in both low-dose and standard-dose CT (all p < 0.01). There were no significant differences in the mean APEWA% between different axes in all reconstruction algorithms and in both low-dose and standard-dose CT (A1 and A2, all p > 0.05). There were significant differences in APEWA% between IMR-SP1 and the other algorithms in all the airway tubes in both low-dose and standard-dose CT (Fig. 7). VME70 showed significantly higher APEWA% compared with IMR-R1 and IMR-SP1 in both low-dose and standard-dose CT (all p < 0.001).
Fig. 5. APEWA% of across all airway tubes (all of A1, A2, and A3 airway tubes) on (A) low-dose CT and (B) standard-dose CT.
Mean APEWA% of across all airway tubes was lowest in IMR-SP1 in both low-dose and standard-dose settings. Mean APEWA% decreased as WA% increased. APEWA% = absolute percentage measurement error of wall area percentage, WA% = wall area percentage
Table 3. APEWA% according to Different Air Columns, CT Tube Current, and Reconstruction Algorithms.
| Airway Tube | FBP | iDose4 | IMR-R1 | IMR-SP1 | VME70 | P† | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low Dose | Standard Dose | Low Dose | Standard Dose | Low Dose | Standard Dose | Low Dose | Standard Dose | Low Dose | Standard Dose | Low Dose | Standard Dose | |
| Across all airway tubes | 0.242 ± 0.002 | 0.221 ± 0.001* | 0.242 ± 0.001 | 0.221 ± 0.001* | 0.151 ± 0.002 | 0.158 ± 0.001* | 0.067 ± 0.002 | 0.060 ± 0.003* | 0.251 ± 0.002 | 0.221 ± 0.001* | < 0.001 | < 0.001 |
| A1 | 0.210 ± 0.003 | 0.190 ± 0.002* | 0.209 ± 0.003 | 0.190 ± 0.002* | 0.113 ± 0.003 | 0.112 ± 0.002 | 0.014 ± 0.003 | 0.011 ± 0.004 | 0.218 ± 0.003 | 0.190 ± 0.003* | 0.003 | 0.011 |
| A2 | 0.190 ± 0.003 | 0.168 ± 0.002* | 0.189 ± 0.003 | 0.168 ± 0.002* | 0.096 ± 0.003 | 0.108 ± 0.002* | 0.010 ± 0.003 | 0.013 ± 0.004 | 0.202 ± 0.003 | 0.168 ± 0.003* | 0.029 | 0.045 |
| A3 | 0.327 ± 0.003 | 0.306 ± 0.002* | 0.327 ± 0.003 | 0.305 ± 0.002* | 0.243 ± 0.003 | 0.253 ± 0.002* | 0.176 ± 0.003 | 0.156 ± 0.004* | 0.334 ± 0.003 | 0.306 ± 0.003* | 0.016 | 0.032 |
APEWA% are expressed as mean ± standard deviation. *Showing significant difference between APE between low dose and standard dose (p < 0.05), †Showing significant difference between reconstruction algorithms. APEWA% = absolute percentage measurement error of wall area percentage, IMR-SPI = sharp plus level 1
Fig. 6. Absolute percentage measurement error of each airway tube (each A1, A2, and A3 airway tube) on (A) low-dose CT and (B) standard-dose CT.
Mean APEWA% decreased as WA% increased.
Fig. 7. Mean APEWA% in each airway tube on (A) low-dose and (B) standard-dose CT.
Significant differences in APEWA% from IMR-SP1 are marked with asterisk (*).
The mean APEWA% of the airway tube with a lower WA% (A3) was significantly lower in IMR-SP1 at the standard dose than at the low dose (p = 0.046), although there were no significant differences in the mean APEWA% of two airway tubes which had same WA% but different axes (A1 and A2) between low-dose and standard-dose IMR-SP1 (all p > 0.05).
DISCUSSION
Our study demonstrated that IMR was the most accurate modality for measurement of both EV and airway wall thickness. However, VME70 did not show significantly better accuracy compared with other algorithms.
There have been few studies that compared MIR with FBP and HIR for emphysema detection and accurate measurement of EV and airway thickness (9,16,17). Choo et al. (9) demonstrated that IR could affect quantitative measurements of lung and airways: MIR seemed to provide the most accurate emphysema and AMs, compared with FBP and adaptive statistical IR. Therefore, they emphasized that care should be taken in selecting the appropriate IR algorithms. However, this study was limited by the fact that there was no reference for emphysema quantification. Gomez-Cardona et al. (16) showed that the optimal reconstruction algorithm for airway thickness measurement was MIR using an airway phantom. In their study, MIR enabled a significant reduction in both the relative bias and angular standard deviation of airway wall thickness.
Our study also showed that MIR provided the most accurate measurement values for both EV and airway wall thickness. Among MIR, EV was the most accurate in the IMR-R1 algorithm, whereas airway wall thickness was the most accurate in the IMR-SP1 algorithm. We used three different image definitions for MIR in this study: body routine (IMR-R1) and body soft tissue (IMR-ST1) for EV analysis, and body routine (IMR-R1) and sharp plus (IMR-SP1) for airway wall thickness. Body routine (IMR-R1) was the closest to filter B, which was used for FBP and HIR in our study. As sharp kernels are usually employed for lung parenchyma, image noise is too high at reduced dose levels; therefore, a soft tissue filter (filter B or IMR-R1) is recommended by the vendor and is routinely used for both soft tissue and parenchyma analyses (17,18). In the measurement of airway wall thickness, a sharp filter provided the most accurate value, which is recommended for analysis of airway thickness by the vendor. Our study revealed the usefulness of these filters in IMR for accurate measurement of EV and airway wall thickness. There have not been any previous articles on filter differences in IMR. We recommend use of these filters when analyzing EV or airway wall thickness using IMR.
We found no significant differences between IMR-R1 and IMR-ST1 or VME70 when measuring the 30-mm air column, whose volume was 28.416 mm3, although there were significant differences between IMR-R1 and FBP or iDose4. However, IMR-R1 had significantly different accuracy from the other algorithms when measuring the 5-mm and 10-mm air columns; the EVs were 0.789 mm3 and 3.157 mm3, respectively. These results demonstrate that IMR-R1, IMR-ST1, or VME70 could be used when the EV was larger than 28.416 mm3, but IMR can measure more accurate values when the EV is smaller than 3.157 mm3. It is important to measure small-volume emphysema accurately to understand the distribution of the low-attenuation areas in the emphysema quantification study, in addition to accurately measuring the EV (19,20,21). Such information is useful for classification or monitoring of patients with emphysema.
The major concern in CT quantification of EV and airway wall thickness is exposure to radiation, and these patients are more likely to undergo multiple CT scans than other patients. Our study showed that there was no significant difference in EV measurements between standard-dose and low-dose CT using IMR. Therefore, the EV could be followed with low-dose CT reconstructed with IMR. However, airway wall measurements for airways with a WA% of 40.83 or less (WA% of A3 was 40.83) may be inaccurate if low-dose CT is used, even though MIR is used. Therefore, when attempting to perform low-dose CT quantification, the reconstruction algorithm should be appropriately reconstructed.
Our study also demonstrated the measurement accuracy of EV and airway wall thickness in the VME70 algorithm, which is known to reduce metallic artifact and improve image quality. However, no studies have yet shown CT quantification in VME. Disappointingly, the VME did not appear suitable for CT quantification of EV and airway wall thickness.
Our study has several limitations. First, there are inherent limitations of phantom studies. All air columns and airway tubes were cylindrical in shape within a chest phantom, eliminating complicating factors in real clinical situations such as different emphysema morphology, breathing and cardiac motion artifacts, and different body sizes. Therefore, further clinical studies will be needed. Second, the number of air columns and airway tubes was limited. We believe that a larger number of air columns and airway tubes with various diameters and wall thicknesses are required for more precise evaluation of measurement feasibility. Third, we only observed the at the 70-keV setting, but we were not able to study what the impact would be in other keV settings. Therefore, further research from this perspective will be needed in the future.
In conclusion, IMR significantly improves the accuracy of emphysema and AMs compared with FBP and HIR in both low-dose and standard-dose CT. The EV was most accurate in IMR-R1, and the airway wall thickness was most accurately measured in IMR-SP1. However, VME70 did not result in any improvement of measurement accuracy.
Footnotes
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (NRF-2016R1A2B4012155).
This study was supported by Dong-Kook Pharmaceutical (Q1524331), and Reyon Pharmaceutical and Philips Company (I0902491).
This study was supported by a Research Grant from Korea University (K1518111, L1600241, K1722091).
References
- 1.Lynch DA, Al-Qaisi MA. Quantitative computed tomography in chronic obstructive pulmonary disease. J Thorac Imaging. 2013;28:284–290. doi: 10.1097/RTI.0b013e318298733c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim SS, Jin GY, Li YZ, Lee JE, Shin HS. CT Quantification of Lungs and Airways in Normal Korean Subjects. Korean J Radiol. 2017;18:739–748. doi: 10.3348/kjr.2017.18.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks RA, Di Chiro G. Theory of image reconstruction in computed tomography. Radiology. 1975;117(3 Pt 1):561–572. doi: 10.1148/117.3.561. [DOI] [PubMed] [Google Scholar]
- 4.Baumueller S, Winklehner A, Karlo C, Goetti R, Flohr T, Russi EW, et al. Low-dose CT of the lung: potential value of iterative reconstructions. Eur Radiol. 2012;22:2597–2606. doi: 10.1007/s00330-012-2524-0. [DOI] [PubMed] [Google Scholar]
- 5.Yang WJ, Yan FH, Liu B, Pang LF, Hou L, Zhang H, et al. Can sinogram-affirmed iterative (SAFIRE) reconstruction improve imaging quality on low-dose lung CT screening compared with traditional filtered back projection (FBP) reconstruction? J Comput Assist Tomogr. 2013;37:301–305. doi: 10.1097/RCT.0b013e31827b8c66. [DOI] [PubMed] [Google Scholar]
- 6.Lim HJ, Chung MJ, Shin KE, Hwang HS, Lee KS. The impact of iterative reconstruction in low-dose computed tomography on the evaluation of diffuse interstitial lung disease. Korean J Radiol. 2016;17:950–960. doi: 10.3348/kjr.2016.17.6.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katsura M, Matsuda I, Akahane M, Sato J, Akai H, Yasaka K, et al. Model-based iterative reconstruction technique for radiation dose reduction in chest CT: comparison with the adaptive statistical iterative reconstruction technique. Eur Radiol. 2012;22:1613–1623. doi: 10.1007/s00330-012-2452-z. [DOI] [PubMed] [Google Scholar]
- 8.Yuki H, Oda S, Utsunomiya D, Funama Y, Kidoh M, Namimoto T, et al. Clinical impact of model-based type iterative reconstruction with fast reconstruction time on image quality of low-dose screening chest CT. Acta Radiol. 2016;57:295–302. doi: 10.1177/0284185115575537. [DOI] [PubMed] [Google Scholar]
- 9.Choo JY, Goo JM, Lee CH, Park CM, Park SJ, Shim MS. Quantitative analysis of emphysema and airway measurements according to iterative reconstruction algorithms: comparison of filtered back projection, adaptive statistical iterative reconstruction and model-based iterative reconstruction. Eur Radiol. 2014;24:799–806. doi: 10.1007/s00330-013-3078-5. [DOI] [PubMed] [Google Scholar]
- 10.Mets OM, Willemink MJ, de Kort FP, Mol CP, Leiner T, Oudkerk M, et al. The effect of iterative reconstruction on computed tomography assessment of emphysema, air trapping and airway dimensions. Eur Radiol. 2012;22:2103–2109. doi: 10.1007/s00330-012-2489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishio M, Matsumoto S, Ohno Y, Sugihara N, Inokawa H, Yoshikawa T, et al. Emphysema quantification by low-dose CT: potential impact of adaptive iterative dose reduction using 3D processing. AJR Am J Roentgenol. 2012;199:595–601. doi: 10.2214/AJR.11.8174. [DOI] [PubMed] [Google Scholar]
- 12.Leng S, Yu L, Fletcher JG, McCollough CH. Maximizing iodine contrast-to-noise ratios in abdominal CT imaging through use of energy domain noise reduction and virtual monoenergetic dual-energy CT. Radiology. 2015;276:562–570. doi: 10.1148/radiol.2015140857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goo HW, Goo JM. Dual-energy CT: new horizon in medical imaging. Korean J Radiol. 2017;18:555–569. doi: 10.3348/kjr.2017.18.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaup M, Scholtz JE, Engler A, Albrecht MH, Bauer RW, Kerl JM, et al. Dual-energy computed tomography virtual monoenergetic imaging of lung cancer: assessment of optimal energy levels. J Comput Assist Tomogr. 2016;40:80–85. doi: 10.1097/RCT.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 15.Frellesen C, Kaup M, Wichmann JL, Hüsers K, Scholtz JE, Albrecht MH, et al. Noise-optimized advanced image-based virtual monoenergetic imaging for improved visualization of lung cancer: comparison with traditional virtual monoenergetic imaging. Eur J Radiol. 2016;85:665–672. doi: 10.1016/j.ejrad.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Cardona D, Nagle SK, Li K, Robinson TE, Chen GH. Influence of radiation dose and reconstruction algorithm in MDCT assessment of airway wall thickness: a phantom study. Med Phys. 2015;42:5919–5927. doi: 10.1118/1.4930797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Margerie-Mellon C, de Bazelaire C, Montlahuc C, Lambert J, Martineau A, Coulon P, et al. Reducing radiation dose at chest CT: comparison among model-based type iterative reconstruction, hybrid iterative reconstruction, and filtered back projection. Acad Radiol. 2016;23:1246–1254. doi: 10.1016/j.acra.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Nakajo C, Heinzer S, Montandon S, Dunet V, Bize P, Feldman A, et al. Chest CT at a dose below 0.3 mSv: impact of iterative reconstruction on image quality and lung analysis. Acta Radiol. 2016;57:311–317. doi: 10.1177/0284185115578469. [DOI] [PubMed] [Google Scholar]
- 19.Blechschmidt RA, Werthschützky R, Lörcher U. Automated CT image evaluation of the lung: a morphology-based concept. IEEE Trans Med Imaging. 2001;20:434–442. doi: 10.1109/42.925296. [DOI] [PubMed] [Google Scholar]
- 20.Mishima M, Hirai T, Itoh H, Nakano Y, Sakai H, Muro S, et al. Complexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A. 1999;96:8829–8834. doi: 10.1073/pnas.96.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madani A, Van Muylem A, de Maertelaer V, Zanen J, Gevenois PA. Pulmonary emphysema: size distribution of emphysematous spaces on multidetector CT images--comparison with macroscopic and microscopic morphometry. Radiology. 2008;248:1036–1041. doi: 10.1148/radiol.2483071434. [DOI] [PubMed] [Google Scholar]