Abstract
Purpose
Acute kidney injury (AKI) frequently occurs after heart transplantation (HTx), but its relation to preoperative right heart hemodynamic (RHH) parameters remains unknown. Therefore, we aimed to determine their predictive properties for postoperative AKI severity within 30 days after HTx.
Methods
From 1984 to 2016, all consecutive HTx recipients (n = 595) in our tertiary referral center were included and analyzed for the occurrence of postoperative AKI staged by the kidney disease improving global outcome criteria. The effects of preoperative RHH parameters on postoperative AKI were calculated using logistic regression, and predictive accuracy was assessed using integrated discrimination improvement (IDI), net reclassification improvement (NRI), and area under the receiver operating characteristic curves (AUC).
Results
Postoperative AKI occurred in 430 (72%) patients including 278 (47%) stage 1, 66 (11%) stage 2, and 86 (14%) stage 3 cases. Renal replacement therapy (RRT) was administered in 41 (7%) patients. Patients with higher AKI stages had also higher baseline right atrial pressure (RAP; median 7, 7, 8, and in RRT 11 mmHg, p trend = 0.021), RAP-to-pulmonary capillary wedge pressure ratio (median 0.37, 0.36, 0.40, 0.47, p trend = 0.009), and lower pulmonary artery pulsatility index (PAPi) values (median 2.83, 3.17, 2.54, 2.31, p trend = 0.012). Higher RAP and lower PAPi values independently predicted AKI severity [adjusted odds ratio (OR) per doubling of RAP 1.16 (1.02–1.32), p = 0.029; of PAPi 0.85 (0.75–0.96), p = 0.008]. Based on IDI, NRI, and delta AUC, inclusion of these parameters improved the models’ predictive accuracy.
Conclusions
Preoperative PAPi and RAP strongly predict the development of AKI early after HTx and can be used as early AKI predictors.
Electronic supplementary material
The online version of this article (10.1007/s00134-018-5159-z) contains supplementary material, which is available to authorized users.
Keywords: Right heart hemodynamics, Pulmonary artery pulsatility index, Acute kidney injury, Heart transplantation, Right atrial pressure, Mortality
Take-home message
| Novel composite right heart hemodynamic parameter pulmonary artery pulsatility index (PAPi), may outperform currently assessed parameters like transpulmonary gradient, diastolic pulmonary gradient and pulmonary vascular resistance. PAPi and right atrial pressure can be used as predictors for early AKI in patients undergoing heart transplantation, and early prediction of AKI could be used to timely treat high-risk patients. |
Introduction
Heart transplantation (HTx) remains the gold standard therapy for patients with end-stage heart failure (HF), improving both their survival and quality of life [1]. Recent advances in immunosuppressive therapy and treatment protocols have significantly improved the long-term outcome in HTx recipients despite the propensity for accepting older donors [2]. However, the short-term outcome during the early postoperative phase has remained complex, affecting both morbidity and mortality [2, 3].
Acute kidney injury (AKI) occurs frequently after HTx, ranging from 22 to 76% and carries unfavorable prognosis [4–7]. In addition to anesthesia- and surgery-related factors that can precipitate AKI, postoperatively used nephrotoxic drugs (e.g., CNI) and hemodynamic instability may also lead to AKI [4].
It is known that preexisting pulmonary hypertension increases right ventricular afterload that can lead to right ventricular failure (RVF) [8]. Importantly, RVF can critically diminish renal function by increasing renal venous pressure, causing congestive AKI [9, 10]. Consequently, the right heart hemodynamic (RHH) parameters have been routinely assessed in all HTx candidates [11]. However, it is unclear how these RHH parameters relate to RVF and, more importantly, to AKI early after HTx. Finally, the question remains whether and to what extent is the relationship between preoperative RHH parameters and the occurrence of postoperative AKI explained by the occurrence of RVF along that pathway.
Recently, new composite hemodynamic parameters such as the pulmonary artery pulsatility index (PAPi), the right atrial pressure-to-pulmonary capillary wedge pressure ratio (RAP-to-PCWP), and diastolic pulmonary gradient (DPG) are considered to be predictors of RVF [12–15]. However, their relationships with postoperative AKI early after HTx remain unknown.
The aim of this study was to determine the predictive properties of routine and novel RHH parameters measured at the time of transplantation listing in relation to AKI early after HTx. Preliminary results have been previously reported [16].
Methods
Study population
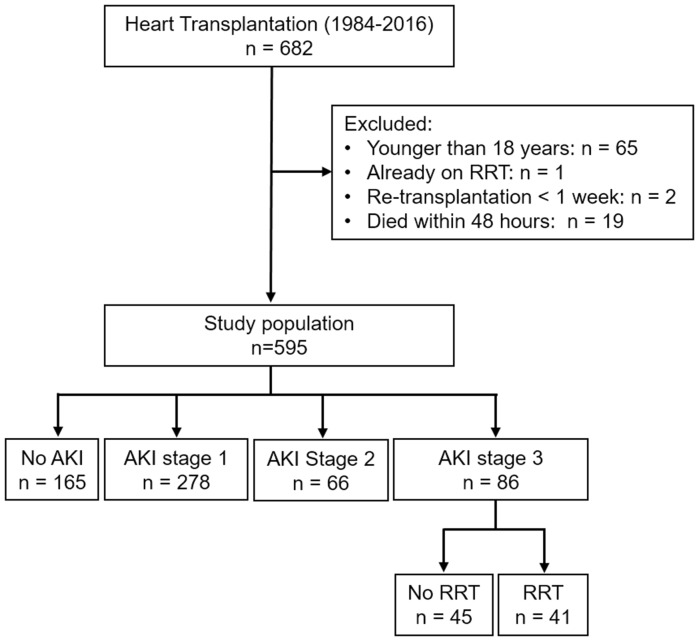
Data of all consecutive HTx in the Erasmus Medical Center, Rotterdam, have been collected prospectively since the first transplantation in June 1984 [2, 4]. We included all adult (≥ 18 years) patients transplanted between 1984 and December 2016. Patients were excluded if age < 18 years at the time of transplantation, were on renal replacement therapy (RRT) before transplantation, died within 48 h, or were re-transplanted within 7 days after transplantation (Fig. 1). No patients underwent simultaneous heart/kidney transplantation. Patient data were obtained from the hospital database, electronic records, and by chart review.
Fig. 1.
Flowchart of study population according to postoperative AKI severity. AKI acute kidney injury, RRT renal replacement therapy
Immunosuppressive protocol
From 1984 to 1999, immunosuppressive therapy consisted of calcineurin inhibitor (CNI) cyclosporine A and tacrolimus thereafter. In patients who did not receive induction therapy, CNI was initiated peri-operatively or immediately after HTx. Induction therapy was used to delay the starting of CNI, especially in patients with already impaired kidneys and/or postoperative hemodynamic instability, to postpone CNI nephrotoxicity [4]. Induction therapy consisted of anti-CD3 (1987–1994), anti-IL-2 (1987–1994), horse polyclonal anti-thymocyte globulin (1987–2008), and rabbit anti-thymocyte globulin (2008 and thereafter).
Preoperative right heart catheterization parameters
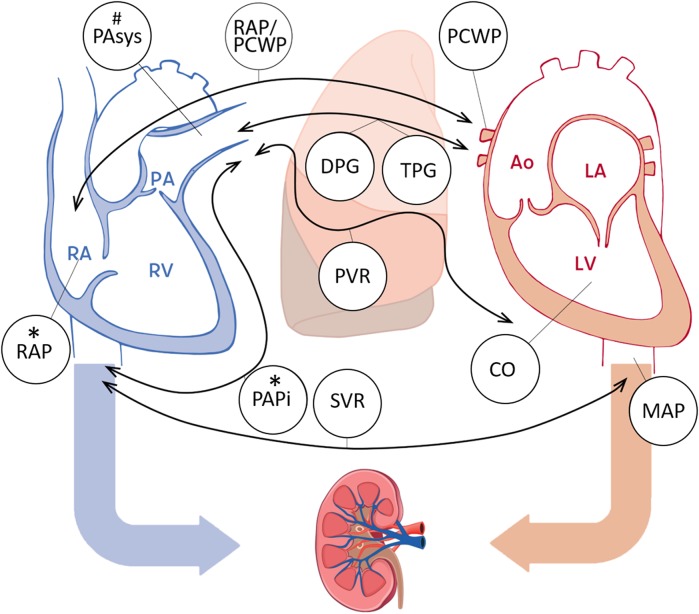
All HTx candidates underwent right heart catheterization during the screening for transplantation listing. If a patient’s clinical status deteriorated with suspicion of pulmonary hypertension while on the waiting list, an additional catheterization was performed where the most recent data prior to HTx were used for our analysis. Procedural data were extracted from the catheterization reports and included the following parameters: RAP, PCWP, pulmonary artery (PA) systolic, diastolic, and mean pressures, systemic arterial systolic, diastolic, and mean pressures, cardiac output, pulmonary vascular resistance, systemic vascular resistance, PAPi, transpulmonary gradient (TPG), and DPG (Fig. 2) [13, 15].
Fig. 2.
Preoperative hemodynamic parameters and their relation to postoperative right ventricular failure and acute kidney injury early (≤ 30 days) after heart transplantation. An illustration of the assessed hemodynamic parameters of the heart including RAP; PA systolic, diastolic, and mean pressures ; PAPi ; PCWP; RAP-to-PCWP ratio; TPG ; DPG ; cardiac output (CO); pulmonary vascular resistance (PVR) ; systolic, diastolic, and mean arterial pressure (MAP) ; systemic vascular resistance (SVR) . The asterisk indicates a significant predictor of acute kidney injury (AKI). The hash symbol indicates a significant predictor of right ventricular failure (RVF). RA right atrium, RV right ventricle, PA pulmonary artery, LA left atrium, LV left ventricle, Ao aorta
Renal function assessment
Serum creatinine was measured as part of routine clinical care at baseline, daily from postoperative day 0 until day 7, and at 1, 3, 6, 9, and 12 months. Baseline creatinine was defined as the most recent outpatient value up to 6 months before transplantation. If unavailable, creatinine values at hospital admission were accepted as the baseline. Estimated glomerular filtration rate (eGFR) was assessed by the CKD-EPI equation [17], and categorization was performed by National Kidney Foundation-Kidney Disease Outcome Quality Initiative clinical practice guidelines [18].
Follow-up and study endpoints
The primary endpoint was AKI severity as defined by kidney disease improving global outcome (KDIGO) criteria during the first month after HTx. AKI stage 1 was defined as serum creatinine increased by ≥ 0.3 mg/dl (≥ 26.5 µmol/l) within 48 h or by 1.5–1.9 times baseline; AKI stage 2 as serum creatinine increased 2.0–2.9 times from baseline; and AKI stage 3 as serum creatinine increased 3.0 times from baseline or by ≥ 4.0 mg/dl (≥ 353.6 µmol/l) or starting RRT [19]. The time interval between HTx and the RRT was recorded within the first month. RRT requirement at 1 year was evaluated in all survivors.
The secondary endpoints were postoperative RVF and 1-year survival. RVF was defined as need of postoperative RVAD or as reported in the medical reports by the attending physicians. Post-discharge survival status was obtained from our hospital’s electronic patient file and was completed for all patients.
Statistical analysis
For reasons of uniformity, all continuous variables are presented as median (interquartile range, IQR) and categorical variables are presented as numbers and percentages. The distributions of continuous variables were tested for normality by the Kolmogorov–Smirnov test, and, if skewed, were 2log-transformed. For continuous variables, the linear trend across AKI stages was performed by analysis of variance (ANOVA) or the Kruskal–Wallis test, when appropriate; categorical variables were tested by the χ2 trend test.
Ordinal logistic regression analysis was performed to relate perioperative data to postoperative AKI severity (i.e., deterioration to any level of AKI). Covariates that were univariably associated with AKI severity (exploratory p < 0.10) were entered into a multivariable model, applying proportional odds ordinal regression with a full likelihood ratio method. All analyses were performed in the total cohort, and subsequently in the subgroup of patients with RAP ≥ 6 mmHg (previously determined as the cut-off for the opening of the collapsed vein) [20, 21]. A multiplicative interaction between dichotomized RAP and PAPi was also explored.
We assessed predictive accuracy for the most severe AKI (stage 3) before and after adding significant hemodynamic parameters (p < 0.05) into the clinical model using the delta between the areas under the two receiver operating characteristic curves (AUC), integrated discrimination improvement (IDI), and net reclassification improvement (NRI) [22, 23]. Clinical variables found to be univariably associated with AKI stage 3 (exploratory p < 0.10) were entered into a multivariable model using the stepwise backward likelihood ratio method. Only clinical predictors with p < 0.05 in the multivariable model were used to assess the models’ predictive accuracy.
Binary logistic regression analysis was applied to assess the association between RRT administration within 30 days after HTx and chronic RRT dependency at 1 year, and to relate preoperative data to the onset of RVF.
For 1-year survival, we performed the log-rank test and estimated event-time distributions across AKI stages and temporal RRT requirements using the Kaplan–Meier method. Cox regression analysis was performed to assess hazard ratios with 95% confidence intervals for 1-year survival.
All analyses were performed with a complete dataset using SPSS software (SPSS 20.0; IBMCorp., Armonk, NY, USA) and R statistical software using packages ‘pROC’, “Hmisc”, and “effects” [22, 24, 25]. All tests were two-tailed, and p values < 0.05 were considered statistically significant.
Results
Incidence and temporal trends of postoperative AKI
From 1984 to 2016, 682 patients underwent HTx at the Erasmus MC, of which 595 patients were included in this study (Fig. 1). Of 595 patients, 430 (72%) developed AKI, including 278 (47%) stage 1, 66 (11%) stage 2, and 86 (14%) stage 3 AKI. Of those who developed AKI stage 3, 41 (7%) required RRT which lasted for a median of 7 days (IQR 5–13) and had a 3.3-times (95%CI 1.6–6.6, p = 0.001) higher crude risk of chronic RRT in the first year than those who did not require such treatment. Figure S1 displays the time distribution for the occurrence of AKI with the highest peaks for all three stages on the seventh day.
We found a tendency towards a higher incidence of overall AKI noticeable in recent years. This tendency was accompanied by a trend in a lower baseline eGFR (median eGFR per 6 5-year intervals 69, 67, 67, 56, 69, 56 years, p trend < 0.001), an increasing incidence of diabetes (0, 3, 10, 9, 13, 13%, p trend < 0.001), and older donors (24, 28, 35, 38, 45, 46 years, p trend < 0.001). We also found a tendency towards a higher incidence of AKI stage 3, but only when defined as a requirement for RRT (Fig. S2).
Demographic and perioperative data
Table 1 shows baseline characteristics and perioperative data stratified by AKI stages. Patients who had a higher AKI stage also had a higher baseline BMI (median 22.6, 23.2, 22.9, 24.2 kg/m2, p trend < 0.001), a lower baseline eGFR (71, 60, 67, 56 ml/min/1.73 m2, p trend < 0.001), and more frequent diabetes (4, 7, 8, 13%, p trend = 0.015). They also received a heart from older donors (31, 34, 37, 39 years, p trend < 0.001) with more frequently female gender (46, 46, 58, 59%, p trend = 0.019) and postoperatively were more frequently diagnosed with RVF (7, 5, 12, 28%, p trend < 0.001). These patients had a longer hospital stay (20, 24, 24, 37 days, p trend < 0.001) and were less likely to have received induction therapy (90, 80, 71, 67%, p trend < 0.001). A trend was also seen in higher in-hospital mortality with higher AKI stages (2, 5, 14, 9%, p trend = 0.003).
Table 1.
Baseline characteristics and perioperative data according to postoperative AKI stages
| n (%) | No AKI 165 (28) | AKI stage 1 278 (48) | AKI stage 2 66 (11) | AKI stage 3 86 (14) | p value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years | 51 (45–56) | 51 (43–57) | 51 (43–57) | 48 (41–55) | 0.23 |
| Male sex | 127 (77) | 208 (75) | 49 (74) | 67 (78) | 0.95 |
| BMI, kg/m2 | 22.6 (20.1–24.5) | 23.2 (21.0–25.2) | 22.9 (20.8–25.8) | 24.2 (22.1–26.8) | < 0.001* |
| Renal function | |||||
| eGFR, ml/min/1.73m2 | 71 (58–88) | 60 (47–79) | 67 (60–79) | 56 (43–70) | < 0.001* |
| eGFR ≥ 90 | 38 (23) | 37 (13) | 11 (17) | 2 (2) | < 0.001* |
| eGFR 60–89 | 79 (48) | 103 (37) | 40 (60) | 33 (38) | |
| eGFR < 60 | 48 (29) | 138 (50) | 15 (23) | 51 (60) | |
| eGFR 45–59 | 31 (19) | 81 (29) | 11 (17) | 27(32) | |
| eGFR < 45 | 17 (10) | 57 (21) | 4 (6) | 24 (28) | |
| Medical history | |||||
| Prior cardiac surgery | 45 (27) | 89 (32) | 15 (23) | 25 (29) | 0.90 |
| Diabetes mellitus | 7 (4) | 19 (7) | 5 (8) | 11 (13) | 0.015* |
| Hypertension | 17 (10) | 29 (10) | 5 (8) | 8 (9) | 0.65 |
| Donor characteristics | |||||
| Age, years | 31 (20–42) | 34 (22–45) | 37 (24–45) | 39 (27–49) | < 0.001* |
| Male sex | 89 (54) | 149 (54) | 28 (42) | 35 (41) | 0.019* |
| Cause of death | 0.61 | ||||
| Trauma | 74 (45) | 117 (42) | 26 (39) | 36 (42) | |
| CVA | 83 (50) | 149 (54) | 38 (58) | 4 (51) | |
| Other | 6 (4) | 11 (4) | 2 (3) | 6 (7) | |
| Unknown | 2 (1) | 1 (0) | 0 (0) | 0 (0) | |
| Time of ischemia donor heart, minutes | 165 (139–196) | 171 (143–206) | 170 (147–195) | 176 (150–210) | 0.09 |
| Urgency status on waiting list | 0.78 | ||||
| Elective | 78 (47) | 166 (60) | 41 (62) | 38 (44) | |
| Urgent | 58 (35) | 73 (26) | 14 (21) | 31 (36) | |
| Unknown | 29 (18) | 39 (14) | 11 (17) | 17 (20) | |
| Preoperative hemodynamic parameters at the time of transplantation listing | |||||
| Days before HTx | 182 (81–331) | 275 (123–545) | 273 (117–505) | 213 (100–534) | 0.15 |
| Heart rate, beats/min | 80 (68–100) | 80 (70–92) | 73 (61–94) | 72 (67–90) | 0.06 |
| Systolic AP, mmHg | 99 (90–106) | 97 (90–105) | 95 (84–105) | 97 (87–107) | 0.27 |
| Diastolic AP, mmHg | 63 (57–70) | 61 (56–69) | 62 (54–69) | 62 (56–72) | 0.91 |
| Mean AP, mmHg | 74 (68–80) | 73 (67–81) | 73 (64–81) | 75 (66–83) | 0.61 |
| Cardiac output, L/min | 3.8 (3.1–4.6) | 4.0 (3.3–4.7) | 3.8 (3.2–4.5) | 3.8 (3.0–4.5) | 0.98 |
| PVR, dync/s/cm2 | 172 (115–230) | 149 (96–224) | 154 (93–245) | 144 (82–226) | 0.44 |
| SVR, dync/s/cm2 | 1442 (1192–1764) | 1286 (1086–1671) | 1398 (1216–1605) | 1333 (1042–1630) | 0.14 |
| RAP, mmHg | 7 (5–12) | 7 (4–11) | 8 (5–13) | 11 (5–17) | 0.021* |
| PA systolic, mmHg | 44 (32–55) | 42 (30–52) | 44 (34–53) | 45 (29–59) | 0.93 |
| PA diastolic, mmHg | 23 (15–30) | 21 (14–29) | 21 (15–30) | 23 (15–29) | 0.78 |
| PA mean, mmHg | 30 (21–39) | 28 (19–36) | 27 (21–37) | 31 (20–38) | 0.84 |
| PCWP, mmHg | 21 (14–29) | 20 (13–26) | 20 (14–27) | 22 (13–29) | 0.81 |
| TPG, mmHg | 8.3 (5.0–11.0) | 7.7 (4.3–10.7) | 7.2 (4.4–10.3) | 7.3 (4.3–10.3) | 0.25 |
| DPG, mmHg | 1.0 (–2.0–4.0) | 1.0 (–2.0–4.0) | 0.0 (–2.0–3.0) | 0.0 (–2.0–4.0) | 0.93 |
| PAPi | 2.83 (1.89–5.81) | 3.17 (1.61–5.67) | 2.54 (1.82–5.60) | 2.31 (1.01–4.57) | 0.012* |
| RAP-to-PCWP ratio | 0.37 (0.24–0.57) | 0.36 (0.23–0.52) | 0.40 (0.25–0.53) | 0.47 (0.29–0.74) | 0.009* |
| Preoperative hemodynamic support | |||||
| Inotropes | 41 (25) | 59 (21) | 15 (23) | 29 (34) | 0.16 |
| IABP/ECMO | 16 (10) | 20 (7) | 5 (8) | 10 (12) | 0.68 |
| LVAD | 14 (8) | 15 (5) | 1 (1) | 5 (6) | |
| Postoperative complications | |||||
| Right ventricle failure | 11 (7) | 14 (5) | 8 (12) | 24(28) | < 0.001* |
| Re-thoracotomy | 12 (7) | 18 (6) | 9 (14) | 12 (14) | 0.06 |
| Primary graft failure | 3 (2) | 4 (1) | 4 (6) | 2 (2) | 0.14 |
| Othera | 7 (4) | 14 (5) | 1 (1) | 7 (8) | |
| Immunosuppressive therapy | |||||
| Induction therapy (yes) | 148 (90) | 222 (80) | 47 (71) | 58 (67) | < 0.001* |
| ATG use | 89 (54) | 151 (54) | 29 (44) | 46 (53) | 0.58 |
| Anti-CD3 | 45 (27) | 41 (15) | 5 (7) | 5 (6) | < 0.001* |
| Anti-IL2 | 14 (9) | 30 (11) | 13 (20) | 7 (8) | 0.49 |
| Postoperative delay CNIb, days | 3 (2–5) | 3 (1–4) | 2 (0–3) | 2 (0–5) | 0.35 |
| Hospital stay | |||||
| Days in ICU | 3 (2–4) | 3 (2–4) | 4 (3–6) | 8 (4–14) | < 0.001* |
| Days in hospital | 20 (16–29) | 24 (17–33) | 24 (17–32) | 37 (23–58) | < 0.001* |
| In-hospital mortality | 4 (2) | 15 (5) | 9 (14) | 8 (9) | 0.003* |
Due to uniformity, all continuous data are presented as median and IQR; all categorical data as number and percentage
eGFR estimated glomerular filtration rate, HTx heart transplantation, AP arterial pressure, PVR pulmonary vascular resistance, SVR systemic vascular resistance, RAP right atrial pressure, PA pulmonary artery, PCWP pulmonary capillary wedge pressure, TPG trans-pulmonary gradient, DPG diastolic pulmonary gradient, PAPi pulmonary artery pulsatility index, IABP intra-aortic balloon pump, LVAD left ventricular assist device, ECMO extracorporeal membrane oxygenator, ICU intensive care unit, ATG anti-thymocyte globulins
*p value for a linear trend < 0.05 is statistically significant
aOther includes: perioperative bleeding, cardiac arrest, dosing of inotropes, pacemaker malfunction, acute rejection, and instability of unknown cause
bPostoperative delay after heart transplantation before starting CNI
Relationship of preoperative RHH parameters with postoperative AKI severity
Figure 2 summarizes the investigated hemodynamic parameters. Table 1 shows the values of RHH parameters according to the AKI stages. Patients with a higher AKI stage also had a higher baseline RAP (median 7, 7, 8, 11 mmHg, p trend = 0.021) and RAP-to-PCWP ratio (0.37, 0.36, 0.40, 0.47, p trend = 0.009) and lower PAPi values (2.83, 3.17, 2.54, 2.31, p trend = 0.012).
Table 2 and Fig. S3 display the associations of the significant RHH parameters with the risk of postoperative AKI severity. In the total cohort, higher RAP and lower PAPi values were associated with AKI severity independently of the patient’s BMI, baseline eGFR, diabetes, donor’s age and sex, ischemia time of the donor’s heart, time from right heart catheterization to HTx, postoperative RVF, and the postoperative use of induction therapy [adjusted OR (95%CI) per doubling RAP 1.16 (1.02–1.32), p = 0.029; PAPi 0.85 (0.75–0.96), p = 0.008]. Moreover, we found a significant multiplicative interaction between RAP ≥ 6 mmHg and PAPi values (p interaction = 0.034), indicating even more pronounced association between lower PAPi values and higher probability of AKI severity in patients with elevated RAP [adjusted OR per doubling of PAPi 0.70 (0.56–0.87), p = 0.002] (Table 2, Fig. S4).
Table 2.
Associations between preoperative hemodynamic parameters and postoperative AKI severity early after heart transplantation
| Univariable model | Multivariable modelf | |||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Total cohorta | ||||
| PAPic | 0.88 (0.79–0.99) | 0.043* | 0.85 (0.75–0.96) | 0.008* |
| RAPc | 1.12 (0.99–1.28) | 0.07 | 1.16 (1.02–1.32) | 0.029* |
| RAP-to-PCWPc | 1.19 (1.01–1.39) | 0.033* | 1.15 (0.98–1.35) | 0.10 |
| Heart rated | 0.90 (0.81–1.00) | 0.06 | 0.98 (0.87–1.11) | 0.75 |
| SVRe | 0.96 (0.91–1.00) | 0.06 | 0.96 (0.92–1.01) | 0.13 |
| Subgroup RAP ≥ 6 mmHgb | ||||
| PAPic | 0.74 (0.60–0.92) | 0.006* | 0.70 (0.56–0.87) | 0.002* |
| RAPc | 1.62 (1.17–2.25) | 0.004* | 1.78 (1.27–2.50) | 0.001* |
| RAP-to-PCWPc | 1.44 (1.06–1.96) | 0.019* | 1.31 (0.95–1.08) | 0.10 |
| Heart rated | 0.88 (0.77–1.01) | 0.08 | 0.95 (0.82–1.11) | 0.52 |
| SVRe | 0.98 (0.92–1.03) | 0.42 | 0.99 (0.93–1.05) | 0.78 |
OR (95% CI) indicates proportional odds ratio with 95% confidence interval; for other abbreviations, please see Table 1
*p value < 0.05 is statistically significant
aTotal n = 595, no AKI = 165, AKI stage 1 = 278, AKI stage 2 = 66, AKI stage 3 = 86
bTotal n = 340, no AKI = 94, AKI stage 1 = 147, AKI stage 2 = 42, AKI stage 3 = 57
cOR are given per doubling of a preoperative hemodynamic parameter. OR are interpreted as the odds of having a more severe renal injury for any level of AKI (stage 3, stage 2, stage 1, and no AKI). For example, if RAP increases from 7 to 14 mmHg (i.e., doubled), the odds of having AKI stage 3 versus combined AKI stages ≤ 2 and no AKI are 1.12 times greater. Likewise, the odds of having AKI stages ≥ 2 versus combined AKI stage 1 and no AKI are 1.12 times greater. Finally, the odds of having AKI of any stage versus no AKI are 1.12 times greater
dOR are given per 10-unit increase in preoperative heart rate (interpretation is the same as under c)
eOR are given per 100 dyne/sec/cm2 Increase in preoperative SVR (interpretation is the same as under c)
fPreoperative hemodynamic parameters were adjusted for all variables with p < 0.10 in univariable analysis and included patient’s BMI, baseline eGFR, diabetes, donor’s age and sex, ischemia time of donor’s heart, time from catheterization to HTx, postoperative RVF, and the postoperative use of induction therapy. Associations between these variables and postoperative AKI stages are presented in Table S1
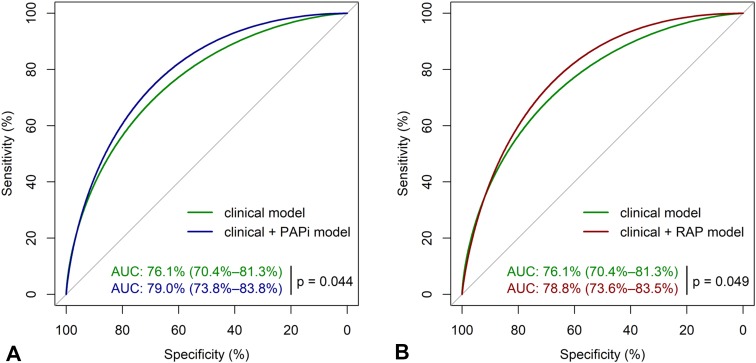
For predicting AKI stage 3, adding each of the hemodynamic parameters, PAPi and RAP, significantly improved the models’ predictive accuracy compared to the best clinical model (PAPi IDI = 0.03, p = 0.013 and total continuous NRI = 0.320, p = 0.007 with 25% reclassification for events and 7% reclassification for non-events; RAP IDI = 0.02, p = 0.040 and NRI = 0.278, p = 0.018 with 25% reclassification for events and 3% reclassification for non-events). In Fig. 3, AUC analysis also showed significant discriminatory improvement [clinical model AUC 76.1% (95%CI 70.4–81.3), clinical model + PAPi 79.0% (73.8–83.8), p delta = 0.044; clinical model + RAP 78.8% (73.6–83.5), p delta = 0.049; PAPi alone 60.7% (53.8–67.1); RAP alone 62.2% (54.6–69.0)]. Based on Youden’s index, the best cut-off point for predicting AKI stage 3 was PAPi < 1.05 and RAP > 11 mmHg. Finally, the associations of clinical data with postoperative AKI severity are shown in Table S1.
Fig. 3.
AUC analysis for the prediction of postoperative AKI stage 3. PAPi pulmonary artery pulsatility index, RAP right atrial pressure, AUC area under the receiver operating characteristic (ROC) curve with p value for the difference between different models. Only predictors that remained significant (p < 0.05) in the final model were used to assess discriminative power, and those were patient body mass index, baseline estimated glomerular filtration rate (eGFR), postoperative right ventricular failure, and the postoperative use of induction therapy. a The ROC curves of clinical model (green) and clinical model + PAPi (blue) for the prediction of AKI stage 3 with AUC and corresponding 95% confidence intervals; b The ROC curves of clinical model (green) and clinical model + RAP (red) for the prediction of AKI stage 3
Predictors of RVF and its relation to AKI
Of 595 patients, 57 (10%) experienced RVF early after HTx. Table S2 shows the predictors of early RVF among which the most significant clinical predictors were the patients’ impaired baseline eGFR and older donors; higher pulmonary artery systolic pressure was the only preoperative RHH parameter that predicted RVF. Furthermore, the occurrence of RVF was strongly associated with AKI severity (Table S1).
Relationship of AKI with 1-year mortality
In total, 51 deaths occurred during the first year after HTx with a cumulative mortality of 5, 7, 15, and 14% for those without AKI and with AKI stages 1, 2, and 3, respectively (log-rank test, p trend = 0.021; Fig. S5a). The cumulative mortality was also higher in patients who received RRT during the first month than in those who had not (22 vs. 8%; log-rank test, p = 0.001, Fig. S5b).
Discussion
To the best of our knowledge, this study is the first to assess the predictive properties of different preoperative hemodynamic parameters in relation to the occurrence of postoperative AKI severity within 30 days after HTx. We found that lower PAPi and higher RAP values predict AKI severity independently of the recipient BMI, baseline eGFR, diabetes, donor age and sex, ischemia time of the donor’s heart, time from right heart catheterization to HTx, postoperative RVF, and the use of induction therapy. These hemodynamic parameters routinely collected at the time of transplantation listing could be used to predict AKI severity early after HTx.
Although renal injury after HTx has traditionally been attributed to the impaired arterial perfusion and CNI nephrotoxicity [4], our results illustrate strong evidence of the independent relationship between preoperative right-sided hemodynamics and AKI severity after HTx. One of the potential explanations for this relationship may be the long-standing venous congestion that chronically compromises the kidneys. Subsequently, the kidneys may become more vulnerable to the development of AKI, especially in cases of postoperative hemodynamic instability with hypotensive episodes, or in the settings of de novo RVF during the adaptation period of the new heart. However, we found that a de novo RVF significantly contributed to the development of AKI, but could not entirely explain the relationships of preoperative RAP and PAPi with the postoperative renal injury. Several pathophysiological mechanisms may be responsible for this peculiar renal vulnerability, including attenuated vascular reflexes, elevated renal interstitial and intra-tubular pressure, activation of the renin-angiotensin-aldosterone system, and chronic venous pressure-induced tubule-glomerular feedback dysfunction [26, 27]. Moreover, we found a significant interaction between RAP and PAPi, indicating that probabilities of AKI (especially stages 2 and 3) are markedly increased with lower PAPi values, but mostly in patients with elevated RAP (≥ 6 mmHg). These findings are supported by Damman et al., who found that eGFR starts to significantly decline when RAP increases above 6 mmHg [28]. Altogether, it appears that preoperatively compromised right-sided venous pressures deserve clinical attention in the context of postoperative AKI and may be even more important for kidney functioning after HTx than low systemic pressures.
Acute or chronic cardiac dysfunction has negative effects on kidney function and vice versa. This complex cross-talk was recently described as cardio-renal syndrome (CRS) [29]. Chronic HF leading to chronic renal congestion in the pre-HTx period is classified as CRS type 2. On the other hand, early AKI post-HTx could be considered as CRS type 1. Recognition of post-HTx CRS may provide possibilities of prevention and treatment strategies in the settings of HTx. Importantly, early prediction of postoperative AKI based on preoperative RAP and PAPi could help to timely and more proactively intervene in the patients who are at high risk, in terms of giving more attention to the perioperative volume overload, postponing the introduction of nephrotoxic CNI, prolonging the support of the right ventricle with inotropes, no ventilation, and early introduction of pulmonary vasodilators (e.g., sildenafil) [11, 27, 30–33]. Furthermore, these patients could possibly benefit from functional kidney stress tests to assess the renal functional reserve and identify patients who will progress to AKI post-HTx [34, 35]. In addition to decreasing values, PAPi can also go in the opposite direction. Hence, the recovery of PAPi values may indicate improvement of right-sided pressure, which may be used to optimize perioperative hemodynamic support to preserve the kidneys from injury. However, prospective studies, preferably interventional in nature, are needed to elucidate this promising concept.
The overall AKI incidence was 72%, which fits at the high-end of the reported incidence range of 22–76% in HTx recipients [4–7]. This widespread distribution probably results from the large heterogeneity between studies and deserves closer attention. Previously, most studies reported the older risk injury failure loss end-stage renal disease criteria or the acute kidney injury network criteria [6, 36]. However, we used the newer KDIGO criteria to define AKI stages [37]. Second, time intervals for the occurrence of AKI were different from the present study [4–7, 38, 39]. We targeted 1 month as the clinically relevant time interval, whereas previous studies mainly focused on the first week [4, 6, 7, 38]. Our results show that although all AKI stages peaked on day 7, the risk of AKI remains through the first month. This late postoperative peak of AKI could be attributed to the delay in starting of CNI. For this purpose, we use induction therapy, especially in patients with impaired kidneys and/or hemodynamic instability early after HTx, to postpone CNI nephrotoxicity. In our data, the use of induction therapy was protective for the occurrence of AKI. Another important and more worrisome observation is that AKI incidence has increased in recent years. This is probably explained by an increasing proportion of HTx recipients being listed with more comorbidities such as chronic renal failure and diabetes, older heart donors, and probable changes in clinical management with more proactive treatment protocols [2, 40].
Limitations
First, this was a single-center study, and, therefore, the clinical management and treatment modalities may differ from other transplant centers. However, patient selection for transplantation listing and cardiac procedures were performed according to generally accepted international criteria [11]. Second, its retrospective nature precluded the inclusion and evaluation of additional parameters such as echocardiography. Furthermore, we were not able to analyze the postoperative RHH parameters due to unavailability and incompleteness of the historical data. Therefore, postoperative RVF could only been retrieved as reported or the need for postoperative RVAD. Further studies are needed to analyze the incremental value of post-HTx RHH parameters. Third, we did not include urine output in the definition of AKI because these patients were on intensive diuretic therapy, which would lead to misinterpretation of the urine output values. Altogether, this study is currently the largest HTx cohort in which the associations between preoperative hemodynamics and postoperative AKI were investigated.
Conclusion
Preoperative PAPi and RAP strongly predict the postoperative AKI early after HTx and can be used as early AKI predictors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
Goksel Guven and Milos Brankovic contributed equally.
Electronic supplementary material
The online version of this article (10.1007/s00134-018-5159-z) contains supplementary material, which is available to authorized users.
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology F, American Heart Association Task Force on Practice G 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Zijlstra LE, Constantinescu AA, Manintveld O, Birim O, Hesselink DA, van Thiel R, van Domburg R, Balk AH, Caliskan K. Improved long-term survival in Dutchheart transplant patients despite increasing donor age: the Rotterdam experience. Transpl Int. 2015;28:962–971. doi: 10.1111/tri.12503. [DOI] [PubMed] [Google Scholar]
- 3.Dellgren G, Geiran O, Lemstrom K, Gustafsson F, Eiskjaer H, Koul B, Hagerman I, Selimovic N, Nordic Thoracic Transplant Study G Three decades of heart transplantation in Scandinavia: long-term follow-up. Eur J Heart Fail. 2013;15:308–315. doi: 10.1093/eurjhf/hfs160. [DOI] [PubMed] [Google Scholar]
- 4.Fortrie G, Manintveld OC, Caliskan K, Bekkers JA, Betjes MG. Acute kidney injury as a complication of cardiac transplantation: incidence, risk factors, and impact on 1-year mortality and renal function. Transplantation. 2016;100:1740–1749. doi: 10.1097/TP.0000000000000956. [DOI] [PubMed] [Google Scholar]
- 5.Turker M, Zeyneloglu P, Sezgin A, Pirat A, Arslan G. RIFLE criteria for acute kidney dysfunction following heart transplantation: incidence and risk factors. Transplant Proc. 2013;45:3534–3537. doi: 10.1016/j.transproceed.2013.08.100. [DOI] [PubMed] [Google Scholar]
- 6.Schiferer A, Zuckermann A, Dunkler D, Eskandary F, Bernardi M, Hiesmayr M, Lassnigg A, Hutschala D. Acute kidney injury and outcome after heart transplantation: large differences in performance of scoring systems. Transplantation. 2016;100:2439–2446. doi: 10.1097/TP.0000000000001030. [DOI] [PubMed] [Google Scholar]
- 7.Gude E, Andreassen AK, Arora S, Gullestad L, Grov I, Hartmann A, Leivestad T, Fiane AE, Geiran OR, Vardal M, Simonsen S. Acute renal failure early after heart transplantation: risk factors and clinical consequences. Clin Transplant. 2010;24:E207–E213. doi: 10.1111/j.1399-0012.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- 8.Naeije R, Manes A. The right ventricle in pulmonary arterial hypertension. Eur Respir Rev. 2014;23:476–487. doi: 10.1183/09059180.00007414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perner A, Prowle J, Joannidis M, Young P, Hjortrup PB, Pettila V. Fluid management in acute kidney injury. Intensive Care Med. 2017;43:807–815. doi: 10.1007/s00134-017-4817-x. [DOI] [PubMed] [Google Scholar]
- 10.Ross EA. Congestive renal failure: the pathophysiology and treatment of renal venous hypertension. J Card Fail. 2012;18:930–938. doi: 10.1016/j.cardfail.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, Danziger-Isakov L, Kirklin JK, Kirk R, Kushwaha SS, Lund LH, Potena L, Ross HJ, Taylor DO, Verschuuren EA, Zuckermann A, International Society for Heart Lung Transplantation Infectious Diseases C, International Society for Heart Lung Transplantation Pediatric Transplantation C, International Society for Heart Lung Transplantation Heart F, Transplantation C The 2016 international society for heart lung transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant. 2016;35:1–23. doi: 10.1016/j.healun.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 12.Kang G, Ha R, Banerjee D. Pulmonary artery pulsatility index predicts right ventricular failure after left ventricular assist device implantation. J Heart Lung Transplant. 2016;35:67–73. doi: 10.1016/j.healun.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Korabathina R, Heffernan KS, Paruchuri V, Patel AR, Mudd JO, Prutkin JM, Orr NM, Weintraub A, Kimmelstiel CD, Kapur NK. The pulmonary artery pulsatility index identifies severe right ventricular dysfunction in acute inferior myocardial infarction. Catheter Cardiovasc Interv. 2012;80:593–600. doi: 10.1002/ccd.23309. [DOI] [PubMed] [Google Scholar]
- 14.Morine KJ, Kiernan MS, Pham DT, Paruchuri V, Denofrio D, Kapur NK. Pulmonary artery pulsatility index is associated with right ventricular failure after left ventricular assist device surgery. J Card Fail. 2016;22:110–116. doi: 10.1016/j.cardfail.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H, Luis Zamorano J. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 16.Guven G, Manintveld O, Brankovic M, Brugts J, Constantinescu A, Akin S, Hesselink D, Birim O, Caliskan K. Predictive value of right heart hemodynamics on the development of acute kidney injury early after heart transplantation. Eur Heart J. 2017;38(Supplement):1224–1225. [Google Scholar]
- 17.McAlister FA, Ezekowitz J, Tarantini L, Squire I, Komajda M, Bayes-Genis A, Gotsman I, Whalley G, Earle N, Poppe KK, Doughty RN. Renal dysfunction in patients with heart failure with preserved versus reduced ejection fraction: impact of the new chronic kidney disease—epidemiology collaboration group formula. Circ Heart Fail. 2012;5:309–314. doi: 10.1161/CIRCHEARTFAILURE.111.966242. [DOI] [PubMed] [Google Scholar]
- 18.National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 19.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Herzog CA, Joannidis M, Kribben A, Levey AS, MacLeod AM. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 20.Guyton AC, Lindsey AW, Abernathy B, Richardson T. Venous return at various right atrial pressures and the normal venous return curve. Am J Physiol. 1957;189:609–615. doi: 10.1152/ajplegacy.1957.189.3.609. [DOI] [PubMed] [Google Scholar]
- 21.Jardin F, Bourdarias JP. Right heart catheterization at bedside: a critical view. Intensive Care Med. 1995;21:291–295. doi: 10.1007/BF01705405. [DOI] [PubMed] [Google Scholar]
- 22.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, Müller M. pROC: an open-source package for R and S + to analyze and compare ROC curves. BMC Bioinform. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 24.Alzola C, Harrell F (2004) An introduction to S and the Hmisc and design libraries at http://biostat.mc.vanderbilt.edu/twiki/pub/Main/RS/sintro.pdf for extensive documentation and examples for the Hmisc package. Accessed 15 June 2017
- 25.Fox J. Effect displays in R for generalised linear models. J Stat Softw. 2003;8:1–9. doi: 10.18637/jss.v008.i15. [DOI] [Google Scholar]
- 26.Angelini A, Castellani C, Virzi GM, Fedrigo M, Thiene G, Valente M, Ronco C, Vescovo G. The role of congestion in cardiorenal syndrome type 2: new pathophysiological insights into an experimental model of heart failure. Cardiorenal Med. 2015;6:61–72. doi: 10.1159/000440775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gambardella I, Gaudino M, Ronco C, Lau C, Ivascu N, Girardi LN. Congestive kidney failure in cardiac surgery: the relationship between central venous pressure and acute kidney injury. Interact Cardiovasc Thorac Surg. 2016;23:800–805. doi: 10.1093/icvts/ivw229. [DOI] [PubMed] [Google Scholar]
- 28.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 29.Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House AA, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P, Acute Dialysis Quality Initiative consensus g Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31:703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joannidis M, Druml W, Forni LG, Groeneveld ABJ, Honore PM, Hoste E, Ostermann M, Oudemans-van Straaten HM, Schetz M. Prevention of acute kidney injury and protection of renal function in the intensive care unit: update 2017: expert opinion of the working group on prevention, AKI section, European Society of Intensive Care Medicine. Intensive Care Med. 2017;43:730–749. doi: 10.1007/s00134-017-4832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, Zarbock A. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the prevAKI randomized controlled trial. Intensive Care Med. 2017;43:1551–1561. doi: 10.1007/s00134-016-4670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pickkers P, Ostermann M, Joannidis M, Zarbock A, Hoste E, Bellomo R, Prowle J, Darmon M, Bonventre JV, Forni L, Bagshaw SM, Schetz M. The intensive care medicine agenda on acute kidney injury. Intensive Care Med. 2017;43:1198–1209. doi: 10.1007/s00134-017-4687-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobashigawa J, Zuckermann A, Macdonald P, Leprince P, Esmailian F, Luu M, Mancini D, Patel J, Razi R, Reichenspurner H, Russell S, Segovia J, Smedira N, Stehlik J, Wagner F, Consensus Conference p Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J Heart Lung Transpl. 2014;33:327–340. doi: 10.1016/j.healun.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 34.Chawla LS, Davison DL, Brasha-Mitchell E, Koyner JL, Arthur JM, Shaw AD, Tumlin JA, Trevino SA, Kimmel PL, Seneff MG. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care. 2013;17:R207. doi: 10.1186/cc13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ronco C, Bellomo R, Kellum J. Understanding renal functional reserve. Intensive Care Med. 2017;43:917–920. doi: 10.1007/s00134-017-4691-6. [DOI] [PubMed] [Google Scholar]
- 36.Joannidis M, Metnitz B, Bauer P, Schusterschitz N, Moreno R, Druml W, Metnitz PG. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009;35:1692–1702. doi: 10.1007/s00134-009-1530-4. [DOI] [PubMed] [Google Scholar]
- 37.Kellum JA, Lameire N, Group KAGW Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1) Crit Care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tjahjono R, Connellan M, Granger E. Predictors of acute kidney injury in cardiac transplantation. Transpl Proc. 2016;48:167–172. doi: 10.1016/j.transproceed.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Janus N, Launay-Vacher V, Sebbag L, Despins P, Epailly E, Pavie A, Obadia JF, Pattier S, Varnous S, Pezzella V, Trillaud L, Deray G, Guillemain R. Renal insufficiency, mortality, and drug management in heart transplant. Results of the CARIN study. Transpl Int. 2014;27:931–938. doi: 10.1111/tri.12359. [DOI] [PubMed] [Google Scholar]
- 40.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honore PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI–EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.