Abstract
Pancreatic ductal adenocarcinoma (PDA) is a worldwide health problem. Early diagnosis and assessment may enhance the quality of life and survival of patients. The present study investigated the potential correlations between the gene and protein expression of laminin-332 (LM-332 or laminin-5) and clinicopathological factors as well as evaluating its influence on the survival of patients with PDA. The expression of LM-332 subunit mRNAs in pancreatic carcinoma specimens from 37 patients was investigated by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis. Using immunohistochemical methods, the protein expressions of the three chains of LM-322 (LNα3, LNβ3 and LNγ2) were determined in 96 pancreatic carcinoma specimens, for association analysis with clinicopathological characteristics from patient data. The results of the prognosis analysis of three mRNAs expression datasets were validated in The Cancer Genome Atlas datasets. RT-qPCR results indicated that the overall relative values of LNα3 and LNγ2 mRNAs were increased in pancreatic carcinoma compared with the control. In immunostaining analyses LNα3 and LNγ2 expression was observed in all tumor tissues from the 96 patient samples. The expression levels of LNα3, LNβ3 and LNγ2 were associated with each other. LNα3 and LNγ2 positivity was significantly associated with differentiation, depth of invasion and advanced stage (P<0.05). The samples were classified into three groups: Basement membrane (B) type, cytoplasmic (C) type and mixed (M) type, according to their LNγ2 immunohistochemical expression patterns. The B type correlated significantly with differentiation (P=0.010) and the M type was significantly associated with hepatic metastasis (P=0.031). Patients with B-type LNγ2 demonstrated significantly better outcomes than patients with the C or M type (P=0.012 and P=0.003, respectively). Overexpression of the α3, β3 and γ2 chains of LM-332 may serve an important role in the progression and prognosis of PDA.
Keywords: pancreatic ductal adenocarcinoma, laminin-332, laminin α3, laminin β3, laminin γ2
Introduction
Laminins are major components of the extracellular matrix (ECM). They localize to the basement membrane, and play essential roles in cell adhesion, differentiation, migration, and mechanosignal transduction. The laminin molecule is a cruciform heterotrimer assembled from α, β, and γ glycoprotein chains, encoded in humans by five α, three β, and three γ genes (1). To date, 16 distinct laminin isoforms have been identified in mammals (2).
Laminin-332 (LM-332) is a major member of the laminin family, consisting of LNα3, β3, and γ2 chains, encoded by the LAMA3, LAMB3, and LAMC2 genes, respectively. The three chains are expressed from the three genes separately, and subsequent formation of the heterotrimer is now considered an essential step in the production of LM-332 (3). Unlike the α3 and β3 chains, the γ2 chain is unique in the LM-332 trimer (4). LM-332 has been demonstrated to facilitate diverse actions in cultured cells, including roles in adhesion, scattering and migration, polarity, proliferation, and apoptosis, through focal adhesion and hemidesmosomes formed via an interaction between α3β1 integrin and α6β4 integrin (5,6). Moreover, these integrins also interact with molecules involved in important signal transduction pathways (7,8), which have important roles in tumor invasion and metastasis (9,10). These properties of LM-332 suggest that it may play an important role in carcinogenesis.
Although there are only a few reports concerning the expression of LNα3 and LNβ3 in human cancers, LNγ2 has been studied previously. Several immunohistochemistry investigations have indicated that LNγ2 is localized at the leading edge of invading carcinomas and its expression correlates positively with invasiveness and poor patient survival (11). Shinichiro (12) reported that the cytoplasmic expression of LNγ2 demonstrates high invasive potential of tumors and is correlated with distant metastasis, especially hepatic metastasis, and with a poor prognosis. However, coexpression of the α3/β3/γ2 chains of LM-332 has not been reported in patients with pancreatic ductal adenocarcinoma (PDA). Accordingly, further study is required to identify the expression of the three subunits of LM-332 in PDA.
In a previous investigation, we demonstrated (through immunostaining) that LNβ3 was expressed in all patients with PDA and was related to differentiation, advanced stage, and survival time (13). In the present study, we expanded the scope of this exploration, including two other chains (LNα3 and LNγ2). Firstly, we analyzed the mRNA expression of LAMA3 and LAMC2 genes in pairs of pancreatic carcinoma and non-tumor pancreatic tissues from 37 patients. Secondly, we immunohistochemically examined the expression of LNα3 and LNγ2 in 96 tissue samples of PDA and assessed the potential relationships among the three subunits. Finally, we compared the expression levels of the three subunits and assessed the potential relationships between clinical and pathological features in patients with PDA postoperation.
Patients and methods
Patients and sample collection
Fresh specimens of PDA and non-tumor pancreatic tissues were obtained from patients (n=37) undergoing surgical resection at the Department of Hepatobiliary and Pancreatic Surgery, The First Affiliated Hospital, College of Medicine, Zhejiang University, between February 2010 and March 2013. These experiments were approved by our institutional review board. Tissue specimens were snap-frozen in liquid nitrogen and stored at −80°C.
Formalin-fixed, paraffin wax-embedded sections of 96 resected specimens were used for immunohistochemical staining. All 96 paraffin wax blocks were confirmed to contain tumor tissue by two pathologists; among them, 90 included adjacent normal pancreatic ductal tissue and 6 did not.
The following clinical data were collected: Patient age, gender, and outcome; the presence/absence of metastasis; and tumor location, size, margin status, TNM stage, degree of differentiation, and invasion degree and location (bile duct/duodenal, lymph node, serosa, portal vein, hepatic, perineural, vascular). No particular procedure was used to select the cases.
Patients were informed about the project and gave their written consent to participate in the study.
Follow up
Overall survival was measured from the time of surgery to the time of death or the last follow-up visit. Dates of death were determined from patient hospital records or follow-up telephone calls. The median survival time was 7.5 months, and the longest survival time was 35 months at the last follow-up visit.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR). Total RNA was extracted from pancreatic cancer tissues and adjacent tissues using the TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and cDNA was synthesized from total RNA (2 µg) using iScript cDNA Synthesis (Bio-Rad Laboratories, Inc., Hercules, CA, USA). qPCR was performed with an ABI PRISM 7900 Sequence Detection System (Applied Biosystems; Thermo Fisher Scientific, Inc.) using the iTaq universal SYBR-Green supermix (Bio-Rad Laboratories, Inc.). Amplification reactions included 1 µl cDNA template, 0.3 µl each of the forward and reverse primers (10 µM), 0.2 µl 50X ROX Reference Dye II (Takara Biotechnology Co., Ltd., Dalian, China), and 5 µl 2X SYBR Premix DimerEraser in a total volume of 10 µl. The primers were as follows: LAMA3, 5′-AAAGCGTATGTGGATAAATGTGG-3′ (forward) and 5′-CGGAAAGCAGGCGTAGAAA-3′ (reverse); LAMC2, 5′-TTCTACAACGATCCGCACGAC-3′ (forward) and 5′-ACACCACCTCCTCCGTCTCC-3′ (reverse); and β-actin, 5′-CTTAGTTGCGTTACACCCTTTC-3′ (forward) and 5′-GAGTTAAAAGCAGCCCTGGT-3′ (reverse). Amplification of the transcripts involved an initial denaturation at 95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec, 55°C for 30 sec, and 72°C for 34 sec. The Cquantification cycle (Cq) comparison method was used for relative quantification. β-actin was used as the internal control for normalization. All qPCRs were performed in triplicate. Results were calculated using the 2−ΔΔCq method (14).
Immunohistochemistry
Formalin-fixed, paraffin wax-embedded tumor tissues from 96 patients were sectioned (4 µm thick), mounted on poly L-lysine-coated glass slides, and allowed to dry overnight at 65°C. Briefly, slides were deparaffinized in two xylene washes and transferred through three changes in 95% ethanol, and then transferred to water. For antigen retrieval (α3, γ2), the slides were boiled in a pressure cooker containing 0.01 mol/l sodium citrate (pH 6.0) at maximum heat for 3 min and then cooled over 20 min to room temperature. Endogenous peroxidase activity was blocked in 1.5% methanol/hydrogen peroxide for 8 min at room temperature. Following incubation, the slides were washed three times in PBS for 2 min each. Then, the slides were incubated with the primary antibody: α3 antibodies (cat. no. sc-20143; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 1:100 dilution or γ2 (cat. no. sc-25341; Santa Cruz Biotechnology, Inc.) at 1:250 dilution overnight at 4°C. After washing three times in PBS for 2 min each, the bound primary antibody was detected using a ready-to-use secondary antibody kit (cat. no. K5007; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) for 30 min at room temperature, then the slides were washed three times in PBS for 2 min each and the chromogenic substrate 3,3′-diaminobenzidine tetrahydrochloride (DAB) was added. The specimens were counterstained with hematoxylin, mounted, and examined by light microscopy.
The percentage of tumor cells was scored as follows: 0, ≤5% tumor cells; 1, 6–25% tumor cells; 2, 26–50% tumor cells; and 3, >51% tumor cells. Scoring criteria for staining intensity were as follows: 0, no staining; 1, weak staining (light yellow); 2, moderate staining (yellow/brown); and 3, strong staining (brown). The staining index was evaluated as the product of the percentage of positive tumor cells and staining intensity scores. Using this method, we evaluated the expression of LNα3 and LNγ2 in the tumor and adjacent normal pancreatic ductal tissue by determining the staining index with scores of 0, 1, 2, 3, 4, 6, or 9. 0–1 is negative (−); 2–3 is weak-positive (+); 4–6 is the medium positive (+ +); >6 is strongly positive (+ + +) (15). In the statistical analyses, an optimal cutoff value was assessed as follows: A staining index score of >6 was used to indicate tumors with high LNα3 and LNγ2 expression, and a staining index score of ≤6 was used to define low LNα3 and LNγ2 expression.
According to the locations of LNγ2 immunohistochemical expression patterns, samples were classified into three groups, as follows: i) basement membrane (B) type: The LNγ2 was predominantly present in the basement membrane ECM and showed a continuous linear structure (>10% of ECM stained LNγ2-positive); ii) cytoplasmic (C) type: The LNγ2 was present in the cytoplasm of cancer cells (>10% of cytoplasm of cancer cells stained LNγ2-positive); and iii) mixed (M) type: The LNγ2 was present in the ECM and cytoplasm of cancer cells (>10% of ECM and cytoplasm of cancer cells stained LNγ2-positive).
LAMA3, LAMB3 and LAMC2 mRNA prognosis analysis of TCGA
The results of prognosis analysis of three mRNAs expression datasets were validated in the TCGA datasets. TCGA-pancreatic cancer mRNA data and clinical data (level 3) of the corresponding patients (178 tumor tissue) were downloaded from the TCGA Data portal. The expression analyses were carried out using BRB-ArrayTools (version 4.5; National Cancer Institute, Bethesda, MD, USA) (16). We identified three genes whose expression was significantly related to survival of the patient by survival analysis function of BRB array tools based on univariate proportional hazards models. We divide the gene expression level for low or high using the median value as the cutoff.
Statistical analysis
All statistical analyses were performed using the SPSS software (version 21.0; SPSS, Inc., Chicago, IL, USA). Differences in relative values of the three genes between the pancreatic carcinoma specimens and non-tumor pancreatic tissues were assessed using paired-sample t-tests. The relationship between immunohistochemical expression of three chains in the cancer tissues and clinicopathological characteristics was analyzed using a χ2 (two-tailed) test or Fisher's exact test. Furthermore, the Kaplan-Meier method with a log-rank analysis was used to assess the correlation between expression levels of the three protein chains and survival rate. The Cox proportional hazards regression model was used for multivariate analyses. P<0.05 was considered to indicate a statistically significant difference. P-values between 0.05 and 0.10 were considered to indicate a trend towards an association.
The LNβ3 data are from results of our previous study using the same samples.
Results
mRNA expression of LAMA3 and LAMC2 between pancreatic adenocarcinoma and non-tumor pancreatic tissues
In this investigation, 37 pairs of primary pancreatic adenocarcinoma and corresponding non-tumor pancreatic tissues were chosen randomly for DNA analysis by QRT-PCR. The relative values of LNα3 and LNγ2 mRNA showed differential expression between pancreatic carcinoma and non-tumor pancreatic tissues: 1.560±1.511 and 0.996±1.112 in the former, and 2.701±2.863 and 1.592±1.745 in the latter. Like LAMB3, although the overall expression levels of LAMA3 and LAMC2 were increased compared to non-tumor tissues, some showed loss of expression or downregulation, so no statistically significant association was found (P=0.089 and P=0.054, respectively).
Overexpression of LNα3 and LNγ2 in PDA
The expression of LNβ3 in PDA, as assessed by immunohistochemistry, has been observed in 83 of 96 (86.5%) cases in our previous study (13). In the present study, staining for LNα3 and LNγ2 were negative, weakly positive, or moderately positive, while strong staining for (high expression of) LNα3 and LNγ2 was not observed in normal pancreatic ducts (Table I). Although the expression intensity varied, expression of LNα3 and LNγ2 was found in all tumor tissues. Strong staining for (high expression of) LNα3 was observed in 65 of 96 (67.7%) cases and strong staining for (high expression of) LNγ2 was observed in 49 (51.0%) patients. Because there was no adjacent normal pancreatic ductal tissue in six cases, expression for LNα3 and LNγ2 was not assessed in those cases.
Table I.
Expression of LNα3 and LNγ2 in tissue n (%).
| LNα3, n (%) | LNγ2, n (%) | |||||
|---|---|---|---|---|---|---|
| Tissue case | Low | High | P-value | Low | High | P-value |
| Tumor 96 | 31 (32.3) | 65 (67.7) | <0.001 | 47 (49.0) | 49 (51.0) | <0.001 |
| Normal 90 | 90 (100) | 0 (0) | 90 (100) | 0 (0) | ||
P-values were calculated using paired-sample t-tests, where appropriate. LNα3, laminin α3; LNγ2, laminin γ2.
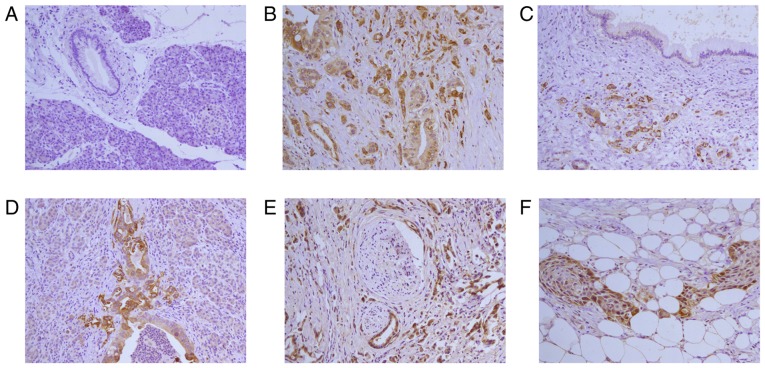
Fig. 1 shows the expression results for LNα3 in PDA. In normal pancreatic ducts, staining for LNα3 was negative. In carcinoma tissues, staining was found predominantly in the cytoplasm of cancer cells and at the invasive front; budding cancer cells often showed more intense cytoplasmic staining. The expression of LNα3 increased with the degree of differentiation. The cytoplasmic immunoreactivity of adenocarcinoma with squamous metaplasia was more intense than that in squamous metaplasia areas. The immunoreactivity was predominantly at the edge of cancer nests and weakly in the center of cancer nests. In the ECM of carcinoma tissues, LNα3 expression, when present in tumor cells, was often surrounded by a discontinuous staining pattern, with a floccular or lamellar structure.
Figure 1.
Immunohistochemistry for LNα3 in pancreatic ductal adenocarcinoma tissues. (A) Pancreatic tissue is negative for staining. (B) Poorly differentiated adenocarcinoma is positive for staining. Intensity of staining for LNα3 in Poorly differentiated domain is more strongly than moderately differentiated domain. (C) Poorly differentiated adenocarcinoma is strong positive for staining. Proliferative duct is negative for staining. (D) Poorly differentiated pancreatic ductal adenocarcinoma positive for staining. Tumor budding was seen in the invasive fronts. Expression of LNα3 is strong. (E) Perineural invasion is observed in poorly differentiated adenocarcinoma. Strong (high expression) stains for LNα3 is shown in tumor cells. (F) Peripancreatic adipo tissue invasion is observed in adenocarcinoma with squamous metaplasia. Strong (high expression) stains for LNα3 is predominantly expressed in cancer cells contacting the stroma at the edge of cancer nests and weakly stains was detected in the center of cancer nests. Magnification, ×200. LNα3, laminin α3.
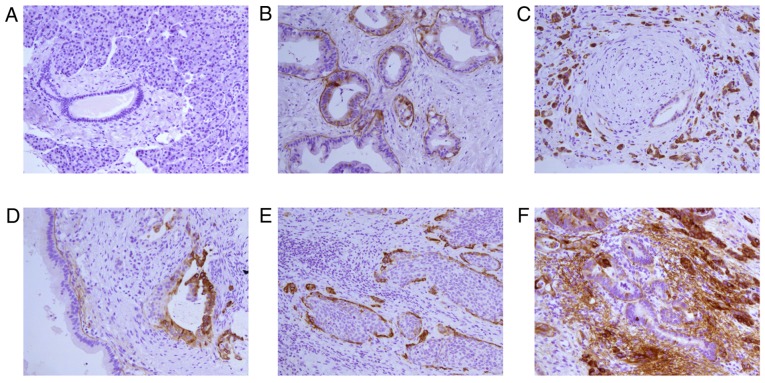
Fig. 2 shows the expressions of immunohistochemistry for LNγ2 in PDA. In normal pancreatic ducts, LNγ2 was negative. In carcinoma tissues, LNγ2 was overexpressed that similar to LNα3 and LNβ3. We also observed significant expression of LNγ2 in the basement membrane surrounding PDAs in some cases. Accordingly, LNγ2 expression patterns were divided into three types: B type (21, 21.9%), C type (59, 61.5%), and M type (16, 16.7%).
Figure 2.
Immunohistochemistry for LNγ2 in pancreatic ductal adenocarcinoma tissues. (A) Peritumoral pancreatic ductal and acinar is negative for staining. (B) Well differentiated adenocarcinoma is positive for staining mainly in the basement membrane. Please note that most of the basement membrane around the duct stained LNγ2 is continuous linear structure. (C) Perineural invasion is observed in poorly differentiated adenocarcinoma. Strong (high expression) stains for LNγ2 is shown in cytoplasm of cancer cells. Please note that the basement membrane around a well-differentiated tubular is positive for staining. (D) The basement membrane in well differentiated adenocarcinoma stained LNγ2 is continuous linear structure. Please note that cytoplasm of cancer cells in tumor budding is stained strongly and the structure of basement membrane is absence. (E) Adenocarcinoma is accompanied with squamous metaplasia. Strong (high expression) stains for LNγ2 is predominantly expressed in the cytoplasm of cancer cells contacting the stroma at the edge of cancer nests and negative stains was detected in the center of cancer nests. (F) The cytoplasm of well differentiated glandular in the center of adenocarcinoma stained LNγ2 is very weakly and the basement membrane is continuous linear structure. The cytoplasm of moderately-poorly differentiated glandular at the edge of adenocarcinoma stained LNγ2 is strongly, the structure of basement membrane is absence and a lot of linear and flocculent basement membrane-like material is observed in extracellular matrix of adenocarcinoma. Magnification, ×200. LNγ2, laminin γ2.
In total, LNα3 and LNγ2 overexpression in pancreatic adenocarcinoma tissues was significant, compared to normal pancreatic tissue (P<0.001 and <0.001 respectively; Table I).
Relationships among LNα3, LNβ3, and LNγ2 expression
Of the 96 cases, 31 (32.3%) showed low expression and 65 (67.7%) showed high expression of LNα3, 13 (13.5%) showed low expression and 83 (86.5%) showed high expression of LNβ3, and 47 (49.0%) showed low expression and 49 (51.0%) showed high expression of LNγ2. The expression levels of LNα3, LNβ3, and LNγ2 were significantly associated with each other (Table II).
Table II.
The association between LNα3, LNβ3 and LNγ2 expression in pancreatic ductal carcinoma n (%).
| LNβ3 | LNγ2 | |||||
|---|---|---|---|---|---|---|
| Expression | Low | High | P-value | Low | High | P-value |
| LNα3 | <0.001 | <0.001 | ||||
| Low | 13 | 18 | 30 | 1 | ||
| High | 0 | 65 | 17 | 48 | ||
| LNβ3 | / | <0.001 | ||||
| Low | / | / | 13 | 0 | ||
| High | / | / | 34 | 39 | ||
P-values were calculated using paired-sample t-tests, where appropriate. LNα3, laminin α3; LNβ3, laminin β3; LNγ2, laminin γ2.
Association among LNα3 and LNγ2 expression and clinicopathological characteristics
According to the staining intensity of LNα3 and LNγ2 in the 96 patient samples with pancreatic ductal carcinoma, the clinical data detailed above were examined (Table III). LNα3 positivity was significantly associated with tumor differentiation, depth of invasion, and advanced stage (P<0.05). LNγ2 positivity was significantly correlated with differentiation, invasion into the serosa, depth of invasion, and TNM stage (P<0.05). Cases with LNα3 positivity had a higher tendency for serosa invasion than those negative for LNα3 (P=0.088).
Table III.
Clinicopathological characteristics based on staining intensity of LNα3, LNγ2 and expression patterns of LNγ2 (%).
| LNα3 | LNγ2 | Pattern of LN | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case (n=96) | Low | High | P-value | Low | High | P-value | B-type | C-type | M-type | P-value | |
| Gender | 0.356 | 0.152 | 0.364 | ||||||||
| M | 62 (64.6) | 18 (29.0) | 44 (71.0) | 27 (43.5) | 35 (56.5) | 11 (17.7) | 41 (66.1) | 10 (16.1) | |||
| F | 34 (35.4) | 13 (38.2) | 21 (61.8) | 20 (58.2) | 14 (41.2) | 10 (29.4) | 18 (52.9) | 6 (17.6) | |||
| Tumor size | 0.999 | 0.712 | 0.649 | ||||||||
| ≤2 cm | 7 (7.3) | 2 (28.6) | 5 (71.4) | 4 (57.1) | 3 (42.9) | 1 (14.3) | 4 (57.1) | 2 (28.6) | |||
| >2 cm | 89 (92.7) | 29 (32.6) | 60 (67.4) | 43 (48.3) | 46 (51.7) | 20 (22.5) | 55 (61.8) | 14 (15.7) | |||
| Location | 0.223 | 0.426 | 0.317 | ||||||||
| Head | 55 (57.3) | 15 (27.3) | 40 (72.7) | 25 (45.5) | 30 (54.5) | 9 (16.4) | 36 (65.5) | 10 (18.2) | |||
| Body or tail | 41 (42.7) | 16 (39.0) | 25 (61.0) | 22 (53.7) | 19 (46.3) | 12 (29.3) | 23 (56.1) | 6 (14.6) | |||
| Bile duct invasion | 0.464 | 0.792 | 0.792 | ||||||||
| Absent | 60 (62.5) | 21 (35.0) | 39 (65.0) | 30 (50.0) | 30 (50.0) | 15 (25.0) | 37 (61.7) | 8 (13.3) | |||
| Present | 36 (37.5) | 10 (27.8) | 26 (72.7) | 17 (47.2) | 19 (52.8) | 6 (16.7) | 22 (61.1) | 8 (22.2) | |||
| Duodenal invasion | 0.404 | 0.921 | 0.632 | ||||||||
| Absent | 69 (71.9) | 24 (77.4) | 45 (69.2) | 34 (49.3) | 35 (50.7) | 16 (23.2) | 43 (62.3) | 10 (14.5) | |||
| Present | 27 (28.1) | 7 (25.9) | 20 (74.1) | 13 (48.1) | 14 (51.9) | 5 (18.5) | 16 (59.3) | 6 (22.2) | |||
| Differentiation | 0.001 | 0.000 | 0.010 | ||||||||
| Well | 2 (2.1) | 2 (100.0) | 0 (0.0) | 2 (100.0) | 0 (0.0) | 2 (100.0) | 0 (0.0) | 0 (0.0) | |||
| Moderate | 37 (38.5) | 18 (48.6) | 19 (51.4) | 27 (73.0) | 10 (27.0) | 12 (32.4) | 21 (56.8) | 4 (10.8) | |||
| Poor | 57 (59.4) | 11 (19.3) | 46 (80.7) | 18 (31.6) | 39 (68.4) | 7 (12.3) | 38 (66.7) | 12 (21.1) | |||
| Lymph nodes invasion | 0.356 | 0.563 | 0.160 | ||||||||
| Absent | 34 (35.4) | 13 (38.2) | 21 (61.8) | 18 (52.9) | 16 (47.1) | 11 (32.4) | 19 (55.9) | 4 (11.8) | |||
| Present | 62 (64.6) | 18 (29.0) | 44 (71.0) | 29 (46.8) | 33 (53.2) | 10 (16.1) | 40 (64.5) | 12 (19.4) | |||
| Perineural invasion | 0.347 | 0.896 | 0.587 | ||||||||
| Absent | 28 (29.2) | 11 (39.3) | 17 (60.7) | 14 (50.0) | 14 (50.0) | 8 (28.6) | 16 (57.1) | 4 (14.3) | |||
| Present | 68 (70.8) | 20 (29.4) | 48 (70.6) | 33 (48.5) | 35 (51.5) | 13 (19.1) | 43 (63.2) | 12 (17.6) | |||
| Vascular invasion | 0.650 | 0.671 | 0.347 | ||||||||
| Absent | 78 (81.3) | 26 (33.3) | 52 (66.7) | 39 (50.0) | 39 (50.0) | 17 (21.8) | 50 (64.1) | 11 (14.1) | |||
| Present | 18 (18.8) | 5 (27.8) | 13 (72.2) | 8 (44.4) | 10 (55.6) | 4 (22.2) | 9 (50.0) | 5 (27.8) | |||
| Invasion to the serosa | 0.088 | 0.038 | 0.749 | ||||||||
| Absent | 53 (55.2) | 21 (39.6) | 32 (60.4) | 31 (58.5) | 22 (41.5) | 13 (24.5) | 32 (60.4) | 8 (15.1) | |||
| Present | 43 (44.8) | 10 (23.3) | 33 (76.7) | 16 (37.2) | 27 (62.8) | 8 (18.6) | 27 (62.8) | 8 (18.6) | |||
| Invasion to the serosa | 0.088 | 0.038 | 0.749 | ||||||||
| Absent | 53 (55.2) | 21 (39.6) | 32 (60.4) | 31 (58.5) | 22 (41.5) | 13 (24.5) | 32 (60.4) | 8 (15.1) | |||
| Present | 43 (44.8) | 10 (23.3) | 33 (76.7) | 16 (37.2) | 27 (62.8) | 8 (18.6) | 27 (62.8) | 8 (18.6) | |||
| Portal vein invasion | 0.971 | 0.564 | 0.864 | ||||||||
| Absent | 71 (74.0) | 23 (32.4) | 48 (67.6) | 36 (50.7) | 35 (49.3) | 16 (22.5) | 44 (62.0) | 11 (15.5) | |||
| Present | 25 (26.0) | 8 (32.0) | 17 (68.0) | 11 (44.0) | 14 (56.0) | 5 (20.0) | 15 (60.0) | 5 (20.0) | |||
| Margin status | 0.494 | 0.805 | 0.334 | ||||||||
| Absent | 85 (88.5) | 29 (34.1) | 56 (65.9) | 42 (49.4) | 43 (50.6) | 20 (23.5) | 50 (58.8) | 15 (17.6) | |||
| Present | 11 (11.5) | 2 (18.2) | 9 (81.8) | 5 (45.5) | 6 (54.5) | 1 (9.1) | 9 (81.8) | 1 (9.1) | |||
| Depth of invasion | 0.050 | 0.037 | 0.249 | ||||||||
| T1 | 6 (6.3) | 3 (50.0) | 3 (50.0) | 4 (66.7) | 2 (33.3) | 1 (16.7) | 3 (50.0) | 2 (33.3) | |||
| T2 | 39 (40.6) | 17 (43.6) | 22 (56.4) | 25 (64.1) | 14 (35.9) | 10 (25.6) | 27 (69.2) | 2 (5.1) | |||
| T3 | 31 (32.3) | 9 (29.0) | 22 (71.0) | 12 (38.7) | 19 (61.3) | 7 (22.6) | 18 (58.1) | 6 (19.4) | |||
| T4 | 20 (20.8) | 2 (10.0) | 18 (90.0) | 6 (30.0) | 14 (70.0) | 3 (15.0) | 11 (55.0) | 6 (30.0) | |||
| Hepatic metastasis | 0.999 | 0.357 | 0.031 | ||||||||
| Absent | 84 (87.5) | 27 (32.1) | 57 (67.9) | 43 (51.2) | 41 (48.8) | 18 (21.4) | 55 (65.5) | 11 (13.1) | |||
| Present | 12 (12.5) | 4 (33.3) | 8 (66.7) | 4 (33.3) | 8 (66.7) | 3 (25.0) | 4 (33.3) | 5 (41.7) | |||
| Stage | 0.014 | 0.014 | 0.103 | ||||||||
| 0+ I+ II | 64 (66.7) | 26 (40.6) | 38 (59.4) | 37 (57.8) | 27 (42.2) | 15 (23.4) | 42 (65.6) | 7 (10.9) | |||
| III + IV | 32 (33.3) | 5 (15.6) | 27 (84.4) | 10 (31.3) | 22 (68.8) | 6 (18.8) | 17 (53.1) | 9 (28.1) | |||
P-values were calculated using a χ2 (two-tailed) test or Fisher's exact test, where appropriate. LNα3, laminin α3; LNγ2, laminin γ2; M, male; F, female; T, tumor; B-type, basement membrane type; C-type, cytoplasmic type; M-type, mixed type; M, male; F, female.
Association between LNγ2 expression patterns and clinicopathological characteristics
Table III shows the associations between LNγ2 expression patterns and clinicopathological characteristics. Only the B-type pattern correlated significantly with differentiation (P=0.010). There were significant differences between the enhanced LNγ2 expression in the basement membrane and the increase in differentiation, whereas no significant differences in histology were observed between C and M types. In addition, only the M-type pattern was significantly associated with hepatic metastasis (P=0.031). In this type, it was easy to find hepatic metastasis, whereas no significant differences in hepatic metastasis were observed in the C and B type patterns.
Survival
The median survival time was 7.911 vs. 18.434 months with strong vs. weak LNα3 expression by immunohistochemistry, respectively (Gehan test score, u=4.941, P=0.026; Table IV). The 1-year survival rate was shorter when LNα3 was highly expressed (21 vs. 57%, respectively). Patient outcomes for those with high expression were significantly worse than for those with low expression using the Kaplan-Meier method with log-rank analysis (P=0.008; Fig. 3A).
Table IV.
The survival time of LNα3, LNγ2 all three subunits expression and the three expression patterns of LNγ2.
| Group | Case, n | Median survival time (months) | 1 year survival (%) | u-value | P-value |
|---|---|---|---|---|---|
| LNα3 | 4.941 | 0.026 | |||
| Low | 26 | 18.434 | 57 | ||
| High | 52 | 7.911 | 21 | ||
| LNγ2 | 8.248 | 0.004 | |||
| Low | 38 | 18.961 | 60 | ||
| High | 40 | 7.234 | 14 | ||
| LNα3/LNβ3/LNγ2 | 9.996 | 0.002 | |||
| Others | 39 | 19.373 | 61 | ||
| Patterns of LNγ2 | |||||
| B-type | 17 | 34.000 | 70 | 4.059 | 0.044 |
| C-type | 46 | 10.540 | 32 | ||
| B-type | 17 | 34.000 | 70 | 6.247 | 0.012 |
| M-type | 15 | 6.271 | 9 | ||
| C-type | 46 | 10.540 | 32 | 1.861 | 0.173 |
| M-type | 15 | 6.271 | 9 |
Gehan test score was used for univariate analyses. LNα3, laminin α3; LNβ3, laminin β3; LNγ2, laminin γ2; B-type, basement membrane type; C-type, cytoplasmic type; M-type, mixed type.
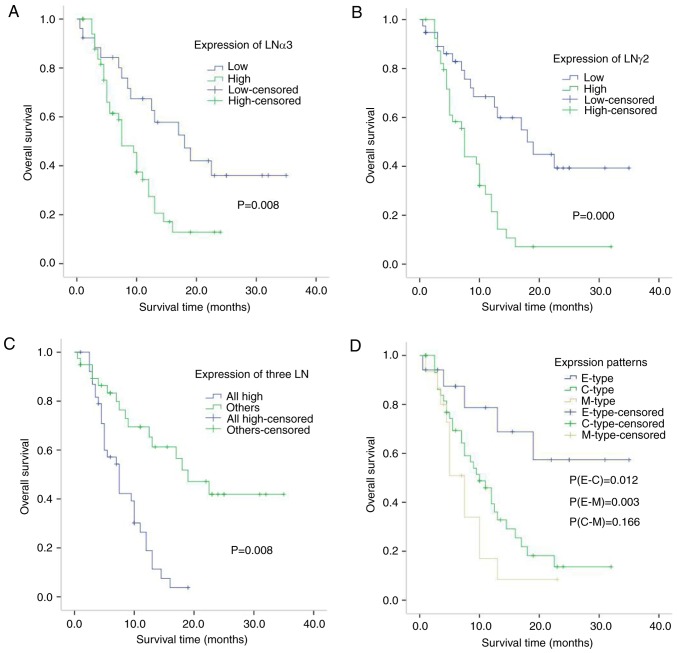
Figure 3.
Correlation between LAMA3, LAMC2, three LN and three patterns of LAMC2 immunohistochemical expression in pancreatic cancer patients. (A) Kaplan-Meier plots for overall survival for a discriminatory median LAMA3 immunohistochemical expression, (B) LAMC2 (C) three LN and (D) three patterns of LAMC2. P-values were calculated using the log-rank test. LNα3 (laminin α3) and γ2 (laminin γ2) chains are encoded by the LAMA3 and LAMC2 genes, respectively.
In our previous study, Patient outcomes for those with high expression were significantly worse than for those with low expression using the Kaplan-Meier method with log-rank analysis (13).
The median survival time was 7.234 vs. 18.961 months with strong vs. weak LNγ2 expression by immunohistochemistry, respectively (Gehan test score, u=8.248, P=0.004; Table IV). The 1-year survival rate was shorter when LNγ2 was highly expressed (14 vs. 60%, respectively). Patient outcomes for those with high expression were significantly worse than for those with low expression using the Kaplan-Meier method with log-rank analysis (P<0.001; Fig. 3B).
The median survival time was 7.044 vs. 19.373 months when all three subunits were highly expressed vs. other expression patterns, respectively (Gehan test score, u=9.996, P=0.002; Table IV). The 1-year survival rate was shorter when all three subunits were highly expressed (11 vs. 61%, respectively). Patient outcomes for those with high expression of all three subunits were significantly worse than for those with other expression patterns using the Kaplan-Meier method with log-rank analysis (P<0.001; Fig. 3C).
The median survival time differed with the three expression patterns of LNγ2 (B type=34.000 months, C type=10.540 months, and M type=6.271 months). The 1-year survival rate also varied (B type, 70%, C type, 32%, and M type, 9%). Patients with the B-type pattern showed better outcomes than patients with the C or M types (Gehan test score, u=4.059 and 6.247, P=0.044 and 0.012, respectively). Using the Kaplan-Meier method with a log-rank analysis, case outcomes were significantly better for those with the B-type pattern than for those with the C or M type (P=0.012 and P=0.003, respectively; Fig. 3D).
Consistent with our results, the prognostic value of LAMA3 and LAMB3 in pancreatic cancer were verified by the Cancer Genome Atlas (TCGA). The result demonstrated that high mRNA expression of LAMA3 and LAMB3 are correlated to poorer overall survival (P=0.001 and P=0.002; Fig. 4A and B respectively) in 178 tumor patients. The LAMC2 mRNA prognosis result showed that high mRNA expression of LAMA2 was correlated to poorer overall survival, but not significantly in TCGA pancreatic cancer datasets (P=0.181; Fig. 4C).
Figure 4.
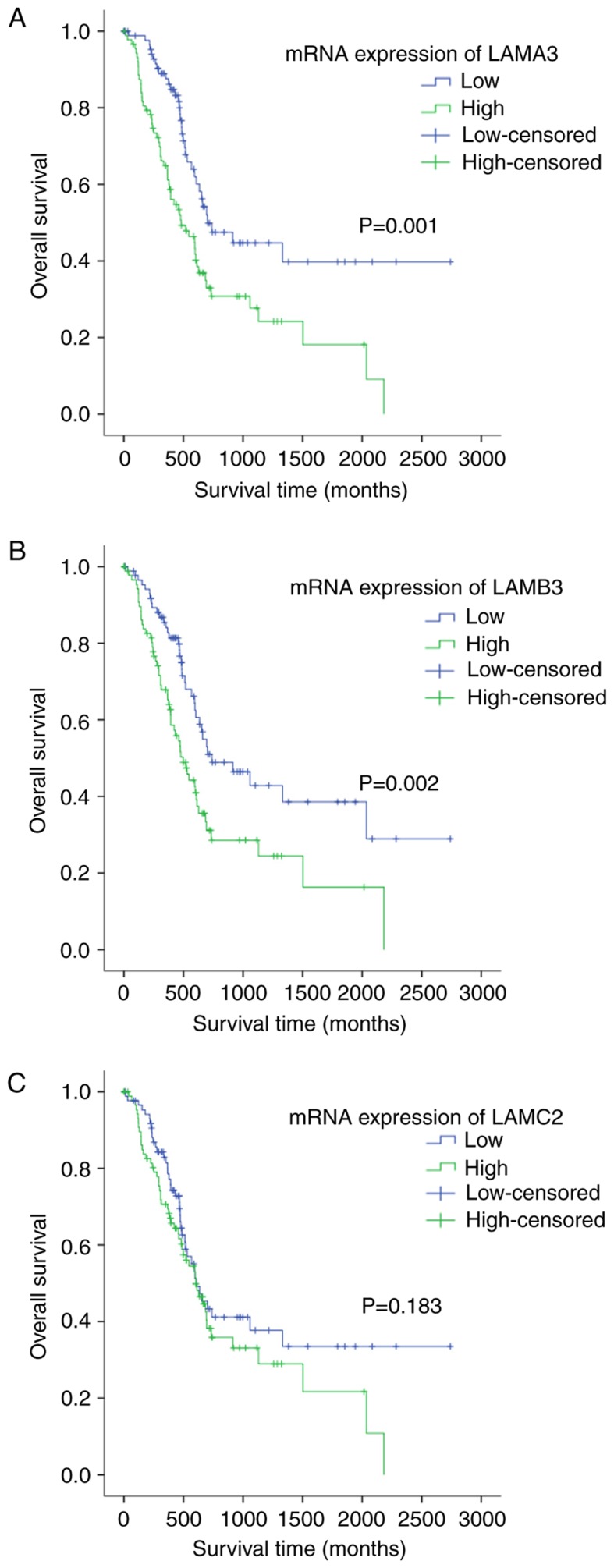
Correlation between LAMA3, LAMB3 and LAMC2 mRNA expression and prognosis in pancreatic cancer patients. (A) Kaplan-Meier plots for overall survival for a discriminatory median LAMA3 mRNA expression, from TCGA sequencing data to assess prognostic accuracy, (B) LAMB3 and (C) LAMC2. P-values were calculated using the log-rank test. LNα3 (laminin α3), β3 (laminin β3) and γ2 (laminin γ2) chains are encoded by the LAMA3, LAMB3, and LAMC2 genes, respectively.
In univariate analyses, we determined the 9 most influential prognostic factors in patients with pancreatic adenocarcinoma (P≤0.05): Tumor location, duodenal invasion, depth of invasion, metastasis, TNM stage, LNα3/β3/γ2 protein expression levels, and LNγ2 expression patterns. Then these 9 factors were used in a multivariate model; however, none of them were significant predictive factors in patients with pancreatic cancer (Table V).
Table V.
Potential predictors of overall survival in 96 patients with pancreatic cancer who underwent resection.
| P-value | ||
|---|---|---|
| Variable | Univariate | Multivariate |
| Location | 0.024 | 0.077 |
| Duodenal invasion | 0.016 | 0.776 |
| Depth of invasion | 0.002 | 0.260 |
| Hepatic metastasis | <0.001 | 0.186 |
| Stage | <0.001 | 0.068 |
| LNα3 (low vs. high) | 0.008 | 0.549 |
| LNβ3 (low vs. high) | 0.016 | 0.429 |
| LNγ2 (low vs. high) | <0.001 | 0.377 |
| Expression patterns of LNγ2 | 0.007 | 0.245 |
The Cox proportional hazards regression model was used for multivariate analyses. LNα3, laminin α3; LNβ3, laminin β3; LNγ2, laminin γ2.
Discussion
The Co-expression of the α3, β3, and γ2 subunits of LM332 in human cancers rarely reported previously, especially in PDA. Generally, tumors derived from tissues normally express LM-332 might have high expression level of LM-332, such as cutaneous, esophageal, thyroid, and colon carcinomas (17–19). However, there is also generally decreased LM-332 expression in some tumors, such as advanced breast and prostate cancers (20,21).
The mechanism of the downregulation of the laminin-5-encoding genes (LAMA3, LAMB3, and LAMC2) was not clearly understood until recently. Several researchs showed that expression of the laminin-5-encoding genes was lost partially in lung, breast, prostate, and bladder cancers, and that one or more of the genes were methylated in cancer cell lines and tumors, with significant associations between the two (22–25). In those studies, subgroups with a high Gleason score, a high preoperative serum prostate-specific antigen, and with an advanced stage had significantly higher methylation frequencies for LAMA3 than subgroups with low values. In addition, LAMA3 promoter methylation frequency in breast tumor was associated with increased tumor stage and tumor size.
In present study, the increased expression levels of LAMA3, LAMB3, and LAMC2 were observed in most pancreatic adenocarcinoma tissue when compared with non-tumor tissues (based on QRT-PCR), and in some tissues showed a loss of expression or downregulation. Further research is needed to validate whether loss of LAMB3 genes is associated with promoter methylation and is correlated with clinicopathological features of poor prognosis in pancreatic adenocarcinoma.
Several previous studies of immunohistochemical (3,6,11,12) that focused on the expression of LNγ2 and the LNβ3/γ2 heterodimer of LM-332 in human cancer revealed that the β3 and γ2 chains were assembled into a β3γ2 heterodimer before forming an α3β3γ2 heterotrimer with the α3 subunit. The Co-expression of LNβ3 and LNγ2 also has been detected in hepatocellular carcinoma, squamous cell carcinoma of the tongue, colorectal carcinoma, basal cell carcinoma of the skin, biliary cancer, and gastric carcinoma (3,6,19,26). In biliary cancer, the high positivity of LNγ2 was significantly associated with worse differentiation, deeper depth of invasion (into the serosa), and more advanced stage, while an LNβ3 invasive front-dominant pattern is significantly associated with worse differentiation and more advanced stage (6). In human gastric cancer cell lines, there is a co-expression of LNγ2 and LNβ3 at the protein level, and it is significantly associated with deeper depth of invasion and more advanced tumor stage (3). Our results are consistent with the results before that the expression of three subunits of LM332 increased and play a substantial role in the progression and prognosis of PDA.
We previously reported of staining for LNβ3 in all patients with PDA and found that it was related to worse differentiation, more advanced stage, and shorter survival time (13). In current study, the positivity LNα3 and LNγ2 were significantly associated with worse differentiation, deeper depth of invasion, more advanced stage, and shorter survival time. and that the expression level of LNγ2 was also correlated with depth of invasion. What's more, the expression levels of LNα3, LNβ3, and LNγ2 was significantly associated with each other. Survival outcomes were significantly worse for patients with high expression of all three subunits than for those with other expression patterns. These results suggested that the three genes of LM332 undergo gene transcription by a related mechanism and might play an important role in the progression and prognosis of PDA.
The cytoplasmic expression of three subunits was elevated in all 96 adenocarcinoma tissues and often more intense in areas of the invasive front, cancer cell budding, or poor differentiation, suggesting that accumulation of the three subunits of LM332 may contribute to a more aggressive phenotype of carcinoma cells. Similar expression of LNγ2 protein in cancer tissue has also previously been reported (27,28).
In the nest of adeno-squamous carcinomas, cytoplasmic staining of the three subunits was often more intense at the invasive front and was weak or absent at the center. In esophageal squamous cell carcinoma and lung squamous cell carcinoma, the expression of LNγ2 was strong in cords or small nests of poor differentiation and was weak or absent in larger nests or large sheets of well-differentiated cells, indicating that LNγ2 expression is associated with worse differentiation (29–31). In present study, not only in squamous carcinomas but also in adenocarcinomas, the high expression of three subunits was associated with worse cancer differentiation, not only in squamous carcinomas but also in adenocarcinomas.
The Laminin expression in the stroma of the tumor differs with type of cancer tissue. In adenomas, the staining expression of LM332 subunits is continuous and even enhanced (32). In carcinomas, the expression of LM332 commonly displayed in a more disrupted pattern, or fragmentation, especially in invasive area (33–35). Until now, there are limited reports concerning the association between expression patterns of LNγ2 and its prognosis. Ito et al (29) classified the expression patterns of LNγ2 in esophageal cancer into two types: E type, with staining of the ECM such as the basement membrane and matrix, and C type, with cytoplasmic staining of cancer cells; the C-type pattern was associated with unfavorable outcomes. Masuda et al (30) described three types in lung squamous cell carcinoma: B ype, in which LNγ2 was present in the basement membrane; C type, in which it was present in the intracellular matrix; and F type, in which it was present in the cytoplasm and in part of the peripheral nest; only the F type was associated with a poor prognosis.
To the best of our knowledge, there is no previous report on expression patterns of LNγ2 being correlated with prognosis of PDA. Similar to Masuda et al (30), we classified LNγ2 expression in PDA into B-, C-, and M-types. Our results indicated that most of the basement membrane around the duct stained with LNγ2 was a continuous linear structure in well-differentiated adenocarcinomas. The C and M types showed no significant difference in tumor differentiation, While significant difference was observed between M-type and the other types in hepatic metastasis.
In the survival analysis, outcome of those with B-type patterns was significantly better than those with C- or M-type. The results demonstrate that the basement membrane structure in well-differentiated adenocarcinoma was maintained and that the continuous structures prohibited the invasion and metastasis of tumor cells, while the basement membranous structure in poorly differentiated adenocarcinoma was disrupted and was associated with poor prognosis in patients with PDA.
Laminins are essential components of the ECM, localized to the epithelial basement membrane. The interactions between tumor cell and laminins in tumor tissue are more complex. The expressions of laminins in the tumor and endothelial cells are upregulated, while the laminins stimulate the surrounding stromal cells to express matrix metalloproteinases (MMPs), promoting invasive growth of tumor cells by degrading surrounding ECM barriers and allowing new vascular budding (36). Oka et al (6) suggested that the laminins of the basement membrane in tumor tissue were degraded by MMPs secreted by tumor cells or from the ECM, resulting in accumulation of LNγ2 and LNβ3 at the invasive front, which may play a direct role in tumor invasion processes. Tani et al (37) reported that laminin-5 was synthesized and deposited in the basement membrane in pancreatic carcinomas; invading cells adhere to this newly produced basement membrane and migrate over it.
Based on our results, we suggest that the increased synthesis of the three subunits of LM332 resulted in them becoming deposited at the basement membrane and tumor stroma. The basement membrane in poorly differentiated pancreatic cancer becomes degraded by proteases and displays discontinuities or holes, which could promote the migration and/or invasion of pancreatic cancer cells via an interaction with α3β1 integrin and/or α6β4 integrin. However, the basement membrane showed a continuous linear structure, which may prevent pancreatic cancer cell migration and/or infiltration in well-differentiated adenocarcinoma. Further studies are needed to assess this hypothesis.
In conclusion, the increased expression of three subunits of LM332 might be an clinically survival indicator of PDA. Considering the important role of three subunits in disease progression, they may provide a new molecular target of therapy for pancreatic adenocarcinoma patients.
Acknowledgements
Not applicable.
Funding
The present study was funded by Projects of Science and Technology Plan of JinHua of Zhejiang Province (grant no. 2015-3-005).
Availability of data and materials
All data generated or analyzed during this study are included in the published article.
Authors' contributions
JC and SAY contributed to the conceptualization and design of the study; JC drafted and critically revised the work; XYZ and DKZ performed the experiments. HZ, XML, JSL and XKW acquired, analyzed and interpreted the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All study participants provided written informed consent to participate in the study. The study was approved by the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University.
Consent for publication
All study participants provided written informed consent for the publication of their data.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 2.Marinkovich MP. Laminin 332 in squamous-cell carcinoma. Nature Rev Cancer. 2007;7:370–380. doi: 10.1038/nrc2089. [DOI] [PubMed] [Google Scholar]
- 3.Ii M, Yamamoto H, Taniguchi H, Adachi Y, Nakazawa M, Ohashi H, Tanuma T, Sukawa Y, Suzuki H, Sasaki S, et al. Co-expression of laminin β3 and γ2 chains and epigenetic inactivation of laminin α3 chain in gastric cancer. Int J Oncol. 2011;39:593–599. doi: 10.3892/ijo.2011.1048. [DOI] [PubMed] [Google Scholar]
- 4.Guess CM, Quaranta V. Defining the role of laminin-332 in carcinoma. Matrix Biol. 2009;28:445–455. doi: 10.1016/j.matbio.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamashita H1, Tripathi M, Harris MP, Liu S, Weidow B, Zent R, Quaranta V. The role of a recombinant fragment of laminin-332 in integrin α3β1-dependent cell binding, spreading and migration. Biomaterials. 2010;31:5110–5121. doi: 10.1016/j.biomaterials.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oka T, Yamamoto H, Sasaki S, Ii M, Hizaki K, Taniguchi H, Adachi Y, Imai K, Shinomura Y. Overexpression of β3/γ2 chains of laminin-5 and MMP7 in biliary cancer. World J Gastroenterol. 2009;15:3865–3873. doi: 10.3748/wjg.15.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Kariya Y, Kariya Y, Gu J. Roles of laminin-332 and alpha6beta4 integrin in tumor progression. Mini Rev Med Chem. 2009;9:1284–1291. doi: 10.2174/138955709789878114. [DOI] [PubMed] [Google Scholar]
- 9.Kariya Y, Miyazaki K. The basement membrane protein laminin-5 acts soluble cell motility factor. Exp Cell Res. 2004;297:508–520. doi: 10.1016/j.yexcr.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 10.Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Puri C, Tacchetti C, Giancotti FG. Targeted deletion of the integrin beta4 signaling domain suppresses laminin-5-dependent nuclear entry of mitogenactivated protein kinases and NF-kappaB, causing defects in epidermal growth and migration. Mol Cell Biol. 2005;25:6090–6102. doi: 10.1128/MCB.25.17.7926.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Junior Marangon H, Rocha VN, Leite CF, de Aguiar MC, Souza PE, Horta MC. Laminin-5 gamma 2 chain expression is associated with intensity of tumor budding and density of stromal myofibroblasts in oral squamous cell carcinoma. J Oral Pathol Med. 2014;43:199–204. doi: 10.1111/jop.12121. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi S, Hasebe T, Oda T, Sasaki S, Kinoshita T, Konishi M, Ochiai T, Ochiai A. Cytoplasmic expression of laminin gamma2 chain correlates with postoperative hepatic metastasis and poor prognosis in patients with pancreatic ductal adenocarcinoma. Cancer. 2002;94:1894–1901. doi: 10.1002/cncr.10395. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Wang W, Wei J, Zhou D, Zhao X, Song W, Sun Q, Huang P, Zheng S. Overexpression of β3 chains of laminin-332 is associated with clinicopathologic features and decreased survival in patients with pancreatic adenocarcinoma. Appl Immunohistochem Mol Morphol. 2015;23:516–521. doi: 10.1097/PAI.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) methods. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Li L, Lin H. A multianalysis study on clinicopathologic factors related to lymph node metastasis in gastric cancer. Chin J Oncol. 2001;23:399–402. [PubMed] [Google Scholar]
- 16.Zhao Y, Simon R. BRB-array tools data archive for human cancer gene expression: A unique and efficient data sharing resource. Cancer Inform. 2008;6:9–15. doi: 10.4137/CIN.S448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernard P1, Antonicelli F, Bedane C, Joly P, Le Roux-Villet C, Duvert-Lehembre S, Rousselle P, Prost-Squarcioni C. Prevalence and clinical significance of anti-laminin 332 autoantibodies detected by a novel enzyme-linked immunosorbent assay in mucous membrane pemphigoid. JAMA Dermatol. 2013;149:533–540. doi: 10.1001/jamadermatol.2013.1434. [DOI] [PubMed] [Google Scholar]
- 18.Oh KH, Choi J, Woo JS, Baek SK, Jung KY, Koh MJ, Kim YS, Kwon SY. Role of laminin 332 in lymph node metastasis of papillary thyroid carcinoma. Auris Nasus Larynx. 2017;44:729–734. doi: 10.1016/j.anl.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Pelissier-Rota M, Chartier NT, Bonaz B, Jacquier-Sarlin MR. A crosstalk between muscarinic and CRF2 receptors regulates cellular adhesion properties of human colon cancer cells. Biochim Biophys Acta. 2017;1864:1246–1259. doi: 10.1016/j.bbamcr.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter PM, Sivadas P, Hua SS, Xiao C, Gutierrez AB, Ngo T, Gershon PD. Migration of breast cancer cell lines in response to pulmonary laminin 332. Cancer Med. 2017;6:220–234. doi: 10.1002/cam4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao J, Jackson L, Calaluce R, McDaniel K, Dalkin BL, Nagle RB. Investigation into the mechanism of the loss of laminin 5(alpha3beta3gamma2) expression in prostate cancer. Am J Pathol. 2001;158:1129–1135. doi: 10.1016/S0002-9440(10)64060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sathyanarayana UG, Toyooka S, Padar A, Takahashi T, Brambilla E, Minna JD, Gazdar AF. Epigenetic inactivation of laminin-5-encoding genes in lung cancers. Clin Cancer Res. 2003;9:2665–2672. [PubMed] [Google Scholar]
- 23.Sathyanarayana UG, Padar A, Huang CX, Suzuki M, Shigematsu H, Bekele BN, Gazdar AF. Aberrant promoter methylation and silencing of laminin-5-encoding genes in breast carcinoma. Clin Cancer Res. 2003;9:6389–6394. [PubMed] [Google Scholar]
- 24.Sathyanarayana UG, Padar A, Suzuki M, Maruyama R, Shigematsu H, Hsieh JT, Frenkel EP, Gazdar AF. Aberrant promoter methylation of laminin-5-encoding genes in prostate cancers and its relationship to clinicopathological features. Clin Cancer Res. 2003;9:6395–6400. [PubMed] [Google Scholar]
- 25.Sathyanarayana UG, Maruyama R, Padar A, Suzuki M, Bondaruk J, Sagalowsky A, Minna JD, Frenkel EP, Grossman HB, Czerniak B, Gazdar AF. Molecular detection of noninvasive and invasive bladder tumor tissues and exfoliated cells by aberrant promoter methylation of laminin-5 encoding genes. Cancer Res. 2004;64:1425–1430. doi: 10.1158/0008-5472.CAN-03-0701. [DOI] [PubMed] [Google Scholar]
- 26.Akimoto S, Nakanishi Y, Sakamoto M, Kanai Y, Hirohashi S. Laminin 5 beta3 and gamma2 chains are frequently coexpressed in cancer cells. Pathol Int. 2004;54:688–692. doi: 10.1111/j.1440-1827.2004.01681.x. [DOI] [PubMed] [Google Scholar]
- 27.Kamada M, Koshikawa N, Mineqishi T, Kawada C, Karashima T, Shuin T, Seiki M. Urinary laminin-γ2 is a novel biomarker of non-muscle invasive urothelial carcinoma. Cancer Sci. 2015;106:1730–1737. doi: 10.1111/cas.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okado Y, Aoki M, Hamasaki M, Koga K, Sueta T, Shiratsuchi H, Oda Y, Nakagawa T, Nabeshima K. Tumor budding and laminin5-γ2 in squamous cell carcinoma of the external auditory canal are associated with shorter survival. Springerplus. 2015;4:814. doi: 10.1186/s40064-015-1620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito E, Ozawa S, Kijima H, Kazuno A, Miyako H, Nishi T, Chino O, Shimada H, Tanaka M, Inoue S, et al. Clinicopathological significance of laminin-5γ2 chain expression in superficial esophageal cancer. Dis Esophagus. 2014;27:463–469. doi: 10.1111/j.1442-2050.2012.01416.x. [DOI] [PubMed] [Google Scholar]
- 30.Masuda R, Kijima H, Imamura N, Aruga N, Nakazato K, Oiwa K, Nakano T, Watanabe H, Ikoma Y, Tanaka M, et al. Laminin-5γ2 chain expression is associated with tumor cell invasiveness and prognosis of lung squamous cell carcinoma. Biomed Res. 2012;33:309–317. doi: 10.2220/biomedres.33.309. [DOI] [PubMed] [Google Scholar]
- 31.Xue LY, Zou SM, Zheng S, Liu XY, Wen P, Yuan YL, Lin DM, Lu N. Expressions of the γ2 chain of laminin-5 and secreted protein acidic and rich in cysteine in esophageal squamous cell carcinoma and their relation to prognosis. Chin J Cancer. 2011;30:69–78. doi: 10.5732/cjc.010.10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haas KM, Berndt A, Stiller KJ, Hyckel P, Kosmehl H. A comparative quantitative analysis of laminin-5 in the basement membrane of normal, hyperplastic and malignant oral mucosa by confocal immunofluorescence imaging. J Histochem Cytochem. 2001;49:1261–1268. doi: 10.1177/002215540104901008. [DOI] [PubMed] [Google Scholar]
- 33.Kang SG, Ha YR, Ko YH, Kang SH, Joo KJ, Cho HY, Park HS, Kim CH, Kwon SY, Kim JJ, et al. Effect of laminin 332 on motility and invasion in bladder cancer. Kaohsiung J Med Sci. 2013;29:422–429. doi: 10.1016/j.kjms.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Rahman F, Rao NN, Tippu SR, Patil S, Agarwal S, Srivastava S. The expression of laminin-5 in severe dysplasia/carcinoma in situ and early invasive squamous cell carcinoma: An immunohistochemical study. Minerva Stomatol. 2013;62:139–146. [PubMed] [Google Scholar]
- 35.Kinoshita T, Hanazawa T, Nohata N, Kikkawa N, Enokida H, Yoshino H, Yamasaki T, Hidaka H, Nakagawa M, Okamoto Y, Seki N. Tumor suppressive microRNA-218 inhibits cancer cell migration and invasion through targeting laminin-332 in head and neck squamous cell carcinoma. Oncotarget. 2012;3:1386–1400. doi: 10.18632/oncotarget.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyazaki K. Laminin-5 (laminin-332): Unique biological activity and role in tumor growth and invasion. Cancer Sci. 2006;97:91–98. doi: 10.1111/j.1349-7006.2006.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tani T, Lumme A, Linnala A, Kivilaakso E, Kiviluoto T, Burgeson RE, Kangas L, Leivo I, Virtanen I. Pancreatic carcinomas deposit laminin-5, preferably adhere to laminin-5 and migrate on the newly deposited basement membrane. Am J Pathol. 1997;151:1289–1302. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in the published article.