Abstract
Background
Chronic obstructive pulmonary disease (COPD) is predicted to become the fifth leading cause of disability and the third leading cause of death around the world by 2020. Though it is potentially treatable and preventable, evidence of brain structural alterations in COPD remains sparse and conflicting. We aim to investigate the effect of Liuweibuqi capsules on CD4+CD25+ Forkhead box protein 3+ (Foxp3+) regulatory T cells (Tregs), helper T cells (Th) and lung function in patients with stable COPD complicated with lung Qi deficiency.
Methods
COPD patients with lung Qi deficiency [458] were assigned into non-smoking COPD (NS-COPD), non-smoking control (NS-control), smoking COPD (S-COPD) and smoking control (S-control) groups, and healthy volunteers [245] into the non-smoking healthy (NSH) and smoking healthy (SH) groups. Levels of inflammatory cytokines were detected by Enzyme-linked immunoassay (ELISA). Contents of inflammatory cells, inflammatory marker, and CD4+CD25+Fox3+Tregs were measured by flow cytometry. FEV1/FVC (%) and FEV1 (%) were detected by pulmonary function test apparatus. Correlation between FEV1 (%) and Th1, Th2, Th17, Th1/Th2 or CD4+CD25+Fox3+Tregs was analyzed by Spearman rank correlation test. The related factors affecting treatment efficacy was assessed by logistic analysis.
Results
COPD patients and smoking people showed higher level of INF-γ, IL-4, IL-17, Th1, Th2, Th17 and Th1/Th2 but lower level of CD4+CD25+Fox3+Tregs. Liuweibuqi capsules could decrease level of inflammatory cells, cytokines, and markers (especially Th17 and IL-17), and increase level of CD4+CD25+Fox3+Tregs. FEV1 (%) negatively correlated with Th1, Th2, Th17 and Th1/Th2 but positively correlated with CD4+CD25+Fox3+Tregs, and smoking may strengthen their correlation, but Liuweibuqi capsules may weaker their correlation. Levels of inflammatory cytokines, cells, marker, CD4+CD25+Fox3+Tregs, FEV1/FVC (%), FEV1 (%), smoking and Liuweibuqi capsules are factors affecting efficacy.
Conclusions
Taken together, our data support the notion that smoking is an important factor to induce and aggravate COPD. Liuweibuqi capsules could stimulate proliferation of CD4+CD25+Fox3+Tregs and decrease Th17 expression to improve the lung function in stable COPD patients with lung Qi deficiency, and it had obvious efficacy for smoking COPD patients.
Keywords: Liuweibuqi capsules, chronic obstructive pulmonary disease (COPD), lung Qi deficiency, regulatory T cells, helper T cells
Introduction
Chronic obstructive pulmonary disease (COPD) is defined as an airflow obstruction with several different but overlapping obstructive syndromes (1). COPD has been thought to be one of major disease prevention goal for public health (2). It was predicted that morbidity of COPD would continue to increase and may be the third leading lethal disease by 2030 (3). The common obstructive syndromes of COPD are chronic bronchitis, emphysema and reversible or irreversible small airways obstruction (4). COPD patients often suffer wheezing, expiratory dyspnea, cough, chest tightness and sputum production (5). Cigarette smoke has been reported as the main cause of COPD, other factors include ambient air pollution, airway hyper-responsiveness and allergy (6,7). Treatment of COPD over the past decades of management includes pharmacological and nonpharmacological interventions with the targets to control symptoms and exacerbations, improve functional performance and quality of life, and decrease risk of hospitalizations, emergency care, and mortality (8,9). However, limitation of clinical practice causes unsatisfactory result as compared with the expectations (10). Qi deficiency and qi-yin deficiency are the most common syndromes, relating to Lung, spleen and kidney (11). And study has shown that the morbidity of lung cancer is implicated in Qi deficiency, which is the internal cause of viscera imbalance (12).
Currently, traditional Chinese medicine (TCM) therapies are widespread in both developing and developed areas (13). Liuweibuqi capsules, a Chinese patent medicine, consist of Radix Ginseng (Renshen), Radix Astragali Mongolici (Huangqi), Alpiniae Oxyphyllae Fructus (Yizhi), Rhizoma Polygonati Odorati (Yuzhu), Cortex Cinnamomi (Rougui), Pericarpium Citri Reticulatae (Chenpi) (14). Renshen is one of the most popular TCM which could inhibit metastasis of melanoma in the lung (15,16). Astragaloside IV is one of the components of Huangqi which has been found a broad application prospects, especially in cardiovascular diseases and cancers (17). Yizhi is in favor of anti-aging activity and an anti-neoplastic medicine (18,19). Yuzhu is regarded as a traditional medicine which has functions of removing dryness, replenishing vital essence, promoting secretion of fluid and quenching thirst, and has been used to treat many diseases, especially tuberculosis (20). Rougui have been documented that it takes part in a variety of biological activities in vitro, one of its function is to induce apoptosis via reactive oxygen species and anti-inflammatory diseases (21). Chenpi has been commonly used as one of the herbal materials to eliminate phlegm and strengthen spleen, which is also an anti-inflammatory medicine (22). However, few studies investigate the relationship among Liuweibuqi capsules, inflammatory cells, cytokines and indicator, immune regulatory cells, and lung function in stable COPD patients with lung Qi deficiency. Therefore, from a microcosmic perspective, we conducted this experiment to investigate their relationship with the perspective to provide a clinical reference in treating COPD patients with lung Qi deficiency.
Methods
Ethical statement
This study was approved by ethics committee of the First Affiliated Hospital of Anhui Medical University (2012-006). All subjects voluntarily participated in the experiment were fully informed of gains and risks and signed informed consents. This experiment was in accordance with the Nuremberg criteria and the Helsinki declaration.
Subjects
We randomly selected 458 stable COPD patients with lung Qi deficiency (11) (with over 3-year disease history) who were admitted into the Respiratory Department of the First Affiliated Hospital of Anhui Medical University from January 2012 to December 2016. Non-smoking COPD patients with lung Qi deficiency were 230 cases including 126 males and 104 females aged from 39–76 years (mean age of 56.80±9.78 years old), and COPD patients with over 10-year smoking duration were 228 cases including 122 males and 106 females aged from 35–74 years (mean age of 59.32±7.41 years old). We also selected 121 non-smoking healthy volunteers including 62 males and 59 females aged from 31–79 years (mean age of 55.85±10.36 years old) and 124 healthy volunteers with over 10-year smoking duration including 71 males and 53 females aged from 45–67 years (mean age of 56.51±4.45 years old).
Inclusion and exclusion criteria
COPD was diagnosed in accordance with Diagnostic guidelines for chronic obstructive pulmonary disease which was a diagnostic standard of COPD regulated by Chinese Thoracic Society in 2011. The inclusion criteria include COPD patients at stable phase with lung function at I–II grade (50%< FEV1) (23) and lung Qi deficiency syndrome; patients aged 35–76 years old and voluntarily participated and signed informed consents; patients with normal blood and urine routine, normal blood-fat and blood glucose, normal liver function, renal function and electrocardiogram. Exclusion criteria include people with lung disease such as tuberculosis or lung cancer; critical patients with cardiac dysfunction, pulmonary encephalopathy or immunological disease; patients with serious complication such as cardiovascular, hematopoietic, digestive, urinary or endocrine metabolic systems; pregnant woman or suckling period woman; patients with mentally ill.
Therapeutic regimens
Non-smoking COPD patients were randomly grouped into non-smoking COPD patients (NS-COPD group, 114 cases) and non-smoking controls (NS-control group, 116 cases); smoking COPD patients were randomly assigned into smoking COPD patients (S-COPD group, 110 cases) and smoking controls (S-control group, 118 cases). Before experiment, the NS-COPD and NS-control groups were of no significant differences in the baseline characteristics and blood indexes, same as the S-COPD and S-control groups (P>0.05). The NS-COPD and S-COPD groups were dosed with Liuweibuqi capsules (the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine, 0.4 g/capsule), and each patient took three capsules in each morning and evening for two courses of treatment (30 days for one course of treatment); patients in the NS-control and S-control groups were dosed with Roflumilast (Jiangsu Hengrui Medicine Co., Ltd, Lianyungang, Jiangsu, China, 0.5 g/tablet), and each patient took one tablet each time, once a day for two courses of treatment (30 days for one course of treatment). Non-smoking healthy people (NSH group) and smoking healthy people (SH group) were in orthobiosis and in regular check.
Enzyme-linked immunoassay (ELISA)
Venous blood (10 mL) from the elbow was extracted from patients before and after experiment (from healthy people during physical examination). Then the blood was centrifuged for 10 min at a radius of 8 cm and speed of 3,000 rpm. After centrifugation, the serum was separated and stored at −20 °C. ELISA kit (Quantikine, R&D Systems, USA) were used to detect the levels of serum cytokines INF-γ (ab9829, Abcam, Cambridge, UK), IL-4 (ab215089, Abcam, Cambridge, UK) and IL-17 (ab119535, Abcam, Cambridge, UK). The specific experimental steps were as follows: standard samples were diluted with double broth dilution method and added into the blank wells, human serum of the correspondent groups was added to the other wells (100 µL for each well). Each group was set up three duplicated wells which were incubated at 37 °C for 90 min. The plate was washed for 5 times, and the blank wells were added with biotin-labeled antibody diluent. The other wells were added with biotinylated antibody working fluid (100 µL for each well), sealed with sealing adhesive, and incubated at 37 °C for 60 min. After that, the plate was washed for 5 times, the blank wells were added with enzyme combination diluent and the other wells were added with enzyme combination working solution (100 µL for each well), and then sealed with sealing adhesive, incubated at 37 °C for 30 min. The plate was washed for 5 times, the wells were added with chromogenic substrate (100 µL for each well), and then sealed with sealing adhesive, incubated at 37 °C for 15 min. Each well was then added with stop buffer (100 µL), and optical density (OD) value was measured at 450 nm by ELx808™ Absorbance Microplate Reader (BioTek, Winooski, VT, USA). Finally, the standard curve was drawn, and the levels of INF-γ, IL-4 and IL-7 in each group were calculated by standard curve.
Flow cytometry
Venous blood (2 mL) from the elbow was extracted from patients before and after experiment (from healthy people during physical examination). The blood was added with anticoagulant and labeled according to the groups. Then the blood was centrifuged at 3,000 rpm for 10 min with hematocyte removed and cell concentration was adjusted at 106 cells/mL. The cells were packed into a flow tube with three replicates. Then the cells were added with fluorescein isothiocyanate (FITC)-anti-human CD4 antibody (0.25 µg, Abcam Inc., Cambridge, MA, USA) and phycoerythrin (PE)-anti-human CD25 antibody (1.0 µg, Abcam, USA) for incubation for 30 min in the dark. After that, the cells were washed with precooling phosphate buffer saline (PBS, 0.02 M, PH 7.2) for 2 times (5 min for each time), centrifuged at 2,000 g for 5 min with supernatant removed, added with erythrocyte lysate (1 mL) and incubated for 30 min at 4 °C in the dark. After washed with 2 mL Fix & Perm buffer solution for 2 times, each time for 5 min, the cells were centrifuged at 2,000 g for 5 min with supernatant removed, added with 1 mL Fix & Perm, and incubated for 60 min at 4 °C in the dark. Then the cells were centrifuged at 2000 g for 5 min with supernatant removed, and sealed with 3% bovine serum albumin (BSA, 1 mL) in the dark for 15 min. The cells were then added with PE-anti-human Cy5Foxp3 antibody (5 µg, Abcam Inc., Cambridge, MA, USA) for incubation for 30 min at 4 °C in the dark. The cells then washed with 2 mL permeabilization buffer solution for 2 times, centrifuged at 2,000 g for 5 min with supernatant removed. The cells were re-suspended with flow cytometry staining. The percentage of cells was determined by FACSAria (e140916, BD Biosciences, Franklin Lakes, NJ, USA). The above methods were also used to measure the percentage of Th1, Th2 and Th17 to CD4+Treg.
Measurement of lung function
The forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) (%) and FEV1 (%) of patients before and after treatment and of healthy people during physical examination were detected by pulmonary function test apparatus (AS-507. MINATO, Japan).
Effectiveness evaluation
Markedly effectiveness: patients had obviously relieved or disappeared wheezing symptoms; after activity patients had relieved shortness of breath and patients did not administrate oral and intravenous anti-asthmatic drugs or decreased the dosage of oral and intravenous anti-asthmatic drugs. Effectiveness: patients had relieved wheezing symptoms, shortness of breath, decreased chronic attacks and shortened attack time; patients had decreased dosage of the other anti-asthmatic drugs. Ineffective: patients had no changes in symptoms and dosages compared with that before taking Liuweibuqi capsules.
Statistical analysis
All the data were used SPSS 21 statistical software (IBM Corp., Armonk, NY, USA) for processing, measurement data using form of mean standard ± deviation. Comparison between two groups conducted with the t-test, and comparison among groups with single factor variance analysis. Enumeration data is expressed as a percentage or rate, comparison using χ2 test. Analysis of variance (ANOVA) was used to compare multi-group variables with homogeneity test of variance. When the results of ANOVA showed a significant difference, q test were used to compare groups in pairs, and when it showed no significant difference, rank test of non-parametric test were used to compare groups in pairs. Logistic analysis was used to assess the related factors for affecting the efficacy of Liuweibuqi capsules in treating patients with COPD. Text standard was α=0.05, P<0.05 means difference is of statistically significance.
Results
Baseline characteristics of subjects in six groups
Baseline characteristics of all enrolled subjects were collected when they were admitted in the First Affiliated Hospital of Anhui Medical University (Table 1).
Table 1. Baseline characteristics of all enrolled subjects in six groups.
| Characteristics | NSH | SH | NS-COPD | NS-control | S-COPD | S-control |
|---|---|---|---|---|---|---|
| Cases | 121 | 124 | 114 | 116 | 110 | 118 |
| Mean ages | 55.85±10.36 | 56.51±4.45 | 56.95±10.31 | 56.66±9.28 | 58.82±7.47 | 59.78±7.36 |
| Gender | 62/59 | 71/53 | 62/52 | 64/52 | 59/51 | 63/55 |
| Duration of cigarette smoking | – | 12.21±3.24 | – | – | 14.43±4.17 | 15.09±3.42 |
| Family medical history | – | – | – | – | – | – |
NS-COPD, non-smoking COPD; NS-control, non-smoking control; S-COPD, smoking COPD; S-control, smoking control; NSH, non-smoking healthy people; SH, smoking healthy people; COPD, chronic obstructive pulmonary disease.
Stable COPD patients had higher levels of INF-γ, IL-17 and IL-4
Firstly, levels of inflammatory cytokines (INF-γ, IL-17 and IL-4) before treatment in the NSH, SH, NS-COPD and S-COPD groups were measured, and the results are shown in Figure 1. Compared with the NSH group, serum INF-γ and IL-17 levels increased in the SH group (P<0.05), while IL-4 had no difference in the two groups (P>0.05). Compared with the NSH group, IL-4 had higher level (P<0.05), INF-γ and IL-17 had obvious elevated level (P<0.01) in the NS-COPD and S-COPD groups. Compared with the SH group, INF-γ, IL-17 and IL-4 showed higher level in the NS-COPD group (P<0.05); IL-4 was relative high (P<0.05) but INF-γ, IL-17 are extremely higher (P<0.01) in the S-COPD group. Compared with the NS-COPD group, INF-γ, IL-17 and IL-4 showed higher level in the S-COPD group (P<0.05). The above results indicated that stable COPD patients had higher serum INF-γ, IL-17 and IL-4 levels, and smoking may increase INF-γ, IL-17 and IL-4 levels in both COPD patients and healthy people.
Figure 1.
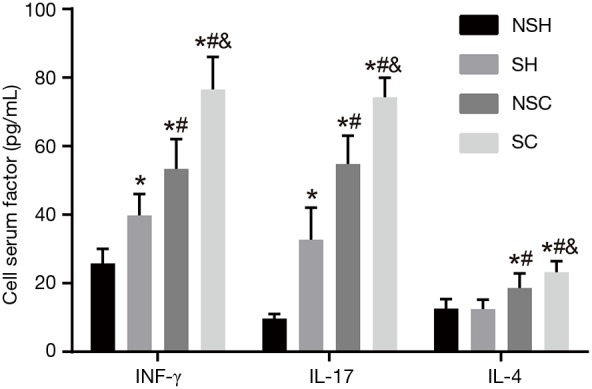
Serum levels of INF-γ, IL-17 and IL-4 in the NSH, SH, NS-COPD and S-COPD groups before treatment. *, P<0.05 vs. the NSH group; #, P<0.05 vs. the SH group; &, P<0.05 vs. the NS-COPD group; number of samples NSH =121, SH =124, NSC =114, SC =110. NS-COPD, non-smoking COPD; S-COPD, smoking COPD; NSH, non-smoking healthy people; SH, smoking healthy people; COPD, chronic obstructive pulmonary disease.
Liuweibuqi capsules lowered levels of INF-γ, IL-17 and IL-4 especially for IL-17
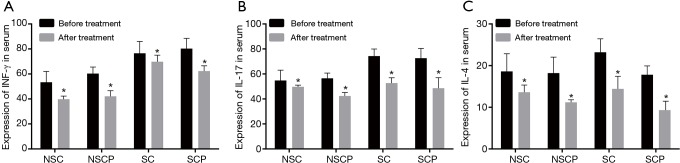
Then levels of inflammatory cytokines (INF-γ, IL-17 and IL-4) before and after treatment in the NS-control, S-control, NS-COPD and S-COPD groups were detected and the findings are shown in Figure 2. After treatment, the levels of INF-γ and IL-4 were decreased in the S-COPD and S-control groups (P<0.05), IL-17 was obviously decreased (P<0.01); the levels of INF-γ, IL-17 and IL-4 was also decreased in the NS-COPD and NS-control groups (P<0.05). These results showed that Liuweibuqi capsules may lower the levels of INF-γ, IL-17 and IL-4, especially for IL-17 level.
Figure 2.
Comparison of serum levels of INF-γ, IL-17 and IL-4 among the NS-COPD, NS-control, S-COPD and S-control groups before and after treatment. (A) Changes of serum levels of INF-γ before and after treatment; (B) changes of serum levels of IL-17 before and after treatment; (C) changes of serum levels of IL-4 before and after treatment. *, P<0.05 vs. the serum factors before treatment; number of samples: NSC =114, NSCP =116, SC =110, SCP =118. NS-COPD, non-smoking COPD; NS-control, non-smoking control; S-COPD, smoking COPD; S-control, smoking control; COPD, chronic obstructive pulmonary disease.
Stable COPD patients had higher levels of Th1, Th2, Th17 and Th1/Th2 and lower level of CD4+CD25+Fox3+Tregs
Next, levels of Th1, Th2, Th17 and Th1/Th2 and CD4+CD25+Fox3+Tregs were determined before treatment (Figure 3). Compared with the NSH group, in the SH group, Th1 and Th1/Th2 showed elevated level (P<0.05) and Th17 showed significantly higher level (P<0.01), Th2 showed no change (P>0.05), and CD4+CD25+Fox3+Tregs showed decreased level (P<0.05); in the NS-COPD group, Th1, Th2, and Th1/Th2 had higher levels (P<0.05) and Th17 showed significantly higher level (P<0.01), CD4+CD25+Fox3+Tregs showed significantly decreased level (P<0.01); in the S-COPD group, Th2 had higher level (P<0.05) and Th1, Th17 and Th1/Th2 showed significantly higher levels (P<0.01), and CD4+CD25+Fox3+Tregs showed significantly decreased level (P<0.01). Compared with the SH group, the NS-COPD group showed higher Th2 level (P<0.05), significantly higher Th17 level (P<0.01), and no changes of Th1 and Th1/Th2 levels; in the S-COPD group, the levels of Th2 and Th1/Th2 was higher (P<0.05), Th1 and Th17 showed significantly higher levels (P<0.01), and level of CD4+CD25+Fox3+Tregs decreased (P<0.05). Compared with the NS-COPD group, the S-COPD group had higher levels of Th17 and Th1/Th2 (P<0.05), significantly higher Th1 level (P<0.01), no significantly changed in Th2 level (P>0.05), decreased level of CD4+CD25+Fox3+Tregs (P<0.05). The above results indicated that stable COPD patients had higher levels of Th1, Th2, Th17 and Th1/Th2 and lower level of CD4+CD25+Fox3+Tregs and smoking may increase the level of Th1, Th2, Th17 and Th1/Th2 but decrease the level of CD4+CD25+Fox3+Tregs in both stable COPD patients and healthy people.
Figure 3.
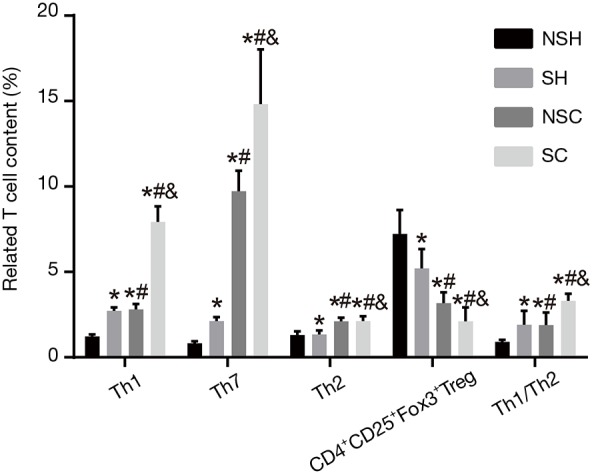
Levels of Th1, Th2, Th17 and Th1/Th2 and CD4+CD25+Fox3+Tregs in the NSH, SH, NS-COPD and S-COPD groups before treatment (%). *, P<0.05 vs. the NSH group; #, P<0.05 vs. the SH group; &, P<0.05 vs. the NS-COPD group; number of samples: NSH =121, SH =124, NSC =114, SC =110. NS-COPD, non-smoking COPD; S-COPD, smoking COPD; NSH, non-smoking healthy people; SH, smoking healthy people; COPD, chronic obstructive pulmonary disease; Tregs, regulatory T cells; Th, helper T cells.
Liuweibuqi capsules decreased levels of Th1, Th2, Th17 and Th1/Th2 and increased CD4+CD25+Fox3+Tregs level
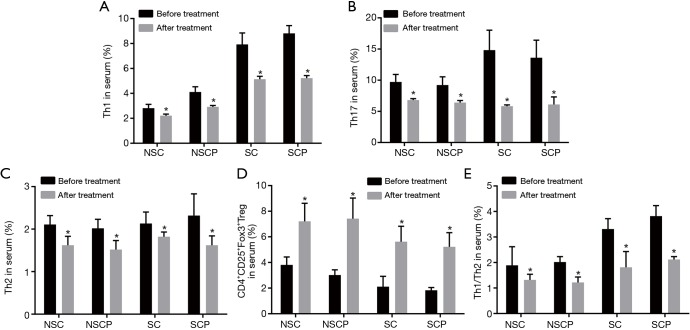
Subsequently, levels of Th1, Th2, Th17, Th1/Th2 and CD4+CD25+Fox3+Tregs before and after treatment were compared (Figure 4). After treatment, the S-COPD and S-control groups had decreased levels of Th1, Th2, Th17 and Th1/Th2 (P<0.05), significantly decreased Th17 level (P<0.01), and significantly increased CD4+CD25+Fox3+Tregs level (P<0.01). The NS-COPD and NS-control groups had decreased levels of Th1, Th17, Th2 and Th1/Th2 (P<0.05), significantly increased CD4+CD25+Fox3+Tregs level (P<0.01). The above results indicated that Liuweibuqi capsules may significantly decrease the levels of Th1, Th2, Th17 and Th1/Th2 especially for Th17 level and improve the level of CD4+CD25+Fox3+Tregs.
Figure 4.
Levels of Th1, Th2, Th17, Th1/Th2 and CD4+CD25+Fox3+Tregs in the NS-COPD, NS-control, S-COPD and S-control groups before and after treatment. (A) Changes of the content of Th1 cells in blood before and after treatment; (B) changes of the content of Th17 cells in blood before and after treatment; (C) changes of the content of Th2 cells in blood before and after treatment; (D) changes of the content of CD4+CD25+Fox3+Tregs cells in blood before and after treatment; (E) changes of the content of Th1/Th2 cells in blood before and after treatment. *, P<0.05 vs. T cells before treatment; number of samples: NSC =114, NSCP =116, SC =110, SCP =118. NS-COPD, non-smoking COPD; NS-control, non-smoking control; S-COPD, smoking COPD; S-control, smoking control; COPD, chronic obstructive pulmonary disease; Tregs, regulatory T cells; Th, helper T cells.
COPD patients had lower lung function and smoking may lower lung function
In the following experiment, lung function indicators FEV1 (%) and FEV1/FVC (%) in the NSH, SH, NS-COPD and S-COPD groups were compared (Figure 5). Compared with the NSH group, the SH group had decreased level of FEV1 (%) and FEV1/FVC (%) (P<0.05); the NS-COPD and S-COPD groups had significant decreased FEV1 (%) and FEV1/FVC (%) (P<0.01). Compared with the SH group, the NS-COPD group had decreased FEV1 (%) and FEV1/FVC (%) (P<0.05) while the S-COPD group had significantly decreased FEV1 (%) and FEV1/FVC (%) (P<0.01). Compared with the NS-COPD group, the S-COPD group had the decreased FEV1 (%) and FEV1/FVC (%) (P<0.05). The above results indicated that the FEV1 (%) and FEV1/FVC (%) in stable COPD patients were decreased, and smoking could further weaker their lung function.
Figure 5.
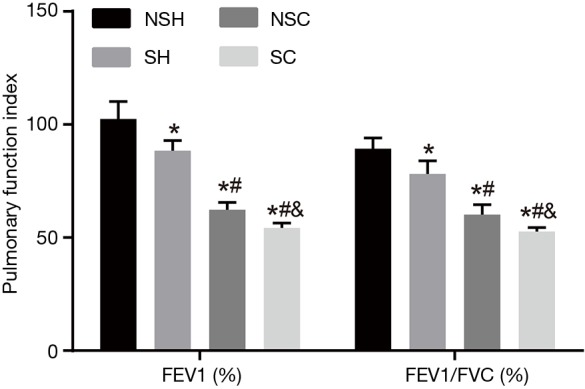
FEV1 (%) and FEV1/FVC (%) in the NSH, SH, NS-COPD and S-COPD groups before treatment. *, P<0.05 vs. the NSH group; #, P<0.05 vs. the SH group; &, P<0.05 vs. the NS-COPD group; number of samples: NSH =121, SH =124, NSC =114, SC =110. NS-COPD, non-smoking COPD; S-COPD, smoking COPD; NSH, non-smoking healthy people; SH, smoking healthy people; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
Liuweibuqi capsules helped COPD patients improve their lung function
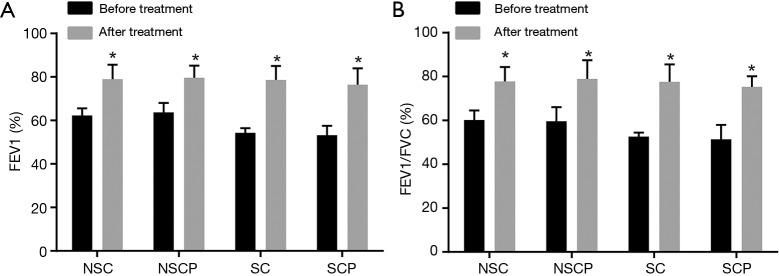
Afterwards, lung function in the NS-COPD, NS-control, S-COPD and S-control groups before and after treatment was compared (Figure 6). After treatment, the NS-COPD and NS-control groups had elevated FEV1 (%) and FEV1/FVC (%) (P<0.05), and the S-COPD and S-control groups had significantly higher FEV1 (%) and FEV1/FVC (%) (P<0.01). All findings verified that Liuweibuqi capsules may have promoting effects on lung function of COPD patients.
Figure 6.
FEV1 (%) and FEV1/FVC (%) in the NS-COPD, NS-control, S-COPD and S-control groups before and after treatment. (A) Changes of pulmonary function index FEV1 (%) before and after treatment in COPD patients; (B) changes of pulmonary function index FEV1/FVC (%) before and after treatment in COPD patients. *, P<0.05 vs. lung function indicators before treatment; number of samples: NSC =114, NSCP =116, SC =110, SCP =118. NS-COPD, non-smoking COPD; NS-control, non-smoking control; S-COPD, smoking COPD; S-control, smoking control; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
Liuweibuqi capsules weakened the correlation between lung function and T cells and smoking strengthened it
Spearman rank correlation test was then conducted and the results showed (Table 2) that FEV1 (%) had negative correlation with Th1, Th2, Th17 and Th1/Th2 but had positive correlation with CD4+CD25+Fox3+Tregs in stable COPD patients. Smoking increases the correlation of FEV1 (%) with Th1, Th2, Th17, Th1/Th2 and CD4+CD25+Fox3+Tregs in both stable COPD patients and healthy people. Liuweibuqi capsules may decrease the correlation of FEV1 (%) with Th1, Th2, Th17, Th1/Th2 and CD4+CD25+Fox3+Tregs in smoking and non-smoking COPD patients. The above results provided evidence that Liuweibuqi capsules may weaken the correlation between lung function and T cells, while smoking strengthened the correlation between lung function and T cells.
Table 2. Correlation of FEV1 and T cells in each group analyzed by Spearman rank correlation test.
| T cells | NSH (r) | SH (r) | NS-COPD (r) | NS-control (r) | S-COPD (r) | S-control (r) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | ||||||
| Th1 | −0.081 | −0.130 | −0.882* | −0.507* | −0.449* | −0.195* | −0.528* | −0.411* | −0.671* | −0.396* | |||
| Th2 | −0.134 | −0.167 | −0.636* | −0.089 | −0.482* | −0.147 | −0.443* | −0.050 | −0.692* | −0.069 | |||
| Th17 | −0.078 | −0.601* | −0.545* | −0.329* | −0.545* | −0.318* | −0.595* | −0.306* | −0.889* | −0.233* | |||
| Th1/Th2 | −0.072 | −0.119 | −0.524* | −0.064 | −0.454* | −0.090 | −0.559* | −0.055 | −0.575* | −0.142 | |||
| CD4+CD25+Fox3+Treg | 0.064 | 0.107 | 0.924* | 0.278* | 0.472* | 0.300* | 0.568* | 0.288* | 0.640* | 0.378* | |||
*, P<0.05, Spearman rank correlation test; number of samples: NSH =121, SH =124, NSC =114, NSCP =116, SC =110, SCP =118. NS-COPD, non-smoking COPD; NS-control, non-smoking controls; S-COPD, smoking COPD; S-control, smoking controls; NSH, non-smoking healthy people; SH, smoking healthy people; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; Tregs, regulatory T cells; Th, helper T cells.
Level of inflammatory cytokines, cells, marker, pulmonary function indicators, smoking and Liuweibuqi capsules were factors affecting efficacy
Finally, 60 days after treatment, the patients are assigned into effectiveness (n=379) and ineffectiveness (n=79) groups. To establish the Logistic regression analysis, the effectiveness of the inflammatory cells as independent variables, level of inflammatory cytokines (INF-γ, IL-17, IL-4), T cells content (Th1, Th17, Th2, CD4+CD25+Fox3+Tregs, Th1/Th2), pulmonary function indicators [FEV1 (%), FEV1/FVC (%)], smoking and Liuweibuqi capsules as the dependent variables. The results (Table 3) showed that the above factors were factors for affecting efficacy (all P<0.05).
Table 3. Logistic analysis for factors affecting efficacy of Liuweibuqi capsules in treating patients with COPD.
| Variables | B | S.E | Wald | df | P | Exp (B) | 95% CI |
|---|---|---|---|---|---|---|---|
| INF-γ | 0.145 | 0.045 | 10.622 | 1 | 0.001 | 1.156 | 1.060–1.262 |
| IL-17 | 0.064 | 0.029 | 4.919 | 1 | 0.027 | 1.066 | 1.008–1.129 |
| IL-4 | 0.234 | 0.110 | 4.560 | 1 | 0.033 | 1.263 | 1.019–1.566 |
| Th1 | 2.193 | 1.019 | 4.630 | 1 | 0.031 | 8.961 | 1.216–66.048 |
| Th17 | 0.491 | 0.204 | 5.797 | 1 | 0.016 | 1.635 | 1.096–2.439 |
| Th2 | 1.789 | 0.888 | 4.059 | 1 | 0.044 | 5.984 | 1.050–34.111 |
| CD4+CD25+Fox3+Tregs | 0.466 | 0.162 | 8.301 | 1 | 0.004 | 1.593 | 1.161–2.187 |
| FEV1 (%) | 0.071 | 0.030 | 5.507 | 1 | 0.001 | 1.074 | 1.012–1.140 |
| FEV1/FVC (%) | 9.368 | 2.606 | 12.918 | 1 | 0.019 | 11,708.259 | 70.769–11,708.259 |
| Smoking | 2.435 | 0.859 | 8.046 | 1 | <0.001 | 11.421 | 2.123–61.458 |
| Liuweibuqi capsules | 0.145 | 0.045 | 10.622 | 1 | 0.005 | 1.156 | 1.060–1.262 |
COPD, chronic obstructive pulmonary disease; Tregs, regulatory T cells; Th, helper T cells; FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
Discussion
As arise of population ages and smoking frequencies, population with COPD worldwide will increase as predicted (24). Previous studies have proved that herbal medicine relieves COPD symptoms effectively, especially for the stable COPD (25,26). In this study, we investigated how Liuweibuqi capsules act on the stable COPD patients with lung Qi deficiency. We found that Liuweibuqi capsules may relieve COPD patients effectively by stimulating the proliferation of CD4+CD25+Fox3+Tregs and increasing the ratio of Th17 so as to balance Th1/Th2 ratio and improve the lung function of COPD patients with lung Qi deficiency.
Firstly, we found that the contents of T cells (Th1, Th2 and Th17), and levels of inflammatory cytokines (INF-γ, IL-4 and IL-17) and inflammatory indicator Th1/Th2 were elevated, but the level of CD4+CD25+Fox3+Tregs was decreased of stable COPD patients. Th17 cells, different from Th1 or Th2 cells, is a subset of T cells produced from IL-17, which regarded as a critical regulator in inflammation and autoimmune diseases (27). Several studies also found that the level of Th1, Th2 and Th17 cells increased in COPD patients (28,29). IFN-γ is produced by Th1 cells, which takes part in the cell-mediated pro-inflammatory activity (30). IL-4 is an immune response-related cytokine produced by Th2 cells, which are involved in inhibiting anti-tumor immunity (31). IL-17 is found secreted from Th17 cells and neutrophils in pulmonary aspergillosis and systemic histoplasmosis (32). An imbalance of Th1/Th2 status may lead to the development of viral or bacterial infections, allergic disease and autoimmune disorders, and COPD is correlated with Th1/Th2 imbalance (28,33). Increased level of IFN-γ, IL-4, IL-17 and Th1/Th2 were also found in COPD patients in previous studies, which are consistent with the results in this study (34-37). CD4+CD25+Fox3+Tregs play an important role in creating a regulatory environment that promotes infectious tolerance and it was found to improve prognoses of acute exacerbation in COPD patients (38,39).
Secondly, smoking could increase the level of inflammatory cells, cytokines and indicator, decrease the CD4+CD25+Fox3+Tregs levels. Tregs have the potential to maintain normal lung function in smokers and may stop the progression of immune responses (40), and the level of Tregs in smokers’ lungs without lung dysfunction is more than that in people without a history of smoking (41). CD8+ T cells are inversely correlated with pulmonary function, and previous study indicated that CD8+Tregs have reverse correlation with smoking (38). Smoking may directly destroy the balance of pro-inflammatory and anti-inflammatory response via inhibiting CD8+ T cell/CD8+Tregs action (42). Cigarette cessation has been proved to be the most successful and cost-effective regimen for suppressing disease progression (43). Lung functions is generally determined by FEV1 (%), and low FEV1 in early adulthood is regarded as the genesis of COPD (44,45). We found that smoking could strengthen the correlation of FEV1 (%) and inflammatory cells, cytokines, indicator and CD4+CD25+Fox3+Tregs.
Thirdly, Liuweibuqi capsules may effectively decrease the level of inflammatory cells (Th1, Th2 and Th17), cytokines (INF-γ, IL-4 and IL-17) and indicator (Th1/Th2), and increase the level of CD4+CD25+Fox3+Tregs, indicating markedly effectiveness of treatment with Liuweibuqi capsules. Recent evidence has revealed that systemic inflammation plays vital roles in development of COPD and there are a large number of chemokines and cytokines that have been correlated to the pathogenesis of COPD, including INF-γ, IL-1β, IL-13 and IL-6 (46). The immune mechanism in COPD includes innate immune reaction and adaptive immune reaction (47). Remedium cardinales of Liuweibuqi capsules are Renshen and Huangqi (14). Ginsenosides are major active components of Ren Shen, which positively functions in anticancer, antiaging, and antiviral activities, and it also have anti-inflammatory function via inhibiting TNF receptor-associated factor 6 (48,49). A study suggested that ginsenoside Rg3 combined with acupuncture may reduce IFN-γ and it may also counteract the disorder Th1/Th2 profile (50). In TCM, one of the original causes of COPD is lung deficiency, the regimen of which is treated with supplementary Qi (14). Furthermore, Renshen and Huangqi have been proved as the popular restoratives which mainly used for symptoms due to Qi deficiency (51). Therefore, we may conclude that Liuweibuqi capsules can inhibit COPD-induced inflammatory response, providing markedly effectiveness on treatment of COPD.
Above all, we inferred that in the one hand, Liuweibuqi capsules lower the pro-inflammatory reaction by inhibiting differentiation from Th1 to Th17 and decreasing IL-17, INF-γ and IL-4 levels; in the other hand, Liuweibuqi capsules enhance the immune tolerance of the organism by increasing the contents of CD4+CD25+Fox3+Tregs cells. The study is only related to the adaptive immune, the mechanism of the innate immune is needed further exploration, so as to fulfill mechanism of Liuweibuqi capsules and COPD.
Acknowledgements
Funding: This work was supported by the National Nature Science Foundation of China (Grant No. 81573942).
Ethical Statement: This study was approved by ethics committee of the First Affiliated Hospital of Anhui Medical University (2012-006). All subjects voluntarily participated in the experiment were fully informed of gains and risks and signed informed consents. This experiment was in accordance with the Nuremberg criteria and the Helsinki declaration.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Louie S, Zeki AA, Schivo M, et al. The asthma-chronic obstructive pulmonary disease overlap syndrome: pharmacotherapeutic considerations. Expert Rev Clin Pharmacol 2013;6:197-219. 10.1586/ecp.13.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rennard SI, Drummond MB. Early chronic obstructive pulmonary disease: definition, assessment, and prevention. Lancet 2015;385:1778-88. 10.1016/S0140-6736(15)60647-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Shergis JL, Wu L, et al. A systematic review and meta-analysis of the herbal formula Buzhong Yiqi Tang for stable chronic obstructive pulmonary disease. Complement Ther Med 2016;29:94-108. 10.1016/j.ctim.2016.09.017 [DOI] [PubMed] [Google Scholar]
- 4.Lynch DA, Austin JH, Hogg JC, et al. CT-Definable Subtypes of Chronic Obstructive Pulmonary Disease: A Statement of the Fleischner Society. Radiology 2015;277:192-205. 10.1148/radiol.2015141579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kankaanranta H, Harju T, Kilpelainen M, et al. Diagnosis and pharmacotherapy of stable chronic obstructive pulmonary disease: the finnish guidelines. Basic Clin Pharmacol Toxicol 2015;116:291-307. 10.1111/bcpt.12366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musafiri S, van Meerbeeck J, Musango L, et al. Prevalence of atopy, asthma and COPD in an urban and a rural area of an African country. Respir Med 2011;105:1596-605. 10.1016/j.rmed.2011.06.013 [DOI] [PubMed] [Google Scholar]
- 7.Hu G, Zhong N, Ran P. Air pollution and COPD in China. J Thorac Dis 2015;7:59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miravitlles M. Prevention of exacerbations of COPD with pharmacotherapy. Eur Respir Rev 2010;19:119-26. 10.1183/09059180.00002810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nyberg A, Lindstrom B, Wadell K. Assessing the effect of high-repetitive single limb exercises (HRSLE) on exercise capacity and quality of life in patients with chronic obstructive pulmonary disease (COPD): study protocol for randomized controlled trial. Trials 2012;13:114. 10.1186/1745-6215-13-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russo R, Coultas D, Ashmore J, et al. Chronic obstructive pulmonary disease self-management activation research trial (COPD-SMART): results of recruitment and baseline patient characteristics. Contemp Clin Trials 2015;41:192-201. 10.1016/j.cct.2015.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li JS, Wang HF. Sequential syndrome differentiation by eliminating pathogen and strengthening vital Qi on the basis of acute exacerbation of chronic obstructive pulmonary disease risk window. Zhongguo Zhong Xi Yi Jie He Za Zhi 2011;31:1276-80. [PubMed] [Google Scholar]
- 12.Yuan F, Zhou Y, Jiang Y, et al. Therapeutic effect and apoptosis mechanism of lung-tonifying and expectorant decoction on lung cancer rats with Qi deficiency and blood stasis. Asian Pac J Trop Med 2015;8:983-8. 10.1016/j.apjtm.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 13.Kakooza-Mwesige A. The importance of botanical treatments in traditional societies and challenges in developing countries. Epilepsy Behav 2015;52:297-307. 10.1016/j.yebeh.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 14.Wang C, Li Z, Liu X, et al. Effect of Liuweibuqi capsule, a Chinese patent medicine, on the JAK1/STAT3 pathway and MMP9/TIMP1 in a chronic obstructive pulmonary disease rat model. J Tradit Chin Med 2015;35:54-62. [DOI] [PubMed]
- 15.Wei X, Chen J, Su F, et al. Stereospecificity of ginsenoside Rg3 in promotion of the immune response to ovalbumin in mice. Int Immunol 2012;24:465-71. 10.1093/intimm/dxs043 [DOI] [PubMed] [Google Scholar]
- 16.Pang B, Zhou Q, Zhao TY, et al. Innovative Thoughts on Treating Diabetes from the Perspective of Traditional Chinese Medicine. Evid Based Complement Alternat Med 2015;2015:905432. 10.1155/2015/905432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren S, Zhang H, Mu Y, et al. Pharmacological effects of Astragaloside IV: a literature review. J Tradit Chin Med 2013;33:413-6. 10.1016/S0254-6272(13)60189-2 [DOI] [PubMed] [Google Scholar]
- 18.Kim EJ, Lee YJ, Ahn YM, et al. Renoprotective effect of Alpiniae oxyphyllae Fructus on ischemia/reperfusion-induced acute renal failure. Arch Pharm Res 2013;36:1004-12. 10.1007/s12272-013-0117-3 [DOI] [PubMed] [Google Scholar]
- 19.Chang YM, Ye CX, Ho TJ, et al. Alpinia oxyphylla Miquel fruit extract activates MAPK-mediated signaling of PAs and MMP2/9 to induce Schwann cell migration and nerve regeneration. Int J Artif Organs 2014;37:402-13. 10.5301/ijao.5000313 [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Feng R, Guo Y, et al. Toxicity studies of Rhizoma Polygonati Odorati. J Ethnopharmacol 2001;74:221-4. 10.1016/S0378-8741(00)00358-5 [DOI] [PubMed] [Google Scholar]
- 21.Hayashi K, Imanishi N, Kashiwayama Y, et al. Inhibitory effect of cinnamaldehyde, derived from Cinnamomi cortex, on the growth of influenza A/PR/8 virus in vitro and in vivo. Antiviral Res 2007;74:1-8. 10.1016/j.antiviral.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 22.Yi LZ, Yuan DL, Liang YZ, et al. Quality control and discrimination of pericarpium citri reticulatae and pericarpium citri reticulatae viride based on high-performance liquid chromatographic fingerprints and multivariate statistical analysis. Anal Chim Acta 2007;588:207-15. 10.1016/j.aca.2007.02.012 [DOI] [PubMed] [Google Scholar]
- 23.Shiffman RN, Shekelle P, Overhage JM, et al. Standardized reporting of clinical practice guidelines: a proposal from the Conference on Guideline Standardization. Ann Intern Med 2003;139:493-8. 10.7326/0003-4819-139-6-200309160-00013 [DOI] [PubMed] [Google Scholar]
- 24.Hong CM, Galvagno SM., Jr Patients with chronic pulmonary disease. Med Clin North Am 2013;97:1095-107. 10.1016/j.mcna.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 25.Li SY, Li JS, Wang MH, et al. Effects of comprehensive therapy based on traditional Chinese medicine patterns in stable chronic obstructive pulmonary disease: a four-center, open-label, randomized, controlled study. BMC Complement Altern Med 2012;12:197. 10.1186/1472-6882-12-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukaida K, Hattori N, Kondo K, et al. A pilot study of the multiherb Kampo medicine bakumondoto for cough in patients with chronic obstructive pulmonary disease. Phytomedicine 2011;18:625-9. 10.1016/j.phymed.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 27.Vargas-Rojas MI, Ramirez-Venegas A, Limon-Camacho L, et al. Increase of Th17 cells in peripheral blood of patients with chronic obstructive pulmonary disease. Respir Med 2011;105:1648-54. 10.1016/j.rmed.2011.05.017 [DOI] [PubMed] [Google Scholar]
- 28.Gu XY, Chu X, Zeng XL, et al. Effects of PM2.5 exposure on the Notch signaling pathway and immune imbalance in chronic obstructive pulmonary disease. Environ Pollut 2017;226:163-73. 10.1016/j.envpol.2017.03.070 [DOI] [PubMed] [Google Scholar]
- 29.Hodge SJ, Hodge GL, Holmes M, et al. Flow cytometric characterization of cell populations in bronchoalveolar lavage and bronchial brushings from patients with chronic obstructive pulmonary disease. Cytometry B Clin Cytom 2004;61:27-34. 10.1002/cyto.b.20020 [DOI] [PubMed] [Google Scholar]
- 30.Xu X, Wang R, Su Q, et al. Expression of Th1- Th2- and Th17-associated cytokines in laryngeal carcinoma. Oncol Lett 2016;12:1941-8. 10.3892/ol.2016.4854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S, Karasuyama H, Lopez AF, et al. IL-4 Derived from Non-T Cells Induces Basophil- and IL-3-independent Th2 Immune Responses. Immune Netw 2013;13:249-56. 10.4110/in.2013.13.6.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor PR, Leal SM, Jr, Sun Y, et al. Aspergillus and Fusarium corneal infections are regulated by Th17 cells and IL-17-producing neutrophils. J Immunol 2014;192:3319-27. 10.4049/jimmunol.1302235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang F, Wang F, An L, et al. Upregulation of Tim-3 on CD4(+) T cells is associated with Th1/Th2 imbalance in patients with allergic asthma. Int J Clin Exp Med 2015;8:3809-16. [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Chu S, Zhong X, et al. Increased expression of CD4+IL-17+ cells in the lung tissue of patients with stable chronic obstructive pulmonary disease (COPD) and smokers. Int Immunopharmacol 2013;15:58-66. 10.1016/j.intimp.2012.10.018 [DOI] [PubMed] [Google Scholar]
- 35.Wang CY, Liu XG, Wang CB, et al. Regulation of Thl/Th2 cells by T cell-mediated transcription factor in rats with chronic obstructive pulmonary disease. Sichuan Da Xue Xue Bao Yi Xue Ban 2014;45:941-5. [PubMed] [Google Scholar]
- 36.Cao Y, Gong W, Zhang H, et al. A comparison of serum and sputum inflammatory mediator profiles in patients with asthma and COPD. J Int Med Res 2012;40:2231-42. 10.1177/030006051204000621 [DOI] [PubMed] [Google Scholar]
- 37.Saturni S, Contoli M, Spanevello A, et al. Models of Respiratory Infections: Virus-Induced Asthma Exacerbations and Beyond. Allergy Asthma Immunol Res 2015;7:525-33. 10.4168/aair.2015.7.6.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiong XZ, Jin Y, Zhou Q, et al. [Correlation between FoxP3(+) regulatory T cells and chronic obstructive pulmonary disease]. Zhonghua Yi Xue Za Zhi 2008;88:471-4. [PubMed] [Google Scholar]
- 39.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol 2008;9:239-44. 10.1038/ni1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L, Ma QL, Yao W, et al. Relationship between the anti-inflammatory properties of salmeterol/fluticasone and the expression of CD4(+)CD25(+)Foxp3(+) regulatory T cells in COPD. Respir Res 2011;12:142. 10.1186/1465-9921-12-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med 2009;360:2445-54. 10.1056/NEJMra0804752 [DOI] [PubMed] [Google Scholar]
- 42.Chen G, Zhou M, Chen L, et al. Cigarette Smoke Disturbs the Survival of CD8+ Tc/Tregs Partially through Muscarinic Receptors-Dependent Mechanisms in Chronic Obstructive Pulmonary Disease. PLoS One 2016;11:e0147232. 10.1371/journal.pone.0147232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narsingam S, Bozarth AL, Abdeljalil A. Updates in the management of stable chronic obstructive pulmonary disease. Postgrad Med 2015;127:758-70. 10.1080/00325481.2015.1084212 [DOI] [PubMed] [Google Scholar]
- 44.Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011;155:179-91. 10.7326/0003-4819-155-3-201108020-00008 [DOI] [PubMed] [Google Scholar]
- 45.Lange P, Celli B, Agusti A, et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N Engl J Med 2015;373:111-22. 10.1056/NEJMoa1411532 [DOI] [PubMed] [Google Scholar]
- 46.Wang C, Ding H, Tang X, et al. Effect of Liuweibuqi Capsules in Pulmonary Alveolar Epithelial Cells and COPD Through JAK/STAT Pathway. Cell Physiol Biochem 2017;43:743-56. 10.1159/000481558 [DOI] [PubMed] [Google Scholar]
- 47.Tsoumakidou M, Tsiligianni I, Tzanakis N. Mechanisms of altered cell immunity and cytotoxicity in COPD. Curr Drug Targets 2011;12:450-9. 10.2174/138945011794751546 [DOI] [PubMed] [Google Scholar]
- 48.Lee KT, Jung TW, Lee HJ, et al. The antidiabetic effect of ginsenoside Rb2 via activation of AMPK. Arch Pharm Res 2011;34:1201-8. 10.1007/s12272-011-0719-6 [DOI] [PubMed] [Google Scholar]
- 49.Kang LJ, Choi YJ, Lee SG. Stimulation of TRAF6/TAK1 degradation and inhibition of JNK/AP-1 signalling by ginsenoside Rg3 attenuates hepatitis B virus replication. Int J Biochem Cell Biol 2013;45:2612-21. 10.1016/j.biocel.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 50.Zhang W, Zhang Y, Ma X, et al. Effects of acupuncturing Pishu combined with Ginsenoside Rg3 on the immune function of rats with chronic fatigue. Int J Clin Exp Med 2015;8:19022-9. [PMC free article] [PubMed] [Google Scholar]
- 51.Liao H, Banbury L. Different Proportions of Huangqi (Radix Astragali Mongolici) and Honghua (Flos Carthami) Injection on alpha-Glucosidase and alpha-Amylase Activities. Evid Based Complement Alternat Med 2015;2015:785193. 10.1155/2015/785193 [DOI] [PMC free article] [PubMed] [Google Scholar]