Abstract
Rationale: The contribution of ventilatory control to the pathogenesis of obstructive sleep apnea (OSA) in preterm-born children is unknown.
Objectives: To characterize phenotypes of ventilatory control that are associated with the presence of OSA in preterm-born children during early childhood.
Methods: Preterm- and term-born children without comorbid conditions were enrolled. They were categorized into an OSA group and a non-OSA group on the basis of polysomnography.
Measurements and Main Results: Loop gain, controller gain, and plant gain, reflecting ventilatory instability, chemoreceptor sensitivity, and blood gas response to a change in ventilation, respectively, were estimated from spontaneous sighs identified during polysomnography. Cardiorespiratory coupling, a measure of brainstem maturation, was estimated by measuring the interval between inspiration and the preceding electrocardiogram R-wave. Cluster analysis was performed to develop phenotypes based on controller gain, plant gain, cardiorespiratory coupling, and gestational age. The study included 92 children, 63 of whom were born preterm (41% OSA) and 29 of whom were born at term (48% OSA). Three phenotypes of ventilatory control were derived with risks for OSA being 8%, 47%, and 77% in clusters 1, 2, and 3, respectively. There was a stepwise decrease in controller gain and an increase in plant gain from clusters 1 to 3. Children in cluster 1 had significantly higher cardiorespiratory coupling and gestational age than clusters 2 and 3. No difference in loop gain was found between clusters.
Conclusions: The risk for OSA could be stratified according to controller gain, plant gain, cardiorespiratory coupling, and gestational age. These findings could guide personalized care for children at risk for OSA.
Keywords: obstructive sleep apnea, loop gain, cardiorespiratory coupling, neonatal prematurity, cluster analysis
At a Glance Commentary
Scientific Knowledge on the Subject
Compared with children born at full term, preterm-born children are three to five times more likely to have obstructive sleep apnea (OSA) during childhood and at least twice as likely to have OSA as adults. Factors important in the development of OSA include the anatomy and muscles of the upper airway, the threshold for arousals from sleep, and ventilatory control reflected by central and peripheral chemoreceptor sensitivity. However, the contribution of ventilatory control to the development of OSA in preterm-born children is not known.
What This Study Adds to the Field
The risk of developing OSA later in life in preterm-born children could be stratified on the basis of gestational age and measures of cardioventilatory control, including chemoreceptor sensitivity, the blood gas response to change in ventilation, and coupling between cardiovascular and respiratory brainstem neurons. These findings could guide personalized care for children at risk of developing OSA.
Preterm birth occurs in 1 of every 8–10 infants born in the United States, leading to increased risk for morbidity and mortality and with an estimated annual economic impact of $26 billion (1). Immaturity of ventilatory control, leading to central and obstructive sleep apnea (OSA), is a major cost-bearing morbidity (2). Compared with children born at full term, those born preterm are three to five times more likely to have OSA during childhood (3–5) and at least twice as likely as adults (6); yet, the developmental mechanisms and neonatal factors are unknown. Factors important in the development of OSA include the anatomy and muscles of the upper airway (7), the threshold for arousals from sleep (8), and ventilatory control reflected by central and peripheral chemoreceptor sensitivity (9, 10). In this study, we aimed to test the hypothesis that preterm-born children who develop OSA later in life have a phenotype of ventilatory control distinct from the phenotypes of preterm-born children without OSA and those born at term.
There are multiple reasons for studying ventilatory control in preterm-born children with OSA. First, it was shown that neuromotor activation of upper airway dilator muscles is driven by ventilatory drive through inputs from the chemoreceptors (11–13). Therefore, a decreased ventilatory drive, and in turn deceased neuromotor activation to these muscles, may result in airway closure and OSA. Second, the normal maturation of ventilatory control that starts in utero and reaches adult degrees weeks to months after birth (14, 15) is interrupted when infants are born prematurely, and this continues postnatally (16, 17). This postnatal maturation of ventilatory control could therefore be modified by the neonatal clinical course, including oxygenation history. Preclinical studies demonstrated that in the postnatal period, the maturation of ventilatory control is influenced by hyperoxia, sustained hypoxia, and intermittent hypoxia (18–23). However, these observations have not been reproduced in humans.
There are multiple barriers to studying ventilatory control in young children born prematurely using the standard approaches and hypoxic and hyperoxic challenges. To overcome these barriers, we adopted loop gain (LG) analysis, which reflects the likelihood of ventilatory control instability. Through this analysis, we were able to examine two important components of ventilatory control noninvasively, namely chemoreceptor sensitivity (controller gain [CG]) (24) and blood gas response to change in ventilation (plant gain [PG]), the circulatory time delay, and the difference between the inspired and expired CO2 and O2. Another important objective of this work was to determine whether persistent functional immaturity of brainstem neurons contributes to the phenotypic characteristics of preterm infants that might place them at future risk for OSA. Cardiorespiratory coupling (CRC) is coordinated by the rostral ventrolateral medulla, which contains cardiovascular system– and respiratory system–related neurons that are intermingled and functionally connected (25).
The overarching aim of this study was to characterize phenotypes of ventilatory control that are associated with the presence of OSA during early childhood in children who were born preterm. Some of the results of these studies were previously reported in the form of an abstract (26).
Methods
Subjects
In the present study, we enrolled preterm- and term-born children with and without OSA. The children were subdivided into a group with OSA, defined as obstructive apnea–hypopnea index (OI) equal to or greater than 2 events per hour, and a group without OSA (NOSA), who had OI less than 2 events per hour. The polysomnographic recordings had to meet quality control measures and register at least two sighs during non-REM (NREM) sleep.
Preterm-born children
Preterm-born children aged 6 months to 7 years without comorbid conditions who had a polysomnogram without oxygen supplementation or positive pressure ventilation were identified from the sleep laboratory registry at Cincinnati Children’s Hospital Medical Center. The age range of premature born children was based on published literature showing that ventilatory control reaches an adult degree of maturation by 6 months of age and the prevalence of OSA tends to decline past the age of 7 years (14, 15, 27). Prematurity was defined as a gestational age (GA) between 24 and 36 weeks.
Term-born children
Term-born children aged 4–11 years were recruited through otolaryngology and pulmonary clinics (children with OSA) and through advertisements by the clinical trial office (NOSA). The group of healthy control subjects included children without night symptoms of sleep apnea, including snoring and a polysomnogram showing an OI of less than 2 events per hour of sleep.
Exclusion criteria
Children with the following chronic medical conditions were excluded: 1) genetic disease; 2) craniofacial or skeletal abnormality; 3) central nervous system disease, such as Chiari malformation, cerebral palsy, intracranial hemorrhage with major neurologic sequelae, birth asphyxia, and congenital central hypoventilation; 4) neuromuscular disease; 5) congenital heart disease; 6) major airway disease; and 7) tracheostomy. The study was approved by Cincinnati Children’s review board.
Polysomnography
Polysomnography (PSG) was performed using a digitized system (TWin software; Grass Technologies) according to the 2012 American Academy of Sleep Medicine guidelines (28). The apnea–hypopnea index (AHI) was defined as central and obstructive apneas and hypopneas and mixed apneas. The obstructive index (OI) was defined as obstructive hypopnea, obstructive apnea, and mixed apnea events.
Measurement of LG, PG, and CG
LG was estimated from sighs identified during the overnight PSG. We identified 5-minute segments containing spontaneous sighs occurring during NREM sleep. These segments included 10 breaths before the sigh and at least 30 breaths after the sigh free from respiratory events, arousals, or low-quality end-tidal carbon dioxide pressure (PetCO2), and the resulting data were saved in European Data Format files. The European Data Format files were uploaded for analysis of the nasal airflow, rib cage, and abdominal volume changes detected by computer-assisted respiratory inductance plethysmography, capnography signaling, and ECG recordings using VivoSense software (version 3.0; Vivonoetics). A breath-by-breath time series describing Vt, inspiratory and expiratory durations, and PetCO2 was generated.
Estimations of LG, PG, and CG were extracted from the spontaneous sigh recordings by using an adaptation of the technique employed by Nava-Guerra and colleagues (24). A slight modification extending the frequencies for periodic breathing between 0.01 and 0.125 Hz was done to account for breathing frequency being considerably faster in young children than in adolescents. The online supplement contains details of this technique.
Measurement of CRC
From the segments described above, we first identified the R-wave peaks from the ECG recordings. We then computed the time interval between the onset of inspiration and the preceding heartbeat (RI−1). Last, the strength of the CRC was quantified from the RI−1 time series by means of the transformed relative Shannon entropy (tRSE) that is built on the seminal work of Shannon on communication theory (29). tRSE is used in signal-processing applications to assess the amount of dispersion of a time series. A low tRSE contains values that are highly dispersed, and a high tRSE signal contains values that are more clustered together, indicating a good degree of synchronization in the cardiorespiratory system (25, 30, 31).
Statistical Analysis
Descriptive statistics for the preterm-born and term-born children within the OSA group were reported as mean ± SD values for continuous variables and as percentages for categorical variables. The P values from the comparison of NOSA versus OSA groups were derived by Student’s t test for continuous data and the chi-square test or Fisher’s exact test for categorical data. When the sample size was smaller, such as the number of term subjects, the Wilcoxon signed-rank test was used for continuous data. A comparison between the demographic and sleep study characteristics between each cluster was performed using the F test (type III test for regression model).
The statistical analyses were performed using SAS version 9.4 software (SAS Institute). The level of significance for statistical inference was set a priori at α = 0.05.
Bayesian profile regression method
One of the main goals of this study was to find distinct phenotypes among preterm- and term-born children who have higher risk of developing OSA later in life. One way to achieve this goal statistically is to find covariate patterns or profiles and examine associations between these profiles and an outcome of interest (i.e., OSA event). The Bayesian profile regression method seems suited well to achieving this goal. This method is used to examine the association between different profiles (or subgroups or clusters) and a response variable of interest and to fit the model as a unit, allowing an individual’s outcome to potentially influence a cluster membership (32, 33). One of the main advantages of this modeling framework over traditional clustering approaches, such as k-means clustering (34) or classification and regression tree–based methods (35), is that the cluster membership is derived mainly from a set of covariates. Subsequently, on the basis of cluster membership and other confounders, a profile regression model is fitted with the outcome variable to assess the risk of being in a specific cluster. The appropriateness of the Bayesian regression model and its superiority to other clustering methods is further discussed in the Supplementary Material 2 section and Table E1 in the online supplement.
The covariates included in the Bayesian profile regression were CG, PG, CRC, and GA. These covariate profiles were clustered into groups and were associated with the binary response variable (i.e., an OSA event). Results were adjusted for age at the time of the study, sex, and race as confounders in the profile regression model. The R package PReMiuM (Profile Regression Mixture Models; R Foundation for Statistical Computing) was used for the Bayesian profile regression model. In application, we generated 20,000 Markov chain Monte Carlo samples, with the first 10,000 used as burn-in and the remaining 10,000 used for posterior inference.
Results
Participants
We identified 121 prematurely born children without major comorbidities or tracheostomy who had undergone PSG at the age of 6 months to 7 years. Fifty-eight children were excluded because their polysomnograms either did not include a minimum of two sighs during NREM sleep or their end-tidal CO2 waveforms in proximity to the sighs did not meet the quality criteria. Sixty-three children born preterm were included in the analysis, and 29 children born at term identified from our research registry were subgrouped into those with OSA and those without OSA. The age range at the time of the study was 4–11 years for term-born children. The distribution of GA of the preterm-born children was as follows: 20 children born between 24 and 28 weeks of GA (10 OSA and 10 NOSA), 21 children born between 29 and 32 weeks of GA (15 NOSA and 6 OSA), and 22 children born between 33 and 36 weeks of GA (12 NOSA and 10 OSA). The indications for the PSG referral in the preterm group were snoring in 84%, stridor in 8%, oxygen desaturation in 5%, and central sleep apnea in 3%.
The demographic characteristics and PSG results are presented in Table 1. In the preterm group, the demographic characteristics did not differ between the OSA and NOSA groups. However, in the term group, there was a higher percentage of white subjects in the NOSA group than in the OSA group. There was no difference between OSA and NOSA groups for both preterm-born and term-born children in the prevalence of chorioamnionitis, preeclampsia, gestational diabetes, intrauterine growth retardation/small for GA, or multiple gestation. In addition, history of adenotonsillectomy before PSG was similar in both groups.
Table 1.
Demographic and Polysomnographic Characteristics of Preterm- and Term-born Children, by Obstructive Sleep Apnea Status
| Variables | Preterm-born Children |
Term-born Children |
||||
|---|---|---|---|---|---|---|
| NOSA Group (n = 37) | OSA Group (n = 26) | P Value | NOSA Group (n = 15) | OSA Group (n = 14) | P Value | |
| Age at time of study, yr | 3.18 ± 1.8 | 3.23 ± 2 | 0.9 | 7.6 ± 2.7 | 6.9 ± 2 | 0.3 |
| Male sex, % | 54% | 54% | 27% | 43% | 0.4 | |
| White race, % | 54% | 46% | 0.6 | 80% | 21% | 0.004* |
| GA, wk | 30.7 ± 3.4 | 31.2 ± 3.7 | 0.5 | 40 ± 0 | 40 ± 0 | 1.00 |
| BMI, z-score | 0.35 ± 1.1 | 0.6 ± 1.7 | 0.66 | 0.4 ± 0.8 | 1.5 ± 1.8 | 0.1 |
| OI, events/h | 0.6 ± 0.6 | 8.5 ± 9.2 | <0.001* | 0.2 ± 0.37 | 12.3 ± 6.7 | <0.001* |
| AHI, events/h | 1.7 ± 1.6 | 9.8 ± 9 | <0.001* | 1.4 ± 2.5 | 12.8 ± 6.7 | <0.001* |
| Arousal index, arousals/h | 11 ± 3.5 | 12.3 ± 5 | 0.08 | 10.7 ± 2.4 | 15.9 ± 5.5 | 0.009* |
| Respiratory arousal index, events/h | 0.6 ± 0.7 | 2.84 ± 2.8 | <0.001* | 0.45 ± 0.45 | 5.5 ± 3.3 | <0.001* |
| Average PetCO2, mm Hg | 40.3 ± 2.7 | 40.3 ± 4.23 | 0.91 | 44.5 ± 2.9 | 42.8 ± 4.9 | 0.47 |
| Average SaO2, % | 98 ± 1 | 97 ± 1 | 0.05 | 98 ± 1 | 98 ± 1 | 0.1 |
| Nadir SaO2, % | 91 ± 4 | 82 ± 11 | 0.002* | 92 ± 4 | 80 ± 1 | 0.004* |
| Hypoventilation† | 0.0% | 7.7% | 1.0 | 6.6% | 7.1% | 1.0 |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index; GA = gestational age; NOSA = no obstructive sleep apnea; OI = obstructive index; OSA = obstructive sleep apnea; PetCO2 = end-tidal carbon dioxide pressure.
Data are presented as mean ± SD for continuous variables and as percentage for categorical variables. The comparison is between OSA and NOSA subjects for each group.
Statistically significant at 5% level of significance.
Hypoventilation is defined as spending at least 25% of total sleep time with end-tidal carbon dioxide pressure greater than 50 mm Hg.
Cluster Analysis
The analysis derived from the four variables (CG, PG, CRC, and GA determined using the Bayesian profile regression method) produced three different clusters with different risks for OSA.
Cluster 1
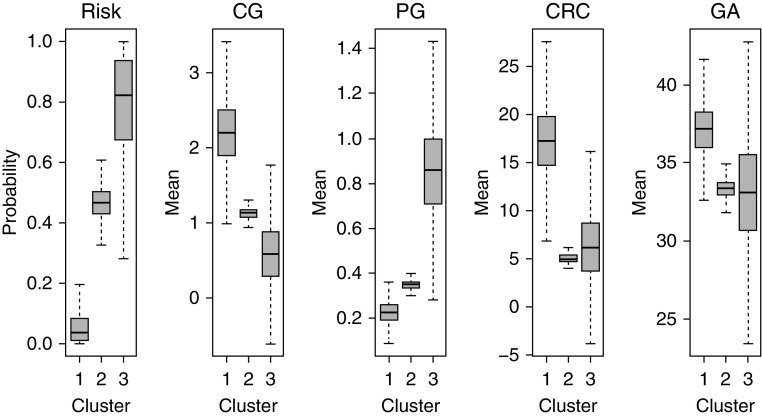
Children in this cluster were more likely to be born at term and had a normal AHI and lower arousal index (Table 2). Although the estimated risk for OSA was 8%, none of the children in this cluster had OSA. Children in this cluster had significantly higher CG and CRC and lower PG than those in the other two clusters (Figure 1).
Table 2.
Demographic and Polysomnographic Characteristics of the Three Distinct Clusters
| Variables | Cluster 1 | Cluster 2 | Cluster 3 | P Value |
|---|---|---|---|---|
| Number of subjects | 11 | 77 | 4 | |
| Gestational age, wk | 38.2 ± 3.2 | 33.2 ± 5.15 | 33.8 ± 5.6 | 0.01* |
| Male sex, % | 45.4% | 51.2% | 50% | 0.92 |
| Age at time of study, yr | 6.2 ± 1.4 | 4.2 ± 2.9 | 3.8 ± 1.34 | 0.08 |
| Prematurity, % | 27% | 74% | 75% | 0.02* |
| Obstructive index, events/h | 0.4 ± 0.2 | 4.9 ± 7.5 | 9.4 ± 7.5 | 0.06 |
| Respiratory arousal index, events/h | 0.16 ± 0 0.23 | 2.18 ± 0.2 | 2 ± 1.8 | 0.10 |
| Arousal index, events/h | 10.4 ± 2.8 | 12.6 ± 4.6 | 12 ± 3.9 | 0.03* |
| Hypoventilation† | 9.1% | 3.9% | 0.0% | 1.0 |
Data are presented as mean ± SD for continuous variables and as percentage for categorical variables. P values were derived by F test (type III test for regression model).
Statistically significant at 5% level of significance.
Hypoventilation is defined as spending at least 25% of the total sleep time with end-tidal carbon dioxide pressure greater than 50 mm Hg.
Figure 1.
Three different phenotypes of ventilatory control, stratified by obstructive sleep apnea risk, cardioventilatory control parameters (CG, PG, and CRC), and GA. In the first panel at left, the three box plots show the estimated median risk of obstructive sleep apnea for each phenotype. In the second, third, fourth, and fifth panels, the three box plots (one for each phenotype) show the estimated posterior median values of CG, PG, CRC, and GA, respectively. In all boxplots, the boxes represent the interquartile range, and the two whiskers depict the minimum and maximum values of the posterior samples derived from the Bayesian profile regression model. The model was adjusted for age at the time of the study, sex, and race. CG = controller gain; CRC = cardiorespiratory coupling; GA = gestational age; PG = plant gain.
Cluster 2
Children in cluster 2 had a higher prevalence of prematurity, as well as a higher AHI and arousal index, than those in cluster 1. The estimated risk for OSA and the prevalence of OSA were both 47%. CG was significantly lower than in cluster 1 and higher than in cluster 3. PG was significantly higher than in cluster 1 and lower than in cluster 3. CRC was significantly lower than in cluster 1 (Figure 1).
Cluster 3
Children in cluster 3 had a higher prevalence of prematurity, as well as a higher AHI and arousal index, than those in cluster 1. Although the estimated risk for OSA was 77%, the prevalence of OSA was 100%. CG was significantly lower and PG significantly higher than in clusters 1 and 2 (Figure 1).
Summary
From clusters 1 to 3, there was a stepwise decrease in CG and an increase in PG. Both CG and PG were statistically different between the three clusters. CRC was higher in cluster 1 than in clusters 2 and 3. GA was similar in clusters 2 and 3. Similar results were found after adjusting for age at the time of PSG, sex, and race.
To determine whether a diagnosis of hypoventilation or the level of PetCO2 altered the clustering of the study population, we ran the model with hypoventilation as a discrete variable or with average PetCO2 as a continuous variable. In both cases, the results produced three clusters with sizes 10, 78, and 4 in clusters 1, 2, and 3, respectively. The categorization of the subjects into the three clusters remained unchanged, except for one subject who was recategorized into cluster 2 instead of cluster 1. There was no difference in LG between clusters (cluster 1, 0.30 ± 0.08; cluster 2, 0.31 ± 0.12; cluster 3, 0.25 ± 0.08; P = 0.52).
Discussion
In this study, we provide novel data demonstrating that preterm-born children with OSA have a distinct phenotype of ventilatory control that is associated with increasing risk of OSA. The parameters that make up the phenotypic characteristics include a measure of chemoreceptor sensitivity (CG); a measure of blood gas response to a change in ventilation (PG); and CRC, a measure of the maturation of cardiorespiratory neurons in the brainstem. The cluster analysis applied in this study demonstrated that although each parameter alone might not predict the risk for OSA, the latter could be stratified when the three parameters CG, PG, and CRC are grouped together with GA. Therefore, the pertinent set of clinical circumstances for the development of OSA in children born preterm is created when decreased chemoreceptor sensitivity, abnormal blood gas response to a change in ventilation, and decreased CRC are present simultaneously. These observations point to a likely abnormal developmental trajectory of ventilatory control and cardiorespiratory neurons in preterm-born children with OSA. The findings of this study may have important clinical implications because the risk of OSA could be estimated from ventilatory control stability and cardioventilatory coupling analyses of short segments of cardiorespiratory recordings.
Our observation that decreased chemoreceptor sensitivity is associated with high risk for the development of OSA is in agreement with the findings of multiple other studies which demonstrated that neuromotor activation of upper airway dilator muscles is driven by ventilatory drive through the inputs from the peripheral and central chemoreceptors (11–13). This is consistent with the hypotheses that OSA has a central nervous system component and that decreased ventilatory drive can result in obstructive apneas (11, 36–39). The basis for this hypothesis lies in the observation that the tone of the upper airway muscles is modulated by respiration (36, 39). Therefore, decreased respiratory drive to these muscles may result in airway closure and obstructive apnea.
Whether the abnormal development of chemoreceptors in preterm-born children is the result of prenatal programming or an effect of the early postnatal clinical course on the developmental trajectory is yet to be determined. Multiple preclinical studies suggest that the postnatal clinical course early in life might indeed modify the normal developmental trajectory of the chemoreceptors. Specifically, work with animal models has shown that a specific oxygenation profile during the neonatal period can modify the sensitivity of the chemoreceptors, particularly the carotid body. Postnatal hyperoxia and chronic hypoxia decrease or blunt the sensitivity of the carotid body, as opposed to intermittent hypoxia that augments its sensitivity. However, these oxygenation profiles, when present later in life, do not have equal effects on chemoreceptor sensitivity (14, 18, 40–43). Collectively, these data suggest that a developmental plasticity of ventilatory control may develop postnatally and could have a long-lasting effect on respiratory stability. However, a cause-and-effect relationship cannot be ascertained from this study. A plausible pathway to decreased chemoreceptor sensitivity is OSA-related intermittent hypercapnia and hypoxemia.
We also observed that in preterm-born children, an elevated PG is associated with increased risk for OSA. PG defines how effectively and quickly a change in ventilation leads to changes in arterial blood gases. An effective response to a change in ventilation creates a damping mechanism whereby large oscillations of blood gases are minimized. However, large swings in blood gases with a change in ventilation and equivalently a high PG occur when the effective gas-exchanging volumes are small enough to reduce the lung stores of CO2 or O2. PG is therefore inversely related to the amount of “damping” in the respiratory system needed to maintain ventilatory stability. The observation of an elevated PG in preterm-born children is not surprising, because several epidemiological studies have demonstrated that lung function of former preterm-born children and adults shows evidence of persistent small airway obstruction, increased air trapping, and reduced vital capacity (44–47). Therefore, the negative impact of chronic lung disease of prematurity on gas exchange and lung volume, together with abnormally decreased ventilatory drive in preterm-born children, might work synergistically to facilitate the development of OSA.
We have also demonstrated that CRC significantly distinguishes between cluster 1, which included mostly term-born children and children without OSA, from clusters 2 and 3, which included mainly preterm-born children with OSA. Thus, a low CRC is associated with low GA and also with OSA status. The mechanisms of the associations between CRC, prematurity, and OSA are too complex to be explained by the data in this study. However, there is evidence that the strength of coupling between respiratory and cardiovascular systems increases with the age of infants together with development of the brainstem, which therefore reflects the maturation of the cardiovascular and respiratory neurons in the brainstem (48).
Whether CRC is simply a measure of functional maturation of the brainstem, or whether it is linked to OSA status independent from prematurity is yet to be determined as a study in adults has shown (49), is yet to be determined. There is evidence that chemoreceptor–baroreceptor interplay driven by the autonomic nervous system has an important role in maintaining respiratory and cardiovascular stability. Studies of mature animals and humans demonstrate the synergistic interplay between ventilatory control and cardiovascular control. This is illustrated by the attenuation of the ventilatory response to peripheral chemoreflex activation by the baroreceptor (50–52) and, conversely, the activation of the peripheral and central chemoreceptors when the baroreceptors are unloaded (53, 54). Likewise, the peripheral chemoreceptors may activate or inhibit arterial baroreflex cardiovascular responses, depending on the nature of hypoxic exposure (50, 55, 56). It is therefore plausible that decreased CRC in preterm-born children is mechanistically linked to the presence of OSA.
To measure ventilatory control instability, we applied the LG analysis overnight polysomnogram recordings. Contrary to what has been described in adults with OSA who have an increase in LG, our pediatric population with OSA did not show a significant difference in LG compared with that in healthy control subjects. Similar observations were made in studies of overweight adolescents (24) and obese women (57), where subjects with OSA also had high PG, low CG, and a low LG. These data suggest that different mechanisms of ventilatory control abnormalities may lead to respiratory instability and OSA. One mechanism was observed in adults with OSA whereby respiratory instability was associated with an increase in CG and overall LG, whereas another mechanism was observed in the pediatric population and obese women whereby CG was diminished and PG was elevated. Further studies are needed to determine a potential causal relationship between decreased chemoreceptor sensitivity and elevated PG.
Various studies in adults used sighs induced by noninvasive ventilation (24, 58, 59). This approach might lead to inaccurately low PG measurements because noninvasive ventilation tends to increase lung volumes. The analysis adopted in the present study estimated LG from the downstream effect of spontaneous sighs (60) on respiratory stability. This likely provides a more accurate estimate of PG.
This study had several limitations that are worth mentioning. The retrospective nature of the study might have introduced a selection bias by enrollment of subjects who had a clinical indication for a polysomnogram. Furthermore, preterm-born children were younger at the time of the study than the term-born children, who had a wide age range. However, age-adjusted cluster analysis did not produce different results. In addition, the contribution of severe lung disease to respiratory instability could not be assessed, because subjects with a tracheostomy or ventilator or oxygen dependency had to be excluded. Finally, the small sample size did not allow the demonstration of a dose-dependent effect of the degree of prematurity on ventilatory control phenotype, despite the fact that the GA of preterm-born children ranged from 24 to 36 weeks.
Conclusions
Preterm-born children have characteristic phenotypes of ventilatory control that allow a stratification of the risk of having OSA later in life. Decreased chemoreceptor sensitivity, abnormal gas exchange, and decreased CRC are associated with a high risk of OSA. Future prospective longitudinal studies that can elucidate the developmental trajectory of ventilatory control and validate the results of the present study are warranted.
Footnotes
Supported by NIH National Institute of Biomedical Imaging and Bioengineering grant P41 EB001978 (M.C.K.).
Author Contributions: K.A.D.: designed the study, analyzed data, and drafted the initial manuscript; M.M.H.: conceptualized the study, analyzed data, and drafted components of the manuscript; L.N.-G. and M.C.K.: programmed the loop gain analysis software and drafted components of the manuscript; Y.X.: analyzed data and drafted components of the manuscript; J.L.C. and M.D.: contributed to interpretation of the data and drafted components of the manuscript; and R.S.A.: conceptualized the study, analyzed data, and drafted and edited the manuscript. All authors contributed to drafting or critical revision of the manuscript for important intellectual content. All authors approved the final manuscript version submitted for publication.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201708-1700OC on January 11, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Committee on Understanding Premature Birth and Assuring Healthy Outcomes, Institute of Medicine Societal costs of preterm birth Behrman RE, Butler AS.editorsPreterm birth: causes, consequences, and prevention. Washington, DC: National Academies Press; 2007398–429. [Google Scholar]
- 2.Levin JC, Jang J, Rhein LM. Apnea in the otherwise healthy, term newborn: national prevalence and utilization during the birth hospitalization. J Pediatr. 2017;181:67–73.e1. doi: 10.1016/j.jpeds.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Rosen CL, Larkin EK, Kirchner HL, Emancipator JL, Bivins SF, Surovec SA, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142:383–389. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 4.Raynes-Greenow CH, Hadfield RM, Cistulli PA, Bowen J, Allen H, Roberts CL. Sleep apnea in early childhood associated with preterm birth but not small for gestational age: a population-based record linkage study. Sleep (Basel) 2012;35:1475–1480. doi: 10.5665/sleep.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hibbs AM, Johnson NL, Rosen CL, Kirchner HL, Martin R, Storfer-Isser A, et al. Prenatal and neonatal risk factors for sleep disordered breathing in school-aged children born preterm. J Pediatr. 2008;153:176–182. doi: 10.1016/j.jpeds.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paavonen EJ, Strang-Karlsson S, Räikkönen K, Heinonen K, Pesonen AK, Hovi P, et al. Very low birth weight increases risk for sleep-disordered breathing in young adulthood: the Helsinki Study of Very Low Birth Weight Adults. Pediatrics. 2007;120:778–784. doi: 10.1542/peds.2007-0540. [DOI] [PubMed] [Google Scholar]
- 7.Wellman A, Jordan AS, Malhotra A, Fogel RB, Katz ES, Schory K, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–1232. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckert DJ, Younes MK. Arousal from sleep: implications for obstructive sleep apnea pathogenesis and treatment. J Appl Physiol (1985) 2014;116:302–313. doi: 10.1152/japplphysiol.00649.2013. [DOI] [PubMed] [Google Scholar]
- 9.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–1370. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 10.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1181–1190. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- 11.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 12.Hicks A, Cori JM, Jordan AS, Nicholas CL, Kubin L, Semmler JG, et al. Mechanisms of the deep, slow-wave, sleep-related increase of upper airway muscle tone in healthy humans. J Appl Physiol (1985) 2017;122:1304–1312. doi: 10.1152/japplphysiol.00872.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol (1985) 2008;105:1389–1405. doi: 10.1152/japplphysiol.90408.2008. [DOI] [PubMed] [Google Scholar]
- 14.Fleming PJ, Goncalves AL, Levine MR, Woollard S. The development of stability of respiration in human infants: changes in ventilatory responses to spontaneous sighs. J Physiol. 1984;347:1–16. doi: 10.1113/jphysiol.1984.sp015049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrington KJ, Finer NN, Wilkinson MH. Progressive shortening of the periodic breathing cycle duration in normal infants. Pediatr Res. 1987;21:247–251. doi: 10.1203/00006450-198703000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Edwards BA, Sands SA, Berger PJ. Postnatal maturation of breathing stability and loop gain: the role of carotid chemoreceptor development. Respir Physiol Neurobiol. 2013;185:144–155. doi: 10.1016/j.resp.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Bates ML, Pillers DA, Palta M, Farrell ET, Eldridge MW. Ventilatory control in infants, children, and adults with bronchopulmonary dysplasia. Respir Physiol Neurobiol. 2013;189:329–337. doi: 10.1016/j.resp.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Attenuation of the hypoxic ventilatory response in adult rats following one month of perinatal hyperoxia. J Physiol. 1996;495:561–571. doi: 10.1113/jphysiol.1996.sp021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Developmental plasticity of the hypoxic ventilatory response. Respir Physiol. 1997;110:261–268. doi: 10.1016/s0034-5687(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 20.Logan S, Tobin KE, Fallon SC, Deng KS, McDonough AB, Bavis RW. Chronic intermittent hyperoxia alters the development of the hypoxic ventilatory response in neonatal rats. Respir Physiol Neurobiol. 2016;220:69–80. doi: 10.1016/j.resp.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bavis RW, van Heerden ES, Brackett DG, Harmeling LH, Johnson SM, Blegen HJ, et al. Postnatal development of eupneic ventilation and metabolism in rats chronically exposed to moderate hyperoxia. Respir Physiol Neurobiol. 2014;198:1–12. doi: 10.1016/j.resp.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill CB, Grandgeorge SH, Bavis RW. Developmental hyperoxia alters CNS mechanisms underlying hypoxic ventilatory depression in neonatal rats. Respir Physiol Neurobiol. 2013;189:498–505. doi: 10.1016/j.resp.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Reeves SR, Gozal D. Developmental plasticity of respiratory control following intermittent hypoxia. Respir Physiol Neurobiol. 2005;149:301–311. doi: 10.1016/j.resp.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Nava-Guerra L, Tran WH, Chalacheva P, Loloyan S, Joshi B, Keens TG, et al. Model-based stability assessment of ventilatory control in overweight adolescents with obstructive sleep apnea during NREM sleep. J Appl Physiol (1985) 2016;121:185–197. doi: 10.1152/japplphysiol.01081.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elder DE, Larsen PD, Galletly DC, Campbell AJ. Cardioventilatory coupling in preterm and term infants: effect of position and sleep state. Respir Physiol Neurobiol. 2010;174:128–134. doi: 10.1016/j.resp.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Armoni Domany K, Nava-Guerra L, McConnell K, Khoo M, Carroll J, Hossain M, et al. Cardiorespiratory control in premature children and the risk of sleep disorder breathing [abstract] Am J Respir Crit Care Med. 2017;195:A7567. [Google Scholar]
- 27.Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–e755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 28.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. American Academy of Sleep Medicine; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shannon CE. The mathematical theory of communication. 1963. MD Comput. 1997;14:306–317. [PubMed] [Google Scholar]
- 30.Friedman L, Dick TE, Jacono FJ, Loparo KA, Yeganeh A, Fishman M, et al. Cardio-ventilatory coupling in young healthy resting subjects. J Appl Physiol (1985) 2012;112:1248–1257. doi: 10.1152/japplphysiol.01424.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galletly DCLP, Larsen PD. Relationship between cardioventilatory coupling and respiratory sinus arrhythmia. Br J Anaesth. 1998;80:164–168. doi: 10.1093/bja/80.2.164. [DOI] [PubMed] [Google Scholar]
- 32.Zou M, Conzen SD. A new dynamic Bayesian network (DBN) approach for identifying gene regulatory networks from time course microarray data. Bioinformatics. 2005;21:71–79. doi: 10.1093/bioinformatics/bth463. [DOI] [PubMed] [Google Scholar]
- 33.Molitor J, Papathomas M, Jerrett M, Richardson S. Bayesian profile regression with an application to the National Survey of Children’s Health. Biostatistics. 2010;11:484–498. doi: 10.1093/biostatistics/kxq013. [DOI] [PubMed] [Google Scholar]
- 34.Kanungo TNN, Piatko CD, Silverman R, Wu AY. An efficient k-means clustering algorithm: analysis and implementation. IEEE Trans Pattern Anal Mach Intell. 2002;24:881–892. [Google Scholar]
- 35.Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med. 2003;26:172–181. doi: 10.1207/S15324796ABM2603_02. [DOI] [PubMed] [Google Scholar]
- 36.Brouillette RT, Thach BT. Control of genioglossus muscle inspiratory activity. J Appl Physiol. 1980;49:801–808. doi: 10.1152/jappl.1980.49.5.801. [DOI] [PubMed] [Google Scholar]
- 37.Martin RJ, Pennock BE, Orr WC, Sanders MH, Rogers RM. Respiratory mechanics and timing during sleep in occlusive sleep apnea. J Appl Physiol Respir Environ Exerc Physiol. 1980;48:432–437. doi: 10.1152/jappl.1980.48.3.432. [DOI] [PubMed] [Google Scholar]
- 38.Onal E, Lopata M. Periodic breathing and the pathogenesis of occlusive sleep apneas. Am Rev Respir Dis. 1982;126:676–680. doi: 10.1164/arrd.1982.126.4.676. [DOI] [PubMed] [Google Scholar]
- 39.Onal E, Burrows DL, Hart RH, Lopata M. Induction of periodic breathing during sleep causes upper airway obstruction in humans. J Appl Physiol (1985) 1986;61:1438–1443. doi: 10.1152/jappl.1986.61.4.1438. [DOI] [PubMed] [Google Scholar]
- 40.Eden GJHM, Hanson MA. Effects of chronic hypoxia from birth on the ventilatory response to acute hypoxia in the newborn rat. J Physiol. 1987;392:11–19. doi: 10.1113/jphysiol.1987.sp016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bavis RW, Fallon SC, Dmitrieff EF. Chronic hyperoxia and the development of the carotid body. Respir Physiol Neurobiol. 2013;185:94–104. doi: 10.1016/j.resp.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuller DD, Bavis RW, Vidruk EH, Wang ZY, Olson EB, Jr, Bisgard GE, et al. Life-long impairment of hypoxic phrenic responses in rats following 1 month of developmental hyperoxia. J Physiol. 2002;538:947–955. doi: 10.1113/jphysiol.2001.012908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pawar A, Peng YJ, Jacono FJ, Prabhakar NR. Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. J Appl Physiol (1985) 2008;104:1287–1294. doi: 10.1152/japplphysiol.00644.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doyle LW, Adams AM, Robertson C, Ranganathan S, Davis NM, Lee KJ, et al. Victorian Infant Collaborative Study Group. Increasing airway obstruction from 8 to 18 years in extremely preterm/low-birthweight survivors born in the surfactant era. Thorax. 2017;72:712–719. doi: 10.1136/thoraxjnl-2016-208524. [DOI] [PubMed] [Google Scholar]
- 45.Martinez FD. Early-life origins of chronic obstructive pulmonary disease. N Engl J Med. 2016;375:871–878. doi: 10.1056/NEJMra1603287. [DOI] [PubMed] [Google Scholar]
- 46.Vollsæter M, Røksund OD, Eide GE, Markestad T, Halvorsen T. Lung function after preterm birth: development from mid-childhood to adulthood. Thorax. 2013;68:767–776. doi: 10.1136/thoraxjnl-2012-202980. [DOI] [PubMed] [Google Scholar]
- 47.Ota C, Baarsma HA, Wagner DE, Hilgendorff A, Königshoff M. Linking bronchopulmonary dysplasia to adult chronic lung diseases: role of WNT signaling. Mol Cell Pediatr. 2016;3:34. doi: 10.1186/s40348-016-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clark MT, Rusin CG, Hudson JL, Lee H, Delos JB, Guin LE, et al. Breath-by-breath analysis of cardiorespiratory interaction for quantifying developmental maturity in premature infants. J Appl Physiol (1985) 2012;112:859–867. doi: 10.1152/japplphysiol.01152.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kabir MM, Dimitri H, Sanders P, Antic R, Nalivaiko E, Abbott D, et al. Cardiorespiratory phase-coupling is reduced in patients with obstructive sleep apnea. PLoS One. 2010;5:e10602. doi: 10.1371/journal.pone.0010602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heistad D, Abboud FM, Mark AL, Schmid PG. Effect of baroreceptor activity on ventilatory response to chemoreceptor stimulation. J Appl Physiol. 1975;39:411–416. doi: 10.1152/jappl.1975.39.3.411. [DOI] [PubMed] [Google Scholar]
- 51.Saupe KW, Smith CA, Henderson KS, Dempsey JA. Respiratory and cardiovascular responses to increased and decreased carotid sinus pressure in sleeping dogs. J Appl Physiol (1985) 1995;78:1688–1698. doi: 10.1152/jappl.1995.78.5.1688. [DOI] [PubMed] [Google Scholar]
- 52.Mifflin SW. Inhibition of chemoreceptor inputs to nucleus of tractus solitarius neurons during baroreceptor stimulation. Am J Physiol. 1993;265:R14–R20. doi: 10.1152/ajpregu.1993.265.1.R14. [DOI] [PubMed] [Google Scholar]
- 53.Taneja I, Medow MS, Clarke DA, Ocon AJ, Stewart JM. Baroreceptor unloading in postural tachycardia syndrome augments peripheral chemoreceptor sensitivity and decreases central chemoreceptor sensitivity. Am J Physiol Heart Circ Physiol. 2011;301:H173–H179. doi: 10.1152/ajpheart.01211.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2. J Physiol. 2010;588:2455–2471. doi: 10.1113/jphysiol.2010.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Somers VK, Mark AL, Abboud FM. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest. 1991;87:1953–1957. doi: 10.1172/JCI115221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cooper VL, Pearson SB, Bowker CM, Elliott MW, Hainsworth R. Interaction of chemoreceptor and baroreceptor reflexes by hypoxia and hypercapnia - a mechanism for promoting hypertension in obstructive sleep apnoea. J Physiol. 2005;568:677–687. doi: 10.1113/jphysiol.2005.094151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bokov P, Essalhi M, Delclaux C. Loop gain in severely obese women with obstructive sleep apnoea. Respir Physiol Neurobiol. 2016;221:49–53. doi: 10.1016/j.resp.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 58.Edwards BA, Sands SA, Feeney C, Skuza EM, Brodecky V, Wilkinson MH, et al. Continuous positive airway pressure reduces loop gain and resolves periodic central apneas in the lamb. Respir Physiol Neurobiol. 2009;168:239–249. doi: 10.1016/j.resp.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 59.O’Donoghue FJ, Catcheside PG, Jordan AS, Bersten AD, McEvoy RD. Effect of CPAP on intrinsic PEEP, inspiratory effort, and lung volume in severe stable COPD. Thorax. 2002;57:533–539. doi: 10.1136/thorax.57.6.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khoo MC, Marmarelis VZ. Estimation of peripheral chemoreflex gain from spontaneous sigh responses. Ann Biomed Eng. 1989;17:557–570. doi: 10.1007/BF02367463. [DOI] [PubMed] [Google Scholar]