Abstract
Full-term pregnancy early in reproductive life is protective against breast cancer in women. Pregnancy also provides protection in animals against carcinogen-induced breast cancer, and this protection can be mimicked by using the hormones estrogen and progesterone. The molecular mechanisms that form the basis for this protective effect have not been elucidated. On the basis of our results, we propose a cell-fate hypothesis. At a critical period in adolescence the hormonal milieu of pregnancy affects the developmental fate of a subset of mammary epithelial cells and its progeny, which results in persistent differences in molecular pathways between the epithelial cells of hormone-treated and mature virgin mammary glands. These changes in turn dictate the proliferative response to carcinogen challenge and include a block in carcinogen-induced increase in mammary epithelial cell proliferation and an increased and sustained expression of nuclear p53 in the hormone-treated mammary gland. This hormone-induced nuclear p53 is transcriptionally active as evidenced by increased expression of mdm2 and p21 (CIP1/WAF1). Importantly, exposure to perphenazine, a compound that induces mammary gland differentiation but does not confer protection, does not induce p53 expression, indicating that p53 is not a differentiation marker. The proliferative block and induction of p53 are operative in both rats and mice, results that support the generality of the proposed hypothesis.
The lifetime risk of developing breast cancer among Western women is ≈10%, and despite advances in therapeutic strategies, breast cancer remains the leading cause of cancer deaths in women in most developed countries (1). Prevention of the disease can be achieved with better understanding of the etiological factors contributing to the development of the disease. There is significant evidence that the timing of normal developmental events like menarche, menopause, and age of first parity have a significant impact on an individual's susceptibility to breast cancer (2, 3). In particular, there is strong epidemiological evidence that women who experience a full-term pregnancy early in their lives have a significantly reduced risk for developing breast cancer (3–5). This is recapitulated in rat models that demonstrate that early full-term pregnancy confers resistance to chemical carcinogen-induced mammary tumorigenesis (6–11). This protection can be mimicked with the hormones estrogen (E) and progesterone (P; refs. 9 and 12) or human CG (13) given either before or immediately after carcinogen challenge to induce a refractory state.
Despite a wealth of literature supporting the role of endocrinological processes in mediating parity-related refractoriness, the cellular and molecular mechanisms that underlie hormone-induced refractoriness are largely unresolved. The utility of the rodent models in which a defined hormonal regimen can be used to mimic the protective effect of pregnancy is well documented (9–17).
Differing hypotheses to explain the protective effects have been proposed (7–10). We have shown that one of the earliest events to occur in the hormone-treated gland is a block to proliferation upon carcinogen challenge (9, 18). On the basis of our published results and the results described in this article we propose a cell-fate hypothesis that hypothesizes that at a critical period in adolescence the hormonal milieu of pregnancy affects the developmental fate of a subset of mammary epithelial cells. The consequence of hormone exposure is the induction of persistent differences in molecular pathways between the epithelial cells in the hormone-treated and mature mammary gland that dictate the proliferative responses upon a carcinogenic insult. Additional data to support this hypothesis have been published recently (19–21).
The tumor suppressor protein p53 protein, which is situated in the hub of cellular DNA damage pathways in higher organisms, is central to the cellular response to a variety of potentially damaging extracellular stimuli including UV light, γ-irradiation, chemical carcinogens, and chemotherapeutic agents (22, 23). Depending on the insult it can evoke cell cycle arrest (24–28), DNA repair (29), or apoptosis (30, 31) through sequence-specific and nonsequence-specific DNA binding and through both positive and negative mechanisms (32). p53 thus maintains the integrity of the genome. The p53 tumor suppressor gene is commonly mutated or altered in expression in human breast tumors (33). Germ line mutations in p53 have been shown to segregate with the Li-Fraumeni cancer susceptibility syndrome (34), which confers on women an increased risk of developing breast cancer compared with the general population (34, 35). In addition mutations in p53 have been detected in over 40% of spontaneous breast carcinomas (36, 37). Deregulation of wild-type p53 also has been shown to play a role in breast cancer. These observations implicate p53 not only in the pathogenesis of the disease but in the susceptibility to it as well.
Because p53 is hormonally responsive (38) and the principal hormones of pregnancy are E and P, we determined the spatial and temporal expression and functional status of p53 in the rat model of hormone-induced protection. We demonstrate that early exposure to pregnancy levels of E and P can induce and sustain chronic nuclear p53 expression such that upon carcinogen challenge p53-dependent responses are elucidated. We further demonstrate that p53 is not induced by perphenazine (PPZ) and hence is not a differentiation marker of the mammary epithelium. The hormone-induced nuclear sequestration of p53 and the block to proliferation observed upon carcinogen challenge in the rat was recapitulated also in the mouse model.
Materials and Methods
Animals.
Thirty-five-day-old female virgin and five-day-timed pregnant Wistar–Furth (WF) rats were purchased from Harlan Breeders (Indianapolis). Animals were housed by using approved American Association of Laboratory Animal Care guidelines under conditions of a 12-h light/dark cycle and permitted ad libitum access to food and water. All experiments were performed in accordance with National Institutes of Health guidelines for experimental animals. Thirty-five-day-old BALB/c females were obtained from breeding pairs in our facility; 6–8-week-old virgins were mated with mature male mice.
Hormonal Manipulations.
Forty-five-day-old WF rats were treated with a defined hormonal regimen to mimic the protective effects of early full-term pregnancy as described previously (9). The preparation and administration of methylnitroso urea (MNU) to induce mammary tumors also has been described. Briefly, 45-day-old WF rats were primed with a 0.1-ml solution of 2.5 μg of estradiol benzoate s.c. to synchronize estrous within and between groups. Three days later the rats were treated with 20 μg of E and 20 mg of P delivered via beeswax pellets implanted s.c. Pellets were replaced after 10 days to provide hormonal stimulation for a total of 21 days. Control age-matched virgin (AMV) animals received blank pellets. After 21 days of hormone stimulation, the pellets were removed, and the mammary glands were allowed to regress for 28 days. On day 97 the animals were administered 50 mg/kg MNU i.p., and mammary tissues were collected at suitable experimental time points. Experiments were repeated three times with five animals per time point in each group.
To distinguish between the processes involved in differentiation and protection caused by E/P, 45-day-old WF rats were treated with PPZ by using a procedure described by Nandi and coworkers (11). Rats were divided into two groups of five animals each: those receiving PPZ and controls. Rats receiving PPZ received a s.c. injection of PPZ (Sigma; 5 mg/kg in 0.03 M HCl) five times per week for a period of 3 weeks. Control animals received 0.03 M HCl alone. At the end of the treatment period the rats were rested again for 28 days to allow the mammary glands to involute.
Five-day-pregnant WF rats and age-matched controls (five each) were obtained from Harlan Breeders. The pregnant rats were allowed to nurse their pups for 7 days, after which the pups were weaned. The mammary glands of the mothers were allowed to involute for 28 days, at which time point they were collected for immunohistochemical staining of p53.
BALB/c mice were treated with a similar hormonal regimen as described for WF rats except that the mice were not primed with estradiol benzoate. E and P concentrations in the beeswax pellets were 50 μg of E and 20 mg of P. The carcinogen used to induce tumors in mice was 7,12-dimethybenz(α)anthracene (DMBA). Each animal received 1 mg of DMBA prepared in cottonseed oil by oral gavage on day 97.
Tissue Collection and Immunohistochemistry (IHC).
The no. 4 abdominal glands (rats and mice) were used for all experiments. Mammary tissues were fixed in 10% neutral buffered formalin for p53 and mdm2 IHC and 4% paraformaldehyde for p21 IHC. Tissues were paraffin-embedded and sectioned. Then 5-μm sections were deparaffinized and subjected to antigen retrieval. Sections were incubated at room temperature for 3 h with rabbit polyclonal CM5 antisera (NovoCastra, Newcastle, U.K.). Each batch of antibody was titrated before use and normally used at 1:250 to 1:500 dilution. p21 IHC in rat tissues was done by using a mouse monoclonal antibody kindly provided by Dr. Ed Harlow (Massachusetts General Hospital Cancer Center, Charlestown, MA). p21 IHC in mouse tissues was assessed by using Ab-5 (1:50; Oncogene Research, Cambridge, MA). Tissues were incubated overnight at 4°C for each p21 antibody. The MDM2 antibody used for immunostaining is the mouse monoclonal antibody SMP14 (Santa Cruz Biotechnology). Immunocomplexes were visualized by using appropriate secondary antibodies and by using the ABC method (Vector Laboratories). Sections were counterstained by using hematoxylin. Tissue sections were incubated without either the primary or secondary antibodies to test for the specificity of each antibody.
Cell Proliferation Analysis in BALB/c Mice Using BrdUrd.
At various time points after DMBA treatment, mice were injected i.p. with BrdUrd (Sigma; 30 mg/kg of body weight) 2 h before killing. The no. 4 abdominal gland was removed, fixed in 4% paraformaldehyde for 2 h at 4°C, washed and saved in 70% ethanol, and then processed and sectioned (5 μm) for BrdUrd IHC. BrdUrd was detected by using the BrdUrd in situ detection kit (PharMingen). There were four mice per time point (97 days, 3 days DMBA, 6 days DMBA, and 10 days DMBA) in each group. Approximately 5,000 cells were counted per group at each time point. The mean ± SEM was determined, and differences between groups were tested for statistical significance by using the two-sided Student's t tests. Results were considered significantly different at P < 0.05.
Results
Localization and Expression of p53 upon Hormone Treatment in the Rat Mammary Gland.
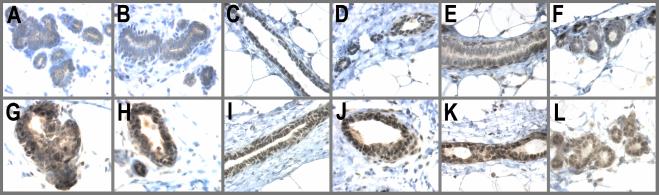
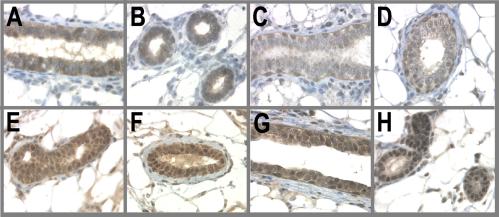
An earlier study has shown that BALB/c mice transiently treated with the hormones human chorionic gonadotropin and pregnant mare serum gonadotropin for 6–12 h resulted in the nuclear accumulation of p53 (38). In this study we wanted to determine whether the pregnancy hormones E and P were able to induce and sustain p53 expression. The results of this analysis clearly demonstrate a striking increase in both the levels and nuclear accumulation of p53 in E/P-treated animals compared with AMV. This increase occurred as early as 3 days after E/P treatment (Fig. 1, G and H). Most importantly, this expression was sustained through the 21 days of hormone stimulation and 28 days of involution; namely, p53 was still present at the time of carcinogen challenge (Fig. 1, I and J). This expression persisted through 3 days after MNU treatment (Fig. 1, K and L) but decreased by day 8 post-MNU treatment (data not shown). Thus, the changes in p53 protein levels and nuclear localization preceded the observed increase in proliferation that is induced by MNU in the AMV but that is blocked by E/P.
Figure 1.
p53 is a hormonally regulated target gene. Animals were treated by the experimental regime described in Materials and Methods. The no. 4 abdominal gland was excised either 3 days after E/P treatment, on day 97, or 3 days after carcinogen administration. Tissues were fixed in 10% neutral buffered formalin and stained for p53. (A and B) AMV, 3 days E/P; (C and D) AMV 97 days; (E and F) AMV, 3 days MNU; (G and H) E/P-treated, 3 days E/P; (I and J) E/P-treated, 97 days; (K and L) E/P-treated, 3 days MNU.
Hormones Induce p53 Activity in the Mammary Gland.
It has been shown in a variety of cell lines and tumors that cytoplasmic sequestration of p53 impairs the ability of p53 to transactivate downstream target genes. Some of the gene targets up-regulated by p53 include p21, MDM2, GADD45, and 14–3-3σ. Therefore, to address the functional status of the hormone-induced p53, we examined the expression of a well characterized downstream target of p53, namely p21 (CIP1/WAF1). Fig. 2 shows that p21 is induced in the E/P-treated mammary glands upon carcinogen challenge but is absent in the AMV. Because p53 also activates the MDM2 promoter, we examined levels of mdm2 in the hormone-treated glands and found mdm2 protein expression also to be elevated (data not shown).
Figure 2.
p21 is a potential downstream mediator of p53 function in hormone-exposed mammary glands. Because p21 is a direct target of p53 action and is a cell cycle inhibitor, p21 expression was examined in the mammary glands of hormone-treated and AMV glands 3 days post-MNU by IHC. (A and B) AMV, 3 days post-MNU; (C and D) E/P-treated, 3 days post-MNU.
Differentiation and p53 Expression.
PPZ is a dopamine receptor inhibitor that causes the acute release of prolactin from the anterior pituitary of rats by blocking the inhibitory influence of dopamine from the hypothalamus. This release of prolactin causes proliferation and differentiation of the mammary gland to a near lactational state after a short period of treatment. Despite inducing morphological and functional differentiation of the mammary gland, PPZ is ineffective in offering protection from MNU-induced carcinogenesis (11). Therefore we used PPZ to test the specificity of the p53 expression that we had observed with E/P treatment.
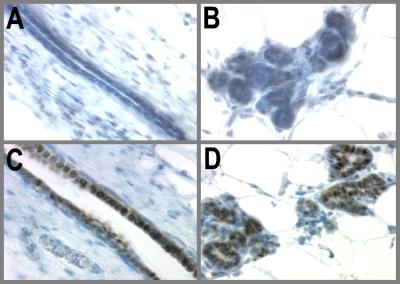
Rats received one injection of PPZ (s.c., 0.5 mg/100 g of body weight) five times a week for 3 weeks. Control animals received one injection of 0.03 N HCl five times for 3 weeks. Mammary glands were allowed to involute for 28 days. Tissues were collected over several time points after PPZ treatment, but only the data for day 97 is shown. Fig. 3 clearly shows that p53 expression in PPZ-treated glands is indistinguishable from the AMV. In both groups p53 staining was very weak and cytoplasmic. Although p53 is induced strongly by E and P, the absence of p53 induction by PPZ clearly indicates that p53 is not a differentiation marker and only occurs after E/P treatment (or pregnancy).
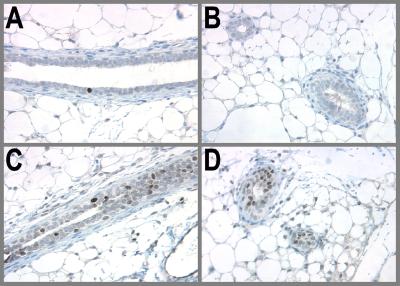
Figure 3.
Treatment with the differentiating agent PPZ does not induce p53 expression. PPZ induces morphological and functional differentiation of the mammary gland but is ineffective in offering protection from MNU-induced carcinogenesis. Rats received one injection of PPZ (s.c, 0.5 mg/100 g of body weight) five times a week for 3 weeks. Control animals received one injection of 0.03 N HCl five times a week for 3 weeks. Glands were allowed to involute for 28 days and then collected for immunohistochemical staining of p53. (A and B) AMV, 97 days; (C and D) PPZ-treated, 97 days.
Pregnancy and p53 Expression.
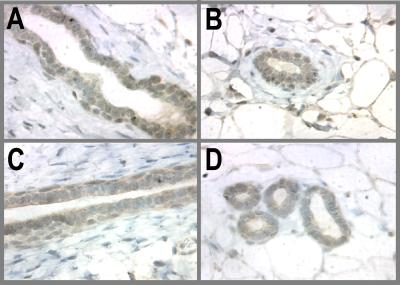
Pregnancy is far more complex than the use of just E/P alone to induce a refractory state. However, because these studies to address the mechanism of protection were initiated on the basis of epidemiological data that an early full-term pregnancy is protective against breast cancer, we examined the p53 status in synchronized pregnant involuted rats compared with that of an age-matched cohort of animals. Pregnant rats were allowed to nurse their pups for 7 days, and the glands were allowed to involute for 28 days and p53 expression was examined. Similar to E/P treatment, the pregnant-involuted mammary glands expressed higher levels of p53 as compared with the AMVs (Fig. 4). This observation further strengthens the notion that p53 is a potential mediator of hormone-induced refractoriness to breast cancer.
Figure 4.
Pregnancy and p53 expression. 5-day-pregnant WF rats and age-matched controls (five each) obtained from Sprague–Dawley, Inc. were allowed to deliver and nurse their pups for 7 days, after which the pups were weaned. The mammary glands of the mothers were allowed to involute for 28 days, at which time point they were collected for immunohistochemical staining of p53. (A and B) AMV; (C and D) pregnant-involuted.
p53 Expression in a Mouse Model for Hormone-Induced Protection Against Breast Cancer.
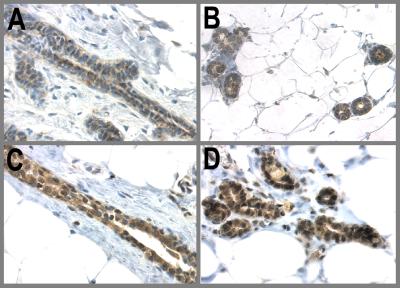
It has been demonstrated that the involuted mammary gland of parous mice exhibits refractoriness to chemical carcinogenesis as compared with the AMV (16). We therefore examined p53 expression in hormone-treated involuted BALB/c mice. After 21 days of hormonal stimulation the mammary glands were allowed to regress for 28 days, and the animals were administered 1 mg of DMBA orally. Control mice received only DMBA. The time points at which tissues were collected were day-97 and 3, 6, and 10 days post-DMBA. Fig. 5 illustrates these results. As in WF rats nuclear p53 expression was induced and sustained in the hormone-treated involuted mammary glands both at the time of carcinogen challenge (Fig. 5 E and F) and 6 days post-DMBA (Fig. 5 G and H). The AMV glands showed weaker cytoplasmic staining of p53 (Fig. 5 A–D). p21 was induced only in the hormone-treated animals after DMBA treatment (Fig. 6), indicating that the p53 was functionally active. In conclusion, the functional state of p53 in the mammary epithelium is regulated by hormonal stimuli both in rats and mice.
Figure 5.
Sustained expression of p53 in hormone-treated mammary glands of BALB/c mice. BALB/c mice were treated with hormones by the experimental regimen described in Materials and Methods. On day 97 they were administered 1 mg of DMBA by oral gavage. The expression and localization of p53 were examined by IHC in the AMV and hormone-treated mammary glands both at the time of (97 day) and 6 days after DMBA treatment. (A and B) AMV, 97 days; (C and D) AMV, 6 days DMBA; (E and F) E/P-treated, 97 days; (G and H) E/P-treated, 6 days DMBA.
Figure 6.
p21 expression in AMV and EP-treated BALB/c mammary glands 6 days post-DMBA. DNA damage activates p53, which in turn would mediate cell cycle arrest by up-regulation of p21 and other genes. We therefore examined p21 expression by IHC in hormone-treated and AMV BALB/c mice 6 days after DMBA challenge. (A and B) AMV, 6 days DMBA; (C and D) E/P-treated, 6 days DMBA.
Cell Proliferation Analysis in Hormone-Treated and Age-Matched Control BALB/c Mice.
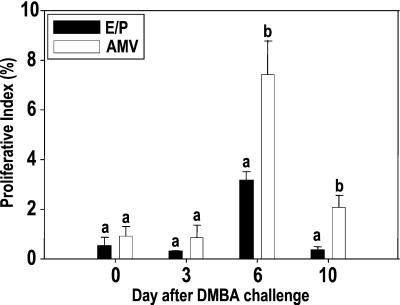
Cell proliferation and cell death are two coordinately regulated processes during mammary gland development and during involution and remodeling. A differential regulation of these processes could explain the difference in sensitivity to carcinogenesis for the hormone-exposed and mature virgin mammary gland. We have shown earlier that in WF rats that at 97 days the proliferation (BrdUrd labeling index) is low in both groups. However, at day 8 post-MNU treatment, the BrdUrd labeling index was still low in the parous-involuted gland and the E/P-treated involuted gland but significantly higher in the AMV. Consequently, we performed a similar analysis by using BALB/c mice and DMBA as the carcinogen. Fig. 7 shows the results of this analysis. On day 97 (day of carcinogen challenge) the proliferation is low in both the hormone-treated (0.50%) and AMV (0.91%) mice. It remained low in both groups 3 days after DMBA challenge (0.28 and 0.86%, respectively). However, at day 6 post-DMBA challenge, the BrdUrd labeling index in the E/P-treated involuted gland was lower (3.16%) than that observed in the AMV (7.41%, P < 0.05). At day 10 post-DMBA the proliferation dropped in both groups but still remained higher in the AMV (2.07%) than the E/P-treated involuted gland (0.33%, P < 0.05). Thus the proliferative block induced by early exposure to hormones upon carcinogen challenge also holds true in mice. The same molecular mechanism is likely to be operative to induce this proliferative block in all animals that have been treated with hormones.
Figure 7.
Proliferation block in hormone-treated BALB/c mice 6 and 10 days after DMBA challenge. BALB/c mice were treated with the hormonal regimen described. On day 97 the control and hormone-treated mice were given 1 mg of DMBA by oral gavage. The mammary glands were collected 0, 3, 6, and 10 days post-DMBA administration. BrdUrd (30 mg/kg of body weight) was administered to the animals 2 h before killing. Cell proliferation in the virgin and hormone-treated glands before and after DMBA treatment was analyzed by BrdUrd IHC. The percentage of BrdUrd-labeled cells was plotted against days after DMBA treatment. Each value represents mean ± SEM. Differences between groups were tested for statistical significance by using the paired Student's t tests. Different superscripts indicate that the values are statistically different within a group.
Discussion
The E/P-treated rat is an established model to study the mechanisms of parity-induced protection against mammary cancer. Despite a wealth of literature supporting the role of endocrinological processes in mediating parity-related refractoriness, the cellular and molecular mechanisms that underlie hormone-induced refractoriness are largely unresolved. Several cellular mechanisms have been proposed for the hormone-induced protection of breast cancer. These include a differentiation hypothesis, in which it is proposed that differentiation of the mammary gland, similar to that induced by pregnancy or hormones, results in the removal of a population of cancer-susceptible cells (present in terminal end buds) and hence confers protection against breast cancer (39–41). A corollary to this hypothesis is that differences in susceptibility to carcinogen-induced tumorigenesis between parous and AMV glands could be explained by the differences in proliferation indices, alterations of the properties associated with carcinogen uptake, binding and metabolism, and an enhanced capacity for DNA repair (40–44). According to an alternative model, the refractory state is intrinsic to the host and is mediated by an altered hormonal milieu in the parous rat mammary gland hormonal environment that results in persistent biochemical alterations in the mammary epithelia (10, 11). These models may not be mutually exclusive. However, several published studies have indicated that protection is independent of the level of differentiation achieved by parity or hormonal stimulation (8, 9, 45). Furthermore, other studies have shown that there is no consistent difference in cellular kinetics between parous and nonparous animals (8, 9).
On the basis of our published results (9, 18–20) and the results described in this article, we developed a cell-fate hypothesis that proposes that at a critical period in adolescence the hormonal milieu of pregnancy affects the developmental fate of a subset of mammary epithelial cells. The consequence of hormone exposure is the induction of some persistent differences in signal transduction and/or gene expression between the epithelial cells in the hormone-treated and the mature virgin mammary gland, leading to permanent changes in cell fate that determine the subsequent proliferation and response of the gland in the face of carcinogen stress. In support of this hypothesis we have shown that an early cellular change observed in mammary cells of the hormone-involuted gland is a proliferation block upon MNU treatment (9). This proliferative block seems to involve the hormone receptor-positive cells because although the majority of the proliferating cells in a mature virgin gland are estrogen receptor-positive (note this is exactly opposite to the immature gland), the few proliferating cells in the hormone treated gland are estrogen receptor-negative. Coexpression of steroid receptor and proliferation may be an early manifestation of an important molecular change in steroid receptor-dependent regulation of proliferation that increases susceptibility to tumor formation (18). At 40 days postcarcinogen treatment there is an increased frequency of estrogen receptor-positive cells in the mammary gland of the AMV and not in the parous rat (D.M., unpublished data). Hormones block this manifestation.
In the current study we show that an important molecular alteration that has occurred in the hormone-treated gland is the induction, sustained expression, activation, and nuclear sequestration of p53 protein. This change was persistent and present at the time of MNU treatment. The p53 protein seems functionally active, because its induction is accompanied by an increase in p21, a key downstream target of p53. Normally, wild-type p53 protein is degraded rapidly, has a short half-life, and maintains a low intracellular level. p53 protein expression is tightly regulated by control of protein production (mRNA production, stability, and efficiency of translation) and by control of its degradation by ubiquitin-mediated proteolysis. In our model stability is unlikely to occur at the level of transcription, because we have shown there is no change in message levels of p53 between the hormone-treated and AMV mammary glands (9). Stability is probably regulated at the posttranscriptional level.
By using an experimental paradigm described earlier by Guzman et al. (11) and recently used in a study by Ginger et al. (20), we show that p53 is not a marker of epithelial cell differentiation per se. Forty-five-day-old WF rats were treated with PPZ for a period of 3 weeks. This treatment results in the increased serum levels of prolactin and P, but not E, which permits differentiation but does not confer protection against carcinogenesis. Earlier studies have shown that similar morphological changes were observed under hormone and PPZ treatment when compared with the AMVs. Thus, although morphologically the hormone-treated and PPZ-treated glands are similar (20), only the hormone-treated gland shows sustained expression of p53 and indicates that p53 induction is not simply a consequence of mammary differentiation.
On the basis of our experimental results, we hypothesize that one mechanism by which hormone-induced protection occurs is the activation of p53 such that upon DNA damage induced by MNU, p53 is functionally active to promote cell cycle arrest and provide temporal assistance for DNA repair. Given the obvious implications of p53 response to mammary tumorigenesis and a recent publication showing p53-dependent global genomic repair of carcinogen-induced DNA adducts in human cells (29), future investigation will focus on the mechanism by which hormones stabilize p53 protein. On the basis of our current findings we hypothesize that E/P either stabilizes p53 expression by posttranslational modifications that alter both its stability and cellular localization and/or E/P modifies the concentration and/or spatiotemporal regulation of one or more established mediators of p53 stability.
There is ample precedence to the important role and state of p53 in both normal mammary gland development (38) and mammary tumorigenesis (46–48). In the normal mammary gland of the mouse, wild-type p53 protein is expressed in the ductal epithelium of the quiescent gland. It was found sequestered within the cytoplasm and unresponsive to ionizing radiation, indicating that p53 was inactive. After short term treatment with placental hormones, exposure to ionizing radiation caused p53 to translocate and accumulate in the nucleus with the induction of p21 and apoptosis (38). Animal models have demonstrated also the potential importance of p53 status in mammary tumorigenesis (46–48). Absence of p53 is sufficient to cause spontaneous development of mammary tumors. Hormone stimulation via pituitary isografts further enhances tumorigenicity of p53 null mammary epithelium to a greater extent than exposure to carcinogen alone. These data provide evidence that p53 plays a pivotal role in regulating hormone-induced molecular processes both in normal development of the mammary gland and in mammary tumorigenesis.
Finally, our data also proves the validity for mouse models to study parity-induced protection against carcinogens. We have shown recently that mice also demonstrated pregnancy-induced resistance to chemical carcinogen-induced breast cancers (16). We now have shown that p53 is activated in both rats and mice in response to E and P, and this expression is sustained to induce p21 upon carcinogen challenge. Furthermore, the proliferative block observed in hormone-treated rats (9) upon carcinogen challenge also is operational in the mouse. With respect to epigenetic changes that occur upon hormonal stimulation, recent studies examining global sequence expression as a function of the hormone-induced refractory state in both the mouse and the rat showed marked similarities in the type and magnitude of the altered genes (20, 21). The capacity to use transgenic and gene knockout mouse models to test the causal role of players in the pathway of E/P-induced refractoriness provides an additional powerful approach toward understanding this important phenomenon.
Acknowledgments
This work was supported by National Cancer Institute Grant PO1CA64225.
Abbreviations
- WF
Wistar–Furth
- MNU
methylnitroso urea
- E
estrogen
- P
progesterone
- AMV
age-matched virgin
- PPZ
perphenazine
- DMBA
7,12-dimethybenz(α)anthracene
- IHC
immunohistochemistry
References
- 1.Pisani P, Parkin D M, Bray F, Ferlay J. Int J Cancer. 1999;83:18–29. doi: 10.1002/(sici)1097-0215(19991210)83:6<870::aid-ijc35>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Martin A M, Weber B L. J Natl Cancer Inst. 2000;92:1126–1135. doi: 10.1093/jnci/92.14.1126. [DOI] [PubMed] [Google Scholar]
- 3.Rosner B, Colditz G A, Willett W C. Am J Epidemiol. 1994;139:819–835. doi: 10.1093/oxfordjournals.aje.a117079. [DOI] [PubMed] [Google Scholar]
- 4.MacMahon B, Cole P, Lin T M, Lowe C R, Mirra A P, Ravnihar B, Salber E J, Valaoras V G, Yuasa S. Bull WHO. 1970;43:209–221. [PMC free article] [PubMed] [Google Scholar]
- 5.Kelsey J L, Gammon M D. Ca Cancer J Clin. 1991;41:146–165. doi: 10.3322/canjclin.41.3.146. [DOI] [PubMed] [Google Scholar]
- 6.Moon R C. Int J Cancer. 1969;4:312–317. doi: 10.1002/ijc.2910040308. [DOI] [PubMed] [Google Scholar]
- 7.Russo J, Russo I H. Am J Pathol. 1980;100:497–512. [PMC free article] [PubMed] [Google Scholar]
- 8.Sinha D K, Pazik J E, Dao T L. Br J Cancer. 1988;57:390–394. doi: 10.1038/bjc.1988.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sivaraman L, Stephens L C, Markaverich B M, Clark J A, Krnacik S, Conneely O M, O'Malley B W, Medina D. Carcinogenesis. 1998;19:1573–1581. doi: 10.1093/carcin/19.9.1573. [DOI] [PubMed] [Google Scholar]
- 10.Thordarson G, Jin E, Guzman R C, Swanson S M, Nandi S, Talamantes F. Carcinogenesis. 1995;16:2847–2853. doi: 10.1093/carcin/16.11.2847. [DOI] [PubMed] [Google Scholar]
- 11.Guzman R C, Yang J, Rajkumar L, Thordarson G, Chen X, Nandi S. Proc Natl Acad Sci USA. 1999;96:2520–2525. doi: 10.1073/pnas.96.5.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grubbs C J, Farnell D R, Hill D L, McDonough K C. J Natl Cancer Inst. 1985;74:927–931. [PubMed] [Google Scholar]
- 13.Russo I H, Koszalka M, Russo J. J Natl Cancer Inst. 1990;82:1286–1289. doi: 10.1093/jnci/82.15.1286. [DOI] [PubMed] [Google Scholar]
- 14.Russo I H, Koszalka M, Russo J. Br J Cancer. 1991;64:481–484. doi: 10.1038/bjc.1991.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russo I H, Koszalka M, Russo J. Carcinogenesis. 1990;11:1849–1855. doi: 10.1093/carcin/11.10.1849. [DOI] [PubMed] [Google Scholar]
- 16.Medina D, Smith G H. J Natl Cancer Inst. 1999;91:967–969. doi: 10.1093/jnci/91.11.967. [DOI] [PubMed] [Google Scholar]
- 17.Grubbs C J, Juliana M M, Whitaker L M. Anticancer Res. 1988;8:113–117. [PubMed] [Google Scholar]
- 18.Sivaraman, L., Zhong, L., Hilsenbeck, S., Gay, J., Conneely, O. M., Medina, D. & O'Malley, B. W. (2001) J. Endocrinol., in press. [DOI] [PubMed]
- 19.Medina D, Sivaraman L, Hilsenbeck S G, Conneely O M, Ginger M, Rosen J, O'Malley O W. Strange International Cancer Prevention Conference, New York, NY. 2001. [DOI] [PubMed] [Google Scholar]
- 20.Ginger, M. G., Gonzalez-Rimbau, M. F., Gay, J. P. & Rosen, J. M. (2001) Endocrinogy, in press. [DOI] [PubMed]
- 21.Chodosh L A, D'Cruz C M, Gardner H P, Ha S I, Marquis S T, Rajan J V, Stairs D B, Wang J Y, Wang M. Cancer Res. 1999;59:1765–1772s. [PubMed] [Google Scholar]
- 22.Liu Y, Kulesz-Martin M. Carcinogenesis. 2001;22:851–860. doi: 10.1093/carcin/22.6.851. [DOI] [PubMed] [Google Scholar]
- 23.Stewart Z A, Pietenpol J A. Chem Res Toxicol. 2001;14:243–263. doi: 10.1021/tx000199t. [DOI] [PubMed] [Google Scholar]
- 24.Kastan M B, Zhan Q, el-Deiry W S, Carrier F, Jacks T, Walsh W V, Plunkett B S, Vogelstein B, Fornace A J., Jr Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal M L, Agarwal A, Taylor W R, Stark G R. Proc Natl Acad Sci USA. 1995;92:8493–8497. doi: 10.1073/pnas.92.18.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aloni-Grinstein R, Schwartz D, Rotter V. EMBO J. 1995;14:1392–1401. doi: 10.1002/j.1460-2075.1995.tb07125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith M L, Chen I T, Zhan Q, Bae I, Chen C Y, Gilmer T M, Kastan M B, O'Connor P M, Fornace A J., Jr Science. 1994;266:1376–1380. doi: 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- 28.el-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 29.Lloyd D R, Hanawalt P C. Cancer Res. 2000;60:517–521. [PubMed] [Google Scholar]
- 30.Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. Nature (London) 1991;352:345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- 31.Miyashita T, Krajewski S, Krajewska M, Wang H G, Lin H K, Liebermann D A, Hoffman B, Reed J C. Oncogene. 1994;9:1799–1805. [PubMed] [Google Scholar]
- 32.Ko L J, Prives C. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 33.Hollstein M, Sidransky D, Vogelstein B, Harris C C. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 34.Malkin D, Li F P, Strong L C, Fraumeni J F, Jr, Nelson C E, Kim D H, Kassel J, Gryka M A, Bischoff F Z, Tainsky M A, et al. Science. 1990;250:1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- 35.Varley J M, McGown G, Thorncroft M, Santibanez-Koref M F, Kelsey A M, Tricker K J, Evans D G, Birch J M. Cancer Res. 1997;57:3245–3252. [PubMed] [Google Scholar]
- 36.Coles C, Condie A, Chetty U, Steel C M, Evans H J, Prosser J. Cancer Res. 1992;52:5291–5298. [PubMed] [Google Scholar]
- 37.Moll U M, Riou G, Levine A J. Proc Natl Acad Sci USA. 1992;89:7262–7266. doi: 10.1073/pnas.89.15.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuperwasser C, Pinkas J, Hurlbut G D, Naber S P, Jerry D J. Cancer Res. 2000;60:2723–2729. [PubMed] [Google Scholar]
- 39.Russo J, Hu Y F, Silva I D, Russo I H. Microsc Res Tech. 2001;52:204–223. doi: 10.1002/1097-0029(20010115)52:2<204::AID-JEMT1006>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 40.Russo J, Russo I H. Medicina (Buenos Aires) 1997;57:81–91. [PubMed] [Google Scholar]
- 41.Russo J, Russo I H. Lab Invest. 1987;57:112–137. [PubMed] [Google Scholar]
- 42.Russo I H, Russo J. J Mammary Gland Biol Neoplasia. 1998;3:49–61. doi: 10.1023/a:1018770218022. [DOI] [PubMed] [Google Scholar]
- 43.Russo I H, Russo J. Environ Health Perspect. 1996;104:938–967. doi: 10.1289/ehp.96104938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tay L K, Russo J. Carcinogenesis. 1981;2:1327–1333. doi: 10.1093/carcin/2.12.1327. [DOI] [PubMed] [Google Scholar]
- 45.Medina D, Peterson L E, Moraes R, Gay J. Cancer Lett. 2001;169:1–6. doi: 10.1016/s0304-3835(01)00507-9. [DOI] [PubMed] [Google Scholar]
- 46.Kuperwasser C, Hurlbut G D, Kittrell F S, Dickinson E S, Laucirica R, Medina D, Naber S P, Jerry D J. Am J Pathol. 2000;157:2151–2159. doi: 10.1016/S0002-9440(10)64853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jerry D J, Kittrell F S, Kuperwasser C, Laucirica R, Dickinson E S, Bonilla P J, Butel J S, Medina D. Oncogene. 2000;19:1052–1058. doi: 10.1038/sj.onc.1203270. [DOI] [PubMed] [Google Scholar]
- 48.Goepfert T M, McCarthy M, Kittrell F S, Stephens C, Ullrich R L, Brinkley B R, Medina D. FASEB J. 2000;14:2221–2229. doi: 10.1096/fj.00-0165com. [DOI] [PubMed] [Google Scholar]