Abstract
The present study aimed to evaluate whether the neoadjuvant chemoradiation response with concurrent thermal therapy for the treatment of rectal cancer can be predicted following the first thermic treatment. Eighty patients with primary rectal adenocarcinoma (≤12 cm from the anal verge) were included in this study. Fifty-four received surgery and pathological response was evaluated. Intensity-modulated radiotherapy was administered conventionally once daily 5 times/week. Neoadjuvant radiotherapy consisted of 50 Gy delivered to the planning target volume in 25 fractions. Concurrent neoadjuvant chemotherapy was delivered in 5-day courses. Capecitabine was administered orally at 1,700 mg/m2/day for 5 days/week. Thermic treatment was performed using the Thermotron-RF 8 and administered once/week for 5 weeks with 50 min irradiation. Patients with a gross tumor volume (GTV) ≤32 cm3 and a radiofrequency (RF) output difference (RO difference) ≥77 Watt/min exhibited pathological complete response (pCR) and CR rates of 50 and 75%, respectively. Those with a GTV ≥80 cm3 and a RO difference ≥77 Watt/min exhibited pCR and CR rates of 42.9 and 42.9%, respectively. The changes in the skin temperature during RF treatment in patients with pCR with a RO difference ≥77 Watt/min increased significantly compared with those of other outcomes, and progressive disease. These data suggest a strategy for predicting which patients will respond best following the first thermic treatment. The results identified that the group of patients with a GTV ≤32 cm3 and a RO difference ≥77 Watt/min (outputable/heatable patients) may respond best.
Keywords: radiofrequency thermal therapy, chemoradiotherapy, rectal cancer, dose-volume histograms, prediction of treatment response
Introduction
Neoadjuvant management philosophy depends on the ability to predict the extent of pathological response using clinical, imaging, and molecular parameters (1–3). Thus far, no parameters have been validated in multi-institutional prospective trials or used prior to starting treatment. New pretreatment or posttreatment clinical, imaging, and molecular data are required to monitor and predict treatment outcomes in radiotherapy.
Neoadjuvant chemoradiation (NACR) for the treatment of rectal cancer significantly increases the rate of pathological complete response (pCR). Moreover, the local recurrence rate is significantly lower among patients who received neoadjuvant radiation, while there is no significant difference with respect to disease-free survival or overall survival (4–8).
In contrast, there has been also reported that NACR reduces the rate of local recurrence and improves local control, which appears to result in improved overall survival (9–14). Moreover, a certain subset of patients may not require surgery at all according to the wait-and-see paradigm (15). On the basis of these favorable results, NACR has been accepted as the standard therapy worldwide except in Japan.
Meanwhile, radiofrequency (RF) hyperthermia using the Thermotron-RF 8 has been administered mainly in Japan but incurs a risk of the hotspot phenomenon, a potentially fatal complication induced by RF treatment itself. To resolve this problem, we established thermal therapy with standardized power escalation principles, i.e., neothermia, and devised a predictive formula for output-limiting symptoms (16,17) which had an adjusted R2 of 0.99 and variance inflation factor (VIF) values <2. This formula, is as follows: Initial energy output at which an output limiting symptom occurred (Watt)=initial time at which an output limiting symptom occurred (min) ×6.162-the thickness of the fat of the abdominal wall (mm) ×17.155 + 967.995.
The present study aimed to evaluate whether NACR response with concurrent thermal therapy for the treatment of rectal cancer can be predicted after the first thermic treatment. Because early assessment of treatment efficacy, and the decision on continuation or cessation of cancer therapy are necessary for the good quality life of the cancer patients.
Materials and methods
Between December 2011 and May 2015, 80 consecutive patients with primary rectal adenocarcinoma localized in the rectum (up to 12 cm from the anal verge) were included. All patients received pre- and post-treatment diagnostic examinations including CT, PET/CT, and MRI at Hidaka Hospital. The extent and location of the tumors were classified according to the tumor-node-metastasis staging (18). We classified the location of the tumor by the results of MRI, CT and colonoscopy.
All patients received NACR with concurrent thermal therapy at Hidaka Hospital. Operations were performed at the Department of General Surgical Science, Gunma University, or the Division of Surgery, Hidaka Hospital. Each resected specimen was evaluated histologically at the Department of Pathology, Gunma University. This study was approved by the ethics committees of Hidaka Hospital and Gunma University. Each patient gave written informed consent. Tumor stages were defined on the basis of colonoscopy, barium enema, CT, or 18F-fluorodeoxyglucose PET/CT, and MRI.
The 80 patients were divided into quartiles with respect to planning target volume (PTV), clinical target volume (CTV), gross tumor volume (GTV), small intestine (n=63), and rectum volume (n=67) as follows: PTV, ≤825, 826–945, 946–1,055, and ≥1,056 cm3; CTV, ≤624, 625–720, 721–841, and ≥842 cm3; GTV, ≤32, 33–50, 51–79, and ≥80 cm3; small intestine volume, ≤265, 266–415, 416–694, and ≥695 cm3; rectum volume, ≤63, 64–80, 81–112, and ≥113 cm3. At present we think that it is difficult to evaluate patients that would assure a statistically powerful and unequivocal result. So we use the quartiles method to classify tumors into 4 groups and finally found the correlation among GTV ≤32, 33–79, and ≥80 cm3 and RO difference RO difference: ≤76 Watt/min and RO difference: ≥77 Watt/min in this study.
Chemoradiotherapy
The GTV was contoured using the Focal Treatment Planning system (Focal, Eindhoven, The Netherlands), taking into consideration clinical information from imaging modalities to identify the primary rectal tumor and enlarged regional lymph nodes. The CTV included the GTV plus a 15-mm margin in the anterior, posterior, and lateral directions and a 25-mm margin in the craniocaudal direction in addition to the entire mesorectum and internal iliac and presacral nodes. The cranial border was S2/3 interspace in order to reduce the irradiated small bowel volume. On the basis of our institutional setup data, the PTV was determined by adding a 3-mm margin around the CTV.
IMRT was administered conventionally, once daily, 5 times/week using TomoTherapy® (Hi-Art® treatment system; ACCURAY®, Inc., Sunnyvale, CA, USA) and neoadjuvant radiotherapy of 50 Gy delivered to the PTV in 25 fractions. The small intestines, bladder, and bilateral femur were contoured and defined as organs at risk (OAR). The doses to OARs were limited as follows: V98 <45 Gy for PTV, V15 <52.5 Gy for PTV, and V10 <55 Gy for PTV. Capecitabine (Cap) was administered orally at a dose of 1,700 mg/m2/day, 5 days per week during the first to fifth weeks of NACR, beginning the day of the start of radiation therapy and ending with the last dose of radiation therapy.
Thermal therapy
Thermic treatment was administered using the Thermotron-RF 8 (Yamamoto Vinita Co., Ltd., Osaka, Japan) once per week for 5 weeks with 50 min irradiation. Precise methods of thermal therapy were described elsewhere (16,17,19,20).
A sensor catheter with four temperature points was attached to the skin on the lateral abdomen of 68 patients. The average surface skin temperature of the four temperature points during each irradiation was measured to calculate the average surface skin temperature of the five thermal treatments.
Evaluation of treatment response and adverse effect
Objective response was evaluated from week 2–18, with a median of 8 weeks, and the timing of surgical resection ranged from weeks 9–43, with a median of 16 weeks after the completion of NACR with concurrent thermal therapy. Response was evaluated according to the response evaluation criteria in solid tumors using MRI, CT, or PET/CT (21). CR was defined as total disappearance of the lesions and partial response (PR) was defined as a 30% decrease in the sum of the diameters of the lesions until the period of the evaluation. Stable disease (SD) was defined as between a 30% decrease and 20% increase in the sum of the diameters of the lesions. Finally, Progressive disease (PD) was defined as a 20% increase in the sum of diameters of the lesions or new distant metastasis. We evaluated CR as the disappearance of the tumor on PET/CT and MRI as well as a positive-to-negative change on PET/CT.
Resected specimens were graded according to the Japanese Classification of Colorectal Carcinoma as follows: Pathological grade 0, no fibrosis in the specimen; grade 1a, denaturation and necrosis of cancer cells in approximately <1/3 of the cancer; grade 1b, denaturation and necrosis in <2/3 of cancer cells plus fusion in >1/3 of the cancer; grade 2, significant denaturation, necrosis, fusion, and loss in >2/3 of the cancer; grade 3 (i.e., pCR), no cancer cells observed in both primary and regional lymph nodes (18). Adverse effects were evaluated on the basis of the Common Terminology Criteria for Adverse Events (4.0) (22).
Statistical analysis
SPSS v21 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. Mean values were compared using paired Students t-test and ANOVA with Tukey-Kramer HSD (honestly significant difference) test for multiple comparisons. Categorical data were analyzed using the χ2 test. All reported P-values are two-tailed and P<0.05 was considered to indicate a statistically significant difference..
In the present study, by using the predictive formula for output-limiting symptoms mentioned above, we used an initial time of 0 min for the time at which an output limiting symptom occurred as a predicted initial RF output (IRO (Watt)), and compared it to the actual observed RF output (i.e. RO difference (Watt/min)=average actual observed RO (Watt/min) during 50 mins RF irradiation-predicted IRO). For RO differences, the quartiles were ≤-153, −152 to −77, −76 to 76, and ≥77 Watt/min at the first thermal treatment.
Results
Patients characteristics
Among the 80 patients, 26 (32.5%), 32 (40%), 9 (11.3%), and 13 (16.3%) achieved CR, PR, SD, and PD, respectively. Consequently, 11 (13.8%), 20 (25%), 18 (22.5%), 5 (6.3%), 12 (15%), and 14 (17.5%) patients were pCR (i.e., grade 3), grade 2, grade 1-0, CR, PR-SD in no resection, and PD included 4 resections, respectively. Reduced tumor burdens were observed in 37/53 (69.8%) patients. In the therapeutic response, there was no significant difference between macroscopic types such as polypoid or ulcerative tumors. Because polypoid tumors were small number.
Table I shows patients characteristics according to the RO differences. There were significant differences in body mass index and internal organs fat area between patients with the RO difference ≤76 Watt/min and ≥77 Watt/min.
Table I.
Characteristics of 80 patients according to the RO differences.
| Difference RO | ||||
|---|---|---|---|---|
| Characteristics | ≤76 Watt/min | ≥77 Watt/min | Total | P-value |
| Total no. of patients | 59 | 21 | 80 | |
| Age, years | 0.301 | |||
| Median | 62 | 65 | 63 | |
| Range | 33–89 | 42–89 | 33–89 | |
| Sex, no. (%) | 0.056 | |||
| Female | 18 (30.5) | 2 (9.5) | 20 | |
| Male | 41 (69.5) | 19 (90.5) | 60 | |
| Distance to anal verge, no. (%) | 0.700 | |||
| 0–3.0 cm | 41 (69.5) | 13 (24.1) | 54 | |
| 3.1–5.0 cm | 11 (18.6) | 4 (26.7) | 15 | |
| ≥5.1 cm | 7 (11.9) | 4 (36.4) | 11 | |
| Tumor location, no. (%) | 0.457 | |||
| Ra | 6 (10.2) | 4 (19.0) | 10 | |
| Rb | 33 (55.9) | 10 (47.6) | 43 | |
| RbP | 20 (33.9) | 7 (33.4) | 27 | |
| Tumor stage, no. (%) | 0.284 | |||
| T2 | 14 (23.7) | 6 (28.6) | 20 | |
| T3 | 36 (61.0) | 9 (42.9) | 45 | |
| T4 | 9 (15.3) | 6 (28.6) | 15 | |
| Lymph node stage, no. (%) | 0.691 | |||
| N0 | 31 (52.5) | 9 (42.9) | 40 | |
| N1 | 26 (44.1) | 11 (52.4) | 37 | |
| N2 | 1 (1.7) | 1 (4.8) | 2 | |
| N3 | 1 (1.7) | 0 (0.0) | 1 | |
| Distant metastasis, no. (%) | 0.451 | |||
| M (−) | 53 (89.8) | 20 (95.2) | 73 | |
| M (+) | 6 (10.2) | 1 (4.8) | 7 | |
| Pretreatment TNM stage, no. (%) | 0.700 | |||
| Stage 1 | 8 (13.6) | 4 (19.0) | 12 | |
| Stage 2 | 19 (32.2) | 5 (23.8) | 24 | |
| Stage 3 | 26 (44.1) | 11 (52.4) | 37 | |
| Stage 4 | 6 (10.2) | 1 (4.8) | 7 | |
| Tumor differentiation, no. (%) | 0.157 | |||
| Well differentiated | 26 (44.1) | 11 (52.4) | 37 | |
| Moderately differentiated | 27 (45.8) | 9 (42.9) | 36 | |
| Poorly differentiated | 6 (10.2) | 0 (0.0) | 6 | |
| Undifferentiated | 0 (0.0) | 1 (4.8) | 1 | |
| Type of surgery, no. (%) | 0.985 | |||
| APR | 9 (15.3) | 3 (14.3) | 12 | |
| LAR | 10 (16.9) | 3 (14.3) | 13 | |
| sLAR | 11 (18.6) | 4 (19.0) | 15 | |
| ISR | 5 (8.5) | 2 (9.5) | 7 | |
| Local incision | 5 (8.5) | 1 (4.8) | 6 | |
| No resection | 1 (1.7) | 1 (4.8) | 2 | |
| No surgery | 18 (30.5) | 7 (33.3) | 25 | |
| BMI, SD | 23.6, 3.2 | 21.3, 3 | 0.001 | |
| Internal organs fat area (cm2), SD | 103.1, 55.8 | 72.9, 50.4 | 0.013 | |
ISR, intersphincteric resection; sLAR, super low anterior resection; LAR, low anterior resection; APR, abdominoperineal resection; SD, standard deviation; TNM, tumor node metastasis; BMI, body mass index.
Treatment response according to the GTVs
Fig. 1 shows the results of treatment response according to the GTVs (≤32 cm3, 33–79 cm3, and ≥80 cm3) among 80 patients (A) and RECIST criteria (B). The highest CR and pCR were shown 85.0 and 30%, respectively, in patients with a GTV ≤32 cm3. There were significant differences in treatment responses among GTVs in (A) and (B) (χ2=30.423, P=0.001 and χ2=34.385, P<0.0001, respectively).
Figure 1.
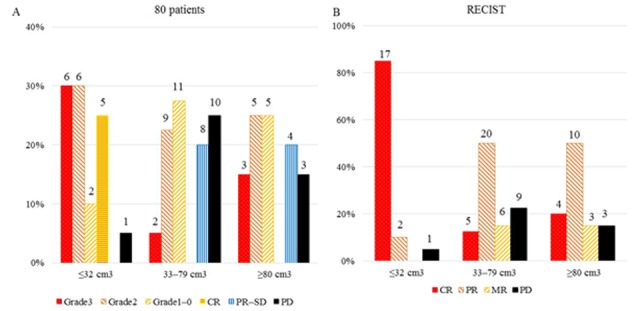
Results of treatment response according to the GTVs among (A) 80 patients and (B) RECIST criteria. Grade 3, grade 2, grade 1-0: Resection; CR, PR-SD: No resection; PD: Included 4 resections.
Treatment response according to the RO differences
Fig. 2 shows the results of treatment response according to the RO differences (≤-153 Watt/min, −152-76 Watt/min, and ≥77 Watt/min among 80 patients (A) and RECIST criteria (B). The highest CR and pCR were shown 38.1 and 23.8%, respectively, in patients with a RO difference ≥77 Watt/min. There was no significant difference in treatment responses among RO differences in (A) and (B).
Figure 2.
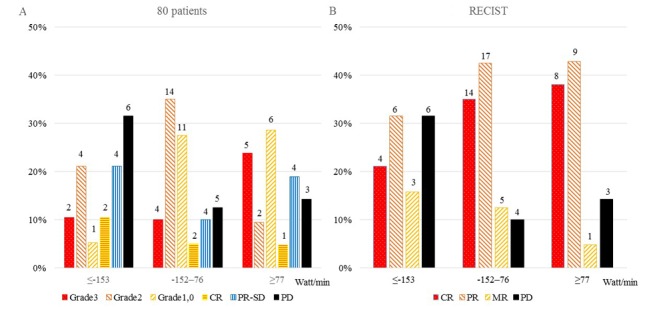
Results of treatment response according to the RO differences among (A) 80 patients and (B) RECIST criteria. Grade 3, grade 2, grade 1-0: Resection; CR, PR-SD: no resection; PD: Included 4 resections.
Treatment response of according to the RO difference
Figs. 3 and 4 shows the results of treatment response of RECIST criteria (Fig. 3) and 80 patients (Fig. 4) according to the RO difference (≤76 Watt/min (A) vs. ≥77 Watt/min (B)) and GTV (≤32 cm3, 33–79 cm3, ≥80 cm3). The highest rates of CR (87.5%) were observed in patients with a GTV ≤32 cm3 and a RO difference ≤76 Watt/min (Fig. 3), while a RO difference ≥77 Watt/min group showed higher rates of pCR in those with a GTV ≤32 cm3 or ≥80 cm3 (50.0% or 42.9%, respectively) than the RO difference ≤76 Watt/min group (Fig. 4). There was a significant difference in treatment responses among GTVs in RECIST criteria patients with a RO difference ≤76 Watt/min group (Fig. 3A) (χ2=34.526, P<0.0001) and not in those with a RO difference ≥77 Watt/min group (Fig. 3B). There was also a significant difference in treatment responses among GTVs in 80 patients with a RO difference ≤76 Watt/min group (Fig. 4A) (χ2=24.365, P=0.007) and not in those with a RO difference ≥77 Watt/min group (Fig. 4B).
Figure 3.
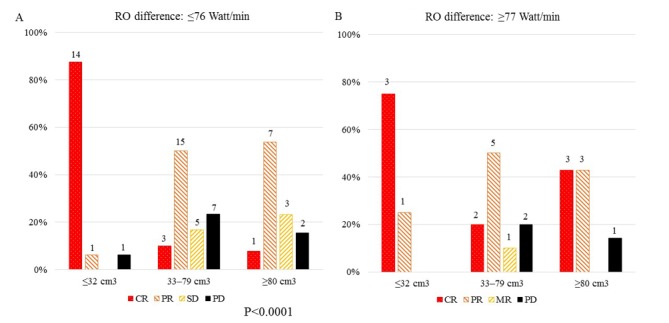
Results of treatment response (RECIST criteria) according to the RO differences, (A) ≤76 Watt/min vs. (B) ≥77 Watt/min and GTVs (≤32 cm3, 33–79 cm3, ≥80 cm3).
Figure 4.
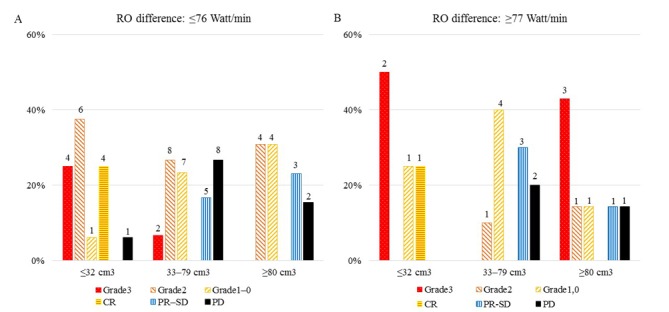
Results of treatment response of 80 patients according to the RO differences, (A) ≤76 Watt/min vs. (B) ≥77 Watt/min and GTVs (≤32 cm3, 33–79 cm3, ≥80 cm3). Grade 3, grade 2, grade 1-0: resection; CR, PR-SD: No resection; PD: Included 4 resections.
Changes of the surface skin temperature
Changes of the surface skin temperature during the 50 min of irradiation are shown in Fig. 5. Skin temperature significantly changed in patients with a pathological grade 3 tumor compared both to those who had PD and other outcomes, only in the RO difference ≥77 Watt/min group (Tukey-Kramer HSD test, P<0.001). In the RO difference ≤76 Watt/min group there was no significant difference among patients with grade 3, other outcomes and PD.
Figure 5.
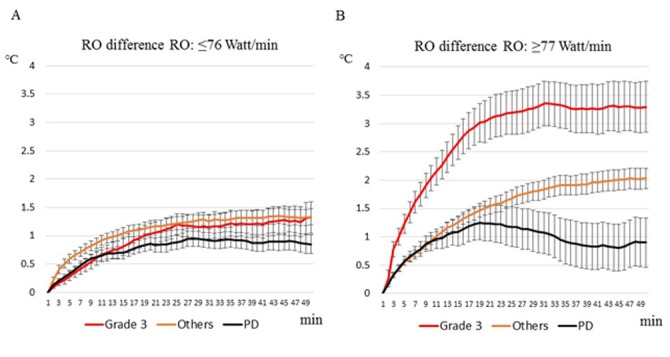
Changes of the surface skin temperature during the 50 min of irradiation according to treatment response and RO difference, (A) ≤76 Watt/min vs. (B) ≥77 Watt/min. Others include patients with grade 2-0 CR and PR-SD. Data in the figure are presented as means with standard error (SEM).
Toxicity
NACR with concurrent thermal therapy was tolerated well, with 96.3% of patients receiving the full dose of chemotherapy and 100% receiving the full dose of radiotherapy with 5 sessions of thermal therapy. Grade 3 toxicity was observed in 7/80 (8.8%) patients. Six of them were classified as non-hematologic toxicity. The remaining one patient had hematologic toxicity as anemia. There was no significant difference between hematologic and non-hematologic toxicity.
During thermic treatment, 72 (90%) patients experienced at least one instance of a troublesome hotspot phenomenon such as pain/irritable sensations (85%) and subcutaneous induration (6.3%).
Discussion
The positive outcome of IMRT plus Cap has been demonstrated by pCR rates ranging from 14.1–30.6%, with grade 3 toxicity rates from 11.1–17.6% (6,8,23–27). These results suggest IMRT has the potential for higher tumor control rates and/or less toxicity for patients. Nevertheless, more accurate and precise delineation of the target is required. Recentry, Valentini et al (28) recommended PTV used is 10–15 mm in view of rectal motion.
In trials of preoperative helical tomotherapy for rectal cancer, De Ridder et al (29) reported that the metabolic response rate was 45% in the non-boosted group compared with 77% in the boosted group. Huang et al (30) reported that there was no significant differences between patients who received tomotherapy or other therapy with respect to pCR (14.3% vs. 8.8%), T downstaging (60% vs. 61.4%), N downstaging in patients with cN1-2 (69.6% vs. 79.1%), ypT0-2N0 (57.1% vs. 43.9%), or sphincter preservation rate for low-lying rectal cancer (85.2% vs. 80.0%). Four patients in the tomotherapy group (11.1%) and 10 in the other therapy group (16.7%) developed G3 acute toxicities during CRT.
From the viewpoint of hyperthermia, Maluta et al (31) reported that hyperthermia plus chemoradiation resulted in 23.6% patients achieving pCR and 5.2% patients with PD. Schroeder et al (32) retrospectively compared neoadjuvant radiation with concurrent 5-FU-based chemotherapy with and without hyperthermia in 106 rectal cancer patients; pCR was achieved by 6.7 and 16.4% of patients, respectively. The rate of sphincter-sparing surgery was 57% in the hyperthermal radiochemotherapy group compared with 35% in the radiochemotherapy group.
Among the nonrandomized and randomized studies mentioned above, none describe the associations of outcomes among dosimetric parameters such as physical volumes of TVs of tumors or the associations between physical volumes of TVs and patient outcomes.
Although our idea based on the results of NACR with concurrent thermal therapy is novel, one possible explanation is that both radiation and RF treatment have electromagnetic effects owing to low-energy, low-frequency (8 MHz) waves and high-energy, high-frequency (>3×1019 Hz) waves on cancer cells, respectively. This results in concurrent RF treatment appearing to have a potential filter effect on the results of radiation treatment, because outputable/heatable conditions may be associated with good response to chemoradiation (20).
The present study has some limitations. First, the small sample size meant there was no comparison group that received radiotherapy with concurrent Cap. Second, the post-treatment follow-up duration was short. Therefore, further studies should be performed to confirm these results.
The results show that the associations between parameters of TVs, patients state and change of body temperature are good predictors of outcomes in patients with rectal cancer who receive NACR with concurrent thermal therapy. First, GTV ≤32 cm3 patients are indicated for this NACR with or without concurrent thermic treatment and second, large tumor patients (GTV ≥80 cm3) need to treat this NACR with concurrent thermic treatment. And also our idea might be able to use for early assessment of treatment efficacy, and will help the decision of the continuation or cessation of this treatment modality.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors contributions
HS contributed significantly to writing of the manuscript and analysis of clinical data. MM, KO, AT, HK and TT contributed to the design and implementation of the research, and to the analysis of the results. KO contributed to the conception of the study and performed statistical analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The Ethics Committee for Human Studies at Hidaka Hospital (Takasaki, Gunma, Japan) approved the present study, which was conducted in accordance with The Declaration of Helsinki. Participants were fully informed of the procedures, and written informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Powathil GG, Adamson DJ, Chaplain MA. Towards predicting the response of a solid tumour to chemotherapy and radiotherapy treatments: clinical insights from a computational model. PLoS Comput Biol. 2013;9:e1003120. doi: 10.1371/journal.pcbi.1003120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holck S, Nielsen HJ, Pedersen N, Larsson LI. Phospho-ERK1/2 levels in cancer cell nuclei predict responsiveness to radiochemotherapy of rectal adenocarcinoma. Oncotarget. 2015;6:34321–34328. doi: 10.18632/oncotarget.5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Z, Shu Y, Zhou H, Zhang W, Wang H. Radiogenomics helps to achieve personalized therapy by evaluating patient responses to radiation treatment. Carcinogenesis. 2015;36:307–317. doi: 10.1093/carcin/bgv007. [DOI] [PubMed] [Google Scholar]
- 4.Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, Bardet E, Beny A, Ollier JC, Bolla M, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: Long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15:184–190. doi: 10.1016/S1470-2045(13)70599-0. [DOI] [PubMed] [Google Scholar]
- 5.Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 6.Park JH, Yoon SM, Yu CS, Kim JH, Kim TW, Kim JC. Randomized phase 3 trial comparing preoperative and postoperative chemoradiotherapy with capecitabine for locally advanced rectal cancer. Cancer. 2011;117:3703–3712. doi: 10.1002/cncr.25943. [DOI] [PubMed] [Google Scholar]
- 7.Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, Rutten H, Pahlman L, Glimelius B, Leer JW, et al. The TME trial after a median follow-up of 6 years: Increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693–701. doi: 10.1097/01.sla.0000257358.56863.ce. [DOI] [PubMed] [Google Scholar]
- 8.Das P, Lin EH, Bhatia S, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ, Hoff PM, Eng C, Wolff RA, et al. Preoperative chemoradiotherapy with capecitabine versus protracted infusion 5-fluorouracil for rectal cancer: A matched pair analysis. Int J Radiat Oncol Biol Phys. 2006;66:1378–1383. doi: 10.1016/j.ijrobp.2006.07.1374. [DOI] [PubMed] [Google Scholar]
- 9.Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 10.Yeo SG, Kim DY, Kim TH, Chang HJ, Oh JH, Park W, Choi DH, Nam H, Kim JS, Cho MJ, et al. Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: Long-term outcomes and prognostic significance of pathologic nodal status (KROG 09–01) Ann Surg. 2010;252:998–1004. doi: 10.1097/SLA.0b013e3181f3f1b1. [DOI] [PubMed] [Google Scholar]
- 11.Park IJ, You YN, Agarwal A, Skibber JM, Rodriguez-Bigas MA, Eng C, Feig BW, Das P, Krishnan S, Crane CH, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30:1770–1776. doi: 10.1200/JCO.2011.39.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C, Becker H, Ghadimi M, Mrak K, Merkel S, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: Updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol. 2014;32:1554–1562. doi: 10.1200/JCO.2013.54.3769. [DOI] [PubMed] [Google Scholar]
- 13.Zhu J, Gu W, Lian P, Sheng W, Cai G, Shi D, Cai S, Zhang Z. A phase II trial of neoadjuvant IMRT-based chemoradiotherapy followed by one cycle of capecitabine for stage II/III rectal adenocarcinoma. Radiat Oncol. 2013;8:130. doi: 10.1186/1748-717X-8-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roh MS, Colangelo LH, OConnell MJ, Yothers G, Deutsch M, Allegra CJ, Kahlenberg MS, Baez-Diaz L, Ursiny CS, Petrelli NJ, Wolmark N. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124–5130. doi: 10.1200/JCO.2009.22.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, Jr, Silva e Sousa AH, Jr, Campos FG, Kiss DR, Gama-Rodrigues J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann Surg. 2004;240:711–718. doi: 10.1097/01.sla.0000141194.27992.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shoji H, Motegi M, Osawa K, Okonogi N, Okazaki A, Andou Y, Asao T, Kuwano H, Takahashi T, Ogoshi K. Does standardization of radiofrequency hyperthermia benefit patients with malignancies? Ann Cancer Res Ther. 2014;22:28–35. doi: 10.4993/acrt.22.28. [DOI] [Google Scholar]
- 17.Shoji H, Motegi M, Osawa K, Okonogi N, Okazaki A, Andou Y, Asao T, Kuwano H, Takahashi T, Ogoshi K. Output-limiting symptoms induced by radiofrequency hyperthermia. Are they predictable? Int J Hyperthermia. 2016;32:199–203. doi: 10.3109/02656736.2015.1107760. [DOI] [PubMed] [Google Scholar]
- 18.Japanese Society for Cancer of the Colon and Rectum: Japanese classification of colon carcinoma. 8th edition. Kanehara, Tokyo: 2013. [Google Scholar]
- 19.Shoji H, Motegi M, Osawa K, Okonogi N, Okazaki A, Andou Y, Asao T, Kuwano H, Takahashi T, Ogoshi K. A novel strategy of radiofrequency hyperthermia (neothermia) in combination with preoperative chemoradiotherapy for the treatment of advanced rectal cancer: A pilot study. Cancer Med. 2015;4:834–843. doi: 10.1002/cam4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shoji H, Motegi M, Osawa K, Okonogi N, Okazaki A, Andou Y, Asao T, Kuwano H, Takahashi T, Ogoshi K. Radiofrequency thermal treatment with chemoradiotherapy for advanced rectal cancer. Oncol Rep. 2016;35:2569–2575. doi: 10.3892/or.2016.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40. [Feb 14;2017 ];US and National Cancer Institute Cancer Therapy Evaluation Program: Common Terminology Criteria for Adverse Events (CTCAE) [Google Scholar]
- 23.Krishnan S, Janjan NA, Skibber JM, Rodriguez-Bigas MA, Wolff RA, Das P, Delclos ME, Chang GJ, Hoff PM, Eng C, et al. Phase II study of capecitabine (Xeloda) and concomitant boost radiotherapy in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66:762–771. doi: 10.1016/j.ijrobp.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 24.Yerushalmi R, Idelevich E, Dror Y, Stemmer SM, Figer A, Sulkes A, Brenner B, Loven D, Dreznik Z, Nudelman I, et al. Preoperative chemoradiation in rectal cancer: Retrospective comparison between capecitabine and continuous infusion of 5-fluorouracil. J Surg Oncol. 2006;93:529–533. doi: 10.1002/jso.20503. [DOI] [PubMed] [Google Scholar]
- 25.De Paoli A, Chiara S, Luppi G, Friso ML, Beretta GD, Del Prete S, Pasetto L, Santantonio M, Sarti E, Mantello G, et al. Capecitabine in combination with preoperative radiation therapy in locally advanced, resectable, rectal cancer: A multicentric phase II study. Ann Oncol. 2006;17:246–251. doi: 10.1093/annonc/mdj041. [DOI] [PubMed] [Google Scholar]
- 26.Craven I, Crellin A, Cooper R, Melcher A, Byrne P, Sebag-Montefiore D. Preoperative radiotherapy combined with 5 days per week capecitabine chemotherapy in locally advanced rectal cancer. Br J Cancer. 2007;97:1333–1337. doi: 10.1038/sj.bjc.6604042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elshazly WG, Farouk M, Samy M. Preoperative concomitant radiotherapy with oral capecitabine in advanced rectal cancer within 6 cm from anal verge. Int J Colorectal Dis. 2009;24:401–407. doi: 10.1007/s00384-008-0623-9. [DOI] [PubMed] [Google Scholar]
- 28.Valentini V, Gambacorta MA, Barbaro B, Chiloiro G, Coco C, Das P, Fanfani F, Joye I, Kachnic L, Maingon P, et al. International consensus guidelines on clinical target volume delineation in rectal cancer. Radiother Oncol. 2016;120:195–201. doi: 10.1016/j.radonc.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 29.De Ridder M, Tournel K, Van Nieuwenhove Y, Engels B, Hoorens A, Everaert H, de Beeck Op B, Vinh-Hung V, De Grève J, Delvaux G, et al. Phase II study of preoperative helical tomotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2008;70:728–734. doi: 10.1016/j.ijrobp.2007.07.2332. [DOI] [PubMed] [Google Scholar]
- 30.Huang MY, Chen CF, Huang CM, Tsai HL, Yeh YS, Ma CJ, Wu CH, Lu CY, Chai CY, Huang CJ, Wang JY. Helical tomotherapy combined with capecitabine in the preoperative treatment of locally advanced rectal cancer. Biomed Res Int. 2014;2014:352083. doi: 10.1155/2014/352083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maluta S, Romano M, Dalloglio S, Genna M, Oliani C, Pioli F, Gabbani M, Marciai N, Palazzi M. Regional hyperthermia added to intensified preoperative chemo-radiation in locally advanced adenocarcinoma of middle and lower rectum. Int J Hyperthermia. 2010;26:108–117. doi: 10.3109/02656730903333958. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder C, Gani C, Lamprecht U, von Weyhern CH, Weinmann M, Bamberg M, Berger B. Pathological complete response and sphincter-sparing surgery after neoadjuvant radiochemotherapy with regional hyperthermia for locally advanced rectal cancer compared with radiochemotherapy alone. Int J Hyperthermia. 2012;28:707–714. doi: 10.3109/02656736.2012.722263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


