Abstract
Research of dopaminergic deficits has focused on the ventral striatum (VS) with many studies elucidating altered resting state functional connectivity (rsFC) in individuals with cocaine dependence (CD). The VS comprises functional subregions and delineation of subregional changes in rsFC requires careful consideration of the differences between addicted and healthy populations. In the current study, we parcellated the VS using whole-brain rsFC differences between CD and non-drug-using controls (HC). Voxels with similar rsFC changes formed functional clusters. The results showed that the VS was divided into 3 subclusters, in the area of the dorsal-anterior VS (daVS), dorsal posterior VS (dpVS), and ventral VS (vVS), each in association with different patterns of rsFC. The three subregions shared reduced rsFC with bilateral hippocampal/parahippocampal gyri (HG/PHG) but also showed distinct changes, including reduced vVS rsFC with ventromedial prefrontal cortex (vmPFC) and increased daVS rsFC with visual cortex in CD as compared to HC. Across CD, daVS visual cortical connectivity was positively correlated with amount of prior-month cocaine use and cocaine craving, and vVS vmPFC connectivity was negatively correlated with the extent of depression and anxiety. These findings suggest a distinct pattern of altered VS subregional rsFC in cocaine dependence, and some of the changes have eluded analyses using the whole VS as a seed region. The findings may provide new insight to delineating VS circuit deficits in cocaine dependence and provide an alternative analytical framework to address functional dysconnectivity in other mental illnesses.
Introduction
Abundant research of dopaminergic deficits in addiction has focused on the ventral striatum (VS)1. The VS receives projections from the dopaminergic midbrain and processes reward-related stimuli, including those associated with drugs of abuse1,2. For instance, positron emission tomography (PET) imaging studies showed that the extracellular concentration of dopamine in the VS increased with drug administration, in link with experienced euphoria3. As with dopamine receptor blockade, dopamine-specific VS lesioning disrupted self-administration of intravenous cocaine4. In a study combining PET and magnetic resonance imaging, dopamine D2 receptor availability in the VS was significantly related to the blood oxygenation level-dependent (BOLD) responses to monetary reward in individuals with cocaine dependence (CD)5. Other studies showed that the VS responded to both acute cocaine administration and cue-elicited craving6–9.
Individuals with cocaine addiction demonstrated altered functional connectivities of the VS10–19, including reduced resting state functional connectivity (rsFC) between the VS and precuneus/posterior cingulate cortex17. These studies employed various templates of the VS as the seed region. On the other hand, the inferior and superior VS each showed connectivity with the medial and lateral orbitofrontal cortex20 and the ventral tegmental area (VTA) showed reduced rsFC with right dorsal posterior VS but not with the entire VS in CD12. These findings suggest distinct VS subregional connectivities, which may differ between CD and non-drug using healthy controls (HC) in specific patterns. Importantly, as a result of the chronic influence of cocaine, VS functional subregions may not follow the same boundary in CD as in HC. It would require a new approach to investigate how VS connectivities are altered in cocaine addiction or other conditions that implicate dopaminergic dysfunction.
Functional subdivisions of a brain region can be parcellated via unique patterns of connectivities21. Specifically, low-frequency BOLD signal fluctuations reflect connectivity between functionally related brain regions, which characterizes the intrinsic organization of the brain22. As regions with similar functionality tend to be correlated in their spontaneous BOLD activity, investigators described subareal functional boundaries for many cortical and subcortical structures. For instance, our previous study showed that the precuneus could be divided into dorsal-anterior, dosal-posterior, and ventral subregions by rsFC, each with distinct and sometimes an opposing pattern of regional connectivities23. It is argued that although not necessarily in agreement with anatomical demarcations, these functional boundaries may better explain regional activations to task challenges and disambiguate seeming discrepancies across studies24.
The current work aimed to address VS subareal rsFC in cocaine addiction. Considering that altered connectivity patterns of the VS may influence functional clustering of the subregions, we employed the connectivity differences between CD and HC to parcellate the VS. Thus, voxels with a similar difference pattern will be grouped into one cluster and a comparison of whole-brain connectivity of these clusters would reflect how CD and HC differ in VS connectivity. We also compared these results with those obtained with the entire VS as a seed region and examined how these regional connectivity differences relate to clinical characteristics.
Materials and methods
Subjects, informed consent, and assessment
Sixty-six recently abstinent participants (44 men) with cocaine dependence (CD) and 66 age- and gender-matched healthy control (HC) subjects (36 men) participated in the study (Supplementary Table 1). CD met criteria for current cocaine dependence, as diagnosed by the Structured Clinical Interview for DSM-IV25. Recent cocaine use was confirmed by urine toxicology screens. CD were drug-free while staying in an inpatient unit prior to the current fMRI study. All subjects were physically healthy with no major medical illnesses or current use of prescription medications. None reported having a history of head injury or neurological illness. Other exclusion criteria included dependence on another psychoactive substance (except nicotine) and current or past history of psychotic disorders. Individuals with current depressive or anxiety symptoms requiring treatment or currently being treated for these symptoms were excluded as well. The Human Investigation committee at Yale University School of Medicine approved all study procedures, and all subjects signed an informed consent prior to study participation.
CD’s were assessed with the Beck Depression Inventory26 and the State-Trait Anxiety Inventory27 at admission. The mean ( ± SD) BDI (9.5 ± 8.1) and STAI state (33.8 ± 11.0) and trait (38.3 ± 9.8) scores were within the range reported previously for individuals with cocaine dependence28,29. Cocaine craving was assessed with the cocaine craving questionnaire, brief version (CCQ-Brief), for all participants every two to three days30. The CCQ-Brief is a 10-item questionnaire, abbreviated from the CCQ-Now. It is highly correlated with the CCQ-Now and other cocaine craving measures30. Each item was rated on a scale from 1 to 7, with a higher total score (ranging from 10 to 70) indicating greater craving. CD showed a CCQ score of 23.8 ± 10.4 across all assessments.
Imaging protocol
Conventional T1-weighted spin echo sagittal anatomical images were acquired for slice localization using a 3 T scanner (Siemens Trio). Anatomical images of the functional slice locations were next obtained with spin echo imaging in the axial plane parallel to the AC–PC line with TR = 300 ms, TE = 2.5 ms, bandwidth = 300 Hz/pixel, flip angle = 60°, field of view = 220 × 220 mm, matrix = 256 × 256, 32 slices with slice thickness = 4 mm and no gap. Functional, blood oxygenation level-dependent (BOLD) signals were then acquired with a single-shot gradient echo echoplanar imaging (EPI) sequence during a resting state (one scan per participant; duration: 10 minutes; eyes closed). Thirty-two axial slices parallel to the AC–PC line covering the whole brain were acquired with TR = 2000 ms, TE = 25 ms, bandwidth = 2004 Hz/pixel, flip angle = 85°, field of view = 220 × 220 mm, matrix = 64 × 64, 32 slices with slice thickness = 4 mm and no gap.
Imaging data preprocessing
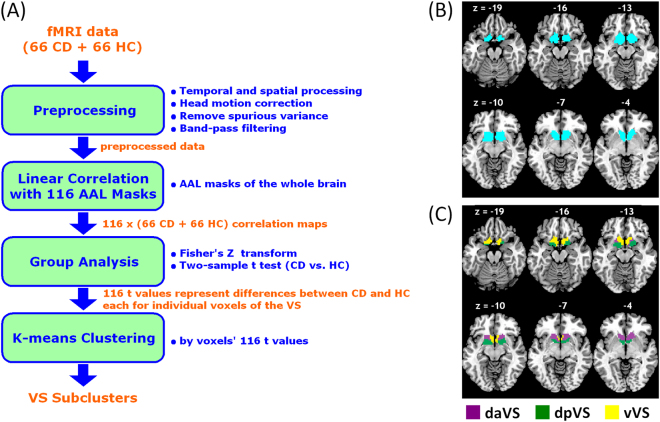
Figure 1a illustrates the step by step procedures in data analysis. The data were processed with Statistical Parametric Mapping (SPM8, Wellcome Department of Imaging Neuroscience, University College London, U.K.). Images from the first five TRs at the beginning of each session were discarded. Images of each individual subject were first realigned (motion corrected) and corrected for slice timing. A mean functional image volume was constructed from the realigned image volumes. These mean images were co-registered with the high-resolution structural image and segmented for normalization with affine registration followed by nonlinear transformation. The normalization parameters determined for the structure volume were then applied to the corresponding functional image volumes for each subject. Finally, the images were smoothed with a Gaussian kernel of 4 mm at Full Width at Half Maximum.
Fig. 1. Data analytic procedures and subregions of the ventral striatum.
(a) A flow chart of data analysis. (b) Seed region: the ventral striatum (VS). (c) K-means clustering segments the VS based on connectivity differences between CD and HC of individual voxels within the region. Three subclusters were represented with different colors as dorsal-anterior VS (daVS), dorsal posterior VS (dpVS), and ventral VS (vVS)
Additional preprocessing was applied to reduce spurious BOLD variances that were unlikely to reflect neuronal activity. The sources of spurious variance were removed through linear regression by including the signal from the ventricular system, white matter, and whole brain, in addition to the six parameters obtained by rigid body head motion correction22,31. First-order derivatives of the whole brain, ventricular and white matter signals were also included in the regression.
Cordes and colleagues suggested that BOLD fluctuations below a frequency of 0.1 Hz contribute to regionally specific BOLD correlations32. Thus, we applied a temporal band-pass filter (0.009 Hz < f < 0.08 Hz) to the time course in order to obtain low-frequency fluctuations, as in previous studies22.
Head motion
As extensively investigated in previous study, micro head motion (>0.1 mm) is an important source of spurious correlations in resting state functional connectivity analysis33. Therefore, we applied a “scrubbing” method proposed by Power and colleagues34 and successfully applied in previous studies35 to remove time points affected by head motions, prior to temporal band-pass filtering. Briefly, for every time point t, we computed the framewise displacement given by, where (dx, dy, dz) and (α, β, γ) are the translational and rotational movements, respectively34. The second head movement metric was the root mean square variance (DVARS) of the differences in % BOLD intensity I(t) between consecutive time points across brain voxels, computed as: , where the brackets indicate the mean across brain voxels. Finally, to compute each subject’s correlation map, we removed every time point with FD(t) > 0.5 mm or DVARS(t) > 0.5%34,35. On average, 1% of the time points were removed across subjects. CD and HC did not differ in either the mean FD (p = 0.76) or DVARS (p = 0.57).
Parcelation of the VS based on functional connectivity differences between CD and HC
As with our previous studies36,37, we used a ventral striatum (VS) mask (Fig. 1b) generated by cytoarchitectonic and topographical criteria38. We employed 116 anatomical masks from the Automated Anatomical Labeling or AAL Atlas based on a Montreal Neurological Institute (MNI) template39. We averaged the BOLD time courses of voxels within each of the 116 regions and computed the correlation coefficient between the averaged time course of each AAL region and the time courses of each individual voxel of the VS for individual subjects. To assess and compare the resting state “correlograms (correlation matrices),” we converted these correlation matrices, which were not normally distributed, to z score maps by Fisher’s z transform:. The z maps were used in group, random effect analyses.
A two-sample t test was first applied to the “z maps” to compare 66 CD with 66 HC for each of the 116 correlograms. Voxels within the VS mask were subject to rsFC based segmentation, with each voxel represented by 116 t values of the two-sample t test. A K-means algorithm was applied to cluster the voxels within the VS on the bases of the 116 t values.
As an unsupervised learning algorithm, K-means clustering classifies a given data set into an a-priori set of K clusters by minimizing an objective squared error function as shown in Eq. (1):.
| 1 |
where is a distance measure between a data point and the cluster center 40. The algorithm was executed by:
Placing K points into the space represented by the objects that are being clustered. These points represent initial group centroids.
Assigning each object to the group that has the closest centroid.
Recalculating the positions of the K centroids, when all objects have been assigned.
Repeating Steps 2 and 3 until the centroids no longer move. This produces a separation of the objects into groups from which the metric to be minimized can be calculated.
In order to determine the optimal number of clusters that best described the data set, we used the Bayesian Information Criterion (BIC), which is widely used for model identification in time series and linear regression:
| 2 |
where n is the number of observations (=116); k is the number of class; RSS is the residual sum of squares from the K-means model. Given any two clustering number k’s, the one with lower BIC value was preferred. Furthermore, because the K-means algorithm is sensitive to the initial, randomly selected cluster centers, we repeated this algorithm 1,000 times to alleviate the effect of the initial conditions. K was tested for values from 2 to 20.
For VS subregions as well as the whole VS, we computed the whole-brain connectivity maps each for CD and HC, and converted to z score maps. In second level analysis, we performed one-sample t test each on the Z maps of those seeds for CD and HC groups and two-sample t test with age as covariate comparing the two groups. In addition, the VS and hypothalamus are reciprocally connected2. Both the VS and hypothalamus receive projections from the dopaminergic midbrain and are implicated in processing of reward-related stimuli, including those associated with drugs of abuse2,41–44. Examining the VS hypothalamus connectivity may provide useful information as to how this understudied circuit is impacted by cocaine addiction3,42,45–47. Thus, we also conducted a region of interest analysis, using small volume correction for a hypothalamus mask48.
Results
VS functional subclusters and connectivity differences between CD and HC
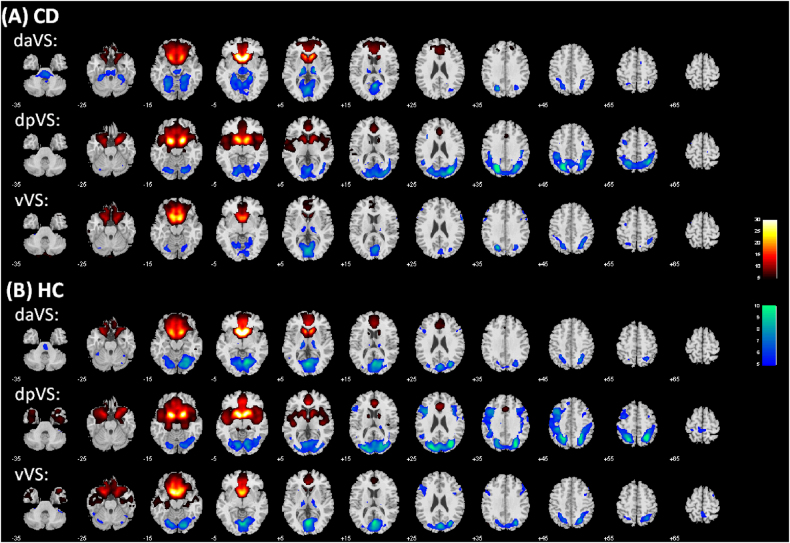
The results of 1,000 runs of K-means clustering suggested an optimal cluster number of 3 according to the BIC (Supplementary Figure 1). Figure 1c showed the three clusters as dorsal anterior (daVS), dorsal posterior (dpVS), and ventral VS (vVS). Figure 2 showed the whole-brain connectivity maps of the three clusters each for CD and HC.
Fig. 2. Ventral striatum subregional connectivity.
One-sample t test results of functional connectivity maps of the dorsal anterior VS (daVS), dorsal posterior VS (dpVS), and ventral VS (vVS) each for (a) CD and (b) HC. p < 0.05, corrected for familywise error of multiple comparisons
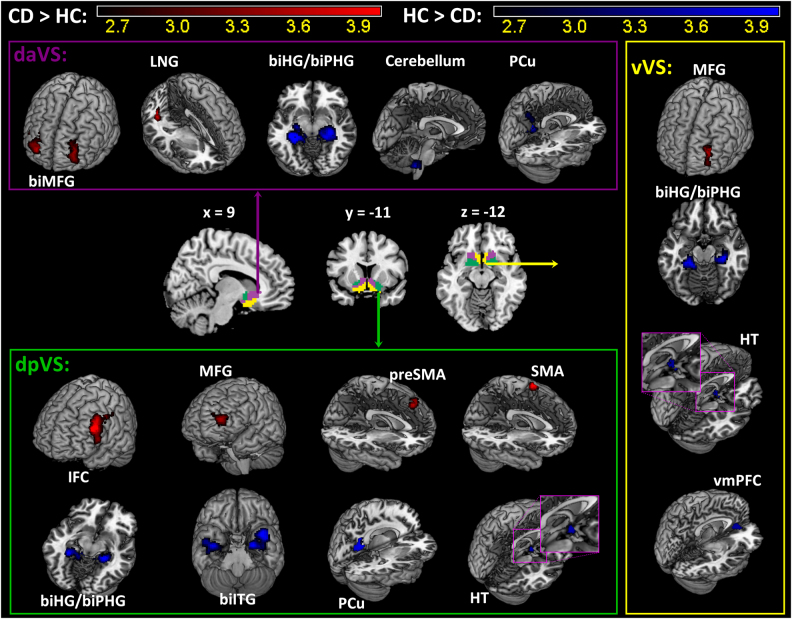
We then examined differences in connectivity of each of the three subclusters in CD as compared to HC in a two-sample t test with age as a covariate at voxel p < 0.005, uncorrected, in combination with cluster p < 0.05, FWE corrected, on the basis of Gaussian random field theory as implemented in SPM. The results are shown in Fig. 3 and Supplementary Table 2. Compared to HC, CD showed increased left middle frontal gyrus (MFG) connectivity and decreased bilateral hippocampal/parahippocampal gyri (HG/PHG) connectivity for all three subclusters. The subclusters also showed distinct differences in connectivity between CD and HC. Both daVS and dpVS showed decreased rsFC with the precuneus, and both dpVS and vVS showed decreased rsFC with the hypothalamus, in CD as compared to HC. Further, the daVS showed increased rsFC with the right MFG and lingual gyrus, and decreased rsFC with the cerebellum, in CD as compared to HC. The dpVS showed increased rsFC with the left inferior frontal cortex (IFC), supplementary motor area (SMA), and pre-SMA, and decreased rsFC with the bilateral inferior temporal gyri (ITG). The vVS showed decreased rsFC with the ventromedial prefrontal cortex (vmPFC) in CD as compared to HC.
Fig. 3. Altered functional connectivity in CD compared to HC is shown for each of the three subclusters of the VS.
Warm color: CD > HC; cool color: HC > CD. Details can be found in Supplementary Table 2. LNG: Lingual gyrus; biHG: bilateral hippocampus; biPHG: bilateral parahippocampus; PCu: precuneus; IFC: inferior frontal cortex; MFG: middle frontal gyrus; SMA: supplementary motor area; preSMA: pre-supplementary motor area; biITG: bilateral inferior temporal gyrus; vmPFC: ventromedial prefrontal gyrus; HT: hypothalamus
Relationship to clinical characteristics
For CD, we examined whether these connectivity changes are related to clinical characteristics with a linear regression of the connectivity strength (z score) of each ROI against recent cocaine use (amount of cocaine use in the past month, grams), craving (CCQ score), the extent of depression (BDI score) and of anxiety (STAI state and trait score). Because of the 17 identified connectivity pairs and five clinical measures, we evaluated the results at a corrected p = 0.05 / (17 × 5) = 0.00059.
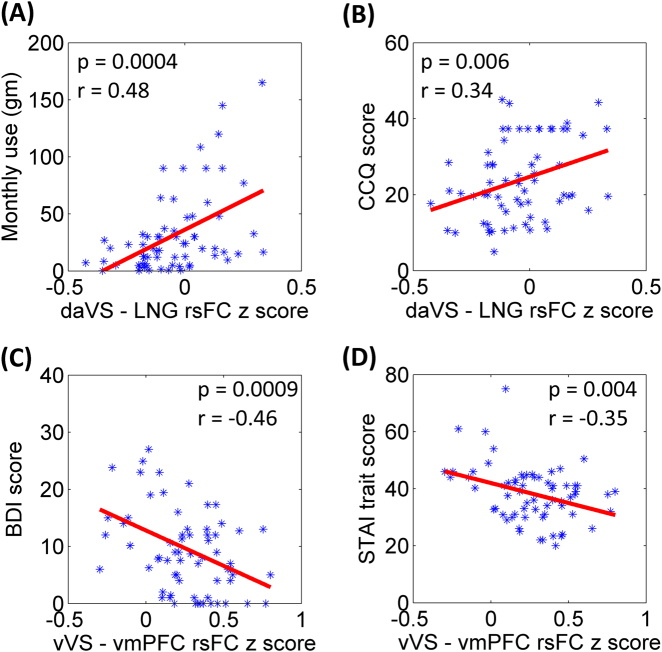
Across CD, the daVS and lingual gyrus connectivity strength was positively correlated with amount of cocaine use in the past month (p = 0.0004, r = 0.48), and, at a trend level, averaged CCQ score (p = 0.006, r = 0.34). The vVS–vmPFC connectivity was negatively correlated with the BDI score (p = 0.0009, r = −0.46) and, at a trend level, STAI trait score (p = 0.004, r = −0.35). Figure 4 summarizes these results.
Fig. 4. Correlation of VS connectivity with clinical measures.
Dorsal anterior VS (daVS) connectivity with the lingual gyrus (LNG) was positively correlated with (a) the average monthly use of cocaine (grams) in the prior year and with (b) craving score as assessed by the Cocaine Craving Questionnaire (CCQ). Ventral VS (vVS) connectivity with the ventromedial prefrontal cortex (vmPFC) was negatively correlated with (c) depression score, as assessed by the Beck Depression Inventory (BDI) and with (d) trait anxiety score, as assessed by the Spielberger State Trait Anxiety Inventory (STAI). Correlations in (b) and (d) were significant only at a trend level, with correction for multiple comparisons
Connectivity of the whole VS
For comparison, we examined how CD and HC differed in rsFC with the whole VS as the seed. Figure 5 shows the one-sample t test results of whole-brain rsFC each for CD and HC as well as two-sample t test results of CD vs. HC. In both CD and HC, VS showed positive connectivity with the dorsal and ventral medial prefrontal cortex, caudate, and insula, and negative connectivity with the thalamus, inferior parietal gyrus, and visual cortex. Compared to HC, CD showed increased VS connectivity with the bilateral middle frontal gyri (x = −21, y = 38, z = 16, peak voxel Z = 3.84, volume = 5,832 mm3; x = 27, y = 32, z = 16, peak voxel Z = 3.49, volume = 4,833 mm3), decreased VS connectivity with the bilateral HG/PHG (x = −30, y = −34, z = −8, peak voxel Z = 4.64, volume = 11,016 mm3; x = 30, y = −28, z = −14, peak voxel Z = 5.02, volume = 8,775 mm3), at a threshold of voxel p < 0.005 uncorrected in combination with cluster-level p < 0.05, FWE corrected. At a relaxed threshold – voxel p < 0.005 uncorrected and AlphaSim p < 0.05 corrected-CD additionally showed increased VS connectivity with the left IFC (x = −42, y = 17, z = 25, peak voxel Z = 3.96, volume = 1,431 mm3) and decreased VS connectivity with the precuneus (x = −15, y = −49, z = 16, peak voxel Z = 4.18, volume = 3078 mm3).
Fig. 5. Ventral striatum connectivity in CD and HC.
One sample t test of whole-brain functional connectivity with the entire VS as the seed each in (a) CD and (b) HC, p < 0.05 FWE; as well as (c) the two-sample t test results of CD vs. HC, p < 0.005 uncorrected
We further examined whether these connectivity differences are related to clinical characteristics. The VS – precuneus connectivity was correlated with amount of cocaine use in the past month at p = 0.03, r = 0.27, and all other correlations were at p’s > 0.17, none of which were significant considering multiple comparisons.
Discussion
We demonstrated that the VS could be separated into dorsal anterior (daVS), dorsal posterior (dpVS), and ventral (vVS) subregions on the basis of whole-brain resting state functional connectivity (rsFC) differences between CD and HC. These subregions reflect how the VS is functionally organized in terms of the differences between CD and HC in voxelwise connectivity. CD showed increased connectivity with the left middle frontal gyrus (MFG) and decreased connectivity in the bilateral hippocampal/parahippocampal gyri (HG/PHG) for all three subclusters. As expected, these changes were also reflected in the findings with the whole VS as the seed.
In addition to the shared patterns of change, the daVS, dpVS, and vVS each showed specific alterations in connectivity. Moreover, the altered connectivities were associated with the clinical measures in CD. The strength of daVS connectivity with the lingual gyrus was positively correlated with amount of cocaine use in the prior month and cocaine craving. The vVS–vmPFC connectivity was negatively correlated with depression and anxiety. Together, the findings may provide new insight to delineating circuit level deficits in cocaine dependence. We highlighted some of the major findings in discussion.
Decreased VS–hippocampus/parahippocampus (HG/PHG) rsFC in cocaine addiction
CD showed decreased connectivity of all three VS subergions with bilateral HG/PHG. The VS and HG/PHG are reciprocally connected as part of the limbic circuit2,49, and as with the VS, the hippocampus responds to cue-induced cocaine craving7,50,51. PET imaging reported cocaine cue-induced dopamine release in both HG and VS52. With fMRI, a double-blind study evaluated subjective effects of rush, high, low, and craving following cocaine (0.6 mg/kg) or saline infusion in cocaine addicted participants9. Cocaine-induced signal increases were observed in a host of brain regions including both HG and VS. Notably, activations of the HG, VS and some of the lateral prefrontal cortical regions are more correlated with craving than with rush ratings while other brain regions showed the opposite pattern of response.
In animal studies, cocaine decreased cerebral metabolism in both HG and VS in monkeys53. Unilateral infusion of N-methyl-d-aspartate in the HG increased dopamine efflux in the VS, and this effect was greater in rats receiving repeated cocaine as compared to controls receiving saline injections49. The results suggested that HG–VS circuit was impacted by repeated exposure to cocaine. Repeated cocaine exposure potentiated hippocampal inputs to the VS in in-vitro studies54–56. Further, following withdrawal from repeated cocaine administration, rats showed attenuated long-term potentiation in the HG–VS circuit57. Together, these studies suggest a critical role of the HG–VS circuit in supporting dopaminergic signaling and responses to cocaine craving. The current findings of decreased HG/PHG–VS connectivity may reflect an attenuated circuit activity in CD when they became abstinent recently. It would be of great interest to investigate whether diminished HG/PHG–VS connectivity may conduce to longer-term abstinence in a longitudinal study.
VS-prefrontal cortical rsFC in cocaine addiction
As compared to HC, CD showed decreased functional connectivity between vVS and vmPFC, and the vVS–vmPFC connectivity was negatively correlated with depression and anxiety in CD. The vmPFC responds to drug cues in numerous imaging studies of substance abuse58. Anatomically and functionally interconnected2, the vmPFC and nucleus accumbens (NAc) are both implicated in the etiological processes of drug addiction59. For instance, vmPFC glutamatergic projections to the NAc were involved in reinstatement of cocaine seeking60,61. Increased Fos expression in the vmPFC, including those neurons projecting to NAc, was associated with context-induced reinstatement of heroin seeking62. In human imaging, transcranial direct current (in contrast with sham) stimulation of dlPFC enhanced the integrity of the white matter tracks connecting the vmPFC and NAc and alleviated drug craving63. Of direct relevance to the current finding, both the vmPFC and VS are involved in emotional regulation and the pathogenesis of depression64. Reduced gray matter volumes of both vmPFC and VS have been reported in depression, and deep brain stimulation may alter the activity of the vmPFC and VS in the treatment of depression65. A recent meta-analysis showed increased activities in both vmPFC and VS in relation to self-referential processing in depressed patients as compared with healthy controls66. Together with these earlier studies, the current findings suggest compromised vVS–vmPFC connectivity in association with negative mood and perhaps other emotion regulation deficits in cocaine dependence.
Notably, two earlier studies reported decreased67 and increased19 VS-vmPFC connectivity, respectively, in cocaine addiction, each seemingly in accord and in contrast with the current findings. However, the latter study employed ventral caudate as the seed region, whereas in the current work the vVS cluster is located at a more ventral location. In the former study, the vmFPC appeared to be in a location more ventral and posterior to the cluster we reported here. As with the VS, the vmPFC is known for subareal functional heterogeneity68. These disparities highlight the importance to carefully define functional subdivisions of an anatomical area.
We observed increased VS–MFC and dpVS–left IFC connectivity in CD. Studies have reported aberrant MFC/IFC activation during emotion processing in addicted individuals58,69. The VS responds to drug reward70 but also to negative emotions, noxious stimulation and pain71–77. In rodents, appetitive and defensive behavior is supported rostrocaudally in the NAc. Environmental manipulation (home vs. a new, overstimulated cage) can shrink or expand these response zones and prefrontal glutamatergic inputs were critical in determining the boundary72. Thus, increased VS–MFC/IFC connectivity may impact motivated behavior, perhaps in favor of overcharged negative responses, in CD. Further, the MFC/IFC is part of the task control circuit and disrupted in addicted individuals42. A previous study showed that the reduction in D2R signaling in the VS leads to reduced activity in the IFC78. Deactivations of both the VS and IFC were observed during decision making in relapsed cocaine users as compared to abstinent individuals79. Cocaine-induced craving correlated positively with activity in both VS and IFC during cocaine self-administration in non-treatment-seeking CD8. Increased MFC/IFC VS connectivity may be associated with deficits of affective and craving control in dependent cocaine users.
VS-visual cortical connectivity in cocaine addiction
The daVS – visual cortex connectivity was positively correlated with the amount of cocaine use in the past month as well as the CCQ score. Although not typically a focus of the addiction literature, the visual cortex was often reported to be activated during exposure to drug cues51,80,81. A recent meta-analysis showed that 86% of published functional imaging studies of addiction reported significant drug cue-induced activity in the visual cortex80. Treatment-seeking participants as well as participants with strong motivation to quit demonstrated decreased cocaine cue-induced visual cortical activation, as compared to non-treatment-seeking and less motivated participants82. The current finding of daVS visual cortical connectivity in positive correlation with recent cocaine use and craving is consistent with this literature. Notably, changes in VS-visual cortical connectivity eluded analyses with the whole VS as a seed region.
Other changes of VS rsFC in cocaine addiction
Both dpVS and vVS showed decreased rsFC with the hypothalamus in CD as compared to HC. The VS and hypothalamus both receive projections from the dopaminergic midbrain and respond to reward and motivated behavior2. Previous studies have implicated the hypothalamus in drug addiction83,84. In rodents, the transition from controlled to compulsive cocaine self-administration was associated with substantial remodeling of hypothalamic circuitry85–87. In humans, cocaine addicted individuals showed decreased hypothalamus activation viewing erotic vs. neutral pictures as compared to non-drug using controls88. Further, hypothalamus response to monetary reward vs. non-reward was negatively correlated with the duration of abstinence from cocaine use89. In both studies, the peak activities were located more in medial than in lateral hypothalamus (MNI coordinates: x = 0, y = 2, z = −14 and x = 3, y = 2, z = −14), consistent with an earlier report that the VS was more heavily connected to the medial than to lateral hypothalamus90. Decreased VS hypothalamus connectivity may have implications for altered motivational processes in cocaine addiction.
Compared to HC, CD showed decreased rsFC between daVS and cerebellum. Although the cerebellum is not conventionally considered as part of addiction circuit, it has been related to addiction by its conspicuous response to methylphenidate91. As the authors suggested, the cerebellar response could be explained as a downstream effect from dopaminergic stimulation of the striatum, as methylphenidate-induced increases in cerebellar activity is predicted by dopamine D2 receptor levels in striatum91,92. Although rarely discussed in detail, many studies showed cerebellar activation in association with cocaine administration or cue-induced cocaine craving8,93–97. More broadly, voxel-based morphometry98–100 showed decreased gray matter volume in the cerebellum in CD as compared to HC. In particular, decreases of gray matter in both striatum and cerebellum were associated with long-term exposure to cocaine99. The current findings add to this literature and research is needed to understand a more focused role of the cerebellum in addiction.
Limitations of the study and conclusions
A few important limitations need to be considered. First, VS mapping based on connectivity differences builds on the rationale that rsFC's differ between CD and HC. Thus, the VS functional divisions as demonstrated here should be considered as specific to cocaine addiction. It remains to be seen whether these functional clusters apply to other substance use disorders or mental illnesses that implicate dopaminergic dysfunction. Second, the current work addressed rsFC and it remains unclear how the findings may apply to studies of cognitive and affective challenges in cocaine-dependent individuals. In summary, we parcellated the VS into three subclusters based on the differences in whole brain functional connectivity between CD and HC. The three VS subregions showed both shared and distinct patterns of altered connectivity. Changes in VS connectivity with the hippocampal and parahippocampal gyrus supports the importance of contextual memory in shaping habitual cocaine use; changes in VS connectivity with the visual cortex may reflect craving and drug seeking motivation; and changes in VS connectivity with the vmPFC are associated with depression and anxiety, a common characteristic of cocaine addicted individuals. Overall, the results confirm ventral striatal functional deficits in relation to cocaine misuse and provide more specific information about the subregional organization of these deficits.
Electronic supplementary material
Acknowledgements
This study was supported by NIH grants DA040032 and DA023248, as well as the Peter McManus Charitable Trust. The funding agencies are otherwise not involved in the study or in the decision to publish the findings.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41398-018-0164-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc. Natl Acad. Sci. USA. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haber, S. N. in Neuroanatomy of Reward: A View from the V entral Striatum (ed Gottfried, J. A.) (CRC Press/Taylor & Francis, Boca Raton, FL, 2011). [PubMed]
- 3.Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56:3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suto N, Wise RA, Vezina P. Dorsal as well as ventral striatal lesions affect levels of intravenous cocaine and morphine self-administration in rats. Neurosci. Lett. 2011;493:29–32. doi: 10.1016/j.neulet.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asensio S, et al. Striatal dopamine D2 receptor availability predicts the thalamic and medial prefrontal responses to reward in cocaine abusers three years later. Synapse. 2010;64:397–402. doi: 10.1002/syn.20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kufahl PR, et al. Neural responses to acute cocaine administration in the human brain detected by fMRI. Neuroimage. 2005;28:904–914. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 7.Kilts CD, Gross RE, Ely TD, Drexler KPG. The neural correlates of cue-induced craving in cocaine-dependent women. Am. J. Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- 8.Risinger RC, et al. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 9.Breiter HC, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/S0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 10.Cisler JM, et al. Altered functional connectivity of the insular cortex across prefrontal networks in cocaine addiction. Psychiatry Res. 2013;213:39–46. doi: 10.1016/j.pscychresns.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding X, Lee SW. Cocaine addiction related reproducible brain regions of abnormal default-mode network functional connectivity: A group ICA study with different model orders. Neurosci. Lett. 2013;548:110–114. doi: 10.1016/j.neulet.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 12.Gu H, et al. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konova AB, Moeller SJ, Tomasi D, Volkow ND, Goldstein RZ. Effects of methylphenidate on resting-state functional connectivity of the mesocorticolimbic dopamine pathways in cocaine addiction. JAMA Psychiatry. 2013;70:857–868. doi: 10.1001/jamapsychiatry.2013.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narayanan A, et al. Functional connectivity during language processing in acute cocaine withdrawal: a pilot study. Neurocase. 2012;18:441–449. doi: 10.1080/13554794.2011.627341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomasi D, et al. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS ONE. 2010;5:e10815. doi: 10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verdejo-Garcia A, et al. Functional alteration in frontolimbic systems relevant to moral judgment in cocaine-dependent subjects. Addict. Biol. 2012;19:272–281. doi: 10.1111/j.1369-1600.2012.00472.x. [DOI] [PubMed] [Google Scholar]
- 17.Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend. 2011;115:137–144. doi: 10.1016/j.drugalcdep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albein-Urios N, et al. Re-appraisal of negative emotions in cocaine dependence: dysfunctional corticolimbic activation and connectivity. Addict. Biol. 2012;19:415–426. doi: 10.1111/j.1369-1600.2012.00497.x. [DOI] [PubMed] [Google Scholar]
- 19.Contreras-Rodriguez O, et al. Cocaine-specific neuroplasticity in the ventral striatum network is linked to delay discounting and drug relapse. Addiction. 2015;110:1953–1962. doi: 10.1111/add.13076. [DOI] [PubMed] [Google Scholar]
- 20.Di Martino A, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb. Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 21.Passingham RE, Stephan KE, Kotter R. The anatomical basis of functional localization in the cortex. Nat. Rev. Neurosci. 2002;3:606–616. doi: 10.1038/nrn893. [DOI] [PubMed] [Google Scholar]
- 22.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Li CS. Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage. 2012;59:3548–3562. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S, Li CS. Functional clustering of the human inferior parietal lobule by whole-brain connectivity mapping of resting-state functional magnetic resonance imaging signals. Brain Connect. 2014;4:53–69. doi: 10.1089/brain.2013.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.First, M., Spitzer, R., Williams, J. & Gibbon, M. Structured Clinical Interview for DSM-IV (SCID) (American Psychiatric Association, Washington, DC, 1995).
- 26.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 27.Speilberger C, Gorsuch R, Lushene R. STAI Manual. Palo Alto, CA: Consulting Psychologist Press; 1970. [Google Scholar]
- 28.Karlsgodt KH, Lukas SE, Elman I. Psychosocial stress and the duration of cocaine use in non-treatment seeking individuals with cocaine dependence. Am. J. Drug Alcohol Abuse. 2003;29:539–551. doi: 10.1081/ADA-120023457. [DOI] [PubMed] [Google Scholar]
- 29.Rubin E, et al. Early abstinence in cocaine dependence: influence of comorbid major depression. Am. J. Addict. 2007;16:283–290. doi: 10.1080/10550490701389880. [DOI] [PubMed] [Google Scholar]
- 30.Sussner BD, et al. The validity and reliability of a brief measure of cocaine craving. Drug Alcohol Depend. 2006;83:233–237. doi: 10.1016/j.drugalcdep.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 31.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. . Natl Acad. Sci. USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cordes D, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am. J. Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 33.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomasi D, Volkow ND. Functional connectivity of substantia nigra and ventral tegmental area: maturation during adolescence and effects of ADHD. Cereb. Cortex. 2014;24:935–944. doi: 10.1093/cercor/bhs382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li CS, et al. Resting state functional connectivity of the basal nucleus of Meynert in humans: in comparison to the ventral striatum and the effects of age. Neuroimage. 2014;97:321–332. doi: 10.1016/j.neuroimage.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang S, Hu S, Chao HH, Li CSR. Hemispheric lateralization of resting-state functional connectivity of the ventral striatum: an exploratory study. Brain. Struct. Funct. 2017;222:2573–2583. doi: 10.1007/s00429-016-1358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaborszky L, et al. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage. 2008;42:1127–1141. doi: 10.1016/j.neuroimage.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 40.Le Cam, L. M. & Neyman, J. Some methods for classification and analysis of multivariate observations. In Proc. 5th Berkeley Symposium of Mathematical Statistics and probability (ed. Le Cam, L. M. & Neyman, J.) (University of California Press, Berkeley, California, 1967).
- 41.Wise RA. Dopamine and reward: The Anhedonia hypothesis 30 years on. Neurotox. Res. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volkow ND, Morales M. The brain on drugs: from reward to addiction. Cell. 2015;162:712–725. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 43.Wise RA. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 44.Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 45.Drevets WC, et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol. Psychiatry. 2001;49:81–96. doi: 10.1016/S0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- 46.Volkow ND, et al. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D-2 receptors. J. Pharmacol. Exp. Ther. 1999;291:409–415. [PubMed] [Google Scholar]
- 47.Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J. Clin. Investig. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baroncini M, et al. MRI atlas of the human hypothalamus. Neuroimage. 2012;59:168–180. doi: 10.1016/j.neuroimage.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 49.Barr JL, Forster GL, Unterwald EM. Repeated cocaine enhances ventral hippocampal-stimulated dopamine efflux in the nucleus accumbens and alters ventral hippocampal NMDA receptor subunit expression. J. Neurochem. 2014;130:583–590. doi: 10.1111/jnc.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Potenza MN, et al. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am. J. Psychiat. 2012;169:406–414. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomasi D, et al. Overlapping patterns of brain activation to food and cocaine cues in cocaine abusers: association to striatal D2/D3 receptors. Hum. Brain Mapp. 2015;36:120–136. doi: 10.1002/hbm.22617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fotros A, et al. Cocaine cue-induced dopamine release in amygdala and hippocampus: a high-resolution PET [F-18]Fallypride study in cocaine-dependent participants. Neuropsychopharmacology. 2013;38:1780–1788. doi: 10.1038/npp.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lyons D, Friedman DP, Nader MA, Porrino LJ. Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys. J. Neurosci. 1996;16:1230–1238. doi: 10.1523/JNEUROSCI.16-03-01230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pascoli V, et al. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509:459–464. doi: 10.1038/nature13257. [DOI] [PubMed] [Google Scholar]
- 55.Britt JP, et al. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muller CP, Carey RJ, Silva MAD, Jocham G, Huston JP. Cocaine increases serotonergic activity in the hippocampus and nucleus accumbens in vivo: 5-HT1a receptor antagonism blocks behavioral but potentiates serotonergic activation. Synapse. 2002;45:67–77. doi: 10.1002/syn.10083. [DOI] [PubMed] [Google Scholar]
- 57.Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: Disruption by cocaine sensitization. Neuron. 2005;47:255–266. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 58.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pickens CL, et al. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park WK, et al. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J. Neurosci. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission Potential therapeutic targets for craving and addiction. Addict. Rev. 2010;1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bossert JM, et al. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J. Neurosci. 2012;32:4982–4991. doi: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakamura-Palacios EM, et al. Ventral medial prefrontal cortex (vmPFC) as a target of the dorsolateral prefrontal modulation by transcranial direct current stimulation (tDCS) in drug addiction. J. Neural Transm. 2016;123:1179–1194. doi: 10.1007/s00702-016-1559-9. [DOI] [PubMed] [Google Scholar]
- 64.Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav. Brain. Res. 2009;201:239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain. Struct. Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuhn S, Gallinat J. Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr. Bull. 2013;39:358–365. doi: 10.1093/schbul/sbr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu YZ, Salmeron BJ, Gu H, Stein EA, Yang YH. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry. 2015;72:584–592. doi: 10.1001/jamapsychiatry.2015.1. [DOI] [PubMed] [Google Scholar]
- 68.Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Mol. Psychiatry. 2012;17:132–141. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Canterberry M, Peltier MR, Brady KT, Hanlon CA. Attenuated neural response to emotional cues in cocaine-dependence: a preliminary analysis of gender differences. Am. J. Drug Alcohol Abuse. 2016;42:577–586. doi: 10.1080/00952990.2016.1192183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kringelbach ML, Berridge KC. The functional neuroanatomy of pleasure and happiness. Discov. Med. 2010;9:579–587. [PMC free article] [PubMed] [Google Scholar]
- 71.Warren BL, et al. Altered gene expression and spine density in nucleus accumbens of adolescent and adult male mice exposed to emotional and physical stress. Dev. Neurosci. 2014;36:250–260. doi: 10.1159/000362875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat. Neurosci. 2008;11:423–425. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwienbacher I, Fendt M, Richardson R, Schnitzler HU. Temporary inactivation of the nucleus accumbens disrupts acquisition and expression of fear-potentiated startle in rats. Brain Res. 2004;1027:87–93. doi: 10.1016/j.brainres.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 74.Pohlack ST, Nees F, Ruttorf M, Schad LR, Flor H. Activation of the ventral striatum during aversive contextual conditioning in humans. Biol. Psychol. 2012;91:74–80. doi: 10.1016/j.biopsycho.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 75.Jensen J, et al. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–1257. doi: 10.1016/S0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- 76.Aharon I, Becerraa L, Chabris CF, Borsooka D. Noxious heat induces fMRI activation in two anatomically distinct clusters within the nucleus accumbens. Neurosci. Lett. 2006;392:159–164. doi: 10.1016/j.neulet.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 77.Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/S0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- 78.Volkow ND, Baler RD. NOW vs LATER brain circuits: implications for obesity and addiction. Trends Neurosci. 2015;38:345–352. doi: 10.1016/j.tins.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 79.Stewart JL, et al. Cocaine dependent individuals with attenuated striatal activation during reinforcement learning are more susceptible to relapse. Psychiat Res. Neuroim. 2014;223:129–139. doi: 10.1016/j.pscychresns.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hanlon CA, Dowdle LT, Naselaris T, Canterberry M, Cortese BM. Visual cortex activation to drug cues: A meta-analysis of functional neuroimaging papers in addiction and substance abuse literature. Drug Alcohol. Depend. 2014;143:206–212. doi: 10.1016/j.drugalcdep.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol. Psychiatry. 2011;70:785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prisciandaro JJ, McRae-Clark AL, Myrick H, Henderson S, Brady KT. Brain activation to cocaine cues and motivation/treatment status. Addict. Biol. 2014;19:240–249. doi: 10.1111/j.1369-1600.2012.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aston-Jones G, et al. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marchant NJ, Millan EZ, McNally GP. The hypothalamus and the neurobiology of drug seeking. Cell. Mol. Life Sci. 2012;69:581–597. doi: 10.1007/s00018-011-0817-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen J, et al. Hypothalamic proteoglycan syndecan-3 is a novel cocaine addiction resilience factor. Nat. Commun. 2013;4:1955. doi: 10.1038/ncomms2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou Y, et al. Effects of cocaine place conditioning, chronic escalating-dose “binge” pattern cocaine administration and acute withdrawal on orexin/hypocretin and preprodynorphin gene expressions in lateral hypothalamus of Fischer and Sprague-Dawley rats. Neuroscience. 2008;153:1225–1234. doi: 10.1016/j.neuroscience.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahmed SH, et al. Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc. . Natl Acad. Sci. USA. 2005;102:11533–11538. doi: 10.1073/pnas.0504438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Asensio S, et al. Altered neural response of the appetitive emotional system in cocaine addiction: an fMRI Study. Addict. Biol. 2010;15:504–516. doi: 10.1111/j.1369-1600.2010.00230.x. [DOI] [PubMed] [Google Scholar]
- 89.Bustamante JC, et al. Abstinence duration modulates striatal functioning during monetary reward processing in cocaine patients. Addict. Biol. 2014;19:885–894. doi: 10.1111/adb.12041. [DOI] [PubMed] [Google Scholar]
- 90.Kullmann S, et al. Resting-state functional connectivity of the human hypothalamus. Hum. Brain. Mapp. 2014;35:6088–6096. doi: 10.1002/hbm.22607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Volkow ND, et al. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: Implications in addiction. Am. J. Psychiat. 1999;156:19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- 92.Volkow ND, et al. Effects of methylphenidate on regional brain glucose metabolism in humans: Relationship to dopamine D-2 receptors. Am. J. Psychiat. 1997;154:50–55. doi: 10.1176/ajp.154.1.50. [DOI] [PubMed] [Google Scholar]
- 93.Anderson CM, et al. Cerebellar vermis involvement in cocaine-related behaviors. Neuropsychopharmacology. 2006;31:1318–1326. doi: 10.1038/sj.npp.1300937. [DOI] [PubMed] [Google Scholar]
- 94.Bonson KR, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- 95.Grant S, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc. . Natl Acad. Sci. USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kilts CD, et al. Neural activity related to drug craving in cocaine addiction. Arch. Gen. Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 97.Wang GJ, et al. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–784. doi: 10.1016/S0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- 98.Sim ME, et al. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32:2229–2237. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- 99.Barros-Loscertales A, et al. Reduced striatal volume in cocaine-dependent patients. Neuroimage. 2011;56:1021–1026. doi: 10.1016/j.neuroimage.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 100.Ide JS, et al. Cerebral gray matter volumes and low-frequency fluctuation of BOLD signals in cocaine dependence: Duration of use and gender difference. Drug Alcohol Depend. 2014;134:51–62. doi: 10.1016/j.drugalcdep.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.