Abstract
Converging evidence from molecular to neuroimaging studies suggests brain energy metabolism abnormalities in both schizophrenia and bipolar disorder. One emerging hypothesis is: decreased oxidative phosphorylation leading to accumulation of lactic acid from glycolysis and subsequent acidification of tissue. In this regard, integrating lactate and pH data from magnetic resonance spectroscopy (MRS) studies in both diseases may help us understand underlying neurobiological mechanisms. In order to achieve this goal, we performed a systematic search of case–control studies examining brain lactate or pH among schizophrenia and/or bipolar patients by using MRS. Medline/Pubmed and EBSCO databases were searched separately for both diseases and outcomes. Our search yielded 33 studies in total composed of 7 lactate and 26 pH studies. In bipolar disorder, 5 out of 6 studies have found elevated lactate levels especially in the cingulate cortex and 4 out of 13 studies reported reduced pH in the frontal lobe. In contrast, in schizophrenia a single study has examined lactate and reported elevation, while only 2 out of 13 studies examining pH have reported reduction in this measure. There were no consistent patterns for the relationship between lactate or pH levels and medication use, disease type, mood state, and other clinical variables. We highlight the need for future studies combining 1H-MRS and 31P-MRS approaches, using longitudinal designs to examine lactate and pH in disease progression across both schizophrenia and bipolar disorders.
Introduction
Schizophrenia and bipolar disorder (BD) are common and severe psychiatric disorders, affecting more than 80 million people worldwide [1], characterized by overlapping genetic background, brain abnormalities, and clinical presentations. Despite progress, neurobiological abnormalities underlying these disorders are not well understood, and available treatments have limited effectiveness in improving the symptoms and functional outcomes [2, 3]. Growing evidence, from molecular to neuroimaging, has recently implicated impaired brain energy metabolism, evidenced by brain glucose utilization abnormalities, mitochondrial dysfunction, and high-energy phosphate (HEP) molecule depletion in their pathophysiology [4]. Although this converging evidence suggests a relationship of brain energy metabolism to pathophysiology in psychotic disorders, the underlying mechanisms remain to be elucidated.
Our understanding of the potential role of lactate in energy metabolism during brain activation has changed radically over the past three decades, shifting from waste product to supplemental fuel and signaling molecule [5]. Schurr et al. [6] were the first to suggest that cerebral energy metabolism can be fueled by lactate under certain conditions like hypoglycemia or ischemia. There is ongoing debate regarding the preference of lactate utilization for energy production by neurons. It has been speculated that lactate is preferred as a substrate when neurons are firing at high frequencies [7]. On the other hand, it may be an “opportunistic” glucose-sparing substrate when it is abundant. Despite growing recognition of the importance of lactate in energy metabolism, it is clear that glucose is the major energy source in normal brain at rest and during activation [5].
Elevated brain lactate concentrations can occur as a result of either increased production by mitochondrial shifts in redox state or decreased clearance by cerebral blood flow alterations and lactate metabolism. Steady-state pHi is dependent on the balance between rates of acid extrusion and loading, which in turn depends on the activities of acid–base transporting proteins [8]. Although intracellular pH regulation is a complex process and not solely regulated by lactate concentration, an important recent meta-analysis [9] confirmed the negative correlation between brain lactate and pH levels. This study, included 10 postmortem brain studies of schizophrenia and bipolar patients, showed lower brain pH levels among patients compared to controls. They also analyzed five neurodevelopmental mouse models of psychiatric disorders (schizophrenia, BD, and autism spectrum disorder), which showed lower pH and higher lactate levels in the brains of model mice together with a significant negative correlation between them.
Among the techniques available for in vivo investigation of human brain, magnetic resonance spectroscopy (MRS) is of special relevance to this field as it can be used to obtain neurochemical information [10]. The proton (1H) nucleus is the most commonly probed due to its high gyromagnetic ratio and widespread development of relevant hardware and techniques. Depending on the specific sequences used, 1H-MRS allows for the quantification of N-acetyl-aspartate, creatine/phosphocreatine (PCr), choline compounds, myoinositol, glutamine/glutamate/GABA as well as lactate. Compared to 1H-MRS, phosphorus (31P)-MRS has lower sensitivity because of the greater mass and lower gyromagnetic ratio of the 31P nucleus. 31P-MRS does not suffer from problems of water and lipid contamination but its utility is limited due to poor spatial resolution, large voxel sizes, long acquisition times, and the need for dedicated hardware. PCr, inorganic phosphate (Pi), three isotopomers of adenosine triphosphate (α-, β-, and γ-ATP), phosphomonoesters and phosphodiesters, intracellular magnesium, and intracellular pH (obtained by using the chemical shift difference between Pi and PCr resonances) can be quantified by 31P-MRS [11]. The combination of these two MRS approaches together with data from molecular, structural, and functional neuroimaging studies may enable us to clarify some of the underlying neurobiological mechanisms of disease in schizophrenia and BD [12].
One emerging hypothesis of metabolic dysfunction in psychotic disorders suggests that impaired oxidative phosphorylation leads to an accumulation of lactate from glycolysis and acidification of tissue (reducing pH), as well as reducing the availability of HEPs [4] (Fig. 1). Integrating such disparate MRS findings as lactate and pH abnormalities in bipolar and schizophrenia patients into a single theory of mitochondrial dysfunction has enormous potential for advancing the study of these disorders, and for developing novel treatment approaches. However, to our knowledge, there has been no systematic review of 31P and 1H-MRS studies of lactate or pH levels in schizophrenia and BD to date. We thus sought to systematically review all controlled studies of lactate and pH levels measured by MRS to (1) estimate the extent to which lactate and pH are altered in schizophrenia and BD, and (2) to investigate the consistency of lactate and pH findings in both schizophrenia and BD.
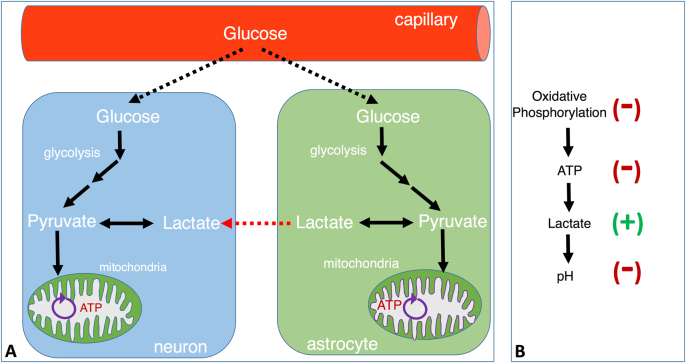
Fig. 1.
Schematic view of astrocyte and neuron glucose metabolism and consequences of reduced oxidative phosphorylation. a Schematic representation of astrocyte and neuron glucose metabolism. Neurons and astrocytes can take up glucose from the circulation. This glucose enters glycolysis pathway and produces pyruvate, which is subsequently converted to either lactate or water and CO2 together with 38 ATP by oxidative phosphorylation within the mitochondria. The lactate produced within astrocytes can also shuttle to neurons through transporters and can be used as an energy substrate following its conversion to pyruvate within the cytoplasm of neurons. This pyruvate is a substrate for oxidative phosphorylation taking part within mitochondria. b The relationship between reduced oxidative phosphorylation and lactate production, pH decrease. Reduced oxidative phosphorylation leads to a shift into glycolysis. Glycolysis results in increased production of lactate, which causes acidification of tissue and decrease in intracellular pH
Methods
Methodological strategies that are used during systematic review procedure were specified in advance and documented in a protocol (https://www.crd.york.ac.uk/PROSPERO/) with a registration number CRD42017082064. We performed a systematic search of case–control studies examining brain lactate or pH in schizophrenia and/or BD patients. Studies were identified by searching electronic databases, scanning reference lists of articles, and consultation with experts in the field. The search was conducted using Medline/Pubmed and EBSCO databases. The last search was done on 26 April 2017. No language, publication date, nor publication status restrictions were imposed. Abstracts of the studies were reviewed and subsequently full texts were obtained. The search was run separately for four different subcategories and the results were merged. The terms used in the search were grouped as follows to capture diagnosis (schizophrenia and BD) and MRS findings (lactate and pH):
(a) Schizophrenia/lactate: (“lactic acid”[MeSH Terms] OR (“lactic”[All Fields] AND “acid”[All Fields]) OR “lactic acid”[All Fields] OR “lactate”[All Fields] OR “lactates”[MeSH Terms] OR “lactates”[All Fields]) AND (“schizophrenia”[All Fields] OR “psychotic”[All Fields] OR “psychosis”[All Fields]) AND (“magnetic resonance spectroscopy”[All Fields] OR “MRS”[All Fields]); (b) Bipolar disorder/lactate: (“lactic acid”[MeSH Terms] OR (“lactic”[All Fields] AND “acid”[All Fields]) OR “lactic acid”[All Fields] OR “lactate”[All Fields] OR “lactates”[MeSH Terms] OR “lactates”[All Fields]) AND (“bipolar”[All Fields] OR “mood disorder”[All Fields]) AND (“magnetic resonance spectroscopy”[All Fields] OR “MRS”[All Fields]; (c) Schizophrenia//pH: (“schizophrenia”[MeSH Terms] OR “schizophrenia”[All Fields] OR “psychosis”[All Fields] OR “psychotic”[All Fields]) AND “pH”[All Fields] AND (“magnetic resonance spectroscopy”[All Fields] OR “MRS”[All Fields]); d) Bipolar disorder/pH: (“bipolar”[All Fields] OR “mood disorder”[All Fields]) AND “pH”[All Fields] AND (“magnetic resonance spectroscopy”[All Fields] OR “MRS”[All Fields]).
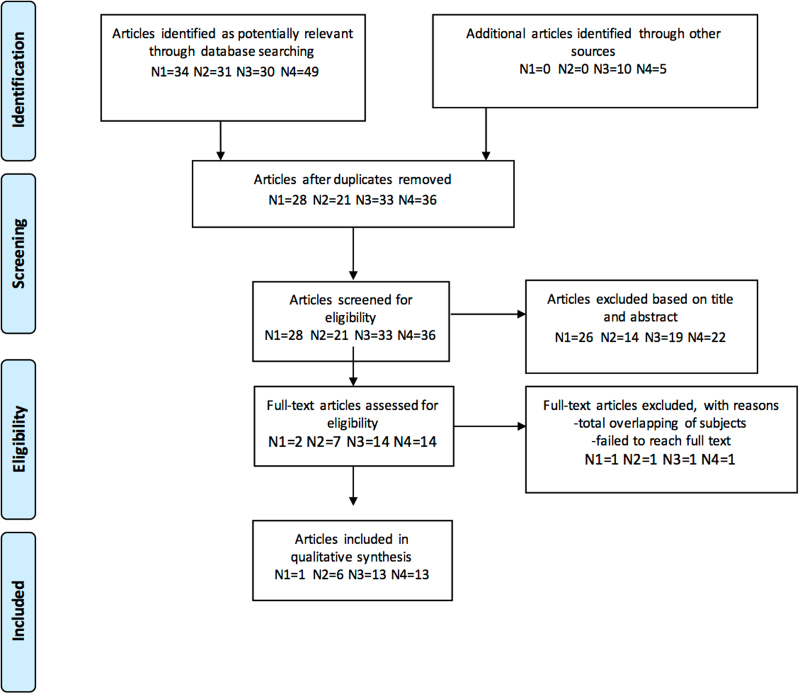
Studies were included if they met all the following criteria: (1) studies included first-episode or chronic adult patients who were diagnosed with schizophrenia and/or schizoaffective and/or BD according to Diagnostic and Statistical Manual of Mental Disorders (Third or Fourth edition); (2) original in vivo 1H-MRS and 31P-MRS studies that measured brain lactate and/or pH levels; and (3) studies included healthy control group for comparison. Figure 2 presents the PRISMA flow diagram showing the study selection process. We extracted results for the following data items: population; disease type; disease state; illness duration; symptom severity; medication use; magnetic field strength; data acquisition and quantitation methods; region of interest; pH; and lactate findings.
Fig. 2.
PRISMA flow chart showing study selection process. This flow chart shows study selection process including identification, screening, eligibility, and inclusion steps
Note that we provided technical details of MRS data acquisition and analysis in the Tables as much as is available in the original manuscripts. Since the level of detail varies between papers, we were not able to provide complete information in each case.
Results
Our systematic search yielded 33 studies in total composed of 7 lactate and 26 pH studies. Lactate studies were published between 2004 and 2017, whereas pH studies were published from 1991 to 2017. The study sample size ranged from 17 to 100 patients. Magnetic field strengths of the studies were between 1.5 and 7 T. Recent studies had higher field strength as expected. Because the study designs, technical details, participants, medication status, and disease state varied markedly, we focused on a qualitative synthesis rather than conducting a meta-analysis.
Lactate
Schizophrenia
Only one recent study [13] examined lactate levels in schizophrenia (Table 1) using 1H-MRS. Data were collected from the anterior cingulate cortex (ACC) of 27 schizophrenia patients (mean illness duration 13.1 years) at 7 T. All but five of the patients were using antipsychotic drugs. Lactate concentrations were elevated in schizophrenia patients and were negatively correlated with general cognitive function and functional capacity. There were no significant relationships between lactate levels and positive/negative symptom severity or chlorpromazine equivalent units.
Table 1.
Schizophrenia and lactate study
| Study | Subjects | Medication | FE/chronic | FS | Method | Region | Lactate |
|---|---|---|---|---|---|---|---|
| Rowland et al. [13] | 27 SZ or SAD 29 NC |
5 MF 22 Med (AP) |
Not mentioned | 7 T | Single-voxel spectroscopy STEAM sequence TE = 14 ms, TR = 3000 ms Voxel size: 30 × 20 × 20 mm3 Quantification: LCModel |
ACC | ↑ |
FE first episode, FS field strength, SZ schizophrenia, SAD schizoaffective disorder, NC normal control, MF medication-free, Med Medicated, AP antipsychotic, STEAM stimulated echo acquisition mode, TE echo time, TR reaction time, LCModel Linear Combination of Model Spectra, ACC anterior cingulate cortex
Bipolar disorder
One study [14] was excluded because of the overlap of subjects with another study [15], which included the same patients, as well as others. Five of the six studies we included reported elevated lactate levels in bipolar patients compared to healthy controls, indicating a distinct pattern (Table 2). The only study [16] that showed a contrary result found no difference during mania but reported a significantly decreased brain lactate levels during euthymia compared to healthy controls. This study was a follow-up study that included only manic bipolar I patients who were all taking medications and were re-examined when they became euthymic.
Table 2.
Bipolar disorder and lactate studies
| Study | Subjects | Medication | FE/chronic | FS | Method | Region | Lactate |
|---|---|---|---|---|---|---|---|
| Machado-Vieira et al. [18] | 20 BD I or II (depressive) 20 NC |
All MF (at least 6 months) |
Illness duration <5 years | 3 T | 2D CSI PRESS sequence TE = 288 ms, TR = 1500 ms Voxel size: 1 × 1 × 2 cm3 Quantification: STEMRW |
Cingulate cortex | ↑ |
| Soeiro-de-Souza et al. [17] | 37 BD I (euthymic) 35 NC |
All medicated | Not mentioned | 3 T | 2D CSI JPRESS sequence Voxel size: 2 × 2 × 4.5 cm3 TE = 31 ms, TR = 1600 ms Quantification: ProFit |
Dorsal ACC | ↑ |
| Xu et al. [19] | 11 BD I (5 depressed + 6 manic) 13 BD II (7 depressed + 6 hypomanic) 20 NC |
All MF (at least 2 weeks) | Not mentioned | 3 T | 2D CSI TE = 30 ms, TR = 1500 ms Sequence: not mentioned Voxel size: 13.75 × 13.75 × 15 mm3 Quantification: LCModel |
ACC, PCC, thalamus | ↑ |
| Chu et al. [20] | 21 BD (16 euthymic + 5 mixt episode) 10 NC |
All medicated | Not mentioned | 4 T | 2D CSI LASER sequence Voxel size: 100 × 80 × 10 mm3 TE = 144 ms, TR = 2000 ms Quantification: LCModel |
ACC, caudate | ↑ |
| Brady et al. [16] | 15 BD I manic (follow-up: 7 euthymic) 6 NC |
All medicated | Not mentioned | 4 T | Single-voxel spectroscopy JPRESS sequence TE = 30–500 ms, TR = 2000 ms Voxel size: 2 × 2 × 2 cm3 Quantification: LCModel |
ACC, POC | Mania → |
| Euthym ↓ | |||||||
| Dager et al. [15] | 28 BD (11 BD I + 17 BD II) (depressed or mixed) 26 NC |
All MF (at least 8 weeks) | Illness duration range: 6 months–33 years | 1.5 T | 2D CSI PEPSI sequence TE = 272 ms, TR = 2 s Voxel size: 1 cm3 Quantification: LCModel |
Frontal lobe, parietal lobe cingulate, caudate putamen, thalamus, insula |
↑ |
BD bipolar disorder, FE first episode, FS field strength, NC normal control, MF medication-free, Med medicated, CSI chemical shift imaging, PRESS point resolution spectroscopy, JPRESS j-resolved point-resolved spectroscopy, LASER localization by adiabatic selective refocusing, TE echo time, RT repetition time, LCModel Linear Combination of Model Spectra, ACC anterior cingulate cortex, PCC posterior cingulate cortex, POC parietal occipital cortex, ProFit prior knowledge fitting, STEMRW Spectroscopy Tool of the Extended MR Workspace R 2.6.3.5, PEPSI proton echo-planar spectroscopic imaging
Disease type
Two studies included only BD I patients [16, 17], three studies included both BD I and BD-II [15, 18, 19], and in one study [20] illness type was not mentioned. The only study that showed reduced lactate levels included only BD I patients. Only a single study [15] compared metabolite levels between BD I and BD-II patients, and found elevated lactate in BD I patients but not in BD II. However, BD I patients had significantly higher scores both in depression and mania scales compared to BD II patients.
Mood state
Three of six studies were uniform in nature regarding mood state [16–18], whereas the other three studies [15, 19, 20] were mixed. Studies that were conducted with a single mood state included either depressive [18], manic [16], or euthymic [17] patients. Among the studies that included patients in different mood states, two [19, 20] mentioned a subgroup analysis for mood state and neither found a correlation between lactate levels and mood state. A follow-up study by Brady et al. [16] compared lactate levels during manic and euthymic periods of patients. During mania, bipolar patients had lactate levels comparable to healthy subjects but during euthymia these levels were significantly reduced.
Medication use
Three studies included only medication-free patients [15, 18, 19] and only one of these [18] included drug-naive patients. The medication-free period ranged between 2 weeks and 6 months in different studies. Studies that did an additional analysis of medication use did not report any difference in lactate levels between drug-free and drug-naive patients, and medication-free and medicated patients. Studies involving medicated patients found no correlation between medication type (mood stabilizer/antipsychotic/antidepressant) and lactate levels. A follow-up study by Machado-Vieira et al. [18] compared baseline and post-treatment (6 weeks of lithium) lactate levels. After lithium treatment, lactate levels were reduced. And this reduction was independent from plasma lithium levels and clinical improvement. One study [14] was excluded because of overlapping of subjects with the study by Dager et al. [15]. Excluded study rescanned 21 bipolar patients after either lithium (n = 12) or valproate (n = 9) treatment and found no significant difference in lactate levels after treatment [14].
Symptom severity
Studies that did an additional analysis [15, 16, 18, 19] showed no correlation between symptom severity and brain lactate levels.
Brain region
The ACC with or without posterior cingulate cortex was the main region of interest in most of the studies. A study [15] with a relatively low field strength investigated a broader area, including the frontal lobe, caudate, putamen, insula, thalamus, parietal, and occipital lobes also showed elevated lactate levels.
pH
Schizophrenia
We excluded a study [21] because 11 of 26 patients were overlapping with the study by Kato et al. [22], which included 27 patients. We identified 13 studies examining pH in schizophrenia and 11 of them reported no difference of brain pH in schizophrenia patients compared to healthy controls (Table 3). A recent study [23] with a relatively higher field strength (4 T) found decreased pH in the frontal lobe of chronically ill patients. This study included patients taking medication and did not show any correlation between antipsychotic dosage and pH levels but they reported a negative correlation between intracellular pH and body mass index (BMI). Another study [24] examined multiple study groups. In the first analysis, clozapine-using patients showed reduced pH levels compared to haloperidol-using patients and healthy controls. In the second analysis, pH did not change when medication-free patients were treated with typical or atypical antipsychotics for 2 weeks. No clear patterns were detected in relation to illness duration, symptomatic status, or brain region in studies of pH in schizophrenia.
Table 3.
Schizophrenia and pH studies
| Study | Subjects | Medication | Fe/chronic | FS (T) | Method | Region | pH |
|---|---|---|---|---|---|---|---|
| Du et al. [23] | 26 SZ or SAD 25 NC |
All medicated | Chronic | 4 | Magnetization transfer MRS Voxel size: 6 × 6 × 4 cm3 Quantification: AMARES |
Frontal lobe | ↓ |
| Shirayama et al. [46] | 11 SZ 15 NC |
All medicated | Chronic | 2 | ISIS sequence TR = 3 s Voxel size: 35 × 45 × 70 mm3 Quantification: in-house method |
PFC | → |
| Riehemann et al. [24] | 51 SZ 32 NC |
8 MF 29 haloperidol 14 clozapine |
Not mentioned | 1.5 | ISIS sequence TR = 3 s Voxel size: 28 × 28 × 50 mm3 Quantification: in-house method |
PFC | Clozapine patients ↓ |
| Volz et al. [47] | 50 SZ 36 NC |
47 medicated 3 MF |
Not mentioned | 1.5 | ISIS sequence TR = 3 s Voxel size: 28 × 28 × 50 mm3 Quantification: in-house method |
Frontal lobe | → |
| Volz et al. [48] | 13 SZ 14 NC |
All medicated | Not mentioned | 1.5 | ISIS sequence TR = 3 s Voxel size: 28 × 28 × 50 mm3 Quantification: in-house method |
DLPFC | → |
| Volz et al. [49] | 60 SZ 36 NC |
10 MF 50 medicated |
Not mentioned | 1.5 | ISIS sequence TR = 3 s Voxel size: 28 × 28 × 50 mm3 Quantification: in-house method |
Frontal lobe | → |
| Kato et al. [22] | 27 SZ 26 NC |
10 MF 17 medicated |
Not mentioned | 1.5 | DRESS sequence TR = 3 s Quantification: in-house method |
Frontal lobe | → |
| Deicken et al. [50] | 20 SZ 16 NC |
6 MF 14 medicated |
Chronic | 2 | TR = 350 ms, TE = 3.5 ms Voxel size:25 cm3 Quantification: in-house method |
Frontal and parietal lobes | → |
| Calabrese et al. [51] | 11 SZ 9 NC |
2 MF 9 medicated |
Chronic | 2 | ISIS sequence TR = 2 s Voxel volume:4.5 × 5.5 × 3.5 cm3 Quantification: in-house method |
Temporal lobe | → |
| Fujimoto et al. [52] | 16 SZ 20 NC |
All medicated | Chronic | 2 | TR = 2 s Voxel size: 3 × 3 × 4 cm3 Quantification: in-house method |
Frontoparietal region | → |
| O’Callaghan et al. [53] | 18 SZ 10 NC |
Medication-free | Chronic | 1.5 | TR = 6 s Quantification: in-house method |
Left temporoparietal region |
→ |
| Pettegrew et al. [54] | 11 SZ 10 NC |
All drug-naive | FE | 1.5 | Voxel size: 20 cm3 TR = 2 s Quantification: GENCAP software |
Dorsal PFC | → |
| Williamson et al. [55] | 10 SZ 7 NC |
All medicated | Chronic | 2 | FROGS pulse sequence TR = 2 s Voxel size: 20 cm3 Quantification: in-house method |
Left DLPFC |
→ |
FE first episode, FS field strength, SZ schizophrenia, SAD schizoaffective disorder, NC normal control, MF medication-free, AP antipsychotic, TE echo time, TR repetition time, ISIS image-selected in vivo spectroscopy, DRESS depth-resolved surface-coil spectroscopy, AMARES advanced method for accurate, robust and efficient spectral fitting, FROGS fast rotating gradient spectroscopy, PFC prefrontal cortex, DLPFC dorsolateral prefrontal cortex
Bipolar disorder
We excluded a study by Kato et al. [25] because 9 of the 10 subjects in that report overlapped with another study by the same group. We also excluded the euthymic patients from a study by Kato et al. [26] in order to avoid duplication because those data were used also in another study by Kato et al. [27]. Four [27–30] of 13 studies reported decreased brain pH in bipolar patients compared to healthy controls (Table 4).
Table 4.
Bipolar disorder and pH studies
| Study | Subjects | Medication | FE/chronic | FS (T) | Method | Region | pH |
|---|---|---|---|---|---|---|---|
| Chouinard et al. [56] | 32 psychosis (17 BD + 4 SZ + 4 SAD) 21 NC |
All medicated | FE | 4 | Magnetization transfer spectroscopy TR = 14 s Voxel volume: 6 × 6 × 4 cm3 Quantification: AMARES |
Frontal lobe | → |
| Du et al. [34] | 20 BD I (all psychotic) 28 NC |
17 medicated | FE | 4 | Voxel size: 6 × 6 × 4 cm3 TR = 14 s Quantification: AMARES |
Frontal lobe | → |
| Shi et al. [36] | 25 BD I (11 depressed + 14 euthymic) 23 NC |
5 MF 20 medicated |
Not mentioned | 3 | MT-ISIS pulse sequence Voxel size: 11 × 8 × 3 cm3 TR = 20.5 s Quantification: AMARES |
Frontal lobe Corpus callosum Thalamus Occipital lobe |
→ |
| Yuksel et al. [57] | 23 BD I (21 euthymic + 2 depressed) 22 NC |
All medicated |
Not mentioned | 4 | TR = 3 s Quantification: AMARES |
Occipital lobe | → |
| Jensen et al. [35] | 11 BD (5 BD I + 6 BD II) (depressive) 9 NC |
All medicated | Not mentioned | 4 | TR = 3 s Voxel volume: 3 × 3 × 3 cm3 Quantification: in-house method |
Whole brain | → |
| Hamakawa et al. [28] | 13 BD 10 NC |
All medicated | Not mentioned | 1.5 | SPECREC sequence TR = 3000 ms Voxel size: 50 × 35 × 30 cm3 Quantification: in-house method |
Basal ganglia Whole brain |
↓ |
| Murashita et al. [31] | 19 BD (13 BD I + 6 BD II) (euthymic) 25 NC |
1 MF 18 medicated |
Not mentioned | 1.5 | TR = 3 s Voxel size: 5 cm3 Processing: OMEGA CSI software Quantification: in-house method |
Occipital lobe | → |
| Kato et al. [29] | 7 BD (4 BD I + 3 BD II) (euthymic) 60 NC |
All MF (at least 10 days) | Not mentioned | 1.5 | DRESS sequence TR = 3 s Quantification: in-house method |
Frontal lobe | ↓ |
| Deicken et al. [32] | 12 BD (euthymic) 16 NC |
All MF | Not mentioned | 2 | TR = 350 ms, TE = 3.5 ms Voxel size: 25 cm3 Quantification: in-house method |
Frontal lobe | → |
| Deicken et al. [33] | 12 BD (euthymic) 14 NC |
All MF (at least 1 week) |
Not mentioned | 2 | TR = 350 ms, TE = 3.5 ms Voxel size: 25 cm3 Quantification: in-house method |
Temporal lobe | → |
| Kato et al. [27] | 40 BD (31 BD I + 9 BD II) (euthymic) 60 NC |
All medicated (all lithium) |
Not mentioned | 1.5 | DRESS sequence TR = 3 s Quantification: in-house method |
Frontal lobe | ↓ |
| Kato et al. [30] | 14 BD I (depressed or euthymic) 15 BD II (depressed, euthymic, or hypomanic) 59 NC |
9 MF 20 medicated |
Not mentioned | 1.5 | DRESS sequence TR = 3 s Quantification: in-house method |
Frontal lobe | BD I euthymic ↓ |
| BD II → | |||||||
| Kato et al. [26] | 17 BD (manic) 17 NC |
All medicated | Not mentioned | 1.5 | DRESS sequence TR = 3 s Quantification: in-house method |
Frontal lobe | → |
BD bipolar disorder, FE first episode, FS field strength, NC normal control, MF medication-free, TE echo time, TR repetition time, MT-ISIS magnetization transfer image-selected in vivo spectroscopy, DRESS depth-resolved surface-coil spectroscopy, AMARES advanced method for accurate, robust and efficient spectral fitting
Disease type
Three [27, 29, 30] out of four studies that reported decreased brain pH were conducted with both bipolar I and II patients, whereas one study [28] did not mention disease subtype. A positive study [27] performed a further analysis and did not find any difference in pH between type I and II patients. There was only a single study [30] that included both types of bipolar disease and reported decreased pH in bipolar I patients but not in bipolar II patients.
Mood state
Seven [26, 27, 29, 31–33] of the studies were uniform in nature regarding mood state, whereas the rest were either mixed or unspecified. Most of the uniform studies were conducted with euthymic patients. All of the four studies with positive results included euthymic patients. Not all the euthymic patients had decreased pH but all the patients who had decreased pH were euthymic. A follow-up study [26] examined patients during a manic episode and re-examined when they became euthymic. Brain pH of manic patients was similar to that of the control group, whereas pH during euthymia decreased compared to both pH of the control group and pH during manic episode. Another follow-up study [30] examined bipolar I patients when they were depressed and euthymic, and found decreased pH while they were euthymic but not depressed.
Medication use
Studies that reported decreased brain pH included patients who were either medication-free, medicated, or both. There was only a single study [30] comparing brain pH of medicated and non-medicated patients. This report concluded that pH was lower in medicated patients than in drug-free patients. Another positive study [27] found no pH difference between patients using antipsychotics or antidepressants and those who were not, however they found a positive correlation between pH and duration of lithium treatment. When the same team investigated the correlation between plasma lithium concentration and brain pH in another study [26] they did not find any correlation. The only study examining lithium effect before and after treatment among six manic patients [26] came up with no significant changes in pH after treatment.
Symptom severity
Most of the studies did not mention performing a subgroup analysis of symptom severity and pH level, and the only three studies that explored manic or depressive symptoms found no correlation to pH.
Brain region
There were seven studies collecting data in the frontal lobe and one study each collecting data in the temporal [33] and occipital lobes [34]. On the other hand, three studies [28, 35, 36] evaluated broader brain areas as detailed in Table 4. The regions of interest included the frontal lobe in all of the studies with a finding of decreased pH.
Discussion
In our systematic review of studies measuring brain lactate and pH in schizophrenia and BD, we found mixed evidence related to bioenergetics abnormalities in both conditions. In BD, 5 out of 6 studies have found elevated lactate levels especially in the cingulate cortex and 4 out of 13 studies reported reduced pH in the frontal lobe. In schizophrenia, a single study has examined lactate and reported elevation, while only 2 out of 13 studies examining pH have reported reduction in this measure.
Mitochondrial and other bioenergetic abnormalities have been found in genetic, postmortem, and neuroimaging studies both in schizophrenia and BD. However, we have yet to put together the greater picture of related and underlying abnormalities of bioenergetic functioning in the brains of patients with schizophrenia and BD. We examined both lactate and pH results, because we consider these two measures to be markers of the same underlying process, specifically, the contributions of glycolysis and oxidative phosphorylation to ATP production. No study to date has collected data on lactate and pH levels together in schizophrenia or BD, with the addition of measures of ATP availability. This review highlights the need for such a study to directly address the proposed lactate–pH hypothesis.
For lactate results, there was only one study in schizophrenia at a very high field strength (7 T), which found elevated lactate levels compared to healthy controls. In this study, elevated ACC lactate level correlated with functioning. This can be interpreted as increased lactate levels reflecting mitochondrial dysfunction causes altered bioenergetics, which may interrupt high-energy-demanding processes like neurotransmission and synaptic plasticity. These altered mechanisms may contribute to cognitive and general functional impairments. In BD, all but one showed increased brain lactate. The study by Brady et al. [16] was the exception as it found no difference in lactate levels between patients and healthy controls when patients were manic. They re-examined the patients when they became euthymic and observed that lactate levels were significantly lower compared to healthy controls. Compared to their euthymic state their lactate levels were higher during mania. Motion artifact can be an issue for all MRS studies of lactate because the lactate resonance at 1.33 ppm is a low-amplitude peak, relatively broad due to J-coupling, mixed with larger resonances of macromolecules and lipids. The low signal-to-noise ratio (SNR) challenge can be compounded by motion, especially in studies of mania.
There were no remarkable patterns concerning the correlation of disease type, mood state, and symptom severity. Most of the studies did not find any difference between medication types. There was no study comparing medicated and medication-free patients. A longitudinal study [18], compared lactate levels before and after lithium treatment (6 weeks of lithium monotherapy), found a significant decrease in cingulate cortex lactate concentrations, which supports the evidence that elevated lactate levels in BD do not arise from medication use. Authors hypothesized that lithium normalizes cell energy metabolism by improving mitochondrial respiration, which leads to reduced lactate production. And these features may contribute to the well-known neuroprotective effects of lithium. A study by Friedman et al. [14] also evaluated patients before and after lithium or valproate treatment and did not find any difference in lactate levels. Both schizophrenia and bipolar studies measuring lactate focused mostly around the cingulate cortex region. Loss of affective control in BD is a key factor and related to the dysfunction of critical structures (e.g., amygdala and caudate) within frontal-subcortical brain networks. Elevated lactate levels observed in ACC and caudate may suggest a possible relationship between affective dysregulation and metabolic abnormalities in these regions but none of the studies showed a correlation between symptom severity and either lactate or pH levels.
The sensitivity of lactate measurement differs across methods and the attribution of a peak to lactate is not straightforward because of lipid contamination from surrounding tissue. Two studies in our review [16, 17] used J-difference spectral lactate editing to address this issue, others [15, 18, 20] used longer echo time while two of them [19, 37] did not mention any lipid suppression method. Data acquisition techniques (single voxel vs chemical shift imaging (CSI)) also can impact lactate measurement. Except for the study by Brady et al. [16], which used single-voxel spectroscopy, all lactate studies used two-dimensional CSI, which provides larger coverage area and higher spatial resolution.
Elevated lactate levels in BD is the most prominent finding of this study. It is should be noted that lactate increase has also been observed in panic disorder (PD). Interventions such as sodium lactate infusion, hyperventilation, visual stimulation all caused greater increase in brain lactate compared to healthy controls [38–40]. More recently it has been suggested that the increased activity-dependent brain lactate accumulation is a trait feature of PD and this increase can be related to altered function of acid-sensitive fear circuits in PD [41].
For studies examining pH in schizophrenia, most of them are published in the 1990s and have lower field strength (1.5 or 2 T). There was only one study performed with 4 T magnetic field, which was also one of two studies that found decreased pH in schizophrenia. Although the study by Riehemann et al. [24] reported a significant pH difference between haloperidol- and clozapine-using patients, longitudinal measures did not confirm this medication effect. This difference may be due to disparate characteristics of patients in these groups. In contrast to schizophrenia, studies in BD, which used higher magnetic field (3 or 4 T), did not find any difference in pH levels. Four studies performed at 1.5 T demonstrated decreased pH especially at frontal lobe and during euthymia.
The study by Du et al. [23] found a negative correlation between BMI and pH levels. This finding may suggest a relationship between brain bioenergetics and peripheral bioenergetic status. Studies have shown that first-episode, drug-naive patients with schizophrenia have higher levels of plasma glucose and insulin levels together with higher insulin resistance [42, 43]. Triglycerides and lipoproteins, such as low-density and high-density lipoproteins, are also abnormal in first-episode, drug-naive patients [44].
The discrepancy of the pH findings might be at least partly due to methodological differences across studies. As seen in Tables 3 and 4, some of the studies used commercial software for quantification, whereas some of them used in-house methods. Intracellular pH is calculated from the chemical shift between PCr and Pi, and the precision of intracellular pH measured by 31P-MRS is dependent on spectral resolution, how many data points are used for data processing, field strength, SNR, and shimming. We calculated an index of pH precision for each study and found a correlation with field strength (data not shown). Thus, data from lower field scanners (1.5 and 2 T) showed larger variation of measurements and lower reliability compared to 3 and 4 T.
Other MR-based approaches can also measure pH in vivo. Recently, 1H MRI pulse sequences have been shown to detect H+ exchange between water and proteins. These techniques include amide proton transfer (APT) and T1 in the rotating frame (T1ρ). APT detects H+ exchange by taking advantage of differences in resonances between amide and water protons. The spin-lock preparation pulse used in T1ρ imaging sensitizes the magnetic resonance (MR) signal to relaxation effects arising from H+ exchange between free water protons and those bound to proteins and macromolecules [45]. These approaches may provide future opportunities for validation of the 31P-MRS results discussed here.
It should be noted that both pH and lactate levels measured by MRS are the average of all cellular components available in the region of interest. Metabolite concentrations can differ between neurons and glial cells and this suggests that the observed findings could be due to differences in cellular composition in schizophrenia and BD. Furthermore, most of the studies did not distinguish white and gray matter differences regarding lactate and pH levels. So, the use of spectroscopic imaging approaches, allowing characterization of gray vs white matter metabolite differences, could additionally contribute to the interpretation of future findings.
This review and our interpretation of findings have certain limitations. First, the literature is small, methods and samples are heterogeneous, and much of it has been acquired at low magnetic field strengths where the intrinsic SNRs are low for both lactate and pH measurements. As seen in all four tables, data acquisition, processing, and quantification methods differ across different studies. Recent studies using modern scanners give higher quality, faster, and more objective results because shimming, scanner adjustments, and water suppression are incorporated into sequence. Second, incomplete reporting of clinical characteristics of patient population (disease duration, disease type, symptom severity, general functionality, etc.) and MRS techniques and procedures hamper our interpretation. Lastly, neuro-metabolite levels and brain pH can be affected by psychotropic medications and several other factors like sleep disturbances, psychological stress, smoking status, peripheral bioenergetic abnormalities, as well as by substances such as caffeine, nicotine, and alcohol. The majority of the studies in this review included patients medicated for various periods of time. Nevertheless, all of the studies with medication-free bipolar patients reported increased lactate, and lactate is decreased by lithium treatment in one study [18]. On the other hand, pH is found to be decreased in a small number of medication-free bipolar patients [29]. A single longitudinal study [26] with a sample size of six compared pre and post lithium treatment pH levels and did not find any changes in pH [27]. Taken together, while the influence of long-term medication cannot be excluded, the literature suggests it is unlikely that lactate abnormalities are solely due to acute medication effects. More studies with medication-free patients which also compare pre- vs post-treatment levels of pH are needed. Additionally, due to its low concentration, lactate measurement with MRS is technically quite challenging and multiple approaches used in different studies to quantify lactate may contribute noise to the literature. Also because of low gyromagnetic ratio of 31P, field strength is critical for good SNR and spectral resolution in 31P-MRS. So, future studies will likely benefit transitioning to high-field scanners.
Overall, our results show that the current literature is limited for now and bears mixed and insufficient findings to drive general conclusions. However, our findings also suggest that a pattern for lactate increase in BD might exist as it has been observed stably in the literature. Therefore, we believe that a thorough investigation of the energetics is necessary and these findings highlight the need for future studies combining 1H-MRS and 31P-MRS approaches, using longitudinal designs to examine lactate and pH in disease progression across both schizophrenia and BDs. Further insights into brain energy mechanisms can potentially lead to novel treatment modalities such as those targeting mitochondrial dysfunction or oxidative phosphorylation.
Acknowledgements
We thank to Dr. Zafer Dogan for his contribution to the manuscript. This review was funded by MH104449(DO) to Dost Ongur, R21MH114020 to Fei Du, NIMH 5T32MH016259(MS) to Virginie-Anne Chouinard and Maria Lorenz Pope Fellowship Award to Cagri Yuksel. No funding body had any involvement in the interpretation of the data, writing of the manuscript or decision to publish.
Competing interests
DÖ served on a Scientific Advisory Board for Neurocrine Inc. in 2017. The reamining authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. WHO Media Centre, Fact Sheets, Mental Health. 2017. http://www.who.int/mediacentre/factsheets/fs396/en/ (accessed on 22 June 2017)
- 2.Swartz MS, Perkins DO, Stroup TS, Davis SM, Capuano G, Rosenheck RA, Investigators C. Effects of antipsychotic medications on psychosocial functioning in patients with chronic schizophrenia: findings from the NIMH CATIE study. Am J Psychiatry. 2007;164:428–36. doi: 10.1176/ajp.2007.164.3.428. [DOI] [PubMed] [Google Scholar]
- 3.Tohen M, Hennen J, Zarate CM, Jr., Baldessarini RJ, Strakowski SM, Stoll AL, Cohen BM. Two-year syndromal and functional recovery in 219 cases of first-episode major affective disorder with psychotic features. Am J Psychiatry. 2000;157:220–8. doi: 10.1176/appi.ajp.157.2.220. [DOI] [PubMed] [Google Scholar]
- 4.Clay HB, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29:311–24. doi: 10.1016/j.ijdevneu.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab. 2012;32:1107–38. doi: 10.1038/jcbfm.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schurr A, West CA, Rigor BM. Lactate-supported synaptic function in the rat hippocampal slice preparation. Science. 1988;240:1326–8. doi: 10.1126/science.3375817. [DOI] [PubMed] [Google Scholar]
- 7.Baltan S. Can lactate serve as an energy substrate for axons in good times and in bad, in sickness and in health? Metab Brain Dis. 2015;30:25–30. doi: 10.1007/s11011-014-9595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruffin VA, Salameh AI, Boron WF, Parker MD. Intracellular pH regulation by acid-base transporters in mammalian neurons. Front Physiol. 2014;5:43. doi: 10.3389/fphys.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagihara H, Catts VS, Katayama Y, Shoji H, Takagi T, Huang FL, Miyakawa T. Decreased brain pH as a shared endophenotype of psychiatric disorders. Neuropsychopharmacology. 2017;43:459–68. doi: 10.1038/npp.2017.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kato T. Mitochondrial dysfunction in bipolar disorder: from 31P-magnetic resonance spectroscopic findings to their molecular mechanisms. Int Rev Neurobiol. 2005;63:21–40. doi: 10.1016/S0074-7742(05)63002-4. [DOI] [PubMed] [Google Scholar]
- 11.Novak J, Wilson M, Macpherson L, Arvanitis TN, Davies NP, Peet AC. Clinical protocols for (3)(1)P MRS of the brain and their use in evaluating optic pathway gliomas in children. Eur J Radiol. 2014;83:e106–12. doi: 10.1016/j.ejrad.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yildiz A, Sachs GS, Dorer DJ, Renshaw PF. 31P Nuclear magnetic resonance spectroscopy findings in bipolar illness: a meta-analysis. Psychiatry Res. 2001;106:181–91. doi: 10.1016/S0925-4927(01)00082-8. [DOI] [PubMed] [Google Scholar]
- 13.Rowland LM, Pradhan S, Korenic S, Wijtenburg SA, Hong LE, Edden RA, Barker PB. Elevated brain lactate in schizophrenia: a 7 T magnetic resonance spectroscopy study. Transl Psychiatry. 2016;6:e967. doi: 10.1038/tp.2016.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman SD, Dager SR, Parow A, Hirashima F, Demopulos C, Stoll AL, Renshaw PF. Lithium and valproic acid treatment effects on brain chemistry in bipolar disorder. Biol Psychiatry. 2004;56:340–8. doi: 10.1016/j.biopsych.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Dager SR, Friedman SD, Parow A, Demopulos C, Stoll AL, Lyoo IK, Renshaw PF. Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry. 2004;61:450–8. doi: 10.1001/archpsyc.61.5.450. [DOI] [PubMed] [Google Scholar]
- 16.Brady RO, Jr., Cooper A, Jensen JE, Tandon N, Cohen B, Renshaw P, Ongur D. A longitudinal pilot proton MRS investigation of the manic and euthymic states of bipolar disorder. Transl Psychiatry. 2012;2:e160. doi: 10.1038/tp.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soeiro-de-Souza MG, Pastorello BF, Leite Cda C, Henning A, Moreno, RA, Garcia Otaduy MC. Dorsal Anterior Cingulate Lactate and Glutathione Levels in Euthymic Bipolar I Disorder: 1H-MRS study. Int J Neuropsychopharmacol. 2016;19. 10.1093/ijnp/pyw032 [DOI] [PMC free article] [PubMed]
- 18.Machado-Vieira R, Zanetti MV, Otaduy MC, De Sousa RT, Soeiro-de-Souza MG, Costa AC, Gattaz WF. Increased brain lactate during depressive episodes and reversal effects by lithium monotherapy in drug-naive bipolar disorder: a 3-T 1H-MRS study. J Clin Psychopharmacol. 2017;37:40–5. doi: 10.1097/JCP.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Dydak U, Harezlak J, Nixon J, Dzemidzic M, Gunn AD, Anand A. Neurochemical abnormalities in unmedicated bipolar depression and mania: a 2D 1H MRS investigation. Psychiatry Res. 2013;213:235–41. doi: 10.1016/j.pscychresns.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu WJ, Delbello MP, Jarvis KB, Norris MM, Kim MJ, Weber W, Adler CM. Magnetic resonance spectroscopy imaging of lactate in patients with bipolar disorder. Psychiatry Res. 2013;213:230–4. doi: 10.1016/j.pscychresns.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Shioiri T, Kato T, Inubushi T, Murashita J, Takahashi S. Correlations of phosphomonoesters measured by phosphorus-31 magnetic resonance spectroscopy in the frontal lobes and negative symptoms in schizophrenia. Psychiatry Res. 1994;55:223–35. doi: 10.1016/0925-4927(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 22.Kato T, Shioiri T, Murashita J, Hamakawa H, Inubushi T, Takahashi S. Lateralized abnormality of high-energy phosphate and bilateral reduction of phosphomonoester measured by phosphorus-31 magnetic resonance spectroscopy of the frontal lobes in schizophrenia. Psychiatry Res. 1995;61:151–60. doi: 10.1016/0925-4927(95)02752-J. [DOI] [PubMed] [Google Scholar]
- 23.Du F, Cooper AJ, Thida T, Sehovic S, Lukas SE, Cohen BM, Ongur D. In vivo evidence for cerebral bioenergetic abnormalities in schizophrenia measured using 31P magnetization transfer spectroscopy. JAMA Psychiatry. 2014;71:19–27. doi: 10.1001/jamapsychiatry.2013.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riehemann S, Hubner G, Smesny S, Volz HP, Sauer H. Do neuroleptics alter the cerebral intracellular pH value in schizophrenics?-a (31)P-MRS study on three different patient groups. Psychiatry Res. 2002;114:113–7. doi: 10.1016/S0925-4927(02)00007-0. [DOI] [PubMed] [Google Scholar]
- 25.Kato T, Takahashi S, Shioiri T, Inubushi T. Brain phosphorous metabolism in depressive disorders detected by phosphorus-31 magnetic resonance spectroscopy. J Affect Disord. 1992;26:223–30. doi: 10.1016/0165-0327(92)90099-R. [DOI] [PubMed] [Google Scholar]
- 26.Kato T, Takahashi S, Shioiri T, Inubushi T. Alterations in brain phosphorous metabolism in bipolar disorder detected by in vivo 31P and 7Li magnetic resonance spectroscopy. J Affect Disord. 1993;27:53–9. doi: 10.1016/0165-0327(93)90097-4. [DOI] [PubMed] [Google Scholar]
- 27.Kato T, Shioiri T, Murashita J, Hamakawa H, Inubushi T, Takahashi S. Phosphorus-31 magnetic resonance spectroscopy and ventricular enlargement in bipolar disorder. Psychiatry Res. 1994;55:41–50. doi: 10.1016/0925-4927(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 28.Hamakawa H, Murashita J, Yamada N, Inubushi T, Kato N, Kato T. Reduced intracellular pH in the basal ganglia and whole brain measured by 31P-MRS in bipolar disorder. Psychiatry Clin Neurosci. 2004;58:82–8. doi: 10.1111/j.1440-1819.2004.01197.x. [DOI] [PubMed] [Google Scholar]
- 29.Kato T, Murashita J, Kamiya A, Shioiri T, Kato N, Inubushi T. Decreased brain intracellular pH measured by 31P-MRS in bipolar disorder: a confirmation in drug-free patients and correlation with white matter hyperintensity. Eur Arch Psychiatry Clin Neurosci. 1998;248:301–6. doi: 10.1007/s004060050054. [DOI] [PubMed] [Google Scholar]
- 30.Kato T, Takahashi S, Shioiri T, Murashita J, Hamakawa H, Inubushi T. Reduction of brain phosphocreatine in bipolar II disorder detected by phosphorus-31 magnetic resonance spectroscopy. J Affect Disord. 1994;31:125–33. doi: 10.1016/0165-0327(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 31.Murashita J, Kato T, Shioiri T, Inubushi T, Kato N. Altered brain energy metabolism in lithium-resistant bipolar disorder detected by photic stimulated 31P-MR spectroscopy. Psychol Med. 2000;30:107–15. doi: 10.1017/S0033291799001439. [DOI] [PubMed] [Google Scholar]
- 32.Deicken RF, Fein G, Weiner MW. Abnormal frontal lobe phosphorous metabolism in bipolar disorder. Am J Psychiatry. 1995;152:915–8. doi: 10.1176/ajp.152.6.915. [DOI] [PubMed] [Google Scholar]
- 33.Deicken RF, Weiner MW, Fein G. Decreased temporal lobe phosphomonoesters in bipolar disorder. J Affect Disord. 1995;33:195–9. doi: 10.1016/0165-0327(94)00089-R. [DOI] [PubMed] [Google Scholar]
- 34.Du F, Yuksel C, Chouinard VA, Huynh P, Ryan K, Cohen BM, Ongur D. Abnormalities in high-energy phosphate metabolism in first-episode bipolar disorder measured using 31P-magnetic resonance spectroscopy. Biol Psychiatry. 2017. 10.1016/j.biopsych.2017.03.025 [DOI] [PMC free article] [PubMed]
- 35.Jensen JE, Daniels M, Haws C, Bolo NR, Lyoo IK, Yoon SJ, Renshaw PF. Triacetyluridine (TAU) decreases depressive symptoms and increases brain pH in bipolar patients. Exp Clin Psychopharmacol. 2008;16:199–206. doi: 10.1037/1064-1297.16.3.199. [DOI] [PubMed] [Google Scholar]
- 36.Shi XF, Carlson PJ, Sung YH, Fiedler KK, Forrest LN, Hellem TL, Kondo DG. Decreased brain PME/PDE ratio in bipolar disorder: a preliminary (31) P magnetic resonance spectroscopy study. Bipolar Disord. 2015;17:743–52. doi: 10.1111/bdi.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim DJ, Lyoo IK, Yoon SJ, Choi T, Lee B, Kim JE, Renshaw PF. Clinical response of quetiapine in rapid cycling manic bipolar patients and lactate level changes in proton magnetic resonance spectroscopy. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1182–8. doi: 10.1016/j.pnpbp.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Layton ME, Friedman SD, Dager SR. Brain metabolic changes during lactate-induced panic: effects of gabapentin treatment. Depress Anxiety. 2001;14:251–4. doi: 10.1002/da.1076. [DOI] [PubMed] [Google Scholar]
- 39.Dager SR, Richards T, Strauss W, Artru A. Single-voxel 1H-MRS investigation of brain metabolic changes during lactate-induced panic. Psychiatry Res. 1997;76:89–99. doi: 10.1016/S0925-4927(97)00066-8. [DOI] [PubMed] [Google Scholar]
- 40.Dager SR, Strauss WL, Marro KI, Richards TL, Metzger GD, Artru AA. Proton magnetic resonance spectroscopy investigation of hyperventilation in subjects with panic disorder and comparison subjects. Am J Psychiatry. 1995;152:666–72. doi: 10.1176/ajp.152.5.666. [DOI] [PubMed] [Google Scholar]
- 41.Maddock RJ, Buonocore MH, Miller AR, Yoon JH, Soosman SK, Unruh AM. Abnormal activity-dependent brain lactate and glutamate+glutamine responses in panic disorder. Biol Psychiatry. 2013;73:1111–9. doi: 10.1016/j.biopsych.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryan MC, Collins P, Thakore JH. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am J Psychiatry. 2003;160:284–9. doi: 10.1176/appi.ajp.160.2.284. [DOI] [PubMed] [Google Scholar]
- 43.Venkatasubramanian G, Chittiprol S, Neelakantachar N, Naveen MN, Thirthall J, Gangadhar BN, Shetty KT. Insulin and insulin-like growth factor-1 abnormalities in antipsychotic-naive schizophrenia. Am J Psychiatry. 2007;164:1557–60. doi: 10.1176/appi.ajp.2007.07020233. [DOI] [PubMed] [Google Scholar]
- 44.Verma SK, Subramaniam M, Liew A, Poon LY. Metabolic risk factors in drug-naive patients with first-episode psychosis. J Clin Psychiatry. 2009;70:997–1000. doi: 10.4088/JCP.08m04508. [DOI] [PubMed] [Google Scholar]
- 45.Magnotta VA, Heo HY, Dlouhy BJ, Dahdaleh NS, Follmer RL, Thedens DR, Wemmie JA. Detecting activity-evoked pH changes in human brain. Proc Natl Acad Sci USA. 2012;109:8270–3. doi: 10.1073/pnas.1205902109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shirayama Y, Yano T, Takahashi K, Takahashi S, Ogino T. In vivo31P NMR spectroscopy shows an increase in glycerophosphorylcholine concentration without alterations in mitochondrial function in the prefrontal cortex of medicated schizophrenic patients at rest. European journal of neuroscience. 2004;20:749–756. doi: 10.1111/j.1460-9568.2004.03524.x. [DOI] [PubMed] [Google Scholar]
- 47.Volz HP, Rzanny R, Rössger G, Hübner G, Kreitschmann-Andermahr I, Kaiser WA, Sauer H. 31Phosphorus magnetic resonance spectroscopy of the dorsolateral prefrontal region in schizophrenics—a study including 50 patients and 36 controls. Biological Psychiatry. 1998;44:399–404. doi: 10.1016/S0006-3223(98)00061-4. [DOI] [PubMed] [Google Scholar]
- 48.Volz HP, Rzanny R, Röβger G, Hübner G, Kreitschmann-Andermahr I, Kaiser WA, Sauer H. Decreased energy demanding processes in the frontal lobes of schizophrenics due to neuroleptics? A 31P-magneto-resonance spectroscopic study. Psychiatry Research: Neuroimaging. 1997;76:123–129. doi: 10.1016/S0925-4927(97)00047-4. [DOI] [PubMed] [Google Scholar]
- 49.Volz HP, Rzanny R, May S, Hegewald H, Preuiβler B, Hajek M, Kaiser WA, Sauer H. 31P magnetic resonance spectroscopy in the dorsolateral prefrontal cortex of schizophrenics with a volume selective technique—preliminary findings. Biological psychiatry. 1997;41:644–648. doi: 10.1016/S0006-3223(96)00062-5. [DOI] [PubMed] [Google Scholar]
- 50.Deicken RF, Calabrese G, Merrin EL, Meyerhoff DJ, Dillon WP, Weiner MW, Fein G. 31Phosphorus magnetic resonance spectroscopy of the frontal and parietal lobes in chronic schizophrenia. Biological psychiatry. 1994;36:503–510. doi: 10.1016/0006-3223(94)90613-0. [DOI] [PubMed] [Google Scholar]
- 51.Calabrese G, Deicken RF, Fein G, Merrin EL, Schoenfeld F, Wiener MW. 31Phosphorus magnetic resonance spectroscopy of the temporal lobes in schizophrenia. Biological Psychiatry. 1992;32:26–32. doi: 10.1016/0006-3223(92)90139-Q. [DOI] [PubMed] [Google Scholar]
- 52.Fujimoto T, Nakano T, Takano T, Hokazono Y, Asakura T, Tsuji T. Study of chronic schizophrenics using 31P magnetic resonance chemical shift imaging. Acta Psychiatrica Scandinavica. 1992;86:455–462. doi: 10.1111/j.1600-0447.1992.tb03297.x. [DOI] [PubMed] [Google Scholar]
- 53.O'Callaghan E, Redmond O, Ennis R, Stack J, Kinsella A, Ennis JT, Waddington JL. Initial investigation of the left temporoparietal region in schizophrenia by 31P magnetic resonance spectroscopy. Biological psychiatry. 1991;29:1149–1152. doi: 10.1016/0006-3223(91)90256-L. [DOI] [PubMed] [Google Scholar]
- 54.Pettegrew JW, Keshavan MS, Panchalingam K, Strychor S, Kaplan DB, Tretta MG, Allen M. Alterations in brain high-energy phosphate and membrane phospholipid metabolism in firstepisode, drug-naive schizophrenics: A pilot study of the dorsal prefrontal cortex by in vivo phosphorus 31 nuclear magnetic resonance spectroscopy. Archives of general psychiatry. 1991;48:563–568. doi: 10.1001/archpsyc.1991.01810300075011. [DOI] [PubMed] [Google Scholar]
- 55.Williamson P, Drost D, Stanley J, Carr T, Morrison S, Merskey H. Localized phosphorus 31 magnetic resonance spectroscopy in chronic schizophrenic patients and normal controls. Archives of general psychiatry. 1991;48:578–578. doi: 10.1001/archpsyc.1991.01810300090013. [DOI] [PubMed] [Google Scholar]
- 56.Chouinard VA, Kim SY, Valeri L, Yuksel C, Ryan KP, Chouinard G, Cohen Bruce M, Du F, Öngür D. Brain bioenergetics and redox state measured by 31P magnetic resonance spectroscopy in unaffected siblings of patients with psychotic disorders. Schizophrenia research. 2017;187:11–16. doi: 10.1016/j.schres.2017.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuksel C, Du F, Ravichandran C, Goldbach JR, Thida T, Lin P, Dora B, Gelda J, O'Connor L, Sehovic S, Gruber S. Abnormal high-energy phosphate molecule metabolism during regional brain activation in patients with bipolar disorder. Molecular psychiatry. 2015;20:1079. doi: 10.1038/mp.2015.13. [DOI] [PubMed] [Google Scholar]