Abstract
Background
The cancer micro-environment is recognized as having an increasing importance in cancer progression. Immune cells originating from the peripheral blood are important elements of this environment. Thrombocytosis, neutrophilia and lymphocytopenia have been found to be negative prognostic indicators in many cancers. This study aims to evaluate the potential of the use of a novel hematological marker, the platelet-neutrophil to lymphocyte ratio (PNLR) as a practical, reliable, and inexpensive prognostic tool in metastatic colorectal adenocarcinomas.
Methods
Charts from 305 patients with colorectal cancer were retrospectively reviewed. Of these, 152 had metastatic disease with complete follow-up data on progression and survival. Data were extracted and stratified by a PNLR cut-off point of 2,000. Baseline parameters of the two groups were evaluated and compared with the χ2 test. Univariate and multivariate Cox proportional-hazards regression analyses were performed on variables of interest.
Results
A total of 102 (67.1%) patients had a PNLR of less than 2,000 while the index for 50 (32.9%) patients was 2,000 or higher. Patients with a PNLR above 2,000 had a shorter median progression-free survival (PFS) [6.5 vs. 13.3 months; hazard ratio (HR), 2.05; 95% CI, 1.32–3.19, P=0.001] than in patients with a PNLR below the threshold. Similar results were observed for median overall survival (OS) (9.6 vs. 21.8 months; HR, 2.33; 95% CI, 1.44–3.79, P=0.001). PNLR had a higher predictive HR than Eastern Cooperative Oncology Group (ECOG) performance status (PS).
Conclusions
In this retrospective analysis of metastatic colorectal cancer patients, PNLR had prognostic value for both OS and PFS. While other variables held significance for poorer prognosis, PNLR had the highest HR and the highest significance in multivariate analysis for both PFS and OS. Thus, it represents a powerful and objective prognostic tool in the evaluation of metastatic colorectal cancer patients that is readily available and does not require any additional expenses.
Keywords: Colorectal cancer, prognosis, metastatic, neutrophilia, lymphocytopenia, thrombocytosis, ratio
Introduction
Colorectal cancer is the most common gastrointestinal malignancy in the western world and remains a prominent cause of cancer morbidity and mortality, despite progress in its management. Affecting approximately 746,000 men and 614,000 women yearly, it is the 3rd most common cancer in the former and the 2nd most common in the latter (1). It is therefore important to find prognostic markers that are practical, reliable, and inexpensive.
The cancer micro-environment is recognized as having an increasing importance in cancer progression (2). Immune and other cells originating from the peripheral blood are important elements of this environment. Furthermore, immune cells have come to the forefront of cancer research with the recent success of immune blockade inhibitors, drugs that potentiate anti-cancer immune function by blocking inhibitory receptors expressed in lymphocytes (e.g., CTLA4, PD-L1) (3). Lymphocytosis, therefore, has been associated with positive prognosis in malignant tumors (4).
Neutrophils, on the other hand, have a more controversial role in cancer. These pro-inflammatory cells may have a pro-tumorigenic effect (2). Due to this, neutrophilia has generally been found to be a negative prognostic factor in malignancies. Cancer is also often associated with thrombocytosis, as the cytokines that stimulate thrombopoiesis are elevated in the circulation of some cancer patients (5,6). Because of this, thrombocytosis has been found to be an adverse prognostic factor in many common cancers, including gastrointestinal cancers (7).
Many studies have reported the use of the platelet to lymphocyte and/or the neutrophil to lymphocyte ratio (PLR and NLR, respectively), culminating in recent systematic reviews and meta-analyses of both these parameters as prognostic tools (8,9). These markers consider the pro-tumorigenic properties of either platelets or neutrophils while factoring in the protective effects of lymphocytes. Nevertheless, no study to date has included all three markers together in colorectal cancer. The current paper aims to evaluate a novel hematologic index, the platelet-NLR (PNLR) as a prognostic tool in metastatic colorectal adenocarcinomas seeking to integrate all three hematologic parameters in an attempt to increase prognostic power.
Methods
Charts from 305 patients with colorectal cancer diagnosed between 2008 and 2014 in our center were retrospectively reviewed. Of these, 152 patients had metastatic disease with complete follow-up data on progression and survival, and were thus included in the analyses. Follow-up was considered complete if the patient was followed until death or if seen within the last 6 months of data collection.
Sex, age, date of metastatic diagnosis or disease recurrence, clinical presentation (high-risk presentation defined as obstruction, perforation, or a change in bowel habits and low-risk presentation defined as diagnosed with screening or bleeding/anemia), site (right colon to splenic flexure, left colon from splenic flexure to sigmoid, and rectum), pathologic grade, previous treatment with adjuvant chemotherapy as well as number of different lines of metastatic treatment, de novo metastatic status, organs involved, blood hematologic and biochemical markers [carcinoembryonic antigen (CEA), lactate dehydrogenase (LDH), albumin, platelets, neutrophils, lymphocytes], diabetes as a co-morbidity, Eastern Cooperative Oncology Group (ECOG) performance status (PS) and whether the patients had a metastasectomy were extracted. All evaluations used recorded values from before the start of any treatment in the metastatic setting.
Overall survival (OS) was defined as the time from metastatic diagnosis to death or censored to last follow-up. Progression-free survival (PFS) was defined as the time from metastatic diagnosis to documented disease progression or death, whichever came first or censored to last follow-up without progression. Strata were created for blood parameters with the following cut-offs: for platelets ≥350×109/L, neutrophils ≥7.5×109/L, lymphocytes ≤1.4×109/L, CEA >5 µg/L, LDH >210 U/L, and albumin <35 g/L. The PNLR was calculated by multiplying the pre-treatment platelet count (×106/mL) by the neutrophil count (×106/mL) divided by the lymphocyte count (×106/mL). A cut-off for PNLR of 2,000 was used for stratification of patients in two prognostic groups. Baseline characteristics of the two groups were compared and significance between the groups was tested using the χ2 test. OS and PFS Kaplan-Meier curves of groups were constructed and compared with the log rank test. Individual univariate analyses were completed on all extracted variables, and significant variables were included in the multivariate analysis model. The Cox proportional-hazards model was used for regression analysis to determine which variables retained significance. It was also used to compute hazard ratios (HR).
The model was assessed for adequacy/goodness of fit with graphical assessment of the proportional-hazards assumption (for all variables) and with the calculation of Harrell’s C concordance statistic for both PFS and OS. All P values were considered significant at values of <0.05.
Data analysis was performed in Microsoft Excel (Microsoft corp., Redmond, WA, USA) and all statistical calculations were performed in STATA 13.1 (StataCorp., TX, USA).
Results
Among the 152 analyzed patients, 102 (67.1%) patients had a PNLR of less than 2,000 while 50 (32.9%) patients were included into the PNLR ≥2,000 group (Table 1). Other baseline characteristics of the analyzed groups are given in Tables 1,2. There was no significant difference between the two groups in the percentage of male patients, patients above age 65, location of the primary tumor, percentage of patients that had more than one line of palliative chemotherapy and percentage of patients with oligometastatic disease. In contrast, more patients in the high PNLR group had a high-risk clinical presentation with obstruction, changes in bowel habits or pain (64.0% vs. 42.6% in the low PNLR group), ECOG PS >1 (62.0% vs. 28.4% in the low PNLR group), de novo metastatic disease (62.0% vs. 30.4% in the low PNLR group), a high LDH above 210 U/L (61.2% vs. 33.7% in the low PNLR group), albumin below 35 g/L (28.6% vs. 12.0% in the low PNLR group), and fewer patients had a metastasectomy as part of their disease management (8.3% vs. 27.1% in the low PNLR group) (Tables 1,2). Regarding treatments, among the 90 patients initially diagnosed with localized disease in the whole cohort, 46 patients (51.1%) had received adjuvant chemotherapy. All of these patients received 5-fluoropyrimidine-based adjuvant chemotherapy, with no significant differences between the groups with high and low PNLR. There was also no significant difference between the groups in the number of lines of palliative chemotherapy that they received.
Table 1. Baseline clinicopathological characteristics of all patients included in the retrospective study and the two groups of patients with PNLR below and equal or above 2,000.
| Clinicopathological characteristics | All patients, N=152 | PNLR <2,000, N=102 (67.1%) | PNLR ≥2,000, N=50 (32.9%) | χ2 P value |
|---|---|---|---|---|
| Age on Dx (recurrence or metastasis), N (%) | 0.412 | |||
| <65 years | 48 (31.6) | 30 (29.4) | 18 (36.0) | |
| ≥65 years | 104 (68.4) | 72 (70.6) | 32 (64.0) | |
| Median (range) | 70.5 (43.0–91.0) | 71.0 (53.0–91.0) | 69.0 (43.0–90.0) | |
| Sex, N (%) | 0.929 | |||
| Male | 95 (62.5) | 64 (62.7) | 31 (62.0) | |
| Female | 57 (37.5) | 38 (37.3) | 19 (38.0) | |
| Clinical presentation, N (%) | N=151 | 0.007 | ||
| Low risk | 75 (49.7) | 58 (57.4) | 17 (34.0) | |
| High risk | 76 (50.3) | 43 (42.6) | 33 (64.0) | |
| ECOG, N (%) | <0.001 | |||
| ≤1 | 92 (60.5) | 73 (71.6) | 19 (38.0) | |
| >1 | 60 (39.5) | 29 (28.4) | 31 (62.0) | |
| Adjuvant chemo, N (%) | N=90 | 0.505 | ||
| Yes | 46 (51.1) | 35 (49.3) | 11 (57.9) | |
| No | 44 (48.9) | 36 (50.7) | 8 (42.1) | |
| Line of palliative chemo, N (%) | 0.477 | |||
| 0–1 | 109 (71.7) | 75 (73.5) | 34 (68.0) | |
| >1 | 43 (28.3) | 27 (26.5) | 16 (32.0) | |
| De novo metastatic, N (%) | <0.001 | |||
| Yes | 62 (40.8) | 31 (30.4) | 31 (62.0) | |
| No | 90 (59.2) | 71 (69.6) | 19 (38.0) | |
| Oligometastatic, N (%) | N=143 | 0.066 | ||
| Yes | 34 (23.8) | 27 (28.4) | 7 (14.6) | |
| No | 109 (76.2) | 68 (71.6) | 41 (85.4) | |
| Metastasectomy, N (%) | N=144 | 0.009 | ||
| Yes | 30 (20.8) | 26 (27.1) | 4 (8.3) | |
| No | 114 (79.2) | 70 (72.9) | 44 (91.7) | |
Numbers in parenthesis refer to percentage, unless otherwise specified. Fifth column contains χ2 P values for comparisons between groups. For some parameters, values were not available for some patients as mentioned in the respective rows. PNLR, platelet-neutrophil to lymphocyte ratio; Dx, diagnosis.
Table 2. Baseline hematological patient characteristics of all patients included in the retrospective study and the two groups of patients with PNLR below and equal or above 2,000.
| Laboratory value | All patients, N=152 | PNLR <2,000, N=102 (67.1%) | PNLR ≥2,000, N=50 (32.9%) | χ2 P value |
|---|---|---|---|---|
| CEA, N (%) | N=139 | 0.225 | ||
| <5 µg/L | 49 (35.3) | 36 (38.7) | 13 (28.3) | |
| ≥5 µg/L | 90 (64.7) | 57 (61.3) | 33 (71.7) | |
| LDH, N (%) | N=150 | 0.001 | ||
| <210 U/L | 86 (57.3) | 67 (66.3) | 19 (38.8) | |
| ≥210 U/L | 64 (42.7) | 34 (33.7) | 30 (61.2) | |
| Albumin, N (%) | N=149 | 0.012 | ||
| <35 g/L | 26 (17.4) | 12 (12.0) | 14 (28.6) | |
| ≥35 g/L | 123 (82.6) | 88 (88.0) | 35 (71.4) | |
| Platelets, N (%) | <0.001 | |||
| <350×109/L | 112 (73.7) | 90 (88.2) | 22 (44.0) | |
| ≥350×109/L | 40 (26.3) | 12 (11.8) | 28 (56.0) | |
| Neutrophils, N (%) | <0.001 | |||
| <7.5×109/L | 113 (74.3) | 95 (93.1) | 18 (36.0) | |
| ≥7.5×109/L | 39 (25.7) | 7 (6.9) | 32 (64.0) | |
| Lymphocytes, N (%) | <0.001 | |||
| <1.4×109/L | 98 (64.5) | 53 (52.0) | 45 (90.0) | |
| ≥1.4×109/L | 54 (35.5) | 49 (48.0) | 5 (10.0) |
Number in parenthesis refers to percentage, unless otherwise specified. Fifth column contains χ2 P values for comparisons between groups. For some parameters, values were not available for some patients as mentioned in the respective rows. PNLR, platelet-neutrophil to lymphocyte ratio.
Both median OS and PFS were significantly shorter in the stratum with a higher PNLR. In terms of median OS, patients with a PNLR ≥2,000 had an OS of 9.6 vs. 21.8 months in the PNLR <2,000 group. A significant difference between the groups was also observed for PFS: 6.5 months in the high PNLR group vs. 13.3 months in the low PNLR group.
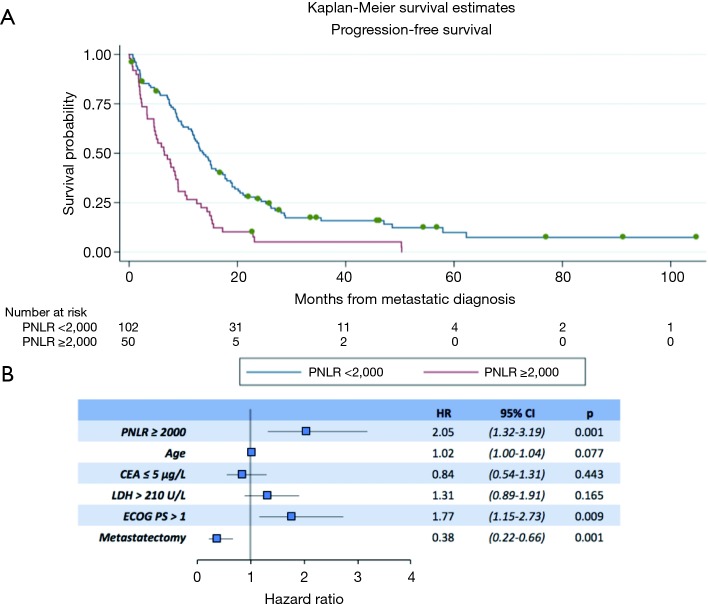
In the univariate analyses, a high PNLR above 2,000 was associated with both worse PFS (log-rank P<0.001, Table 3). Figure 1A presents the Kaplan-Meier PFS curves for the high PNLR and low PNLR groups respectively.
Table 3. Log rank P values of univariate analyses for extracted variables, measured for both OS and PFS.
| Variable | OS log-rank P value | PFS log-rank P value | N |
|---|---|---|---|
| Sex | 0.088 | 0.194 | 152 |
| PNLR ≥2,000 | <0.001 | <0.001 | 152 |
| Age >65 years | <0.001 | 0.040 | 152 |
| High-risk presentation | 0.162 | 0.844 | 151 |
| CEA ≤5 µg/L | <0.001 | <0.001 | 139 |
| LDH >210 U/L | <0.001 | <0.001 | 150 |
| Albumin <35 g/L | 0.007 | 0.155 | 149 |
| Site | 0.119 | 0.283 | 152 |
| De novo metastatic disease | 0.472 | 0.733 | 152 |
| Diabetes | 0.894 | 0.873 | 151 |
| ECOG >1 | <0.001 | <0.001 | 152 |
| Metastasectomy | <0.001 | <0.001 | 144 |
The fourth column refers to the total number of patients included in the univariate analysis for each parameter. OS, overall survival; PFS, progression-free survival; PNLR, platelet-neutrophil to lymphocyte ratio; ECOG, Eastern Cooperative Oncology Group.
Figure 1.
Progression-free survival (PFS) of patients with high and low PNLR. (A) Kaplan-Meier PFS curves in months from the diagnosis of metastatic adenocarcinoma of patients with PNLR below and above the cut-off of 2,000. Log rank test P<0.001; (B) forest plot results of the logistic regression analysis using the Cox-proportional hazards model of PFS as the outcome variable and PNLR, age, CEA (≤5 µg/L), LDH (>210 U/L), metastasectomy and ECOG >1 at presentation of metastatic disease as the predictor variables. PNLR, platelet-neutrophil to lymphocyte ratio; ECOG PS, Eastern Cooperative Oncology Group performance status; CEA, carcinoembryonic antigen; LDH, lactate dehydrogenase; HR, hazard ratio.
Other factors that were significant for PFS in univariate analysis included age above 65 (log-rank P=0.040, Table 3), CEA ≤5 µg/L (log-rank P<0.001), LDH >210 U/L (log-rank P<0.001), ECOG >1 (log-rank P<0.001) and inclusion of metastasectomy in treatment plan (log-rank P<0.001). In contrast, sex, clinical presentation, albumin level, site of the primary tumor, whether the metastatic tumor was diagnosed de novo or recurred from a primary tumor, or the concomitant presence of diabetes were not prognostically associated with PFS.
In a multivariate analysis model that included all factors significant for PFS in univariate analysis, PNLR ≥2,000 (HR, 2.05; 95% CI, 1.32–3.19, P=0.001), ECOG >1 (HR, 1.77; 95% CI, 1.15–2.73, P=0.009), and metastasectomy (HR, 0.38; 95% CI, 0.22–0.66, P=0.001) retained significance as factors predicting PFS (Figure 1B).
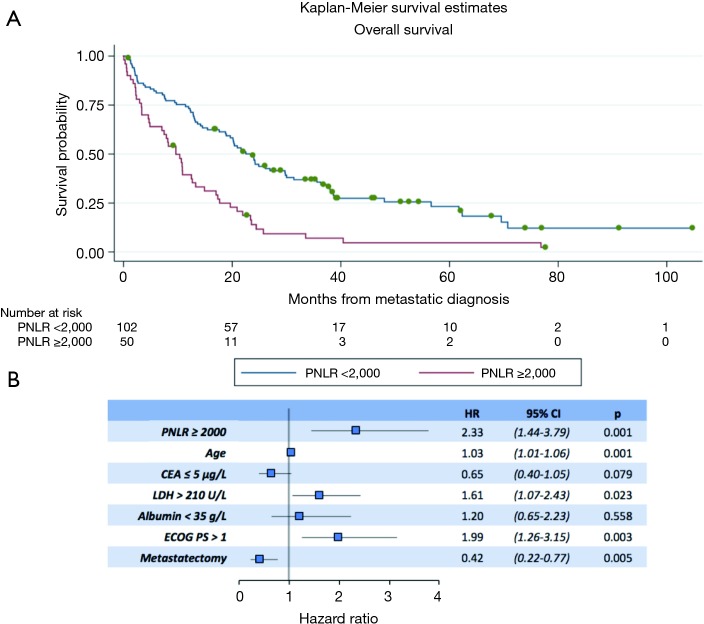
Regarding OS, in the univariate analysis the high PNLR group was also associated with worse outcome (log-rank P<0.001, Table 3). Figure 2A presents the Kaplan-Meier OS curves for the high PNLR and low PNLR groups respectively. Additional factors besides the PNLR that were statistically significant in univariate analysis were comprised of the same variables that were significant in univariate analysis for PFS (age, CEA, LDH, ECOG PS and metastasectomy) and in addition a level of albumin below 35 g/L (Table 3).
Figure 2.
Overall survival (OS) of patients with high and low PNLR. (A) Kaplan-Meier OS curves in months from the diagnosis of metastatic adenocarcinoma of patients with PNLR below and above the cut-off of 2,000. Log-rank test P<0.001; (B) forest plot results of the logistic regression analysis using the Cox-proportional hazards model of OS as the outcome variable and PNLR, age, CEA (≤5 µg/L), LDH (>210 U/L), metastasectomy and ECOG >1 at presentation of metastatic disease as the predictor variables. PNLR, platelet-neutrophil to lymphocyte ratio; ECOG PS, Eastern Cooperative Oncology Group performance status; CEA, carcinoembryonic antigen; LDH, lactate dehydrogenase; HR, hazard ratio.
In multivariate analysis for OS, PNLR ≥2,000 (HR, 2.33; 95% CI, 1.44–3.79, P=0.001), age (HR, 1.03; 95% CI, 1.01–1.06, P=0.001), LDH >210 U/L (HR, 1.61; 95% CI, 1.07–2.43, P=0.023), ECOG >1 (HR, 1.99; 95% CI, 1.26–3.15, P=0.003), and metastasectomy (HR, 0.42; 95% CI, 0.22–0.77, P=0.005) retained significance (Figure 2B).
Goodness of fit for the Cox model was assessed graphically, plotting the logarithm of the cumulative hazard function against the logarithm of time (10). Furthermore, Harrell’s C concordance statistic was 0.733 (95% CI, 0.703–0.763) for PFS and 0.776 (95% CI, 0.744–0.808) for OS, both indicating strong predictive models.
Discussion
Circulating peripheral blood neutrophils, lymphocytes and platelets have all been the subject of research as prognostic elements in cancer. Both neutrophils and lymphocytes are important cells of the immune system and they participate in the formation of the tumor micro-environment. In this micro-environment, neutrophils promote a non-specific pro-inflammatory reaction that mostly contributes to tumor cell survival (11). In addition, myeloid subsets, such as myeloid-derived suppressor cells, impede the anti-tumoral function of lymphocytes (12).
T lymphocytes are the main effector cells of the adaptive immune response and their role in anti-tumor immunity has been recently highlighted with the introduction of immune checkpoint blockers, a new class of anti-neoplastic drugs that work by boosting the lingering response of the immune system to tumor cell neo-antigens. T cells anti-tumoral action is impeded by the synergistic activity of inhibitory cytokines (IL-10 and TGF-β) released by regulatory T-cells (Treg) and continuous activation of the NF-κB pathway in the tumor milieu which have been shown to suppress the immune function of lymphocytes and promote tumor growth (2). In addition, infiltration of tumor beds by immune cells such as effector T lymphocytes is associated with immunogenic tumors and a better response to immune check point inhibitors. Thus, lymphocytopenia may be a marker of worse prognosis given that a lower number of circulating effector cells may lead to a lower supply of immune effectors in the tumor site. Indeed, lymphocytopenia was associated with an adverse OS in early colorectal cancer (13).
Platelets may promote carcinogenesis in several ways. First, circulating tumor cells may use platelets as protective barriers in a complex system of evasion from the attack of immune cells, and possibly as mediators for attachment to endothelial cells when initiating extravasation at metastatic sites (14). Furthermore, platelets have a role in prevention of hemorrhage in newly formed tumor vasculature which is structurally abnormal and lack the stability of local resident vasculature (15). Several cytokines and growth factors contained in the alpha and dense granules of platelets, such as vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), platelet-derived growth factor (PDGF), transforming growth factor β (TGFβ), interleukin 1β (IL-1β), IL-8 and CXC motif containing ligand 12 (CXCL12) may play diverse roles in the tumor micro-environment, including promotion of invasion and metastasis through a positive regulation of the epithelial to mesenchymal transition (EMT) process and immune evasion (16-18).
Given that thrombocytosis, neutrophilia and lymphocytopenia may be associated with poorer prognoses, we hypothesized that creating an index that considers both the pro-tumorigenic effect of platelets and neutrophils with the protective effects of lymphocytes could yield more robust prognostic information than the currently available prognostic markers in metastatic colorectal carcinoma. Combining in an index, all three hematologic measurements may consequently lead to the successful development of an accurate, reliable, and inexpensive prognostic tool.
PNLR index has not been investigated as a marker of prognosis in colorectal cancer and has only been proposed as a prognostic marker for OS in small cell lung cancer (19). Ratios including only two of the three hematologic parameters at a time (NLR and PLR) have been the subjects of more extensive investigations in the prognostic marker literature and remain the primary hematological metrics proposed (8,9). The current study found PNLR with a cut-off value of 2,000 to be a robust prognostic marker in patients with metastatic colorectal cancer producing HR that supersede even those of well-established prognostic markers such as CEA and ECOG PS (20-22). This is a significant finding and seems to correlate well with the aforementioned pathophysiology which provides a rational for the prognostic implications of the PNLR marker and together with the significant HRs revealed in the current study supports its introduction for clinical use, provided a confirmation of its robustness is obtained in other populations. Additional advantages of the proposed PNLR as a prognostic marker include low cost, high prognostic sensitivity, as well as the easy acquisition of blood count measurements in clinical practice that could make this novel marker a potential practical and reliable tool to add to the armamentarium of prognostication.
Some limitations of the current research exist and consist of the retrospective nature of the data acquisition as well as a lack of a defined methodology for obtaining an optimal PNLR cut-off. The currently proposed cut-off is empirical and it was decided based on a combination of the ease of calculation and the comparative good balance of number of patients in the two groups above and below it that resulted. Additional limitations include the fact that patients from a single center were included and the lack of data regarding common molecular lesions in colorectal cancer such as mutations in KRAS, BRAF or microsatellite instability.
Despite these limitations and provided that confirmation in additional patient populations is obtained, the PNLR may become an important prognostic parameter to be considered in colorectal cancer patients. Investigation in other stages of colorectal cancer as well as other malignancies may be warranted.
Clinical practice points
Thrombocytosis, neutrophilia, and lymphopenia have been shown to be pro-tumorigenic and have thus been associated with poorer prognoses in colorectal cancer. In fact, both NLR and PLR are beginning to be established prognostic markers in many types of cancers. Those ratios do not account for the effects of all three hematological parameters concomitantly. This paper studied the prognostic implications of a novel marker: the PNLR. Statistical analysis found significant differences between groups with different PNLR, with poorer prognoses associated with elevated PNLR in metastatic colorectal patients. This could pave the way for a quick, reliable, and inexpensive marker.
Acknowledgements
This research was partly supported by a Dean’s summer student research award of the Northern Ontario School of Medicine, Canada.
Ethical Statement: The study was approved by the Institutional Ethics Board of Sault Area Hospital, Canada (No. 2017-05-05).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Globocan 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- 2.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008; 27:5904-12. 10.1038/onc.2008.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voutsadakis IA. Immune blockade inhibition in breast cancer. Anticancer Res 2016;36:5607-22. 10.21873/anticanres.11145 [DOI] [PubMed] [Google Scholar]
- 4.Shibutani M, Maeda K, Nagahara H, et al. Prognostic significance of the preoperative lymphocyte-to-monoocyte ratio in patients with colorectal cancer. Oncol Lett 2017;13:1000-6. 10.3892/ol.2016.5487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaser A, Brandacher G, Steurer W, et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood 2001;98:2720-5. 10.1182/blood.V98.9.2720 [DOI] [PubMed] [Google Scholar]
- 6.Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med 2012;366:610-8. Erratum in: N Engl J Med 2012;367:1768. 10.1056/NEJMoa1110352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voutsadakis IA. Thrombocytosis as a prognostic marker in gastrointestinal cancers. World J Gastrointest Oncol 2014;6:34-40. 10.4251/wjgo.v6.i2.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li MX, Liu XM, Zhang XF, et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Int J Cancer 2014;134:2403-13. 10.1002/ijc.28536 [DOI] [PubMed] [Google Scholar]
- 9.Tan D, Fu Y, Su Q, et al. Prognostic role of platelet-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e3837. 10.1097/MD.0000000000003837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradburn MJ, Clark TG, Love SB, et al. Survival Analysis Part III: Multivariate data analysis – choosing a model and assessing its adequacy and fit. Br J Cancer 2003;89:605-11. 10.1038/sj.bjc.6601120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uribe-Querol E, Rosales C. Neutrophils in cancer: Two sides of the same coin. J Immunol Res 2015;2015:983698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz-Montero CM, Finke J, Montero AJ. Myeloid-Derived Suppressor Cells in cancer: Therapeutic, predictive, and prognostic implications. Semin Oncol 2014;41:174-84. 10.1053/j.seminoncol.2014.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang L, Zhu J, Jia H, et al. Predictive value of pretreatment lymphocyte count in stage II colorectal cancer and in high-risk patients treated with adjuvant chemotherapy. Oncotarget 2016;7:1014-28. 10.18632/oncotarget.5835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buergy D, Wenz F, Groden C, et al. Tumor-platelet interaction in solid tumors. Int J Cancer 2012;130:2747-60. 10.1002/ijc.27441 [DOI] [PubMed] [Google Scholar]
- 15.Ho-Tin-Noé B, Carbo C, Demers M, et al. Innate immune cells induce hemorrhage in tumors during thrombocytopenia. Am J Pathol 2009;175:1699-708. 10.2353/ajpath.2009.090460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011;20:576-90. 10.1016/j.ccr.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voutsadakis IA. The Ubiquitin-Proteasome System and signal transduction pathways regulating Epithelial Mesenchymal transition of cancer. J Biomed Sci 2012;19:67. 10.1186/1423-0127-19-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopp HG, Placke T, Salih HR. Platelet-derived transforming growth factor-beta down regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res 2009;69:7775-83. 10.1158/0008-5472.CAN-09-2123 [DOI] [PubMed] [Google Scholar]
- 19.Hong X, Cui B, Wang M, et al. Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med 2015;236:297-304. 10.1620/tjem.236.297 [DOI] [PubMed] [Google Scholar]
- 20.Crosara Teixeira M, Marques DF, Ferrari AC, et al. The effects of palliative chemotherapy in metastatic colorectal cancer patients with an ECOG performance status of 3 and 4. Clin Colorectal Cancer 2015;14:52-7. 10.1016/j.clcc.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 21.De Divitiis C, Nasti G, Montano M, et al. Prognostic and predictive response factors in colorectal cancer patients: Between hope and reality. World J Gastroenterol 2014;20:15049-59. 10.3748/wjg.v20.i41.15049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marzouk O, Schofield J. Review of Histopathological and Molecular Prognostic Features in Colorectal Cancer. Cancers 2011;3:2767-810. 10.3390/cancers3022767 [DOI] [PMC free article] [PubMed] [Google Scholar]