Incidence and mortality
Approximately 5–17 cases of adrenal crisis (AC) occur per 100 patient years in patients with primary or secondary adrenal insufficiency (AI) [1, 2]. The mortality rate is estimated to be between 0.5% and 2%. Norwegian data indicate that overall, 1 in 7 patients with Addison’s disease eventually dies from AC. It is estimated that AC will account for 5500–10,600 excess deaths in the European Union in the next decade [3]. Often, these are preventable deaths in relatively young patients in the emergency department (ED) or intensive care unit (ICU) occurring from cardiorespiratory arrest in hypovolemic shock after delayed or insufficient steroid replacement, contributing to the excess mortality in patients at risk.
Adequate treatment of AC using stress-dose intravenous steroids is often delayed, even when AI is known and patients present their emergency card to EDs or retrieval services (European Steroid Emergency Card www.endocrinology.org/adrenal-crisis) [4].
Causes and patients at risk
The main triggers for AC include interruption of glucocorticoid intake, infections, surgery, gastroenteritis, and stress; the most important predictor is a history of AC (OR 2.85) [5, 6].
Primary adrenal insufficiency (PAI) and secondary adrenal insufficiency (SAI) caused by panhypopituitarism or pituitary corticotropic insufficiency may lead to AC. By far the largest group of patients at risk, however, are those with chronic steroid medication (up to 3% of the population) who often develop adrenal suppression over time and therefore may not be able to increase their endogenous cortisol levels sufficiently during acute stress/illness [7]. In critically ill patients, more specific causes include Waterhouse–Friderichsen syndrome and hemorrhagic adrenalitis in sepsis/coagulopathy.
PAI may be caused by autoimmune (Addison’s disease), surgical, hemorrhagic, ischemic, or metastatic etiology.
SAI is caused by traumatic brain injury, tumors, and hemorrhagic/ischemic lesions of the hypothalamic-pituitary axis but also by chronic glucocorticoid treatment lasting more than 3–4 weeks, even including topical administration such as aerosols for asthma and COPD or creams used for skin problems [8]. The latter category likely represents the largest group of patients in the ICU, when infection triggers only an insufficient increase of cortisol levels because of SAI (e.g., transplant recipients on a glucocorticoid-based immunosuppression, or COPD patients with pneumonia). A novel patient group where AC should always be considered are cancer patients on checkpoint inhibitor immunotherapy (including ipilimumab and nivolumab) that may cause severe hypophysitis or adrenalitis [9]. Moreover, several medications interact with steroid metabolism and may cause AC, including antiepileptic drugs, barbiturates, etomidate, antituberculosis and antifungal drugs (fluconazole, ketoconazole, voriconazole) [10].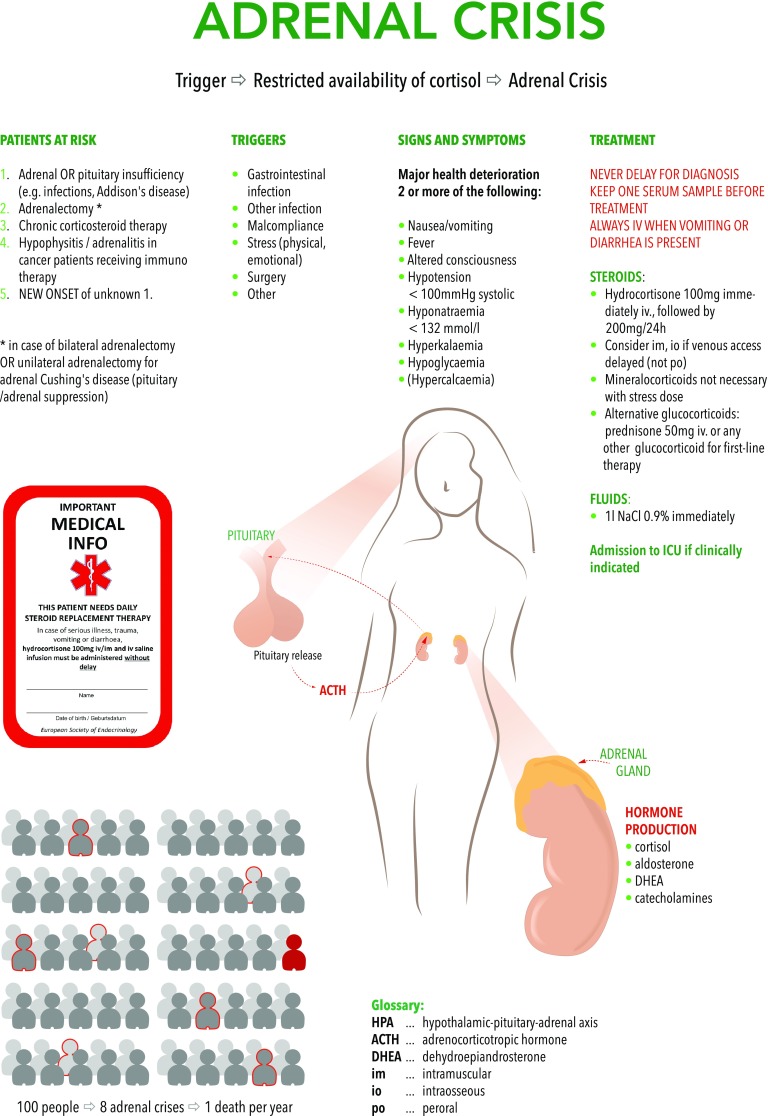
Definition and diagnosis
The definition of AC remains debated, but commonly it is defined as a major acute health deterioration and at least two of the following: hypotension—absolute (systolic blood pressure less than 100 mmHg) or relative (systolic blood pressure reduction greater than 20 mmHg), acute abdominal symptoms, nausea or vomiting, altered mental state, fatigue, fever and laboratory abnormalities (hyponatremia, hyperkalemia, hypoglycemia, rarely hypercalcemia) [11]. In pregnancy, diagnosis of AI is complicated because of endocrine and metabolic changes. AC should be suspected when symptoms are out of proportion in relation to the gestational state and include weight loss, salt-craving, hypoglycemia, and hyponatremia [12].
In the ED or ICU setting, the diagnostic criteria are particularly unsatisfactory, as other serious diseases such as sepsis or cardiogenic shock may well mimic these features or contribute to them, and classic signs (hyperpigmentation in PAI or axillary/pubic hair loss in SAI) may not be present; consequently the diagnosis may be suspected when there is high suspicion level in the context of an “undifferentiated shock”. The medication history has a pivotal role in this syndrome. Further complicating the picture, relative adrenal insufficiency (RAI) or critical illness related corticosteroid insufficiency (CIRCI) may also be present in the context of severely ill patients with multiple end-organ failure, and in such cases, total cortisol level measurement may not be reliable [13] in a context of altered corticosteroid binding protein release and corticosteroid degradation [14].
If AC is possible, a sample for later cortisol/adrenocorticotrophic hormone (ACTH) analysis should be stored but this must never delay treatment. Also, the sensitivity and specificity of total or calculated bioavailable fraction of cortisol are still unknown reflecting the active cellular mediator. The short Synacthen test, a diagnostic tool used under stable conditions (measurement of serum cortisol at baseline and 30 min after i.v. injection of ACTH 250 µg) is of little use in the emergency setting and would unnecessarily delay treatment [15].
Treatment
When recognized, treatment is simple, straightforward, and not different from less severe AC in patients admitted to the ward:
Rapid infusion of 0.9% saline or balanced solutions.
Supraphysiologic doses of i.v. hydrocortisone as soon as possible (initially 100 mg followed by 200 mg over 24 h).
5% glucose may be needed in case of hypoglycemia.
Above a daily dose of 50 mg hydrocortisone, no additional mineralocorticoid replacement is necessary. Usually, the clinical response is rapid and some authors even include an improvement of hypotension within 1 h in the definition of AC.
Summary and suggestions
More often than not, definitive and timely diagnosis of AC is not possible in the ED/ICU because of out-of-hours presentation, unspecific signs/symptoms, and unreliable/unavailable specific laboratory tests.
Given the large number of patients at risk, the poor outcomes when untreated, and the low risk of short-term hydrocortisone treatment in patients without AC, we suggest the following for EDs and ICUs:
Actively consider AC in all acutely ill patients presenting to the ED or the ICU.
Use a low therapeutic threshold to give 100 mg hydrocortisone i.v., especially when hypotension and hyponatremia are present.
If venous access is delayed, use an immediate intraosseous infusion.
In known PAI or SAI, a “show emergency card-to-steroid injection time” < 15 min should be implemented.
When an underlying disease is unknown, keep a serum/plasma sample and consult with endocrinology when possible.
Perform standard testing after complete recovery from critical illness.
Patients with AI must not die from adrenal crisis. It is time we all take the possibility of adrenal crisis dead seriously.
Acknowledgements
This project was carried out during the NEXT ESICM Mentorship Programme (K. Amrein: mentor; G. Martucci: mentee). We thank the European Society of Intensive Care Medicine (ESICM) and specifically the NEXT committee for this initiative. Open access funding provided by Medical University of Graz.
References
- 1.Hahner S, Spinnler C, Fassnacht M, Burger-Stritt S, Lang K, Milovanovic D, Beuschlein F, Willenberg HS, Quinkler M, Allolio B. High incidence of adrenal crisis in educated patients with chronic adrenal insufficiency: a prospective study. J Clin Endocrinol Metabol. 2015;100:407–416. doi: 10.1210/jc.2014-3191. [DOI] [PubMed] [Google Scholar]
- 2.Meyer G, Neumann K, Badenhoop K, Linder R. Increasing prevalence of Addison’s disease in German females: health insurance data 2008–2012. Eur J Endocrinol. 2014;170:367–373. doi: 10.1530/EJE-13-0756. [DOI] [PubMed] [Google Scholar]
- 3.Burman P, Mattsson AF, Johannsson G, Hoybye C, Holmer H, Dahlqvist P, Berinder K, Engstrom BE, Ekman B, Erfurth EM, Svensson J, Wahlberg J, Karlsson FA. Deaths among adult patients with hypopituitarism: hypocortisolism during acute stress, and de novo malignant brain tumors contribute to an increased mortality. J Clin Endocrinol Metabol. 2013;98:1466–1475. doi: 10.1210/jc.2012-4059. [DOI] [PubMed] [Google Scholar]
- 4.Puar TH, Stikkelbroeck NM, Smans LC, Zelissen PM, Hermus AR. Adrenal crisis: still a deadly event in the 21st century. Am J Med. 2016;129:339.e331–339.e339. doi: 10.1016/j.amjmed.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Hahner S, Loeffler M, Bleicken B, Drechsler C, Milovanovic D, Fassnacht M, Ventz M, Quinkler M, Allolio B. Epidemiology of adrenal crisis in chronic adrenal insufficiency: the need for new prevention strategies. Eur J Endocrinol. 2010;162:597–602. doi: 10.1530/EJE-09-0884. [DOI] [PubMed] [Google Scholar]
- 6.Rushworth RL, Torpy DJ, Falhammar H. Adrenal crises: perspectives and research directions. Endocrine. 2017;55:336–345. doi: 10.1007/s12020-016-1204-2. [DOI] [PubMed] [Google Scholar]
- 7.Dinsen S, Baslund B, Klose M, Rasmussen AK, Friis-Hansen L, Hilsted L, Feldt-Rasmussen U. Why glucocorticoid withdrawal may sometimes be as dangerous as the treatment itself. Eur J Intern Med. 2013;24:714–720. doi: 10.1016/j.ejim.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Woods CP, Argese N, Chapman M, Boot C, Webster R, Dabhi V, Grossman AB, Toogood AA, Arlt W, Stewart PM, Crowley RK, Tomlinson JW. Adrenal suppression in patients taking inhaled glucocorticoids is highly prevalent and management can be guided by morning cortisol. Eur J Endocrinol. 2015;173:633–642. doi: 10.1530/EJE-15-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lomax AJ, McNeil C. Acute management of autoimmune toxicity in cancer patients on immunotherapy: common toxicities and the approach for the emergency physician. Emerg Med Australas. 2017;29:245–251. doi: 10.1111/1742-6723.12718. [DOI] [PubMed] [Google Scholar]
- 10.Husebye ES, Allolio B, Arlt W, Badenhoop K, Bensing S, Betterle C, Falorni A, Gan EH, Hulting AL, Kasperlik-Zaluska A, Kampe O, Lovas K, Meyer G, Pearce SH. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J Intern Med. 2014;275:104–115. doi: 10.1111/joim.12162. [DOI] [PubMed] [Google Scholar]
- 11.Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, Husebye ES, Merke DP, Murad MH, Stratakis CA, Torpy DJ. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metabol. 2016;101:364–389. doi: 10.1210/jc.2015-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langlois F, Lim DST, Fleseriu M. Update on adrenal insufficiency: diagnosis and management in pregnancy. Curr Opin Endocrinol Diabetes Obes. 2017;24:184–192. doi: 10.1097/MED.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 13.Annane D, Pastores SM, Rochwerg B, Arlt W, Balk RA, Beishuizen A, Briegel J, Carcillo J, Christ-Crain M, Cooper MS, Marik PE, Umberto Meduri G, Olsen KM, Rodgers S, Russell JA, Van den Berghe G (2017) Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med. doi:10.1007/s00134-017-4919-5 [DOI] [PubMed]
- 14.Venkatesh B, Cohen J. Adrenocortical (dys)function in septic shock—a sick euadrenal state. Best Pract Res Clin Endocrinol Metab. 2011;25:719–733. doi: 10.1016/j.beem.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Venkatesh B, Cohen J, Cooper M. Ten false beliefs about cortisol in critically ill patients. Intensive Care Med. 2015;41:1817–1819. doi: 10.1007/s00134-014-3635-7. [DOI] [PubMed] [Google Scholar]


