Abstract
Objective
The purpose of this study was to compare efficacy, sonication energy efficiency, treatment time and safety of magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU) and those of ultrasound-guided high-intensity focused ultrasound (USgHIFU) for ablation of uterine fibroids.
Materials and Methods
This study included 43 patients with 44 symptomatic uterine fibroids treated with MRgHIFU and 51 patients with 68 symptomatic uterine fibroids treated with USgHIFU. After therapy, contrast-enhanced MRI was conducted and complete ablation was defined as 100% non-perfused volume (NPV) of fibroids. Patients with completely ablated fibroids were selected for the comparison of the treatment data and sonication parameters between MRgHIFU and USgHIFU treated groups.
Results
Thirteen completely ablated fibroids in 10 patients (23.3%, 10/43) were achieved with MRgHIFU and 28 completely ablated fibroids in 22 patients (43.1%, 22/51) were achieved with USgHIFU. In completely ablated fibroids, the energy-efficiency factor (EEF) was 5.1 ± 3.0 J/mm3 and 4.7 ± 2.5 J/mm3 in the MRgHIFU and USgHIFU, respectively (p = 0.165). There was a negative linear correlation between EEF and the NPV of fibroids for MRgHIFU (p = 0.016) and USgHIFU (p = 0.001). The mean treatment time was 174.5 ± 42.2 minutes and 114.4 ± 39.2 minutes in the MRgHIFU and USgHIFU procedures, respectively (p = 0.021). There were no severe adverse events and major complications after treatment.
Conclusion
MRgHIFU and USgHIFU are safe and effective with the equivalent energy efficiency for complete ablation of fibroids. USgHIFU has shorter treatment time than MRgHIFU.
Keywords: Uterine fibroids, High-intensity focused ultrasound ablation, MR imaging, Ultrasound
INTRODUCTION
Recently, magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU) has become a non-invasive therapeutic procedure that offers another promising option for treatment of symptomatic uterine fibroids (1,2,3,4). MRgHIFU is an alternative to the traditional myomectomy without surgical incision and major complication. However, the clinical application of MRgHIFU is limited because of restricted eligibility, requirement for a dedicated MRI device to guide treatment, and lengthy procedure time (5). In the past decade, more than 20000 patients with the malignant or benign tumors have been treated using ultrasound-guided high-intensity focused ultrasound (USgHIFU) with remarkable efficacy (6).
The evaluation of USgHIFU treatment is based on the echogenicity of ultrasound images that monitor the therapeutic accuracy and effectiveness by the change of echogenecity in the targeted region (7,8), that lagged tissue damage. Therefore, the safety of its treatment is of serious concern. In contrast, MRgHIFU has the capability of monitoring temperature elevation to ensure safety and effectiveness, since temperature at the focal region can accurately predict the ablation effect in treatment of uterine fibroids, and provide the feedback to adjust the sonication energy and power. However, there have been no publications on a comparative study between MRgHIFU and USgHIFU for ablation of tumors in the literature to date.
The purpose of this study was to evaluate the efficacy, sonication energy efficiency, treatment time, and safety of USgHIFU compared with those of MRgHIFU for the ablation of uterine fibroids.
MATERIALS AND METHODS
This is a prospective, non-randomized study, approved by the Institutional Ethics Committee (Clinical trial registration number NCT01239641). Written informed consent was obtained from all patients. All subjects were approached by Shanghai Clinical Center of Chinese Academy of Sciences (Shanghai, China).
Patients
The inclusion criteria for MRgHIFU and USgHIFU treatment of uterine fibroids included the following: 1) no more than two fibroids in each patient, determined by MRI; 2) all fibroids hypo-intense on pre-treatment T2-weighted imaging (T2WI). Fibroids were defined as hypo-intense if their pretreatment signal intensity appeared to be equal to or less than that of the skeletal muscle on T2WI (9); 3) all MRgHIFU and USgHIFU procedures were conducted by one interventional radiologist with more than 10 years of experience in image-guided tumor ablation therapy. Each patient underwent only one session of MRgHIFU or USgHIFU treatment.
This study included 94 uterine fibroid patients treated with HIFU. From July 2012 to June 2013, 43 women (41.6 ± 5.5 years) with 44 symptomatic uterine fibroids received MRgHIFU treatment, while 51 women (38.6 ± 7.0 years) with 68 uterine fibroids received USgHIFU treatment. After treatment, the patients with completely ablated fibroids in MRgHIFU and USgHIFU were selected for further analysis.
Pre-Treatment and Post-Treatment Imaging
All patients underwent MRI using a standardized protocol on a 1.5T MRI system or a 3T MRI system (Magnetom Avanto or Verio Tim, Siemens Healthineers, Erlangen, Germany). T2WI was acquired in axial, coronal, and sagittal planes, and T1-weighted imaging (T1WI) was obtained before and after administering gadolinium-diethylenetriaminepentaacetic acid-bis-methylamide (Gd-DTPA-BMA) contrast agent (gadodiamide, Omniscan; 1.0 mmol/mL; 1.0 mmol/kg of body weight; GE Healthcare, Shanghai, China). MR images were used to define the location, number, size, and volume of uterine fibroids. Fibroid volume and non-perfused volume (NPV) were measured by using software program, programmed by the engineers from Chongqing Haifu (HIFU) Tech Co., Ltd., to contour fibroids, and non-perfused region in every slice of MR imaging, then calculated by using the same program. Additionally, the percentage reduction in fibroid volume was calculated using the following formula:
| % reduction = ([total volume at baseline − total volume at 6 months] / total volume at baseline) × 100 |
Therapeutic Equipment
MRgHIFU System
The MRgHIFU procedure was conducted with the clinical extracorporeal JM 5100 MRgHIFU system (Chongqing Haifu Tech Co, Ltd., Chongqing, China) fully integrated into a 1.5T Magnetom Avanto MRI scanner (Siemens Medical Systems, Berlin, Germany) to provide a real-time temperature mapping for treatment control (1). The sonication energy was produced by a focused piezoelectric ceramic composite transducer with a diameter of 18 cm, a focal length of 15 cm, and an operating frequency of 1.0 MHz. The location of the acoustic focus can be electronically controlled, and its diameters were 6 mm along the beam axis and 2 mm in the transverse direction. With use of computer control, the integrated transducer can be moved smoothly in 6 directions.
USgHIFU System
The USgHIFU procedure was conducted with a JC-200 clinical extracorporeal USgHIFU system (Chongqing Haifu Tech Co, Ltd.), which was equipped with a diagnostic ultrasound (MyLab70; Esaote, Genoa, Italy) for real-time guidance. Therapeutic sonication energy was produced by a focused piezoelectric ceramic composite transducer with a physical focus of 22.5 mm3, a diameter of 20 cm an operating frequency of 1.0 MHz, and a focal length of 15 cm. Using computer control, the integrated transducer could be moved smoothly in 6 directions (left and right 120 mm, cranial and caudal 120 mm, and up and down 180 mm). The imaging probe was situated at the center of the high-intensity focused ultrasound transducer.
MRgHIFU and USgHIFU Procedures
Before the treatment, all patients underwent intestinal and skin preparation, and were placed in the prone position. During the HIFU procedure, patients were administered an intravenous sedative and analgesic (0.8–1.0 µg/kg fentanyl; 0.02–0.03 mg/kg midazolam hydrochloride) to maintain conscious sedation. A degassed water balloon was needed to push the bowel away from the acoustic pathway for preventing intestinal damage. Patients were requested to report discomfort.
MRgHIFU Procedure
Before acoustic exposure, the treatment plan was drafted by MRI images. Subsequently, the regions of interest were outlined by the treatment-planning system software. The acoustic power and energy were adjusted according to the real-time temperature elevation on proton resonance frequency-shifted MR imaging that provided the thermal feedback continually during the procedure. When the MR temperature mapping showed that temperature in the focal region increased to 60℃ or higher, we stopped sonicating and moved to the next targeted point. And when region temperature exceeded 70℃, the acoustic power would be decreased. The duration of each acoustic exposure was 2 seconds, and then an interval of 2 seconds or 3 seconds was required as cooling period in MRgHIFU treatment. According to the treatment plan, a variable number of acoustic exposures at each targeted region were conducted to cover entire volumes of the fibroids. The details had been described in our previous publication (1).
USgHIFU Procedure
High-intensity focused ultrasound procedures were conducted under the ultrasound monitoring mode (10). The acoustic power and energy was determined based on the feedback from patients and the changes in gray scale on ultrasound imaging during the procedure. When the gray scale change covered the region of the treated fibroid, 1.2 mL of SonoVue (Bracco Suisse SA, Shanghai, China) solution (reconstituted in a 59-mg vial with addition of 5 mL of normal saline) was administrated intravenously to assess the therapeutic response for terminating the treatment. If any unexpected residual lesion was spotted in the treated lesion, the supplementary sonication at the same session of HIFU treatment could be administered.
Evaluation Parameters
NPV
All patients underwent post-treatment MR imaging within 1 day after HIFU to assess the treatment effectiveness. The tumor volume and NPV were acquired before and after administration of the Gd-DTPA-BMA. The NPV ratio (defined as the NPV divided by the fibroid volume) of the treated fibroid was calculated, and 100% of NPV ratio with no residual portion found in 6 months following-up was defined as the complete ablation.
Treatment Time and Efficiency
Treatment time (minutes) was defined as the duration from the first to last sonication. Sonication time (seconds) was considered the cumulative time of acoustic exposures. Treatment efficiency was expressed as NPV (mm3) per treatment time unit (minute).
Energy-Efficiency Factor
Energy-efficiency factor (EEF, defined as the acoustic energy [J] delivered for ablating 1 mm3 of the fibroid) was the key factor to evaluate treatment energy efficiency. EEF (J/mm3) represents how much energy is required for ablating 1 unit of myomatous lesion.
Adverse Events and Complications
Treatment-related adverse events and complications were recorded after treatment. Complications were classified according to the Society of Interventional Radiology (SIR) Clinical Practice Guidelines (11). Minor complications 1) no therapy, no consequence, 2) nominal therapy, no consequence; includes overnight admission for observation only. Major complications, 3) require therapy, minor hospitalization (48 hours), 4) require major therapy, unplanned increase in level of care, prolonged hospitalization (48 hours), 5) permanent adverse sequelae, and 6) death.
Follow-Up
All patients were followed up for six months to determine the short-term efficacy and the relief of symptoms related to the therapy. All patients received MRI examinations with the same scanning protocol and parameters as those used before treatment.
Patients' symptoms associated with fibroids were prospectively collected and quantitatively evaluated according to eight symptom severity score (SSS). SSS index questions were included in the follow-up Uterine Fibroid Symptom-Quality of Life questionnaire (12). SSS were calculated on a 100-point scale and compared between pre-treatment and the six-month follow-up.
Statistical Analysis
SPSS software (SPSS 18.0; SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Data were presented as mean ± standard deviation. The Student's t test or chi-square test was used for comparison between the two group. The correlation of variables was investigated by conducting linear correlation analysis and Spearman correlation tests. A p value < 0.05 was a statistically significant difference.
RESULTS
Among 43 patients in the MRgHIFU group, the NPV ratios were 100% in 13 fibroids from 10 patients (23.3%, 10/43), 90.0–99.0% in 12 fibroids from 10 patients (23.3%, 10/43), and less than 90.0% in 26 fibroids from 23 patients (53.5%, 23/43). Among 51 patients in USgHIFU group, the NPV ratios were 100% in 28 fibroids from 22 patients (43.1%, 22/51), 90.0–99.0% in 14 fibroids from 9 patients (17.6%, 9/51), and less than 90.0% in 26 fibroids from 20 patients (39.2%, 20/51) (Table 1).
Table 1. Features of Uterine Fibroids for All Subjects.
| Variable | MRgHIFU | USgHIFU | P |
|---|---|---|---|
| Patients (number) | 43 | 51 | |
| Age (years) | 41.6 ± 5.5 | 38.6 ± 7.0 | 0.356 |
| Number of uterine fibroids (n) | 44 | 68 | |
| Volume of uterine fibroids (cm3)* | 95.0 ± 83.8 | 126.9 ± 121.3 | 0.663 |
| Location of uterine fibroids (numbers)† | |||
| Intramural | 22 | 53 | |
| Subserous | 21 | 6 | |
| Submucous | 1 | 9 | |
| Patients with completely ablated fibroid (%) | 10 (23.3%, 10/43) | 22 (43.1%, 22/51) | 0.031 |
*Data showed volume of uterine fibroids in each case, †Data showed number of uterine fibroids. MRgHIFU = magnetic resonance-guided high-intensity focused ultrasound, USgHIFU = ultrasound-guided high-intensity focused ultrasound
Completely Ablated Fibroid(s)
Among the 13 completely ablated fibroids in the MRgHIFU group, 10 were located at the anterior wall of the uterus, one at the posterior wall, and two at the fundus. Among the 28 completely ablated fibroids in the USgHIFU group, 10 were located at the anterior wall of the uterus, 11 at the posterior wall, 6 at the sidewall, and 1 at the fundus. There were no significant differences in the distance from the center of uterine fibroids to the abdominal skin, and the diameter and volume of completely ablated fibroids between MRgHIFU and USgHIFU groups (p > 0.05) (Table 2).
Table 2. Features of Completely Ablated Uterine Fibroids.
| Variable | MRgHIFU | USgHIFU | P |
|---|---|---|---|
| Number of uterine fibroids | 13 | 28 | |
| Diameter of uterine fibroids (cm) | 6.5 ± 1.3 | 6.3 ± 1.0 | 0.633 |
| Average volume of uterine fibroids (cm3) | 127.8 ± 70.2 | 118.9 ± 55.0 | 0.639 |
| Distance from center of uterine fibroids to abdominal skin (mm) | 70.8 ± 18.9 | 70.7 ± 18.2 | 0.957 |
| Location of uterine fibroids (n) | |||
| Anterior wall | 10 | 10 | |
| Posterior wall | 1 | 11 | |
| Sidewall | 0 | 6 | |
| Fundus | 2 | 1 |
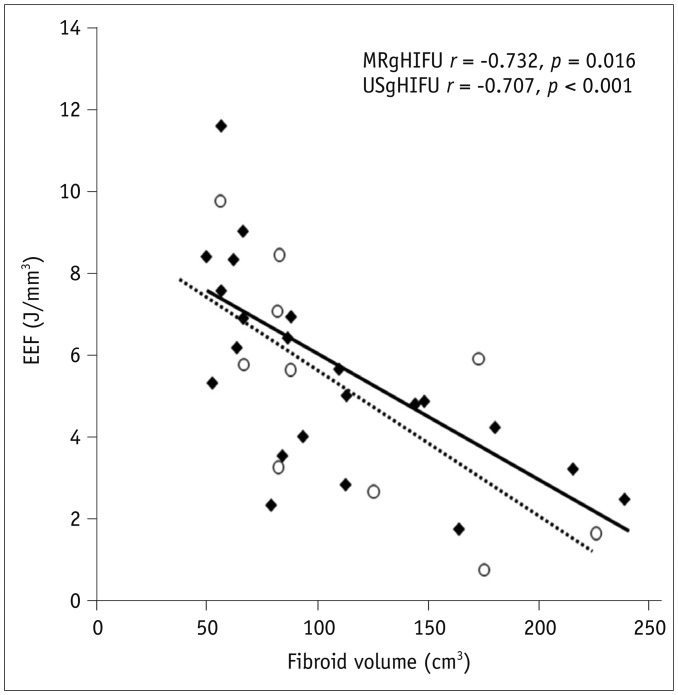
The fibroid NPVs were 127.8 ± 70.2 cm3 and 118.9 ± 55.0 cm3; acoustic energy, 483.0 ± 248.2 kJ and 463.2 ± 156.4 kJ; and EEF, 5.1 ± 3.0 J/mm3 and 4.7 ± 2.5 J/mm3 in MRgHIFU and USgHIFU, respectively, (p > 0.05) (Table 3). There was a linear negative correlation between EEF and the fibroid ablation volume in the two groups (p = 0.016 and p < 0.001) (Fig. 1).
Table 3. Comparison between MRgHIFU and USgHIFU for Complete Ablation of Uterine Fibroids.
| Variable | MRgHIFU (n = 10) | USgHIFU (n = 22) | P |
|---|---|---|---|
| NPV (cm3)* | 127.8 ± 70.2 | 118.9 ± 55.0 | 0.632 |
| Acoustic energy (kJ) | 483.0 ± 248.2 | 463.2 ± 156.4 | 0.412 |
| EEF (J/mm3) | 5.1 ± 3.0 | 4.7 ± 2.5 | 0.165 |
| Acoustic power (W) | 310.2 ± 62.5 | 391.6 ± 16.6 | 0.048 |
| Treatment time (min) | 174.5 ± 42.2 | 114.4 ± 39.2 | 0.021 |
| Treatment speed (cm3/h) | 42.2 ± 25.6 | 70.9 ± 41.9 | 0.018 |
*Sum of fibroid's NPV in each patient. EEF = energy-efficiency factor, NPV = non-perfused volume
Fig. 1. Scatterplot showed EEF distribution was correlated with volume of fibroids completely ablated by MRgHIFU (◆) and USgHIFU (○).
EEF = energy-efficiency factor, MRgHIFU = magnetic resonance-guided high-intensity focused ultrasound, USgHIFU = ultrasound-guided high-intensity focused ultrasound
The acoustic power values were 310.2 ± 62.5 W and 391.6 ± 16.6 W (p = 0.048), the treatment times were 174.5 ± 42.2 minutes and 114.4 ± 39.2 minutes (p = 0.021), and the treatment speed records were 42.2 ± 25.6 cm3/h and 70.9 ± 41.9 cm3/h (p = 0.018) in the MRgHIFU and USgHIFU treatment for complete ablation of fibroids, respectively (Table 3).
Adverse Events and Complications
There were no significant complications (B–F class by the SIR standard) reported and no fever or lower limb numbness occurred in subjects (11). All patients restored normal activities within 2 hours after the procedure. For the patients with completely ablated fibroids, the MRgHIFU group had 2 patients with abnormal vaginal discharge and three patients with mild pain in the lower abdomen, that lasted until the second day after treatment; while the USgHIFU group had 3 patients with abnormal vaginal discharge, 2 patients with mild pain in the lower abdomen and 1 patient feeling mild lower back pain that lasted to the second day after treatment.
6-Month Follow-Up
The mean fibroid volume reductions were 59.1 ± 9.0% and 52.7 ± 11.4% in the MRgHIFU group and USgHIFU group, respectively. The mean transformed SSS decreased from 26.6 ± 4.3 at baseline to 14.6 ± 2.0 in the MRgHIFU group and from 25.3 ± 14.7 at baseline to 15.1 ± 5.1 in the USgHIFU group. There was no significant difference of the fibroid volume reduction ratio and symptom improving score between the two groups (p > 0.05) (Figs. 2, 3).
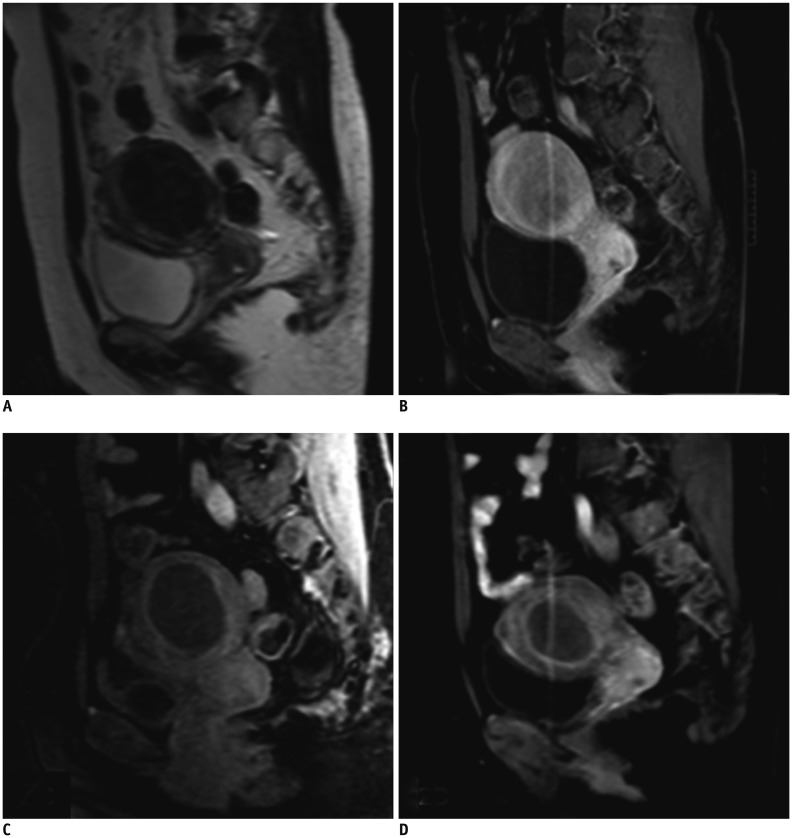
Fig. 2. Patient with symptomatic uterine fibroids treated by MRgHIFU.
Representative images are shown for three different time points. A. Uterine fibroid in 37-year-old woman was hypo-intense on pretreatment T2WI. B. Sagittal contrast-enhanced T1WI showed homogeneous enhancement before MRgHIFU. C. Sagittal contrast-enhanced T1WI showed 100% NPV in fibroid immediately after sonication. D. Fibroid volume shrinkage (65% of baseline) with sustained non-perfused area at 6-month follow-up. NPV = non-perfused volume, T1WI = T1-weighted imaging, T2WI = T2-weighted imaging
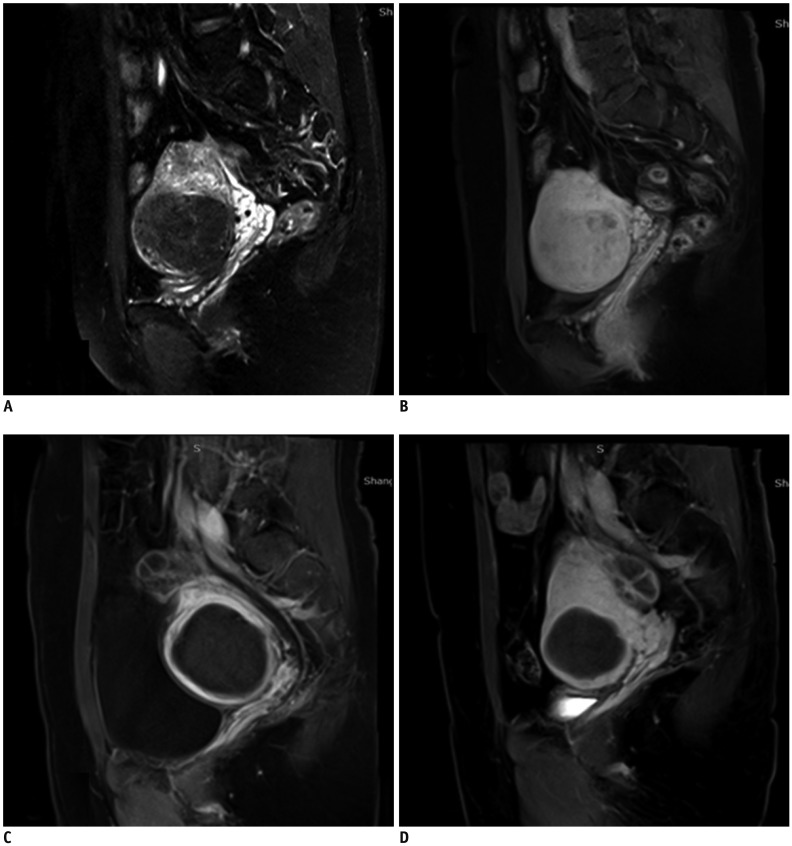
Fig. 3. Patient with symptomatic uterine fibroids treated by USgHIFU.
Representative images are shown for three different time points. A. Uterine fibroid in 36-year-old woman was hypo-intense on pretreatment T2WI. B. Sagittal contrast-enhanced T1WI showed homogeneous enhancement before USgHIFU. C. Sagittal contrast-enhanced T1WI showed 100% NPV in fibroid immediately after treatment. D. Fibroid volume shrinkage (64.5% of baseline) with sustained non-perfused area at 6-month follow-up.
DISCUSSION
As a non-invasive technique, HIFU has been applied in clinical practice for ablation of solid tumors (2). Its basic therapeutic mechanism is the release of sonication energy to a target region to increase local temperature to 60–100℃, resulting in coagulative necrosis of tissue (13). Currently, the HIFU systems are guided by either US or MRI for treatment at a precise focal point in the tissue, without harming overlying and adjacent structures, even those in the path of the ultrasound beam (14).
The temperature mapping sequence was used in MRgHIFU treatment to monitor the temperature elevation in real time, and a temperature of 60℃ or higher could predict the coagulative necrosis at the focal region (15). When the focal temperature was higher than 70℃, the acoustic power and energy would be decreased to prevent the adjacent tissue from diffusing heat resulting from the excessive sonication, that allowed the therapeutic sonication energy to be effective but not excessive so that effectiveness and safety of the treatment were ensured. During the treatment procedure of USgHIFU, however, the changes of echogenecity in the therapeutic area were monitored in real time as a feedback to release the acoustic energy for achieving a safe and effective thermal ablation. Currently there is no consensus regarding that USgHIFU is accurate and safe for the ablation of uterine fibroids. The following two issues should be concerned: 1) Whether the change of ultrasonic echogenecity at the targeted area can predict the effectiveness of the ablation or not; 2) Whether the therapeutic sonication energy was insufficient or excessive, that may result in incomplete or excessive ablation. The real-time temperature mapping of MRgHIFU ensures the acoustic energy released in proportion to avoid over-sonication, and the complete ablation of the fibroids represented the sufficient sonication. Therefore, MRgHIFU can be used as the reference to compare with USgHIFU in the complete ablation of fibroids to evaluate the effectiveness, sonication energy efficiency, treatment time and safety.
In addition to the thermal effect, the acoustic cavitation may be generated by USgHIFU without temperature monitoring since the acoustic power of USgHIFU was higher than that of MRgHIFU in this study. However, the EEF for completely ablating fibroids was 5.1 ± 3.0 J/mm3 in MRgHIFU and 4.7 ± 2.5 J/mm3 in USgHIFU, respectively, there was no significant difference between them. Therefore, the cavitation played only a minor role for enhancing the ablation effect in USgHIFU. Although our results also showed that the volume of the ablated fibroids was negatively correlated with EEF in both groups, that means that EEF was linearly reduced with larger myomatous lesions, there was no significant difference of the average volume of uterine fibroid between the two groups. Figure 1 showed that EEF was correlated with volume of fibroids completely ablated by MRgHIFU and USgHIFU, and both trend-lines were close. We assumed that USgHIFU had the equivalent energy efficiency with MRgHIFU in the treatment of fibroids.
Ideally, HIFU therapy should result in the maximum degree of tumor ablation with the minimum acoustic energy, thereby guaranteeing the safety and effectiveness of the treatment. MRgHIFU procedure has the real-time temperature mapping to monitor the dynamic changes of thermal effect at the focal region and the size of “the temperature-elevated focal spot” (1). The acoustic power and energy to ablate the fibroids could be controlled without over-treatment based on this predictor. In this study, the percentage of patients (23.3%) with completely ablated fibroids treated by MRgHIFU, however, was less than that (43.1%) by USgHIFU. The ultrasound contrast imaging conducted to detect the enhanced portion of treated fibroid for allowing supplementary sonication may contribute to improve the NPV ratio during the USgHIFU procedure. However, the supplementary sonication was not allowed immediately after Gd-DTPA-BMA contrast-enhanced MR imaging, even if the residual was found, because the chelating gadolinium may become Gd3+ after thermal effect, toxic to bone marrow (16,17).
Treatment time of HIFU is a noteworthy issue. Trumm et al. (18) reported that the mean treatment time was 3.3 ± 1.2 hours for the MRgHIFU produced by other commercial manufactures, that was longer than the 2.9 ± 0.7 hours (174.5 ± 42.2 minutes) of the MRgHIFU approved by the China Food and Drug Administration in this study. Our results showed that the treatment time was almost 1 hour shorter in the USgHIFU than that in the MRgHIFU (114.4 ± 39.2 minutes vs. 174.5 ± 42.2 minutes, p = 0.021), and the treatment speed for complete ablation of uterine fibroids in the USgHIFU was 70.9 ± 41.9 cm3/h that was significantly higher than 42.2 ± 25.6 cm3/h of the MRgHIFU. Obviously, the USgHIFU procedure is superior to MRgHIFU in the treatment time, since the ultrasound-guided imaging is more efficient and convenient in HIFU procedure compared to the time-consuming MR imaging procedures.
A previous study showed that uterine fibroid treatment by HIFU had 10.2% complications that included nerve injury, skin burns, hematuria, vertebral burns, and severe abdominal pain (19). Our study demonstrated that HIFU guided by MRI or ultrasound was safe without severe adverse events and major complication. The mean total released ultrasonic energy was close between USgHIFU and MRgHIFU. Therefore, USgHIFU had the acoustic energy release within the range of safety for treatment of the uterine fibroids as MRgHIFU.
The main limitation of this study was small sample size at one medical center, and large multi-center studies are necessary for further investigation since the USgHIFU had a cost-effectiveness advantage. In conclusion, MRgHIFU and USgHIFU are feasible, safe, and effective with the equivalent energy efficiency for complete ablation of T2 hypo-intense fibroids. USgHIFU is superior to MRgHIFU in terms of treatment time.
Acknowledgments
Various types of drugs and all patients were provided by Shanghai Clinical Center of Chinese Academic Sciences.
The clinical extracorporeal JM 2.5C MRgHIFU system and the JC-200 clinical extracorporeal USgHIFU system were provided by Chongqing Haifu Technical Limited Company.
Footnotes
This study was supported in part by the National Key Technology Research and Development Program of China (NO. 2011BAI14B01).
References
- 1.Xu Y, Fu Z, Yang L, Huang Z, Chen WZ, Wang Z. Feasibility, safety, and efficacy of accurate uterine fibroid ablation using magnetic resonance imaging-guided high-intensity focused ultrasound with shot sonication. J Ultrasound Med. 2015;34:2293–2303. doi: 10.7863/ultra.14.12080. [DOI] [PubMed] [Google Scholar]
- 2.Orsi F, Arnone P, Chen W, Zhang L. High intensity focused ultrasound ablation: a new therapeutic option for solid tumors. J Cancer Res Ther. 2010;6:414–420. doi: 10.4103/0973-1482.77064. [DOI] [PubMed] [Google Scholar]
- 3.Jolesz FA, Hynynen K, McDannold N, Freundlich D, Kopelman D. Noninvasive thermal ablation of hepatocellular carcinoma by using magnetic resonance imaging-guided focused ultrasound. Gastroenterology. 2004;127(5 Suppl 1):S242–S247. doi: 10.1053/j.gastro.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Chen WZ, Liu YJ, Hu X, Zhou K, Chen L, et al. Feasibility of magnetic resonance imaging-guided high intensity focused ultrasound therapy for ablating uterine fibroids in patients with bowel lies anterior to uterus. Eur J Radiol. 2010;73:396–403. doi: 10.1016/j.ejrad.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Pron G. Magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU) treatment of symptomatic uterine fibroids: an evidence-based analysis. Ont Health Technol Assess Ser. 2015;15:1–86. [PMC free article] [PubMed] [Google Scholar]
- 6.Yu T, Luo J. Adverse events of extracorporeal ultrasound-guided high intensity focused ultrasound therapy. PLoS One. 2011;6:e26110. doi: 10.1371/journal.pone.0026110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDannold N, Tempany CM, Fennessy FM, So MJ, Rybicki FJ, Stewart EA, et al. Uterine leiomyomas: MR imaging-based thermometry and thermal dosimetry during focused ultrasound thermal ablation. Radiology. 2006;240:263–272. doi: 10.1148/radiol.2401050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart EA, Gedroyc WM, Tempany CM, Quade BJ, Inbar Y, Ehrenstein T, et al. Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol. 2003;189:48–54. doi: 10.1067/mob.2003.345. [DOI] [PubMed] [Google Scholar]
- 9.Li F, Wang Z, Du Y, Ma P, Bai J, Wu F, et al. [Study on therapeutic dosimetry of HIFU ablation tissue] Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2006;23:839–843. [PubMed] [Google Scholar]
- 10.Chen J, Li Y, Wang Z, McCulloch P, Hu L, Chen W, et al. Committee of the Clinical Trial of HIFU versus Surgical Treatment for Fibroids. Evaluation of high-intensity focused ultrasound ablation for uterine fibroids: an IDEAL prospective exploration study. BJOG. 2018;125:354–364. doi: 10.1111/1471-0528.14689. [DOI] [PubMed] [Google Scholar]
- 11.Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–S202. doi: 10.1097/01.rvi.0000094584.83406.3e. [DOI] [PubMed] [Google Scholar]
- 12.Kajiyama K, Yoshinaka K, Takagi S, Matsumoto Y. Micro-bubble enhanced HIFU. Physics Procedia. 2010;3:305–314. [Google Scholar]
- 13.Lynn JG, Zwemer RL, Chick AJ, Miller AE. A new method for the generation and use of focused ultrasound in experimental biology. J Gen Physiol. 1942;26:179–193. doi: 10.1085/jgp.26.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W, Zhu H, Zhang L, Li K, Su H, Jin C, et al. Primary bone malignancy: effective treatment with high-intensity focused ultrasound ablation. Radiology. 2010;255:967–978. doi: 10.1148/radiol.10090374. [DOI] [PubMed] [Google Scholar]
- 15.Venkatesan AM, Partanen A, Pulanic TK, Dreher MR, Fischer J, Zurawin RK, et al. Magnetic resonance imaging-guided volumetric ablation of symptomatic leiomyomata: correlation of imaging with histology. J Vasc Interv Radiol. 2012;23:786–794.e4. doi: 10.1016/j.jvir.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Idée JM, Port M, Robic C, Medina C, Sabatou M, Corot C. Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J Magn Reson Imaging. 2009;30:1249–1258. doi: 10.1002/jmri.21967. [DOI] [PubMed] [Google Scholar]
- 17.Aime S, Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging. 2009;30:1259–1267. doi: 10.1002/jmri.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trumm CG, Stahl R, Clevert DA, Herzog P, Mindjuk I, Kornprobst S, et al. Magnetic resonance imaging-guided focused ultrasound treatment of symptomatic uterine fibroids: impact of technology advancement on ablation volumes in 115 patients. Invest Radiol. 2013;48:359–365. doi: 10.1097/RLI.0b013e3182806904. [DOI] [PubMed] [Google Scholar]
- 19.Hindley J, Gedroyc WM, Regan L, Stewart E, Tempany C, Hynyen K, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: early results. AJR Am J Roentgenol. 2004;183:1713–1719. doi: 10.2214/ajr.183.6.01831713. [DOI] [PubMed] [Google Scholar]