Abstract
Abnormalities in the kynurenine pathway (KP) of tryptophan degradation, leading to the dysfunction of neuroactive KP metabolites in the brain, have been implicated in the pathophysiology of schizophrenia (SZ). One plausible mechanism involves dysregulation of various pro-inflammatory cytokines associated with the disease, which affect indoleamine-2,3-dioxygenase (IDO), a key enzyme for tryptophan to kynurenine conversion. In order to test this hypothesis directly, we measured plasma levels of the major KP metabolites kynurenine and kynurenic acid (KYNA), as well as four major cytokines, in a sample of 106 SZ patients and 104 control participants. In contrast to the replicable findings of elevation of KYNA in the central nervous system in SZ, plasma levels of KYNA were significantly lower in SZ compared to controls (p = .004). Kynurenine levels were significantly correlated with levels of interferon-γ (p < .001), which is involved in the regulation of IDO, in both patients and controls. However, although patients had higher levels of interleukin-6 (IL-6) compared to controls (p = .012), IL-6 levels were not correlated with kynurenine or KYNA, and did not explain group differences in KYNA. Based on the lack of evidence that pro-inflammatory cytokines were significantly related to the KP abnormality in SZ despite an adequate sample size, further studies must consider alternative hypotheses to identify the origins of the KP abnormalities in SZ.
Introduction
The kynurenine pathway (KP) of tryptophan metabolism has emerged as a focus of psychiatric research as this pathway is activated in conditions of inflammation and stress, and several kynurenine metabolites are neuroactive and can modulate oxidative stress, glutamatergic and acetylcholinergic receptors relevant to major mental illnesses [1]. The initial and rate limiting step of the KP is the conversion of tryptophan to kynurenine, catalyzed by two enzymes, tryptophan 2,3-dioxygenase (TDO2) and indoleamine 2,3-dioxygenase (IDO-1 and IDO-2). Kynurenic acid (KYNA), produced from kynurenine by kynurenine aminotransferases, can act as an antagonist at NMDA and α7 nicotinic acetylcholine receptors. These neuroactive properties of KYNA may contribute to cognitive deficits observed in psychiatric disorders such as schizophrenia, thus making the KP an attractive target for development of novel treatment strategies [2–4].
Increased TDO expression [5, 6] and elevated concentrations of kynurenine and KYNA [6–8] have been repeatedly found in post-mortem brain tissue and in cerebrospinal fluid (CSF; [9–11]) of individuals with SZ. In contrast, although increased kynurenine:tryptophan ratios have been reported in the plasma of SZ patients [12–14], findings for peripheral KYNA levels in the disease have so far been inconsistent [15, 16].
One plausible hypothesis for abnormal KP metabolism in SZ is that elevated inflammatory activity drives elevations of kynurenine and KYNA levels through the activation of IDO, which is strongly stimulated by interferon gamma (IFN-γ), but can also be independently induced by other pro-inflammatory cytokines, including interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) [17]. Elevated plasma levels of pro-inflammatory cytokines, including IL-6 and TNF-α, have been found in chronic SZ [18, 19], in first episode SZ, and in individuals with high risk for psychosis [20–22]. Notably, increased levels of IL-6 have also been found in the CSF of SZ patients [23]. On the other hand, although elevations in circulating IFN-γ levels have been noted in individuals with SZ [18], findings from drug-naïve, first episode patients are less consistent [22]. Taken together, the relationship between peripheral cytokines and KP metabolites, and especially their possible relevance for SZ pathophysiology, should be critically examined.
The present study was designed to investigate the question by measuring peripheral levels of kynurenine and KYNA in a large sample of mostly stable, medicated SZ patients and appropriately matched controls, and to determine if disease-related changes may correlate with circulating levels of cytokines. We analyzed four cytokines: IFN-γ, due to the known effects on IDO; TNF-α, and IL-6, pro-inflammatory cytokines that are consistently found to be elevated in schizophrenia patients [18, 22]; and interleukin 10 (IL-10), which tends to have anti-inflammatory effects and served as a control for different inflammatory mechanisms. As some of these cytokines are present in very low concentration in people with no apparent infectious or inflammatory disease, ultra-sensitive assays were used to ascertain their basal levels.
Methods
Participants
Individuals with schizophrenia spectrum disorder (SSD; 106 total, including 81 with schizophrenia and 25 with schizoaffective disorder, age range 17–62) were recruited from the outpatient clinics at the Maryland Psychiatric Research Center and neighboring mental health clinics. Healthy controls (n = 104, age range 14–63) were recruited through media advertisements, with particular effort given to recruiting controls who smoke, in order to frequency-match smoking status. Diagnoses were confirmed with the Structured Clinical Interview (SCID) for DSM-IV in all participants. Exclusion criteria included history of major neurological illnesses and any uncontrolled major medical illnesses, epilepsy, cerebrovascular accident, head injury with cognitive sequelae, and mental retardation. Participants with a history of an autoimmune disorder or chronic infection were not excluded if there were no active symptoms of these illnesses and participants were not being treated for such conditions at time of study. There were 4 SSD participants who reported a history of autoimmune disorder (2 with celiac disease; 1 with Crohn’s disease; 1 with lupus) and 4 SSD participants who reported a history of hepatitis C infection. Exclusion of these participants from the analyses did not materially alter the primary findings, and so data from these participants are included in the results below. Patients and controls with substance dependence within the past 6 months, or current substance use disorder (except nicotine or marijuana) were excluded. Controls had no current DSM-IV Axis I diagnoses and no family history of psychosis in two generations. Except for 4 medication-free participants, all SSD patients were on antipsychotic medications, including 88 taking atypical antipsychotics, 6 taking typical antipsychotics, and 8 taking a combination of antipsychotic types. Of the patients on atypical, 25 were on clozapine. Participants gave written informed consent. This study was approved by the University of Maryland Baltimore IRB.
Clinical assessments
Overall psychiatric symptoms were assessed with the mean of the 20 item Brief Psychiatric Rating Scale (BPRS). Negative symptoms were assessed using the Brief Negative Symptom Scale (BNSS; [24]). To assess cognitive deficits, participants were tested with the Digit Symbol Coding task of the WAIS-3 [25] and the Digit Sequencing task from the Brief Assessment of Cognition in Schizophrenia [26], to assess processing speed and working memory, respectively; deficits in these measures are among the most robust cognitive impairments in schizophrenia [27].
Measurement of kynurenine, KYNA, and cytokine levels
Whole blood was collected in EDTA-containing tubes (Vacutainer) which were immediately centrifuged (1000×g, 10 min). Plasma was then aliquoted into separate tubes and stored at −80 °C until assay. For kynurenine and KYNA measurement, plasma samples were thawed on the day of the assays, and diluted 1:2 (v/v) with ultrapure water. One hundred μl of the sample were acidified with 25 μl of 6% perchloric acid. After centrifugation (16,000×g, 10 min), 30 μl of the supernatant were subjected to high-performance liquid chromatography, and KYNA and kynurenine were isocratically eluted and fluorimetrically detected as previously described [28, 29]. For cytokine assays, samples were analyzed using ultra-sensitive Single Molecule Array (Simoa) technology (Quanterix, Lexington, MA) for the four cytokines. This technology uses antibody-coated paramagnetic beads to capture analytes, after which a biotin-labeled detection antibody is added. Beads are isolated in individual wells, and fluorescence from hydrolysis of a substrate is measured. Lower limits of detection for these molecules using this technology are: 0.006 pg/ml for IL-6; 0.011 pg/ml for TNF-α; 0.0022 pg/ml for IL-10, and 0.0104 pg/ml for IFN-γ, representing superior sensitivities compared to the commonly used multiplex panel or ELISA methods [30]. Intra-assay coefficient of variance was <15% for all cytokines in these assays. The cytokine assays were performed by PBL Assay Science (Piscataway, NJ). All assays were blinded to group or behavior information until after the results were finalized.
Statistical analyses
Plasma levels of kynurenine and KYNA did not significantly deviate from normal distribution as determined by Kolmogorov-Smirnov tests. Independent sample t-tests were used to compare patients and controls for these variables. Levels of all four cytokines measured were heavily skewed, and could not be brought into normal distribution by routine data transformations and therefore nonparametric statistical tests were employed. Group differences on these measures were analyzed using Mann-Whitney tests. Bonferroni corrections for multiple patient-control group comparisons were applied to kynurenine and KYNA plasma measures, such that only group comparisons with p < .025 (.05/2) were considered significant. Similarly, Bonferroni corrections were applied for the cytokine measures, such that only group comparisons with p < .0125 (.05/4) were considered significant. Correlations between cytokine levels and levels of kynurenine and KYNA were examined with Spearman’s rank-correlation coefficients, with Bonferroni correction for 8 comparisons (4 cytokines × 2 KP metabolites), resulting in a threshold p value of .0063. Further analyses explored effects of age, body-mass index (BMI), sex, smoking status, as well as diagnosis × smoking or diagnosis × sex effects. Potential effects from current psychotropic medications were also explored. These exploratory secondary analyses were not corrected for multiple comparisons.
Results
Group differences
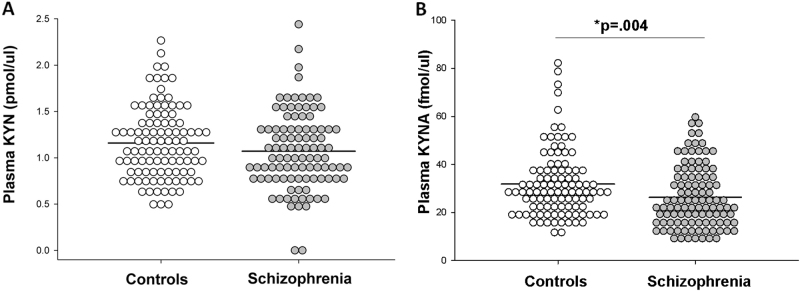
Patient and control groups were not significantly different in age and sex (Table 1). Plasma levels of KYNA were significantly lower in patients compared to controls (t(207)=2.94, p = .004; Fig. 1). Patients had significant elevation in IL-6 (Z = 2.52, p = .012) and IL-10 levels (Z = 2.53, p = .012) compared to controls. There were no significant group differences for kynurenine, TNF-α, or IFN-γ.
Table 1.
Summary of demographic characteristics and plasma measures
| Control (n = 104) | SSD (n = 106) | Test-statistic | p-value | |
|---|---|---|---|---|
| Age (years) | 35.4 ± 14.3 | 36.3 ± 13.4 | t = 0.47 | .64 |
| Smoker/non-smoker | 26/78 | 37/69 | χ2 = 2.45 | .12 |
| Male/female | 59/45 | 69/37 | χ2 = 1.54 | .21 |
| Duration of illness (years) | n/a | 14.1 ± 12.6 | n/a | |
| Body mass index | 26.8 ± 5.3 | 27.9 ± 6.2 | t = 1.39 | .17 |
| Kynurenine (pmol/μl) | 1.16 ± 0.39 | 1.07 ± 0.42 | t = 1.55 | .12 |
| Kynurenic acid (fmol/μl) | 31.8 ± 14.0 | 26.3 ± 12.8 | t = 2.94 | .004 |
| IL-6 (pg/ml) | 4.08 ± 9.5 | 9.11 ± 23.8 | Z = 2.52 | .012 |
| IL-10 (pg/ml) | 2.37 ± 6.7 | 4.16 ± 10.2 | Z = 2.53 | .012 |
| TNF-α (pg/ml) | 7.74 ± 17.0 | 15.27 ± 34.9 | Z = 1.34 | .18 |
| IFN-γ (pg/ml) | 0.44 ± 1.56 | 0.36 ± 0.73 | Z = 0.92 | .34 |
Variance reported as ± standard deviation. Values were mean ± standard deviation
SSDschizophrenia spectrum disorder
Fig. 1.
Density dot plots displaying values of (a) plasma kynurenine (KYN) and (b) kynurenic acid (KYNA) in control and schizophrenia samples. Black lines indicate group means
Relationship of kynurenine, KYNA, and cytokine levels
Kynurenine levels were significantly correlated with levels of IFN-γ in the predicted direction in the combined sample (rho = .28, p < .001) and the relationship was similar in patients (rho = .28, p = .005) and controls (rho = .29, p = .003) examined independently. In comparison, kynurenine was not significantly correlated with IL-6 (p = .75), TNF-α (p = .020) or IL-10 (p = .043) in the combined sample or in each group examined independently (all p’s > .0063, Bonferroni correction). Finally, KYNA was not significantly correlated with any of the cytokines measured (Table 2). Kynurenine and KYNA were highly correlated as expected in the combined sample (r = .68, p < .001) and also in patients (r = .70, p < .001) and controls (r = .65, p < .001) separately.
Table 2.
Spearman’s rank-order correlation coefficients and p values between kynurenine, kynurenic acid (KYNA), and cytokines
| Entire sample | Controls | SSD | ||||
|---|---|---|---|---|---|---|
| Kynurenine | KYNA | Kynurenine | KYNA | Kynurenine | KYNA | |
| IL-6 | .023 n.s. |
.050 n.s. |
.000 n.s. |
.073 n.s. |
.084 n.s. |
.088 n.s. |
| IL-10 |
.143
p = .043 |
.082 n.s. |
.122 n.s. |
.075 n.s. |
.198
p = .049 |
.157 n.s. |
| TNF-α | .164 p = .020 |
.108 n.s. |
.093 n.s. |
.027 n.s. |
.222
p = .027 |
.188 n.s. |
| IFN-γ |
.283
p < .001 |
.103 n.s. |
.292
p = .003 |
.150 n.s. |
.283
p = .005 |
.081 n.s. |
Nominally significant correlations are italicized. Significant correlations passing correction for multiple comparisons are in bold. n.s. = not significant (p > .05)
SSD schizophrenia spectrum disorder
Relationship of kynurenine and KYNA to age, sex, smoking, and medications
Females had lower levels of KYNA (M = 26.6 ± 12.4 fmol/μl) compared to males (M = 30.6 ± 14.3 fmol/μl) (F = 5.70, p = .018) but there was no sex x diagnosis interaction (p = .79). Females had lower kynurenine levels than males but this was not statistically significant (p = .11). Age was not significantly related to kynurenine or KYNA (all p’s > .27). Kynurenine and KYNA levels did not differ between current smokers and non-smokers (p = .92 and .72, respectively), and diagnosis × smoker interactions were not statistically significant (p = .06 and .07, respectively). Body-mass index (BMI) was not correlated with either kynurenine or KYNA (all p’s > .84).
There were positive correlations between dosage of antipsychotic medications (chlorpromazine equivalent, or CPZ) and kynurenine (rho = 0.21, p = .040) and KYNA (rho = 0.21, p = .039). In addition, 24 patients were also on mood stabilizers, 44 on antidepressants, 17 on benozodiazepines, and 17 on anticholinergic medications, all of which are commonly used in managing patients with SSD. To explore the potential effects of different classes of psychotropic medications, linear regression models were run with kynurenine (and then KYNA) as the outcome measure and predictor variables including binary factors denoting typical antipsychotics, atypical antipsychotics other than clozapine, clozapine, mood stabilizers, antidepressants, benzodiazepines, and anticholinergic medications, as well as a variable for total number of medications patients were on at time of study. The overall model was significant for kynurenine (R2 = 0.18, p = .020), where total number of medications was associated with higher levels of kynurenine (β = .44, p = .002), but use of typical antipsychotics predicted lower levels of kynurenine (β = −0.25, p = .046). The model for KYNA found that use of mood stabilizers was associated with lower levels of KYNA (β = −.32, p = .005). No other medication classes were significantly associated with either kynurenine or KYNA. A further exploratory analysis using a one-way ANOVA suggested that the effect of mood stabilizers on KYNA levels may be specific to valproate, as patients taking valproate (n = 11) had significantly lower levels of plasma KYNA (M = 15.9 ± 6.8) compared to patients not on a mood stabilizer (n = 81; M = 27.7 ± 13.2; p = .012). The difference between patients taking valproate versus those taking other mood stabilizers approached significance (n = 12; M = 28.1 ± 10.2; p = .061).
Relationship of plasma measures to clinical characteristics
Within SSD, patients with schizophrenia had lower levels of kynurenine (n = 76; M = 1.01 ± 0.38 µM) than patients with schizoaffective disorder (n = 23; M = 1.26 ± 0.50 µM; F(1,97) = 6.40, p = .013). However, these groups did not differ in levels of KYNA (F(1103) = 0.62, p = .43). Levels of kynurenine or KYNA were not significantly correlated with psychiatric symptoms as measured with BPRS or BNSS, nor were these metabolites correlated with scores for working memory or processing speed in patients (all p’s > .06). Exploratory analyses examining correlations between levels of cytokines and symptom and cognitive measures revealed no significant relationships (all p’s > .13).
Discussion
In the present study, we tested the hypothesis that elevated basal plasma levels of pro-inflammatory cytokines may contribute to abnormal KP metabolism in schizophrenia spectrum disorders. Although some of our findings, for example the positive correlations between IFN-γ and kynurenine and between kynurenine and KYNA, were consistent with predictions, they were seen in both groups, indicating that peripheral IDO activation may not be abnormal in the disease. Consistent with previous studies [18, 19], we found an elevated level of IL-6 in SSD patients; but contrary to our hypothesis, we found no evidence that this was related to altered KP metabolite levels in SSD.
Given the evidence of a possible chronic, low-grade inflammatory state in SSD as indicated by replicated findings of elevated cytokines like IL-6 and TNF-α in plasma [18], we predicted that elevated levels of pro-inflammatory cytokines would affect IDO, leading to increased production of kynurenine and KYNA. Previous studies in other cohorts provided evidence to support this hypothesis. Advanced age (>80 years) was found to be associated with elevated plasma levels of IL-6, C-reactive protein (CRP) and kynurenine, with positive correlations between levels of plasma IL-6, CRP, and kynurenine:tryptophan ratio [31]. A large study of patients with major depressive disorder also found positive correlations between IL-6, CRP, and kynurenine:tryptophan ratio, though only CRP was elevated in the patient group compared to controls [32]. Another study found elevated levels of both IL-6 and KYNA in the CSF of patients with SZ, with a negative correlation between IL-6 and tryptophan:KYNA ratio [13]. The same study also showed increased KYNA production in astrocytes cultured with IL-6 [13]. In the present well-powered study with over 100 SZ patients and relatively well-matched controls, we found only a trend-level correlation (rho = .16, p = .020) between TNF-α and kynurenine levels, but no significant relationship between IL-6 and kynurenine.
In line with previous studies in SZ and schizoaffective disorders [16, 33, 34], we found plasma KYNA to be significantly lower in patients with SSD compared to controls. As also noted by others, this result is inconsistent with findings in post-mortem brains [6–8, 15] and CSF [9–11, 23]. Kynurenine and KYNA were measured only in the periphery in this study, which may not reflect their levels in the brain, as kynurenine can pass the blood brain barrier but KYNA cannot [35]. In contrast, during psychological stress, peripheral KYNA responses as measured by saliva showed significantly elevated KYNA responses in some schizophrenia patients compared with controls [36]. Therefore, it is conceivable that abnormally high KYNA levels are present in the brain, specifically during stress conditions in schizophrenia, but are not readily captured by a cross-sectional, basal plasma assessment.
There are several other limitations to the current study. Plasma samples were not collected under fasting conditions, so there was no control of the basal levels of tryptophan, the parent compound of all KP metabolites. Also, recent studies indicate that aerobic exercise can increase plasma levels of KYNA in humans [37]; in this study we did not account for levels of physical exercise, which may potentially differ between patients and controls. Nicotine may have effects on KP metabolites in the brain or the blood [38, 39]. We matched the proportion of smokers in the samples, and did not observe an overall effect of smoking on plasma kynurenine or KYNA. There was a non-significant trend for SSD patients who smoked to have lower levels of both metabolites, whereas controls who smoked tended to have increased levels. Another potentially relevant limitation, which notably applies to most studies of peripheral KP metabolism in psychiatric patients published to date, is that up to 80% of kynurenine and KYNA in the plasma appear to be bound to albumin or other circulating binding proteins [35, 40]. Thus, as only total plasma levels of these two metabolites were measured in the present study, we may have missed group differences caused by possible differential availability of free kynurenine, which can readily penetrate the blood–brain barrier and then function as a highly effective bioprecursor of KYNA within the brain. Finally, most patients in this study were on psychotropic medications. However, as we observed modest positive correlations between antipsychotic dosage and plasma KYNA levels), antipsychotic medications are unlikely to account for the lower plasma level of KYNA seen in patients compared to controls. Notably, a small number of patients taking valproate had lower levels of plasma KYNA compared to other individuals with SSD, which we speculate could reflect displacement of KYNA from albumin and hence greater renal clearance [41, 42]. Additional studies with assays of free vs. bound tryptophan and its metabolites are needed to determine whether acute or chronic administration of valproate in SSD patients impacts KYNA. Although we did not find significant associations of plasma measures with cognition, it should be noted that only a limited range of cognitive tasks were included in this study. In particular, as animal studies suggest KP abnormalities may impact context-dependent learning and cognitive flexibility in particular [29, 43], the working memory and processing speed tasks used here may not have been sufficiently sensitive to KP changes.
A strength of this study is the use of the Single Molecule Array technology. With the greater sensitivity of this method, less than 6% of the samples had cytokines levels below detectable thresholds, compared to multiplex and ELISA methods, where up to 50% of the samples have undetectable levels (unpublished data). This methodological advance was important, because it permitted more accurate assessments of potential effects of basal circulating cytokines levels on kynurenine and KYNA in plasma. Basal levels of IFN-γ were thus found to be positively correlated with kynurenine levels in both SSD patients and in controls, whereas such correlations could be confidently ruled out for the other cytokines tested. However, our selection of cytokines measured in this study was not exhaustive. In particular, IL-1β is another pro-inflammatory cytokine that is also elevated in plasma in schizophrenia [18, 22] and can induce TDO2 [44]. Notably, a recent study in schizophrenia patients reported a positive correlation between IL-1β and kynurenine levels although IL-1β was not significantly elevated in schizophrenia in that study [45].
In summary, we observed decreased levels of KYNA in the plasma of patients with SSD but no evidence that this was substantially related to abnormal inflammatory cytokines, suggesting that IDO or low-grade cytokine increases in SSD may not be directly responsible for the KP abnormalities observed in this disorder [46–49]. A negative finding using a large sample is important for re-directing the field to focus on other causes that may lead to KP abnormalities in SSD.
Acknowledgements
Funding
Funding support for this work was received from National Institutes of Health (grant numbers K23MH112010, P50 MH103222, U01MH108148, R01MH112180, R01MH085646, R01DA027680, R01MH094520, R01MH096263, T32MH067533).
Conflict of interest
Dr. LEH has received or anticipates receiving research funding or consulting fees from Mitsubishi, Your Energy Systems LLC, Neuralstem, Taisho Pharmaceutical, Sound Pharma, Heptares, Pfizer, and Takeda. All remaining authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–77. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kozak R, Campbell BM, Strick CA, Horner W, Hoffmann WE, Kiss T, et al. Reduction of brain kynurenic acid improves cognitive function. J Neurosci. 2014;34:10592–602. doi: 10.1523/JNEUROSCI.1107-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wonodi I, Schwarcz R. Cortical kynurenine pathway metabolism: a novel target for cognitive enhancement in Schizophrenia. Schizophr Bull. 2010;36:211–8. doi: 10.1093/schbul/sbq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu HQ, Okuyama M, Kajii Y, Pocivavsek A, Bruno JP, Schwarcz R. Targeting kynurenine aminotransferase II in psychiatric diseases: promising effects of an orally active enzyme inhibitor. Schizophr Bull. 2014;40(Suppl 2):S152–8. doi: 10.1093/schbul/sbt157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller CL, Llenos IC, Dulay JR, Barillo MM, Yolken RH, Weis S. Expression of the kynurenine pathway enzyme tryptophan 2,3-dioxygenase is increased in the frontal cortex of individuals with schizophrenia. Neurobiol Dis. 2004;15:618–29. doi: 10.1016/j.nbd.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073-1074:25–37. doi: 10.1016/j.brainres.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 7.Sathyasaikumar KV, Stachowski EK, Wonodi I, Roberts RC, Rassoulpour A, McMahon RP, et al. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull. 2011;37:1147–56. doi: 10.1093/schbul/sbq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521–30. doi: 10.1016/S0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- 9.Erhardt S, Blennow K, Nordin C, Skogh E, Lindström LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313:96–8. doi: 10.1016/S0304-3940(01)02242-X. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson LK, Linderholm KR, Engberg G, Paulson L, Blennow K, Lindström LH, et al. Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr Res. 2005;80:315–22. doi: 10.1016/j.schres.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G, et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull. 2012;38:426–32. doi: 10.1093/schbul/sbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barry S, Clarke G, Scully P, Dinan TG. Kynurenine pathway in psychosis: evidence of increased tryptophan degradation. J Psychopharmacol. 2009;23:287–94. doi: 10.1177/0269881108089583. [DOI] [PubMed] [Google Scholar]
- 13.Schwieler L, Larsson MK, Skogh E, Kegel ME, Orhan F, Abdelmoaty S, et al. Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia–significance for activation of the kynurenine pathway. J Psychiatry Neurosci. 2015;40:126–33. doi: 10.1503/jpn.140126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiappelli J, Postolache TT, Kochunov P, Rowland LM, Wijtenburg SA, Shukla DK, et al. Tryptophan metabolism and white matter integrity in schizophrenia. Neuropsychopharmacology. 2016;41:2587–95. doi: 10.1038/npp.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plitman E, Iwata Y, Caravaggio F, Nakajima S, Chung JK, Gerretsen P, et al. Kynurenic acid in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43:764–77. doi: 10.1093/schbul/sbw221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wurfel BE, Drevets WC, Bliss SA, McMillin JR, Suzuki H, Ford BN, et al. Serum kynurenic acid is reduced in affective psychosis. Transl Psychiatry. 2017;7:e1115. doi: 10.1038/tp.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, et al. The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J Biochem. 2006;139:655–62. doi: 10.1093/jb/mvj072. [DOI] [PubMed] [Google Scholar]
- 18.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–71. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63:801–8. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Noto C, Ota VK, Gouvea ES, Rizzo LB, Spindola LM, Honda PH, et al. Effects of risperidone on cytokine profile in drug-naïve first-episode psychosis. Int J Neuropsychopharmacol. 2015;18:1–8. doi: 10.1093/ijnp/pyu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stojanovic A, Martorell L, Montalvo I, Ortega L, Monseny R, Vilella E, et al. Increased serum interleukin-6 levels in early stages of psychosis: associations with at-risk mental states and the severity of psychotic symptoms. Psychoneuroendocrinology. 2014;41:23–32. doi: 10.1016/j.psyneuen.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Upthegrove R, Manzanares-Teson N, Barnes NM. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res. 2014;155:101–8. doi: 10.1016/j.schres.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Wang AK, Miller BJ. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr Bull. 2017;44:75–83. doi: 10.1093/schbul/sbx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37:300–5. doi: 10.1093/schbul/sbq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wechsler D. Wechsler Adult Intelligence Scale –Third Edition (WAIS-III) San Antonio, TX: Harcourt Assessment; 1997. [Google Scholar]
- 26.Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–97. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry. 2008;64:823–7. doi: 10.1016/j.biopsych.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsson SK, Larsson MK, Erhardt S. Subchronic elevation of brain kynurenic acid augments amphetamine-induced locomotor response in mice. J Neural Transm. 2012;119:155–63. doi: 10.1007/s00702-011-0706-6. [DOI] [PubMed] [Google Scholar]
- 29.Pocivavsek A, Wu HQ, Elmer GI, Bruno JP, Schwarcz R. Pre- and postnatal exposure to kynurenine causes cognitive deficits in adulthood. Eur J Neurosci. 2012;35:1605–12. doi: 10.1111/j.1460-9568.2012.08064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeung D, Ciotti S, Purushothama S, Gharakhani E, Kuesters G, Schlain B, et al. Evaluation of highly sensitive immunoassay technologies for quantitative measurements of sub-pg/mL levels of cytokines in human serum. J Immunol Methods. 2016;437:53–63. doi: 10.1016/j.jim.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Capuron L, Schröcksnadel S, Féart C, Aubert A, Higueret D, Barberger-Gateau P, et al. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry. 2011;70:175–82. doi: 10.1016/j.biopsych.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Quak J, Doornbos B, Roest AM, Duivis HE, Vogelzangs N, Nolen WA, et al. Does tryptophan degradation along the kynurenine pathway mediate the association between pro-inflammatory immune activity and depressive symptoms? Psychoneuroendocrinology. 2014;45:202–10. doi: 10.1016/j.psyneuen.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Myint AM, Schwarz MJ, Verkerk R, Mueller HH, Zach J, Scharpé S, et al. Reversal of imbalance between kynurenic acid and 3-hydroxykynurenine by antipsychotics in medication-naïve and medication-free schizophrenic patients. Brain Behav Immun. 2011;25:1576–81. doi: 10.1016/j.bbi.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Szymona K, Zdzisińska B, Karakuła-Juchnowicz H, Kocki T, Kandefer-Szerszeń M, Flis M, et al. Correlations of kynurenic acid, 3-hydroxykynurenine, sIL-2R, IFN-α, and IL-4 with clinical symptoms during acute relapse of schizophrenia. Neurotox Res. 2017;32:17–26. doi: 10.1007/s12640-017-9714-0. [DOI] [PubMed] [Google Scholar]
- 35.Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–17. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 36.Chiappelli J, Pocivavsek A, Nugent KL, Notarangelo FM, Kochunov P, Rowland LM, et al. Stress induced kynurenic acid as a potential biomarker for schizophrenia patients with distress intolerance. JAMA Psychiatry. 2014;71:761–8. doi: 10.1001/jamapsychiatry.2014.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlittler M, Goiny M, Agudelo LZ, Venckunas T, Brazaitis M, Skurvydas A, et al. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am J Physiol Cell Physiol. 2016;310:C836–40. doi: 10.1152/ajpcell.00053.2016. [DOI] [PubMed] [Google Scholar]
- 38.Rassoulpour A, Wu HQ, Albuquerque EX, Schwarcz R. Prolonged nicotine administration results in biphasic, brain-specific changes in kynurenate levels in the rat. Neuropsychopharmacology. 2005;30:697–704. doi: 10.1038/sj.npp.1300583. [DOI] [PubMed] [Google Scholar]
- 39.Theofylaktopoulou D, Midttun Oslash, Ulvik A, Ueland PM, Tell GS, Vollset SE, et al. A community-based study on determinants of circulating markers of cellular immune activation and kynurenines: the Hordaland Health Study. Clin Exp Immunol. 2013;173:121–30. doi: 10.1111/cei.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holmes EW, Russell PM, Kinzler GJ, Bermes EW., Jr. Inflammation-associated changes in the cellular availability of tryptophan and kynurenine in renal transplant recipients. Clin Chim Acta. 1994;227:1–15. doi: 10.1016/0009-8981(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 41.Maciejak P, Szyndler J, Turzyńska D, Sobolewska A, Kołosowska K, Lehner M, et al. The kynurenine pathway: a missing piece in the puzzle of valproate action? Neuroscience. 2013;234:135–45. doi: 10.1016/j.neuroscience.2012.12.052. [DOI] [PubMed] [Google Scholar]
- 42.Singh T, Goel RK. Adjuvant indoleamine 2,3-dioxygenase enzyme inhibition for comprehensive management of epilepsy and comorbid depression. Eur J Pharmacol. 2016;784:111–20. doi: 10.1016/j.ejphar.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 43.Alexander KS, Wu HQ, Schwarcz R, Bruno JP. Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacol. 2012;220:627–37. doi: 10.1007/s00213-011-2539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urata Y, Koga K, Hirota Y, Akiyama I, Izumi G, Takamura M, et al. IL-1β increases expression of tryptophan 2,3-dioxygenase and stimulates tryptophan catabolism in endometrioma stromal cells. Am J Reprod Immunol. 2014;72:496–503. doi: 10.1111/aji.12282. [DOI] [PubMed] [Google Scholar]
- 45.Joaquim HPG, Costa AC, Gattaz WF, Talib LL. Kynurenine is correlated with IL-1β in plasma of schizophrenia patients. J Neural Transm. 2018;6:1–5. [DOI] [PubMed]
- 46.Kiank C, Zeden JP, Drude S, Domanska G, Fusch G, Otten W, et al. Psychological stress-induced, IDO1-dependent tryptophan catabolism: implications on immunosuppression in mice and humans. PLoS ONE. 2010;5:e11825. doi: 10.1371/journal.pone.0011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lavebratt C, Olsson S, Backlund L, Frisen L, Sellgren C, Priebe L, et al. The KMO allele encoding Arg is associated with psychotic features in bipolar disorder type 1, and with increased CSF KYNA level and reduced KMO expression. Mol Psychiatry. 2014;19:334–41. doi: 10.1038/mp.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277–86. doi: 10.1016/j.pnpbp.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 49.Wonodi I, Stine OC, Sathyasaikumar KV, Roberts RC, Mitchell BD, Hong LE, et al. Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes. Arch Gen Psychiatry. 2011;68:665–74. doi: 10.1001/archgenpsychiatry.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]