Chronic obstructive pulmonary disease (COPD) is a prevalent, heterogeneous disorder with varying presentation and progression but with a limited number of disease-modifying therapies (1). This marked heterogeneity impedes identification of subpopulations at risk for accelerated progression, thwarting therapeutic advances. Most COPD studies have included populations with mean ages older than 60 years (2). However, it is increasingly evident that lung function trajectories in COPD differ significantly and that differences are detectable in young adulthood (3–5). In this Perspective, we highlight the need to distinguish “early disease” from late “mild disease,” propose an operational definition of early COPD for use in research studies, and attempt to unify current views on potential disease mechanisms. We focus on smoking, the chief etiologic factor for COPD in the industrialized world. Whether pathogenic mechanisms and effective treatments are shared with the sizable fraction of COPD in never-smokers or resulting from biomass fuel, electronic nicotine delivery systems, and other exposures, are separate, significant questions. We argue that refocusing investigation on early COPD could revolutionize understanding and therapies of this leading cause of worldwide death.
Limitations of Previous Concepts of COPD Development
Defining early COPD is crucial to design individualized interventions to arrest progression before irreversible damage. Although the degree of airflow obstruction has been used to distinguish mild disease, no accepted definition exists for “early disease”—due to lack of consensus on what constitutes “early” and “disease” in this context. Prior concepts of COPD derived from analysis of older individuals with established disease, emphasized incompletely reversible obstruction, and postulated accelerated decline from normal lung function in early adulthood (6). However, recent data suggest that only half of COPD cases result from accelerated adult loss of lung function related to adult smoking, with the remainder resulting from failure to achieve normal lung function in early adulthood followed by age-appropriate rates of decline (3). If smoking ceases sufficiently early, the rate of spirometric decline appears to return to that of normal aging, and symptoms of cough and sputum reverse (7). Smoking cessation at an older age may fail to prevent spirometric decline at rates faster than normal (8).
However, smoking is now known to lead to COPD via multiple trajectories acting at disparate life stages (2) (Figure 1). Such heterogeneity may explain the absence of accelerated short-term spirometric decline in half of middle-aged patients with COPD (3), who are best classified as having “late mild” disease (2). In the broadest view, COPD pathogenesis may begin before birth, because passive fetal smoke exposure in utero is associated with increased adult COPD risk, independent of later active smoking (9). The same is true for both passive smoke exposure in childhood and active smoking in adolescence (9). Individuals sustaining childhood respiratory impairment are also at increased risk of reduced adult lung function (5). Potential mechanisms include compromised lung development and growth, epigenetic changes, and altered lung microbiome composition (9). Currently, distinguishing between these processes in individuals or untangling their interrelationships is impossible. Although COPD prevention will ultimately require a global understanding of mechanisms potentially spanning generations, these factors are beyond the scope of an operational definition of early COPD to guide development of therapies for use in adults, our focus in this Perspective.
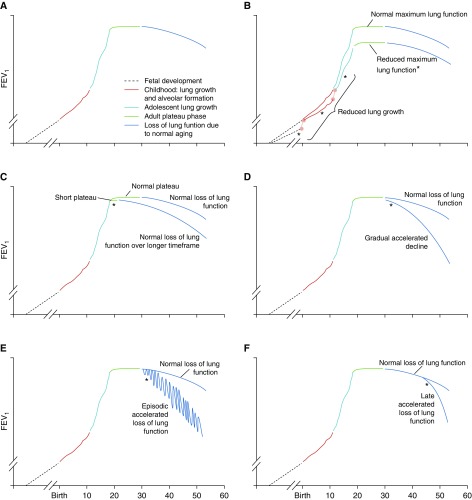
Figure 1.
Proposed trajectories for lung function. (A) Normal lung function natural history; (B) reduced lung growth during fetal development, childhood, or adolescence, which can reduce maximally attained lung function; (C) shortened plateau; (D) accelerated lung function loss during adulthood; (E) episodic loss of lung function without full recovery; (F) late accelerated loss of lung function. *Presence of early disease for each disease natural history. Reproduced by permission from Reference 2.
A further difficulty in defining early COPD is the frequent discordance between spirometric results or patient-reported respiratory symptoms and structural changes in the lung. Three large prospective studies of middle-aged adults recently showed the prognostic significance of symptoms and lung structure independent of airflow limitation (10–12). Cough and sputum production were originally believed to be key COPD risk factors (the “British hypothesis”), but their importance was minimized after the observation that cough and sputum relate only weakly to disease progression (6). Although there does appear to be an association of cough and sputum with excess FEV1 decline, as seen in long-term follow-up of the Copenhagen City Heart Study (4, 13), that study also demonstrated that most individuals who eventually developed airflow obstruction did not report cough and sputum production (13).
Respiratory symptoms clearly herald the presence of a pathological process that will progress in some individuals and are unquestionably burdensome to all. More recent studies demonstrate that symptoms are associated with excess exacerbations and radiographic abnormalities (10, 11, 14). Thus, features inadequately captured by spirometric airflow limitation are now recognized as independent clinical manifestations of COPD-related disease (15).
Proposed Operational Definition of Early COPD
Ideally, early COPD would be defined by detecting the initial events responsible for ultimate development of pathology. Although this is currently not possible, a surrogate form of evidence could be lung pathology unequivocally associated with subsequent accelerated lung function decline leading to objectively confirmed incompletely reversible airflow obstruction and other COPD-related manifestations. Fully validating that approach would require serial invasive sampling in sizeable, long-lasting prospective cohorts. Alternatively, a definition could be based solely on currently available intermediate endpoints, such as symptom presence (5), or changes in lung structure on computed tomography (CT), such as abnormalities in the small and large airways and early emphysema (16), all of which increase progression risk in some (possibly distinct) individuals (17). Two studies of smokers with normal pulmonary function tests demonstrated centriacinar emphysema; the group with CT-detectable emphysema was distinguished by reversible alterations in peripheral pulmonary vascular function (18, 19).
We propose that early changes leading to COPD (“early COPD”) should be studied in those younger than 50 years with 10 or more pack-years smoking history and any of these abnormalities: 1) early airflow limitation (post-bronchodilator FEV1/FVC < lower limit of normal), 2) compatible CT abnormalities, 3) rapid decline in FEV1 (≥60 ml/yr) that is accelerated relative to FVC. This definition (Table 1) assumes the exclusion of other chronic lung diseases, such as pulmonary fibrosis. Elaboration on the rationale for these choices is warranted.
Table 1.
Components of Operational Definition for Early Chronic Obstructive Pulmonary Disease
| Required | One or More of the Following: |
|---|---|
| <50 yr of age | FEV1/FVC less than lower limit of normal |
| ≥10 pack-years smoking history | Compatible computed tomography abnormalities (visual emphysema, air trapping, or bronchial thickening graded mild or worse) |
| Evidence of accelerated FEV1 decline (≥60 ml/yr) |
Exclusion criteria include other known chronic lung diseases, including interstitial lung diseases, but not asthma (see text).
We chose 10 pack-years on the basis of data suggesting that it is both the minimum exposure resulting in lung function decline in early adulthood and the point at which accelerated lung function decline is detectable (3). Pathological studies associate a similar exposure threshold with structural lung abnormalities (20). We acknowledge that this operational definition does not include other environmental exposures implicated in COPD development, including biomass fuel inhalation. Such exposures are more difficult to quantify objectively and require targeted research to define minimum exposure levels.
We chose age younger than 50 years from a pragmatic perspective and a review of older necropsy studies. A recent review of early COPD notes that most COPD studies have focused on subjects 60 years or older (2). This limitation is certainly true of cohorts with detailed imaging and biologic data collection, including COPDGene and SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study), which have large numbers of subjects with “mild” disease (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage 0–1). The mean age of GOLD 0 subjects in these two cohorts at enrollment was ∼60 years, with almost no data among individuals younger than 50 years old. Given their lack of development of airflow obstruction by that age, many will likely never develop significant COPD, precluding these cohorts from defining early COPD pathogenesis. Similarly, older consecutive necropsy studies suggested that likelihood of histological of emphysema (20) or airway remodeling (21) became more prominent in individuals in their 40s to early 50s.
CT abnormalities were included because good evidence associates visually assessed emphysema of moderate severity or greater (22) and greater than 10% low attenuation area (23, 24) with more rapid lung function decline in middle-aged subjects. Airway wall thickening (25) and small airway abnormality (17) are also associated with accelerated spirometric decline. Refining age-specific thresholds for each of these abnormalities will require collection of considerably greater imaging data in younger patient populations. Whether longitudinal changes in imaging parameters, especially parametric response mapping (PRM), described below, can robustly identify clinically relevant disease progression requires additional evaluation.
Regarding rapidity of FEV1 decline, 60 ml/year is likely a specific but potentially insensitive threshold. This rate is roughly double that cited as normal for never-smokers, roughly 25 to 30 ml/year (3, 26–28). Furthermore, in a recent multicohort analysis of lung function trajectory by Lange and colleagues, among those not ultimately developing COPD, less than 3% experienced a rate of decline greater than 2 SDs beyond the mean (24 ± 17 ml/year), or roughly 60 ml/year (3).
Noticeably absent from our operational definition are patient-reported respiratory symptoms, because of their frequent discordance from structural lung changes. A subset of symptomatic individuals probably has pathologic changes that will lead to COPD, but additional research is needed to identify that subgroup unambiguously.
Burden of Early COPD
Our proposed definition depends in part on smoking history and accepts anatomical changes in the absence of airflow obstruction. An 8- to 10-pack-year smoking history has been linked to lung function decline in subjects aged 35 to 53 years (29). Moreover, contemporary studies confirm that “chronic bronchitis” or “chronic mucus hypersecretion” predict future COPD incidence (30), especially among younger adults (4). Studying individuals aged 20 to 44 years, the European Community Respiratory Health Survey (ECRHS) associated chronic cough or phlegm with higher COPD incidence at 9 years of follow-up (31). Likewise, within the Medical Research Council National Survey of Health and Disease (NSHD), smokers reporting chronic cough or phlegm at ages 36 and 43 years were, respectively, 3.70 and 4.11 times more likely to have developed spirometrically defined COPD by age 63 years than asymptomatic counterparts (4).
Respiratory symptoms are common among smokers, reported by 16% during ECRHS and 40% by age 43 years in NSHD (31). At that age, chronic productive cough was present among 13% of smokers but only 2.6% of never-smokers (4). NSHD also demonstrated that the relationship between chronic respiratory symptoms and smoking evolves with age. Its finding of marked symptomatic escalation during midlife plausibly indicates onset of COPD (4). Known worldwide variations in respiratory symptom burdens (32) likely reflect differing prevalence of smoking (33), with which chronic cough and phlegm are closely associated, among both those with (34) and those without (4) COPD. Collectively, these data indicate a strong association of smoking in early adulthood with lung function decline and imply that the impact in susceptible smokers is detectable with exposures as little as 8 to 10 pack-years, when they are aged in their late 30s to early 40s.
Importantly, our early COPD definition does not exclude previous asthma, which recent evidence supports as an important risk factor for development of fixed airflow obstruction (35, 36). Among 15,668 ECRHS subjects, early-onset asthma was observed in 26% of those diagnosed with COPD at a mean age of 37 years, a 20-fold increased risk of adult airway obstruction, compared with those without asthma diagnosis (37). Similarly, in the Childhood Asthma Management Program cohort of 949 subjects enrolled at ages 5 to 12 years, 11% had spirometric values meeting COPD criteria at a mean age of 26 years (38). Childhood asthma affected 12% of the Aberdeen WHEASE (What Happens Eventually to Asthmatic Children: Sociologically and Epidemiologically) study, a general population cohort; those affected were 6.37 times more likely to have spirometric COPD by their seventh decade (35). An acknowledged limitation of these studies is that they defined development of COPD using fixed spirometric airflow obstruction, thus likely encompassing both severe asthma and COPD, which are difficult to distinguish in epidemiologic studies. However, these studies highlight one path toward COPD development.
Severe respiratory infections (especially viral) during infancy impair lung development (39), but their relevance to adult COPD is not well characterized. Smoking during adolescence reduces peak lung function values (40) and may worsen the impact of early life events (5). Nevertheless, patients younger than 50 years old with COPD have similar severity distributions and FEV1 decline trajectories as patients older than 75 years (41). Hence, although adverse early-life exposures are common, their precise contributions to adult COPD remain unclear, merit exploration, but also need not be considered exclusionary in an operational definition of early COPD.
Detecting Early COPD and Evaluating Its Progression
Central to the concept of early COPD is identifying those in whom clinically evident disease will develop over time. Validating this prediction prospectively is one of the field’s most pressing needs. We submit that for research purposes (though not yet for clinical practice), such validation requires multiple endpoints, extending the traditional COPD definition based solely on chronic airflow obstruction (1) and measured by rate of FEV1 decline (42). An analogy is the progression from asymptomatic atherosclerosis to overt ischemic heart disease. Mechanistic insights into the former provided important targets to prevent and treat the latter. Similarly, accepting an asymptomatic preclinical phase in COPD, without equating underlying pathologic processes and clinical disease, is essential to advance from palliative care to prevention.
Airflow obstruction remains an important metric for future studies, despite heterogeneity in spirometric trajectories (3, 43) and controversies regarding optimal spirometric thresholds (2). However, both detecting early small airway disease and assessing its progression might be accomplished noninvasively using more sensitive physiological studies, such as impedance oscillometry, nitrogen washout techniques, and measures of expiratory flow (44). Similarly, altered DlCO has proven predictive of subsequent spirometric obstruction in a small cohort of healthy smokers (45). Despite decades of expertise with these techniques (44), their ability to assess progressive disease in early COPD requires longitudinal evaluation, an important goal for cohort studies.
A complementary approach uses imaging features reflecting anatomic lung abnormalities (46). Qualitative, semiquantitative, and quantitative techniques have all proven valuable to assess the presence and impact of emphysema in large studies (46–48). Each can guide therapeutic decisions (1) and serve as an intermediate outcome of therapy (49). Although directly quantifying airway disease radiographically has been challenging, methods assessing air trapping as a functional measure of small airway abnormality have recently proven promising (46). One such technique, PRM, uses dynamic image registration of paired scans to quantify regional changes in lung density (16). By applying separate density thresholds to the inspiratory and expiratory voxel measurements, this technique can distinguish regions of “normal” lung from “functional small airways disease” (defined as regions of lung >−950 Hounsfield units [HU] on inspiration and <−856 HU on expiration) and “emphysema” (regions of lung <−950 HU on inspiration and <−856 HU on expiration). Its real world relevance is shown by preliminary analyses using ex vivo microCT data of lung explants, in which functional small airways disease PRM (PRMfSAD) has been linked to narrowing and loss of terminal and transitional bronchioles (50).
High-resolution CT imaging might also serve as an outcome biomarker for therapies designed to block progression of early structural airway changes into emphysema. Supporting this possibility, analysis of 5-year COPDGene follow-up data (17) established an association between baseline PRMfSAD and subsequent FEV1 decline, not only in those with established COPD in their early 60s but also among individuals with chronic respiratory symptoms but no airflow limitation (so-called GOLD 0) (mean age, 58 yr). Preliminary analyses of a small group of SPIROMICS subjects (mean ages, early to mid-60s) (51) also suggest an association between baseline PRMfSAD and development of emphysema PRM (PRMemph) 1 year later. Similarly, lower total airway count in a separate older cohort was associated with accelerated loss of FEV1 (52). Collectively, these recent studies complement classic pathological studies that defined the small airways as the earliest site of smoking-induced abnormalities (53–55), evident by ages 40 to 59 years with moderate smoking exposure (21). In addition, dual-energy computed tomography of smokers with pulmonary function test results within the normal range linked centriacinar emphysema to increased perfusion heterogeneity and enlarged segmental-level pulmonary arteries, both reversible with sildenafil (18, 19). All these findings are central to the unified hypothesis of early COPD development presented below. Gaining crucial mechanistic insights required to develop novel, disease-modifying therapies in early COPD could be hastened by coupling this imaging biomarker with thorough profiling of early COPD airway samples.
Finally, we acknowledge that COPD is a systemic disease. Lung injury may be one part of a global vascular process damaging other organs, especially the cardiovascular (56) and renal systems (57). Smoking-induced dysfunction of other organs likely contributes to dyspnea, and in the case of hematopoietic and immune systems might even be crucial to COPD progression. Screening for damage to other organs might help to diagnose early COPD (56) (e.g., patients with COPD frequently have increased albumin-to-creatinine ratios, indicative of renal endothelial injury [57]), but prospective evaluation in young populations is needed.
Possible Host Mechanisms in Early COPD Development and Progression
Recent evidence implies that cigarette smoke exposure induces sequential, stereotypical changes in distal airways culminating, in susceptible individuals, in COPD development (Figure 2). To date, several genetic factors have been identified that increase susceptibility and appear to relate to pathogenesis (58, 59). In addition to alpha-1 antitrypsin deficiency, variants coding for Hedgehog interacting factor, glutathione-S-transferase, transforming growth factor-β1, tumor necrosis factor-α, and superoxide dismutase-3 have been linked to COPD development and may provide insights into both pathogenesis and identifying at-risk individuals (60).
Figure 2.
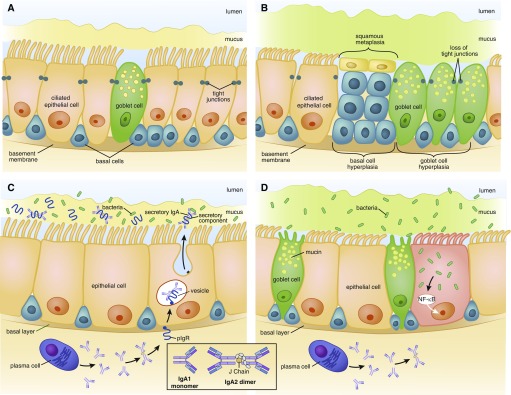
Epigenetic changes induced by smoking lead to progressive small airway damage and inflammation in early chronic obstructive pulmonary disease (COPD). (A and C) Normal small airways; (B and D) small airways in early COPD. (A) Normal distal epithelium contains self-renewing basal cells, which differentiate into ciliated, mucus-producing goblet, and secretory (club) cells, joined by tight junctions that form an impermeable barrier. Mucus is separated from the epithelial surface by a robust aqueous periciliary layer. (B) Smoking induces hyperplasia of basal and goblet cells, squamous metaplasia, loss of club and ciliated cells, decrease in the periciliary layer and ciliary damage and crowding, and junctional barrier loss. (C) In normal small airways, dimeric IgA (structure shown in inset) is transcytosed by the polymeric Ig receptor (pIgR) into the mucosal lumen. pIgR cleavage at the luminal surface liberates secretory IgA, which prevents bacterial invasion. (D) Smoking reduces pIgR expression, leading to localized secretory IgA deficiency in small airways, allowing bacteria to invade and induce sustained airway inflammation. NF-κB = nuclear factor-κB. Illustration by Patricia Ferrer Beals.
Focusing on histologic changes, the earliest detectable step is epigenetic reprogramming of the basal epithelial cells that maintain the epithelial barrier (61, 62). Relative to young nonsmokers (mean age, 40 yr), reprogramming was shown in epithelium in 4th- through 6th-generation and 10th- through 12th-generation airways in smokers without obstruction (mean age, 43 yr) and smokers with COPD (mean age, 52 yr) (63). Epigenetic reprogramming induces in distal airways a proximal airway gene signature (64, 65) that is more prominent in those 44 years or older with FEV1/FVC ratios less than or equal to 0.8 (63). The result is an ecological transition, from a distal epithelial community of diverse cell types, well-suited to local self-defense and efficient clearance (66), to one dominated by mucus hypersecretion, squamous metaplasia and damaged cilia (Figures 2A and 2B). Importantly, this transition initially occurs in the initial absence of inflammatory cell infiltration of the epithelium. Mechanistically, this phenotype is linked to increased signaling in distal airway basal stem cells of epidermal growth factor receptor (EGFR), a pathway implicated in multiple airway changes in smokers (63, 67). Alterations in other pathways crucial to distal lung epithelium, including the Notch (68–73) and Wnt pathways (72–74), may contribute to early bronchoalveolar remodeling, a process termed “accelerated aging” (75).
Epithelial reprogramming also induces multiple alterations to the healthy airway surface liquid (ASL), which comprises an upper mucus layer containing secreted mucins and an underlying, aqueous, periciliary layer (76). Homeostasis depends both on ASL volume (76, 77) and on the relative concentration of the mucus and periciliary layers (78). Smoking changes the balance of mucin gene products and induces mucus hyperconcentration (79) due to smoking-induced cystic fibrosis transmembrane conductance regulator disruption (80). The net effect, mucus that is less readily cleared, leads to the chronic bronchitic phenotype and an age-related risk of COPD development (4) (Figure 2B).
Focal airway injury is central to another eventful consequence of epithelial reprogramming, pIgR (polymeric immunoglobulin receptor) downregulation. Epithelial expression of PIgR is essential to transcytose secretory IgA into the lumens of distal airways (81) (Figure 2C). In areas lacking this crucial opsonin, bacteria selectively invade epithelial cells, inducing focal NF-κB (nuclear factor-κB) activation (82) (Figure 2D). Because NF-κB typically upregulates pIgR, this change is further evidence that epithelial reprogramming drives the earliest changes of COPD. The role of other innate airway defenses in early COPD clearly merits investigation, although, interestingly, β-defensin-1 airway concentrations are increased in established COPD (83, 84) relative to healthy nonsmokers, suggesting a compensatory rather than causal contribution. This response might be an example of how host-derived danger-associated molecular patterns sustain airway inflammation (85).
COPD is characterized by a combination of airway narrowing and frank distal airway disappearance, which silently increases airway resistance and precedes emphysema development (15). Airways are narrowed by both mucus plugging and remodeling (53). The tight association of airway injury with focal loss of luminal secretory IgA staining (82) suggests that inflammation driven by bacterial invasion may be a key trigger, a possibility requiring validation. Remodeling is associated with upregulation of multiple transforming growth factor-β superfamily members (86) and the zinc finger transcription factor Krüppel-like factor 5 (87). Distal airway dropout might represent the extreme of airway remodeling, heightening the importance of understanding fibrotic processes in early COPD.
In addition, global gene analysis demonstrated that in COPD, tissue degradation around small airways predominates over repair (88). Hence, matrix destruction might also cause airway dropout via anoikis, leading to the epithelial apoptosis observed by multiple groups in established disease but unstudied in early COPD. Activated lung NK cells likely also induce epithelial apoptosis (89, 90). These two cytolytic processes (not mutually exclusive and perhaps synergistic) might overwhelm compensatory epithelial cell proliferation in some individuals, leading to progression from distal small airway injury to centrilobular emphysema. Advanced CT analytical techniques (12, 91) demonstrate that significant emphysema can exist despite preserved FEV1% predicted. Hence, centrilobular emphysema is relevant not only to advanced COPD but also to early COPD.
Emphysema might also develop in early COPD because of loss of pulmonary endothelial cell (PEndC)-derived (“angiocrine”) factors (92) essential to sustain epithelial proliferation, especially given the prominent role of increased EGFR signaling in smoking-reprogramed basal epithelial stem cells. The importance of PEndC production of angiocrine factors is supported by the murine model of lung hypertrophy after unilateral adult pneumonectomy. PEndC were essential for the earliest regenerative change, proliferation of cells at the bronchoalveolar duct junction. Coculture experiments supported a role for PEndC elaboration of the membrane-anchored metalloproteinase MMP-14, which unmasks cryptic ligands for EGFR (93). Loss of angiocrine signals might be the “final straw” that overcomes epithelial proliferative capacity and dooms the damaged terminal airway to centrilobular emphysema.
Perhaps the thorniest question regarding mechanisms of early COPD is the precise role of inflammatory cells. Pigmented macrophages prominently accumulated around and within respiratory bronchioles of male smokers dying suddenly at a mean age of 25 years (94); such infiltration is often assumed to affect all young smokers, but the high mean exposure (20.1 ± 4.1 pack-years) in this study must be considered. Relative to smokers without obstruction, COPD is associated with progressive activation of lung dendritic cell subsets in lung parenchyma (95, 96) and loss of cells with a T-regulatory phenotype there (97, 98) and in BAL (99). These changes have motivated years of attempts to modify COPD progression by antiinflammatory therapy, yet it is unproven that every inflammatory cell type infiltrating the lungs in the transition from asymptomatic smoking to early COPD is actually harmful. A key recent advance is recognition of unique innate and innate-like lymphocyte cell types that are largely restricted to mucosal surfaces, including the airways, and appear to play crucial roles in host defense (100, 101). We contend that the role of specific hematopoietic cell types can only be understood in the context of the lung microbiome.
The Lung Microbiome in Early COPD
Differences in lung microbial community structures might explain why not all smokers develop COPD. Bacteria are well established to contribute to established COPD, especially during exacerbations and in the case of potentially pathogenic microorganisms, such as Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis. However, exactly when during early COPD evolution lung microbes transition from exploiting smoking-damaged ASL to driving airway pathology, and which microbes are essential for progression, are among the most important unanswered questions.
Advances in sequence-dependent microbiology have established that the lower respiratory tract of healthy individuals is not sterile and that procedural contamination, although a valid concern, is far less an issue than post-procedure contamination (102, 103). Instead, the healthy lungs sustain a very low burden of bacteria that, with few exceptions, is a neutral subset of those inhabiting the oropharynx (104–106). Thus, the paradigm that COPD is neatly characterized by a change from sterility to bacterial colonization is untenable.
Previous data indicated that smoking in the absence of airflow obstruction does not alter the global community structure of the bacterial lung microbiome as assessed using BAL (102, 107). However, the averaging effect of this technique, relative to protected-specimen brushings, might miss highly localized microbiome changes. A more recent study in younger healthy smokers and nonsmokers showed an association of a lung microbiome enriched for oral bacteria with augmented lung inflammation (108). These considerations support the need to understand local patterns of microbial–host interaction in the lower airways. For example, this could be accomplished by obtaining protected-specimen brush sampling, for microbiome analysis, plus conventional cytological brushing, for genomic and epigenetic analysis, in identical areas of distal human airways (109). Simultaneous analysis of the lower airway virome and mycome is another important goal but may need to await resolution of the technical challenges of high-coverage RNA sequencing and better reference libraries.
Unquestionably, potentially pathogenic microorganisms could perpetuate small airway inflammation by many mechanisms (110–112), including damaging cilia, stimulating mucin production, degrading humoral immunity, and triggering NK cell recognition of infected epithelial cells (113, 114). Bacterial molecules such as endotoxins, membrane lipoproteins, peptidoglycan fragments, and lipoteichoic acid exacerbate inflammation (115). In a recent pilot placebo-controlled randomized trial, macrolide administration in early emphysema was associated with changes in microbiota and decreased levels of inflammatory cytokines in lower airways (116). Importantly, the net antiinflammatory effect seemed to be mediated more by bacterial stress–induced metabolites than by direct macrolide effects on the host. Hence, rather than globally suppressing inflammatory cell function, future therapies to arrest early COPD might focus on containing the microbial invasion that drives inflammation.
Future Therapeutic Trials in Early COPD
To reduce COPD’s long-term societal impact, the goal of interventions must change, from the sole intent of reducing symptoms and exacerbations in advanced disease to halting progression in early disease. Current U.S., European, and Japanese regulatory definitions of disease progression rely on primary outcomes of mortality or rate of FEV1 decline, on the basis of studies in moderate to severe COPD. Such studies typically require 3- to 4-year trials in 8,000 to 16,000 participants (117–120). Focusing on early COPD provides an opportunity to treat the patients most likely to experience long-term benefit while also improving trial efficiency, because FEV1 decline is fastest in patients with GOLD 1 and 2 disease (17). The Lung Health Study, one of the few studies to examine early COPD, demonstrated the benefit of smoking cessation on FEV1 decline over a 5-year period (121). Two recent large studies of milder (although not early) COPD investigated FEV1 decline. The first showed a trend toward attenuation over 2 years of follow-up with long-acting antimuscarinic treatment (122); the second demonstrated significant reduction over up to 3 years of follow-up with a combination of a long-acting β-agonist and inhaled corticosteroid (123).
With improved understanding of COPD subtypes and risk factors for rapid progression, drug development programs may become more efficient. Strategies to enrich a younger trial population for more rapidly progressing subjects include selection for: 1) symptoms plus environmental exposure (pack-year history, occupational risk, geographic location) (4); 2) mild to moderate airflow limitation, the stage at which spirometry deteriorates most rapidly (17, 117, 118, 120); 3) history of lung function decline, retrievable from electronic health records; 4) emphysema or airways disease from thoracic CT imaging (17, 23); 5) blood biomarkers such as sRAGE (soluble form of receptor for advanced glycation end products) for emphysema progression (124) or club cell protein 16 for FEV1 decline (125); and 6) genetic risk factors such as variants in CHRNA3/5, HHIP, and FAM13A (126), possibly combined as genetic risk scores; and other approaches of individualized risk on the basis of anatomic and molecular profiling.
Given the complexity of the experience of the patient with COPD, additional measures of functional impact or systemic manifestations may prove valuable to define early COPD presence or progression (42, 43). Health status is impaired in patients with mild COPD (11, 127) (less prominently than with worse airflow obstruction [42]), but how health status changes longitudinally in those with early disease, who are mostly undiagnosed, remains unclear. In established COPD, acute exacerbations are clinically relevant, but variable, manifestations across a range of spirometric severities (11, 128–131). Longitudinal exacerbation frequency is important in older patients with mild COPD (127). Similar analyses in patients with early COPD are sorely required. Whether abnormalities in muscle function, exercise capacity, or response are relevant manifestations of early COPD (132) will require longitudinal studies.
Appropriate outcome measures must depend on the mechanism of action of specific interventions but should use feasible metrics that document arrest of disease progression. Such metrics might include digital technologies for real-time monitoring of symptoms and health-related quality-of-life measures (St. George’s Respiratory Questionnaire, COPD Assessment Test, and Evaluating Respiratory Symptoms in COPD), lung function decline (mobile spirometry), physical activity limitation (Physical Activity as a Crucial Patient Reported Outcome) (133); exacerbation-tracking (Exacerbations of Chronic Pulmonary Disease Tool questionnaire), and use of composite measures such as the Clinically Important Deterioration (134). However, because individuals with early COPD are largely unstudied, novel instruments using questions more relevant to the health status of this younger population may be needed. Similarly, the utility of blood biomarkers to aid early-phase, proof-of-concept, and dose-ranging trials must also be examined in this population.
We believe that advanced high-resolution CT metrics will play a central role in accelerating progress in early COPD, both to identify high-risk populations and as longitudinal outcome measures. Acceptance of this potentially game-changing modality should increase with availability of ultra–low-dose scanners (135) that alter risk–benefit considerations. Imaging or procedures to stage other chronic diseases are already common practice (e.g., dual-energy X-ray absorptiometry scans for osteoporosis, or endoscopy in inflammatory bowel disease), and might become compelling in early COPD.
Future clinical trials will face unique challenges. Paramount is lack of regulatory acceptance of novel endpoints, beyond use as “exploratory outcomes.” Minimal clinically important differences defined for advanced disease (e.g., changes of 4 for SGRQ; 30 m for 6-min-walk test; 50 ml for FEV1) are likely inappropriate for an early COPD population with preserved lung function. Younger study populations pose a heightened dropout risk, especially in longer studies, because of geomobility and possibly lower compliance. As disease-modifying therapies become available, placebo-controlled trials are less likely to be ethically acceptable; noninferiority trials or superiority trials with an active control arm may become standard for this at-risk population. Trial design and analysis will need to address changing risk profiles (smoking cessation, electronic cigarettes, comorbid conditions). Novel therapies introduced during a study pose the risk of irrelevance, supporting the need for shorter, more agile clinical trials.
This is an exciting time for clinical research in early COPD. The increasingly obvious need for novel therapies has motivated regulators, academia, and industry to work together to accelerate qualification of drug-development tools and approval of new medicines, and, through initiatives like the 21st Century Cures Act, to enhance the ability to collect real-world evidence using medical devices. Widespread acceptance of digital technology by younger subjects may extend to willingness to wear monitoring devices and to participating more interactively in clinical research. Shorter trials are facilitated when an agent’s mechanism of action suggests an immediate effect (e.g., reduced symptoms or exacerbations). Although current regulations require inclusion of multiple doses in phase III trials lacking pharmacodynamic biomarkers for phase II dose selection, this burden may be mitigated via event-driven studies (e.g., SUMMIT [Study to Understand Mortality and Morbidity]) (120) or by advanced predictive analytics and machine learning methodologies to detect therapeutic response.
Conclusions
Although COPD is among the few noncommunicable disorders with increasing worldwide morbidity and mortality, the ability to identify patients at risk for more rapid disease progression is limited. This shortcoming jeopardizes development and validation of disease-modifying therapies for COPD, a crucial unmet clinical need. It is time for the pulmonary community to reconsider its investigational approach. Focusing on younger people to understand early COPD aligns with the goals of the recently released NHLBI COPD National Action Plan to develop strategies to prevent the onset and progression of COPD by studying disease progression.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201710-2028PP on February 6, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 2.Rennard SI, Drummond MB. Early chronic obstructive pulmonary disease: definition, assessment, and prevention. Lancet. 2015;385:1778–1788. doi: 10.1016/S0140-6736(15)60647-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 4.Allinson JP, Hardy R, Donaldson GC, Shaheen SO, Kuh D, Wedzicha JA. The presence of chronic mucus hypersecretion across adult life in relation to chronic obstructive pulmonary disease development. Am J Respir Crit Care Med. 2016;193:662–672. doi: 10.1164/rccm.201511-2210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allinson JP, Hardy R, Donaldson GC, Shaheen SO, Kuh D, Wedzicha JA. Combined impact of smoking and early life exposures on adult lung function trajectories. Am J Respir Crit Care Med. 2017;196:1021–1030. doi: 10.1164/rccm.201703-0506OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ. 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buist AS, Sexton GJ, Nagy JM, Ross BB. The effect of smoking cessation and modification on lung function. Am Rev Respir Dis. 1976;114:115–122. doi: 10.1164/arrd.1976.114.1.115. [DOI] [PubMed] [Google Scholar]
- 8.Kohansal R, Martinez-Camblor P, Agustí A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med. 2009;180:3–10. doi: 10.1164/rccm.200901-0047OC. [DOI] [PubMed] [Google Scholar]
- 9.Martinez FD. Early-life origins of chronic obstructive pulmonary disease. N Engl J Med. 2016;375:871–878. doi: 10.1056/NEJMra1603287. [DOI] [PubMed] [Google Scholar]
- 10.Regan EA, Lynch DA, Curran-Everett D, Curtis JL, Austin JH, Grenier PA, et al. Genetic Epidemiology of COPD (COPDGene) Investigators. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med. 2015;175:1539–1549. doi: 10.1001/jamainternmed.2015.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. SPIROMICS Research Group. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374:1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oelsner EC, Hoffman EA, Folsom AR, Carr JJ, Enright PL, Kawut SM, et al. Association between emphysema-like lung on cardiac computed tomography and mortality in persons without airflow obstruction: a cohort study. Ann Intern Med. 2014;161:863–873. doi: 10.7326/M13-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vestbo J, Prescott E, Lange P Copenhagen City Heart Study Group. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Am J Respir Crit Care Med. 1996;153:1530–1535. doi: 10.1164/ajrccm.153.5.8630597. [DOI] [PubMed] [Google Scholar]
- 14.Oelsner EC, Lima JA, Kawut SM, Burkart KM, Enright PL, Ahmed FS, Barr RG. Noninvasive tests for the diagnostic evaluation of dyspnea among outpatients: the Multi-Ethnic Study of Atherosclerosis Lung Study. Am J Med. 2015;128:171–180.e5. doi: 10.1016/j.amjmed.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365:1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galbán CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18:1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatt SP, Soler X, Wang X, Murray S, Anzueto AR, Beaty TH, et al. COPDGene Investigators. COPDGene Investigators. Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;194:178–184. doi: 10.1164/rccm.201511-2219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alford SK, van Beek EJ, McLennan G, Hoffman EA. Heterogeneity of pulmonary perfusion as a mechanistic image-based phenotype in emphysema susceptible smokers. Proc Natl Acad Sci USA. 2010;107:7485–7490. doi: 10.1073/pnas.0913880107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iyer KS, Newell JD, Jr, Jin D, Fuld MK, Saha PK, Hansdottir S, et al. Quantitative dual-energy computed tomography supports a vascular etiology of smoking-induced inflammatory lung disease. Am J Respir Crit Care Med. 2016;193:652–661. doi: 10.1164/rccm.201506-1196OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson JA, Dunnill MS, Ryder RC. Dependence of the incidence of emphysema on smoking history, age, and sex. Thorax. 1972;27:547–551. doi: 10.1136/thx.27.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auerbach O, Stout AP, Hammond EC, Garfinkel L. Changes in bronchial epithelium in relation to cigarette smoking and in relation to lung cancer. N Engl J Med. 1961;265:253–267. doi: 10.1056/NEJM196108102650601. [DOI] [PubMed] [Google Scholar]
- 22.McAllister DA, Ahmed FS, Austin JH, Henschke CI, Keller BM, Lemeshow A, et al. Emphysema predicts hospitalisation and incident airflow obstruction among older smokers: a prospective cohort study. PLoS One. 2014;9:e93221. doi: 10.1371/journal.pone.0093221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, et al. ECLIPSE Investigators. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365:1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 24.Koo HK, Jin KN, Kim DK, Chung HS, Lee CH. Association of incidental emphysema with annual lung function decline and future development of airflow limitation. Int J Chron Obstruct Pulmon Dis. 2016;11:161–166. doi: 10.2147/COPD.S96809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohamed Hoesein FA, de Jong PA, Lammers JW, Mali WP, Schmidt M, de Koning HJ, et al. Airway wall thickness associated with forced expiratory volume in 1 second decline and development of airflow limitation. Eur Respir J. 2015;45:644–651. doi: 10.1183/09031936.00020714. [DOI] [PubMed] [Google Scholar]
- 26.Burchfiel CM, Marcus EB, Sharp DS, Enright PL, Rodriguez BL, Masaki KH, et al. Characteristics associated with rapid decline in forced expiratory volume. Ann Epidemiol. 1996;6:217–227. doi: 10.1016/1047-2797(96)00007-5. [DOI] [PubMed] [Google Scholar]
- 27.Kerstjens HA, Rijcken B, Schouten JP, Postma DS. Decline of FEV1 by age and smoking status: facts, figures, and fallacies. Thorax. 1997;52:820–827. doi: 10.1136/thx.52.9.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wise RA. The value of forced expiratory volume in 1 second decline in the assessment of chronic obstructive pulmonary disease progression. Am J Med. 2006;119:4–11. doi: 10.1016/j.amjmed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Anderson DO, Ferris BG., Jr Role of tobacco smoking in the causation of chronic respiratory disease. N Engl J Med. 1962;267:787–794. doi: 10.1056/NEJM196210182671601. [DOI] [PubMed] [Google Scholar]
- 30.Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD. Chronic bronchitis before age 50 years predicts incident airflow limitation and mortality risk. Thorax. 2009;64:894–900. doi: 10.1136/thx.2008.110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Marco R, Accordini S, Cerveri I, Corsico A, Antó JM, Künzli N, et al. Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am J Respir Crit Care Med. 2007;175:32–39. doi: 10.1164/rccm.200603-381OC. [DOI] [PubMed] [Google Scholar]
- 32.de Oca MM, Halbert RJ, Lopez MV, Perez-Padilla R, Tálamo C, Moreno D, et al. The chronic bronchitis phenotype in subjects with and without COPD: the PLATINO study. Eur Respir J. 2012;40:28–36. doi: 10.1183/09031936.00141611. [DOI] [PubMed] [Google Scholar]
- 33.GBD 2015 Tobacco Collaborators. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet. 2017;389:1885–1906. doi: 10.1016/S0140-6736(17)30819-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanner RE, Connett JE, Williams DE, Buist AS. Effects of randomized assignment to a smoking cessation intervention and changes in smoking habits on respiratory symptoms in smokers with early chronic obstructive pulmonary disease: the Lung Health Study. Am J Med. 1999;106:410–416. doi: 10.1016/s0002-9343(99)00056-x. [DOI] [PubMed] [Google Scholar]
- 35.Tagiyeva N, Devereux G, Fielding S, Turner S, Douglas G. Outcomes of childhood asthma and wheezy bronchitis: a 50-year cohort study. Am J Respir Crit Care Med. 2016;193:23–30. doi: 10.1164/rccm.201505-0870OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339:1194–1200. doi: 10.1056/NEJM199810223391703. [DOI] [PubMed] [Google Scholar]
- 37.Aanerud M, Carsin AE, Sunyer J, Dratva J, Gislason T, Jarvis D, et al. Interaction between asthma and smoking increases the risk of adult airway obstruction. Eur Respir J. 2015;45:635–643. doi: 10.1183/09031936.00055514. [DOI] [PubMed] [Google Scholar]
- 38.McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, et al. Camp Research Group. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med. 2016;374:1842–1852. doi: 10.1056/NEJMoa1513737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colom AJ, Maffey A, Garcia Bournissen F, Teper A. Pulmonary function of a paediatric cohort of patients with postinfectious bronchiolitis obliterans: a long term follow-up. Thorax. 2015;70:169–174. doi: 10.1136/thoraxjnl-2014-205328. [DOI] [PubMed] [Google Scholar]
- 40.Gold DR, Wang X, Wypij D, Speizer FE, Ware JH, Dockery DW. Effects of cigarette smoking on lung function in adolescent boys and girls. N Engl J Med. 1996;335:931–937. doi: 10.1056/NEJM199609263351304. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez-Salcedo P, Divo M, Casanova C, Pinto-Plata V, de-Torres JP, Cote C, et al. Disease progression in young patients with COPD: rethinking the Fletcher and Peto model. Eur Respir J. 2014;44:324–331. doi: 10.1183/09031936.00208613. [DOI] [PubMed] [Google Scholar]
- 42.Decramer M, Gosselink R, Rutten-Van Mölken M, Buffels J, Van Schayck O, Gevenois PA, et al. Assessment of progression of COPD: report of a workshop held in Leuven, 11-12 March 2004. Thorax. 2005;60:335–342. doi: 10.1136/thx.2004.028712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuwallack RL, Nici L. Modifying the course of chronic obstructive pulmonary disease: looking beyond the FEV1. COPD. 2012;9:637–648. doi: 10.3109/15412555.2012.710668. [DOI] [PubMed] [Google Scholar]
- 44.Hogg JC, Paré PD, Hackett TL. The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Physiol Rev. 2017;97:529–552. doi: 10.1152/physrev.00025.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harvey BG, Strulovici-Barel Y, Kaner RJ, Sanders A, Vincent TL, Mezey JG, et al. Risk of COPD with obstruction in active smokers with normal spirometry and reduced diffusion capacity. Eur Respir J. 2015;46:1589–1597. doi: 10.1183/13993003.02377-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Labaki WW, Martinez CH, Martinez FJ, Galban CJ, Ross BD, Washko GR, et al. The role of chest computed tomography in the evaluation and management of the patient with COPD. Am J Respir Crit Care Med. 2017;196:1372–1379. doi: 10.1164/rccm.201703-0451PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoffman EA, Lynch DA, Barr RG, van Beek EJ, Parraga G IWPFI Investigators. Pulmonary CT and MRI phenotypes that help explain chronic pulmonary obstruction disease pathophysiology and outcomes. J Magn Reson Imaging. 2016;43:544–557. doi: 10.1002/jmri.25010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sieren JP, Newell JD, Jr, Barr RG, Bleecker ER, Burnette N, Carretta EE, et al. SPIROMICS Research Group. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194:794–806. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dirksen A, Dijkman JH, Madsen F, Stoel B, Hutchison DC, Ulrik CS, et al. A randomized clinical trial of alpha(1)-antitrypsin augmentation therapy. Am J Respir Crit Care Med. 1999;160:1468–1472. doi: 10.1164/ajrccm.160.5.9901055. [DOI] [PubMed] [Google Scholar]
- 50.Vasilescu D, Marchetti N, Galban CJ, Meldrum C, Ross BD, Martinez C, et al. The relationship between functional small airways disease and small airways pathology in COPD [abstract] Am J Respir Crit Care Med. 2017;195:A5157. [Google Scholar]
- 51.Boes JL, Hoff BA, Bule M, Johnson TD, Rehemtulla A, Chamberlain R, et al. Parametric response mapping monitors temporal changes on lung CT scans in the subpopulations and intermediate outcome measures in COPD Study (SPIROMICS) Acad Radiol. 2015;22:186–194. doi: 10.1016/j.acra.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirby M, Tanabe N, Tan WC, Zhou G, Obeidat M, Hague CJ, et al. CanCOLD Collaborative Research Group; Canadian Respiratory Research Network; CanCOLD Collaborative Research Group, the Canadian Respiratory Research Network. Total airway count on computed tomography and the risk of chronic obstructive pulmonary disease progression: findings from a population-based study. Am J Respir Crit Care Med. 2018;197:56–65. doi: 10.1164/rccm.201704-0692OC. [DOI] [PubMed] [Google Scholar]
- 53.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009;4:435–459. doi: 10.1146/annurev.pathol.4.110807.092145. [DOI] [PubMed] [Google Scholar]
- 54.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278:1355–1360. doi: 10.1056/NEJM196806202782501. [DOI] [PubMed] [Google Scholar]
- 55.Auerbach O, Forman JB, Gere JB, Kassouny DY, Muehsam GE, Petrick TG, et al. Changes in the bronchial epithelium in relation to smoking and cancer of the lung; a report of progress. N Engl J Med. 1957;256:97–104. doi: 10.1056/NEJM195701172560301. [DOI] [PubMed] [Google Scholar]
- 56.Roversi S, Fabbri LM, Sin DD, Hawkins NM, Agustí A. Chronic obstructive pulmonary disease and cardiac diseases. an urgent need for integrated care. Am J Respir Crit Care Med. 2016;194:1319–1336. doi: 10.1164/rccm.201604-0690SO. [DOI] [PubMed] [Google Scholar]
- 57.Polverino F, Laucho-Contreras ME, Petersen H, Bijol V, Sholl LM, Choi ME, et al. A pilot study linking endothelial injury in lungs and kidneys in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195:1464–1476. doi: 10.1164/rccm.201609-1765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan C, Chang D, Lu G, Deng X. Genetic polymorphism and chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:1385–1393. doi: 10.2147/COPD.S134161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allinson JP, Wedzicha JA. Update in chronic obstructive pulmonary disease 2016. Am J Respir Crit Care Med. 2017;196:414–424. doi: 10.1164/rccm.201703-0588UP. [DOI] [PubMed] [Google Scholar]
- 60.Antuni JD, Barnes PJ. Evaluation of individuals at risk for COPD: beyond the scope of the global initiative for chronic obstructive lung disease. Chronic Obstr Pulm Dis (Miami) 2016;3:653–667. doi: 10.15326/jcopdf.3.3.2016.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heijink IH, Noordhoek JA, Timens W, van Oosterhout AJ, Postma DS. Abnormalities in airway epithelial junction formation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189:1439–1442. doi: 10.1164/rccm.201311-1982LE. [DOI] [PubMed] [Google Scholar]
- 62.Staudt MR, Buro-Auriemma LJ, Walters MS, Salit J, Vincent T, Shaykhiev R, et al. Airway basal stem/progenitor cells have diminished capacity to regenerate airway epithelium in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190:955–958. doi: 10.1164/rccm.201406-1167LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang J, Zuo WL, Fukui T, Chao I, Gomi K, Lee B, et al. Smoking-dependent distal-to-proximal repatterning of the adult human small airway epithelium. Am J Respir Crit Care Med. 2017;196:340–352. doi: 10.1164/rccm.201608-1672OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crystal RG. Airway basal cells: the “smoking gun” of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190:1355–1362. doi: 10.1164/rccm.201408-1492PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shaykhiev R, Crystal RG. Early events in the pathogenesis of chronic obstructive pulmonary disease: smoking-induced reprogramming of airway epithelial basal progenitor cells. Ann Am Thorac Soc. 2014;11:S252–S258. doi: 10.1513/AnnalsATS.201402-049AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337:937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shaykhiev R, Zuo WL, Chao I, Fukui T, Witover B, Brekman A, et al. EGF shifts human airway basal cell fate toward a smoking-associated airway epithelial phenotype. Proc Natl Acad Sci USA. 2013;110:12102–12107. doi: 10.1073/pnas.1303058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tilley AE, Harvey BG, Heguy A, Hackett NR, Wang R, O’Connor TP, et al. Down-regulation of the notch pathway in human airway epithelium in association with smoking and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179:457–466. doi: 10.1164/rccm.200705-795OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell. 2011;8:639–648. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517:621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsao PN, Matsuoka C, Wei SC, Sato A, Sato S, Hasegawa K, et al. Epithelial Notch signaling regulates lung alveolar morphogenesis and airway epithelial integrity. Proc Natl Acad Sci USA. 2016;113:8242–8247. doi: 10.1073/pnas.1511236113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCauley KB, Hawkins F, Serra M, Thomas DC, Jacob A, Kotton DN. Efficient derivation of functional human airway epithelium from pluripotent stem cells via temporal regulation of Wnt signaling. Cell Stem Cell. 2017;20:844–857.e6. doi: 10.1016/j.stem.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baarsma HA, Skronska-Wasek W, Mutze K, Ciolek F, Wagner DE, John-Schuster G, et al. Noncanonical WNT-5A signaling impairs endogenous lung repair in COPD. J Exp Med. 2017;214:143–163. doi: 10.1084/jem.20160675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Skronska-Wasek W, Mutze K, Baarsma HA, Bracke KR, Alsafadi HN, Lehmann M, et al. Reduced Frizzled Receptor 4 expression prevents WNT/beta-catenin-driven alveolar lung repair in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196:172–185. doi: 10.1164/rccm.201605-0904OC. [DOI] [PubMed] [Google Scholar]
- 75.Walters MS, De BP, Salit J, Buro-Auriemma LJ, Wilson T, Rogalski AM, et al. Smoking accelerates aging of the small airway epithelium. Respir Res. 2014;15:94. doi: 10.1186/s12931-014-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boucher RC. An overview of the pathogenesis of cystic fibrosis lung disease. Adv Drug Deliv Rev. 2002;54:1359–1371. doi: 10.1016/s0169-409x(02)00144-8. [DOI] [PubMed] [Google Scholar]
- 77.Tarran R, Grubb BR, Gatzy JT, Davis CW, Boucher RC. The relative roles of passive surface forces and active ion transport in the modulation of airway surface liquid volume and composition. J Gen Physiol. 2001;118:223–236. doi: 10.1085/jgp.118.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boucher RC. Regulation of airway surface liquid volume by human airway epithelia. Pflugers Arch. 2003;445:495–498. doi: 10.1007/s00424-002-0955-1. [DOI] [PubMed] [Google Scholar]
- 79.Kesimer M, Ford AA, Ceppe A, Radicioni G, Cao R, Davis CW, et al. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med. 2017;377:911–922. doi: 10.1056/NEJMoa1701632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dransfield MT, Wilhelm AM, Flanagan B, Courville C, Tidwell SL, Raju SV, et al. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD. Chest. 2013;144:498–506. doi: 10.1378/chest.13-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Curtis JL. A hairline crack in the levee: focal secretory IgA deficiency as a first step toward emphysema. Am J Respir Crit Care Med. 2017;195:970–973. doi: 10.1164/rccm.201612-2509ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Polosukhin VV, Richmond BW, Du RH, Cates JM, Wu P, Nian H, et al. Secretory IgA deficiency in individual small airways is associated with persistent inflammation and remodeling. Am J Respir Crit Care Med. 2017;195:1010–1021. doi: 10.1164/rccm.201604-0759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Andresen E, Günther G, Bullwinkel J, Lange C, Heine H. Increased expression of beta-defensin 1 (DEFB1) in chronic obstructive pulmonary disease. PLoS One. 2011;6:e21898. doi: 10.1371/journal.pone.0021898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baines KJ, Wright TK, Simpson JL, McDonald VM, Wood LG, Parsons KS, et al. Airway β-defensin-1 protein is elevated in COPD and severe asthma. Mediators Inflamm. 2015;2015:407271. doi: 10.1155/2015/407271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pouwels SD, Heijink IH, ten Hacken NH, Vandenabeele P, Krysko DV, Nawijn MC, et al. DAMPs activating innate and adaptive immune responses in COPD. Mucosal Immunol. 2014;7:215–226. doi: 10.1038/mi.2013.77. [DOI] [PubMed] [Google Scholar]
- 86.Verhamme FM, Bracke KR, Joos GF, Brusselle GG. Transforming growth factor-β superfamily in obstructive lung diseases: more suspects than TGF-β alone. Am J Respir Cell Mol Biol. 2015;52:653–662. doi: 10.1165/rcmb.2014-0282RT. [DOI] [PubMed] [Google Scholar]
- 87.Abe K, Sugiura H, Hashimoto Y, Ichikawa T, Koarai A, Yamada M, et al. Possible role of Krüppel-like factor 5 in the remodeling of small airways and pulmonary vessels in chronic obstructive pulmonary disease. Respir Res. 2016;17:7. doi: 10.1186/s12931-016-0322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gosselink JV, Hayashi S, Elliott WM, Xing L, Chan B, Yang L, et al. Differential expression of tissue repair genes in the pathogenesis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:1329–1335. doi: 10.1164/rccm.200812-1902OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Borchers MT, Wesselkamper SC, Curull V, Ramirez-Sarmiento A, Sánchez-Font A, Garcia-Aymerich J, et al. Sustained CTL activation by murine pulmonary epithelial cells promotes the development of COPD-like disease. J Clin Invest. 2009;119:636–649. doi: 10.1172/JCI34462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Freeman CM, Stolberg VR, Crudgington S, Martinez FJ, Han MK, Chensue SW, et al. Human CD56+ cytotoxic lung lymphocytes kill autologous lung cells in chronic obstructive pulmonary disease. PLoS One. 2014;9:e103840. doi: 10.1371/journal.pone.0103840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lo Cascio CM, Quante M, Hoffman EA, Bertoni AG, Aaron CP, Schwartz JE, et al. Percent emphysema and daily motor activity levels in the general population: Multi-Ethnic Study of Atherosclerosis. Chest. 2017;151:1039–1050. doi: 10.1016/j.chest.2016.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529:316–325. doi: 10.1038/nature17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cao Z, Lis R, Ginsberg M, Chavez D, Shido K, Rabbany SY, et al. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat Med. 2016;22:154–162. doi: 10.1038/nm.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Niewoehner DE, Kleinerman J, Rice DB. Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med. 1974;291:755–758. doi: 10.1056/NEJM197410102911503. [DOI] [PubMed] [Google Scholar]
- 95.Freeman CM, Martinez FJ, Han MK, Ames TM, Chensue SW, Todt JC, et al. Lung dendritic cell expression of maturation molecules increases with worsening chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:1179–1188. doi: 10.1164/rccm.200904-0552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Freeman CM, Curtis JL. Lung dendritic cells: shaping immune responses throughout chronic obstructive pulmonary disease progression. Am J Respir Cell Mol Biol. 2017;56:152–159. doi: 10.1165/rcmb.2016-0272TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13:567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 98.Freeman CM, McCubbrey AL, Crudgington S, Nelson J, Martinez FJ, Han MK, et al. Basal gene expression by lung CD4+ T cells in chronic obstructive pulmonary disease identifies independent molecular correlates of airflow obstruction and emphysema extent. PLoS One. 2014;9:e96421. doi: 10.1371/journal.pone.0096421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barceló B, Pons J, Ferrer JM, Sauleda J, Fuster A, Agustí AG. Phenotypic characterisation of T-lymphocytes in COPD: abnormal CD4+CD25+ regulatory T-lymphocyte response to tobacco smoking. Eur Respir J. 2008;31:555–562. doi: 10.1183/09031936.00010407. [DOI] [PubMed] [Google Scholar]
- 100.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 101.Hinks TS. Mucosal-associated invariant T cells in autoimmunity, immune-mediated diseases and airways disease. Immunology. 2016;148:1–12. doi: 10.1111/imm.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Falkowski NR, Huffnagle GB, et al. Bacterial topography of the healthy human lower respiratory tract. MBio. 2017;8:e02287–16. doi: 10.1128/mBio.02287-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Salter SJ, Cox MJ, Turek EM, Calus ST, Cookson WO, Moffatt MF, et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014;12:87. doi: 10.1186/s12915-014-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Beck JM, Huffnagle GB, et al. Spatial variation in the healthy human lung microbiome and the adapted island model of lung biogeography. Ann Am Thorac Soc. 2015;12:821–830. doi: 10.1513/AnnalsATS.201501-029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bassis CM, Erb-Downward JR, Dickson RP, Freeman CM, Schmidt TM, Young VB, et al. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. MBio. 2015;6:e00037. doi: 10.1128/mBio.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Venkataraman A, Bassis CM, Beck JM, Young VB, Curtis JL, Huffnagle GB, et al. Application of a neutral community model to assess structuring of the human lung microbiome. MBio. 2015;6:e02284–14. doi: 10.1128/mBio.02284-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL, et al. Lung HIV Microbiome Project. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med. 2013;187:1067–1075. doi: 10.1164/rccm.201210-1913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Segal LN, Clemente JC, Tsay JC, Koralov SB, Keller BC, Wu BG, et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat Microbiol. 2016;1:16031. doi: 10.1038/nmicrobiol.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grønseth R, Drengenes C, Wiker HG, Tangedal S, Xue Y, Husebø GR, et al. Protected sampling is preferable in bronchoscopic studies of the airway microbiome. ERJ Open Res. 2017;3:00019–2017. doi: 10.1183/23120541.00019-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilkinson TM, Patel IS, Wilks M, Donaldson GC, Wedzicha JA. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:1090–1095. doi: 10.1164/rccm.200210-1179OC. [DOI] [PubMed] [Google Scholar]
- 111.Bresser P, Out TA, van Alphen L, Jansen HM, Lutter R. Airway inflammation in nonobstructive and obstructive chronic bronchitis with chronic Haemophilus influenzae airway infection: comparison with noninfected patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:947–952. doi: 10.1164/ajrccm.162.3.9908103. [DOI] [PubMed] [Google Scholar]
- 112.Sethi S, Maloney J, Grove L, Wrona C, Berenson CS. Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:991–998. doi: 10.1164/rccm.200509-1525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Alphen L, Jansen HM, Dankert J. Virulence factors in the colonization and persistence of bacteria in the airways. Am J Respir Crit Care Med. 1995;151:2094–2099, discussion 2099–2100. doi: 10.1164/ajrccm.151.6.7767563. [DOI] [PubMed] [Google Scholar]
- 114.Foxwell AR, Kyd JM, Cripps AW. Nontypeable Haemophilus influenzae: pathogenesis and prevention. Microbiol Mol Biol Rev. 1998;62:294–308. doi: 10.1128/mmbr.62.2.294-308.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ha U, Lim JH, Jono H, Koga T, Srivastava A, Malley R, et al. A novel role for IkappaB kinase (IKK) alpha and IKKbeta in ERK-dependent up-regulation of MUC5AC mucin transcription by Streptococcus pneumoniae. J Immunol. 2007;178:1736–1747. doi: 10.4049/jimmunol.178.3.1736. [DOI] [PubMed] [Google Scholar]
- 116.Segal LN, Clemente JC, Wu BG, Wikoff WR, Gao Z, Li Y, et al. Randomised, double-blind, placebo-controlled trial with azithromycin selects for anti-inflammatory microbial metabolites in the emphysematous lung. Thorax. 2017;72:13–22. doi: 10.1136/thoraxjnl-2016-208599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vestbo J, Sørensen T, Lange P, Brix A, Torre P, Viskum K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 1999;353:1819–1823. doi: 10.1016/s0140-6736(98)10019-3. [DOI] [PubMed] [Google Scholar]
- 118.Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 119.Vestbo J, Leather D, Diar Bakerly N, New J, Gibson JM, McCorkindale S, et al. Salford Lung Study Investigators. Effectiveness of fluticasone furoate-vilanterol for COPD in clinical practice. N Engl J Med. 2016;375:1253–1260. doi: 10.1056/NEJMoa1608033. [DOI] [PubMed] [Google Scholar]
- 120.Vestbo J, Anderson JA, Brook RD, Calverley PM, Celli BR, Crim C, et al. SUMMIT Investigators. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387:1817–1826. doi: 10.1016/S0140-6736(16)30069-1. [DOI] [PubMed] [Google Scholar]
- 121.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: the Lung Health Study. JAMA. 1994;272:1497–1505. [PubMed] [Google Scholar]
- 122.Zhou Y, Zhong NS, Li X, Chen S, Zheng J, Zhao D, et al. Tiotropium in early-stage chronic obstructive pulmonary disease. N Engl J Med. 2017;377:923–935. doi: 10.1056/NEJMoa1700228. [DOI] [PubMed] [Google Scholar]
- 123.Calverley PMA, Anderson JA, Brook RD, Crim C, Gallot N, Kilbride S, et al. SUMMIT (Study to Understand Mortality and Morbidity) Investigators. Fluticasone furoate, vilanterol, and lung function decline in patients with moderate chronic obstructive pulmonary disease and heightened cardiovascular risk. Am J Respir Crit Care Med. 2018;197:47–55. doi: 10.1164/rccm.201610-2086OC. [DOI] [PubMed] [Google Scholar]
- 124.Yonchuk JG, Silverman EK, Bowler RP, Agustí A, Lomas DA, Miller BE, et al. Circulating soluble receptor for advanced glycation end products (sRAGE) as a biomarker of emphysema and the RAGE axis in the lung. Am J Respir Crit Care Med. 2015;192:785–792. doi: 10.1164/rccm.201501-0137PP. [DOI] [PubMed] [Google Scholar]
- 125.Zemans RL, Jacobson S, Keene J, Kechris K, Miller BE, Tal-Singer R, et al. Multiple biomarkers predict disease severity, progression and mortality in COPD. Respir Res. 2017;18:117. doi: 10.1186/s12931-017-0597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pillai SG, Kong X, Edwards LD, Cho MH, Anderson WH, Coxson HO, et al. ECLIPSE and ICGN Investigators. Loci identified by genome-wide association studies influence different disease-related phenotypes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:1498–1505. doi: 10.1164/rccm.201002-0151OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lawrence PJ, Kolsum U, Gupta V, Donaldson G, Singh R, Barker B, et al. Characteristics and longitudinal progression of chronic obstructive pulmonary disease in GOLD B patients. BMC Pulm Med. 2017;17:42. doi: 10.1186/s12890-017-0384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 129.Han MK, Quibrera PM, Carretta EE, Barr RG, Bleecker ER, Bowler RP, et al. SPIROMICS investigators. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5:619–626. doi: 10.1016/S2213-2600(17)30207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Donaldson GC, Müllerova H, Locantore N, Hurst JR, Calverley PMA, Vestbo J, et al. Factors associated with change in exacerbation frequency in COPD. Respir Res. 2013;14:79. doi: 10.1186/1465-9921-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bhatt SP, Han MK. Reply: FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195:1677. doi: 10.1164/rccm.201701-0127LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Elbehairy AF, Parraga G, Webb KA, Neder JA, O’Donnell DE Canadian Respiratory Research Network (CRRN) Mild chronic obstructive pulmonary disease: why spirometry is not sufficient! Expert Rev Respir Med. 2017;11:549–563. doi: 10.1080/17476348.2017.1334553. [DOI] [PubMed] [Google Scholar]
- 133.Dobbels F, de Jong C, Drost E, Elberse J, Feridou C, Jacobs L, et al. PROactive consortium. The PROactive innovative conceptual framework on physical activity. Eur Respir J. 2014;44:1223–1233. doi: 10.1183/09031936.00004814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Singh D, Maleki-Yazdi MR, Tombs L, Iqbal A, Fahy WA, Naya I. Prevention of clinically important deteriorations in COPD with umeclidinium/vilanterol. Int J Chron Obstruct Pulmon Dis. 2016;11:1413–1424. doi: 10.2147/COPD.S101612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Taylor JT, Poludniowski G, Price T, Waltham C, Allport PP, Casse GL, et al. An experimental demonstration of a new type of proton computed tomography using a novel silicon tracking detector. Med Phys. 2016;43:6129. doi: 10.1118/1.4965809. [DOI] [PubMed] [Google Scholar]