To the Editor:
The T2R38 (taste receptor 2 member 38) bitter taste receptor on respiratory epithelia detects Pseudomonas aeruginosa N-acyl-l-homoserine lactones (AHLs). In vitro, T2R38 activation by AHLs initiates calcium-mediated increases in nitric oxide production and ciliary beat frequency, dependent on polymorphisms in the TAS2R38 gene (1). In patients with chronic rhinosinusitis, the TAS2R38 genotype is proposed to modify mucosal responses to P. aeruginosa (1).
Polymorphisms in the TAS2R38 gene result in two high-frequency haplotypes associated with taste perception of the bitter compound phenylthiocarbamide (2). The “taster” haplotype codes proline-alanine-valine (PAV), and the “nontaster” haplotype codes alanine-valine-isoleucine (AVI) at positions 49, 262, and 296 in the receptor protein. Responses to AHLs in vitro are greatest in PAV/PAV epithelial cells, and this genotype is reported to be protective against P. aeruginosa in the sinonasal airway (1).
P. aeruginosa is the most frequently isolated respiratory pathogen in cystic fibrosis (CF), and chronic infection is associated with accelerated rates of disease progression. Determining the impact of TAS2R38 polymorphisms on P. aeruginosa infection in CF could have implications for patient risk stratification and, as naturally occurring and synthetic agonists to T2R38 are already in clinical use (3), could identify promising therapeutic targets.
We characterized T2R38 localization in the CF airway and investigated the hypothesis that TAS2R38 polymorphisms would modify the prevalence and impact of P. aeruginosa infection in CF. Some of the results of these studies have previously been reported in the form of abstracts (4, 5).
Methods
Nasal and/or bronchial brushings were obtained from four children with CF undergoing bronchoscopy and four healthy adult controls. T2R38 localization was evaluated by immunocytochemistry with antibodies to T2R38 and ciliary proteins as described previously (6). Slides were imaged with a Zeiss LSM-510 confocal microscope, and colocalization was quantified using the JACoP plug-in for ImageJ (7).
DNA was extracted from blood from 271 subjects with CF (>6 yr old) and subjected to PCR for the common TAS2R38 polymorphisms rs713598, rs1726866, and rs10246939. P. aeruginosa infection status was categorized in patients with three or more respiratory cultures during 2014, according to Leeds criteria (8), as chronic (>50% positive), intermittent (≤50% positive), free (previous P. aeruginosa but none for >12 mo), or never. Clinical data were obtained from each patient’s 2014 annual assessment.
Cryopreserved P. aeruginosa isolates from TAS2R38-genotyped patients (matched for age and FEV1) were revived in Luria-Bertani broth in triplicate and filter sterilized. Quantitative analysis of N-butanoyl-l-homoserine lactone (C4-HSL) and N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12-HSL) was performed by liquid chromatography with tandem mass spectrometry. The limits of detection and limits of quantification were defined as signal:noise ratios of 3:1 and 10:1, respectively, as previously described (9).
Power calculations predicted that 250 patients would provide 80% power to detect a difference in chronic P. aeruginosa infection of ≥20% in PAV/PAV compared with other genotypes at an α of 5%. P. aeruginosa infection by TAS2R38 genotype was analyzed by chi-squared analysis and logistic regression. Graphpad Prism 7 and SPSS 23 were used, and the null hypothesis was rejected at P < 0.05.
Ethics review committees approved the protocol (02-019 and 10/H0504/9), and written consent was obtained from the subjects or their parent/guardian.
Results
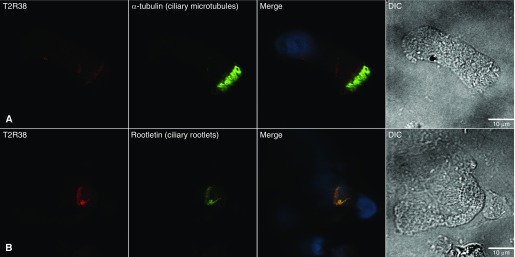
T2R38 immunostaining was present in all nasal (n = 3) and bronchial (n = 3) samples from patients with CF, and in all nasal samples (n = 4) from healthy controls. T2R38 stained proximally to acetylated α-tubulin (ciliary microtubules) and γ-tubulin (ciliary basal bodies), and colocalized with rootletin (ciliary rootlets) in CF and control cells (Figure 1). The thresholded Manders’ correlation coefficients (mean ± SD of four cells) for T2R38 and rootletin were 0.91 ± 0.07, 0.90 ± 0.08, and 0.90 ± 0.04 for control nasal, CF nasal, and CF bronchial cells, respectively, indicating that ≥90% of green (rootletin) pixels were positive for red (T2R38).
Figure 1.
Confocal microscopy images of nasal epithelial cells from a subject with cystic fibrosis. (A and B) Cells were stained with antibodies to T2R38 (red) and acetylated α-tubulin (A) or rootletin (B) (both stained green). Nuclei were stained with DAPI (blue). Colocalized antibodies appear yellow in the merged images. Epithelial cell morphology is shown by differential interference contrast (DIC) images. T2R38 stains proximally to acetylated α-tubulin (ciliary microtubules) and colocalizes with rootletin (ciliary rootlets). The antibodies used in these immunocytochemistry assays were T2R38 (AB130503, Abcam), acetylated α-tubulin (T6793, Sigma), and rootletin (SC-374056, Santa Cruz Biotechnology).
Of 271 patients with CF, 225 had the common AVI/AVI (n = 74), AVI/PAV (n = 110), or PAV/PAV (n = 41) genotypes and three or more respiratory cultures during 2014. Between TAS2R38 genotype groups there was no significant difference in median age, sex, or proportion of p.Phe508del CFTR (cystic fibrosis transmembrane conductance regulator) mutations. There was no association between TAS2R38 genotype and P. aeruginosa infection status (P = 0.46; Table 1). In the logistic regression model with “intermittent and chronic” and “never and free” groups as dependent variables, and age, sex, CFTR genotype, and TAS2R38 genotype as independent variables, only age was associated with intermittent or chronic P. aeruginosa infection (odds ratio, 1.05; 95% confidence interval, 1.03–1.07). There was no association between TAS2R38 genotype and P. aeruginosa infection status when the PAV/PAV genotype was compared against the AVI/AVI or AVI/PAV genotype.
Table 1.
Pseudomonas aeruginosa Infection Category by TAS2R38 Genotype
| AVI/AVI (n = 74) | AVI/PAV (n = 110) | PAV/PAV (n = 41) | |
|---|---|---|---|
| Never, n (%) | 4 (5) | 4 (4) | 3 (7) |
| Free, n (%) | 21 (28) | 36 (32) | 16 (39) |
| Intermittent, n (%) | 11 (15) | 26 (24) | 8 (20) |
| Chronic, n (%) | 38 (51) | 44 (40) | 14 (34) |
Definition of abbreviations: AVI = alanine-valine-isoleucine; PAV = proline-alanine-valine.
Among patients with intermittent or chronic P. aeruginosa infection (n = 141), there was no difference by TAS2R38 genotype in median FEV1% predicted (AVI/AVI, 54.0%; AVI/PAV, 62.0%; PAV/PAV, 53.5%; P = 0.3) or in the proportion of patients from whom mucoid P. aeruginosa was isolated (AVI/AVI, 69%; AVI/PAV, 60%; PAV/PAV, 68%; P = 0.5). In 18 P. aeruginosa isolates from TAS2R38-genotyped patients, there was no difference by genotype in the proportion of isolates in which C4-HSL or 3-oxo-C12-HSL was below the limit of quantification (P = 0.8).
Discussion
We have identified T2R38 in CF nasal and bronchial epithelium, where it localizes to the ciliary rootlet with the same distribution as in non-CF epithelia. Previous studies reported T2R38 localization ranging from the ciliary tip (10) to below the ciliary base (1, 11). Our experiments demonstrate that in fresh, noncultured cells, T2R38 colocalizes with rootletin, a structural component of the ciliary rootlet, originating from the ciliary basal body and extending toward the nucleus (12).
In this study of 225 children and adults with CF, we found no association between the TAS2R38 genotype and P. aeruginosa infection status within the range of differences that our study was powered to detect. Our results show that only age was associated with intermittent or chronic infection, consistent with CF registry data (13). Among patients with intermittent or chronic infection, the lack of any difference in spirometry or prevalence of mucoid P. aeruginosa further supports the lack of a protective effect of the PAV/PAV genotype. Finally, in a small sample of clinical isolates, we observed no relationship between the TAS2R38 genotype and AHL profiles, suggesting that polymorphisms in this receptor do not exert a selective pressure on P. aeruginosa in the CF lung.
Our results indicate that TAS2R38-related differences in sinonasal immunity do not translate to clinically relevant changes in the CF airway, where mucociliary clearance is significantly impaired. We suggest that TAS2R38 genotyping has no prognostic value in patients with CF, nor do our findings indicate that the T2R38 receptor is a promising drug target for CF mucosal immunity.
Footnotes
Supported by the National Institute for Health Research Respiratory Disease Biomedical Research Unit at the Royal Brompton & Harefield NHS Foundation Trust and Imperial College London. The Pseudomonas aeruginosa clinical isolate repository was established as part of the Strategic Research Centre for Pseudomonas Infection in Cystic Fibrosis, funded by the Cystic Fibrosis Trust (UK). The Facility for Imaging by Light Microscopy at Imperial College London is partially supported by funding from the Wellcome Trust (grant 104931/Z/14/Z) and the Biotechnology and Biological Sciences Research Council (grant BB/L015129/1).
Author Contributions: Conception and design: A.R.T., A. Shoemark, and J.C.D.; data collection: A.R.T., A. Shoemark, V.B., R.M., H.L.-P., A. Simbo, and M.M.; analysis and interpretation: A.R.T., A. Shoemark, V.B., and J.C.D.; drafting of the manuscript: A.R.T., A. Shoemark, A.B., E.W.F.W.A., and J.C.D.; and editing and approval of the manuscript: all authors.
Originally Published in Press as DOI: 10.1164/rccm.201711-2365LE on February 26, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Lee RJ, Xiong G, Kofonow JM, Chen B, Lysenko A, Jiang P, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012;122:4145–4159. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221–1225. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- 3.Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, et al. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 2010;35:157–170. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- 4.Turnbull A, Lund-Palau H, Murphy R, Simbo A, Wong K, Bush A, et al. The T2R38 bitter taste receptor as a modifier of host response to Pseudomonas aeruginosa in cystic fibrosis: does T2R38 genotype impact on clinical infection? [abstract] Pediatr Pulmonol. 2016;51:323. [Google Scholar]
- 5.Turnbull A, Shoemark A, Lund-Palau H, Murphy RA, Simbo A, Bush A, et al. Determining the impact of the T2R38 bitter taste receptor on P. aeruginosa infection in the cystic fibrosis airway [abstract] J Cyst Fibros. 2017;16:S88. [Google Scholar]
- 6.Shoemark A, Frost E, Dixon M, Ollosson S, Kilpin K, Patel M, et al. Accuracy of immunofluorescence in the diagnosis of primary ciliary dyskinesia. Am J Respir Crit Care Med. 2017;196:94–101. doi: 10.1164/rccm.201607-1351OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolte S, Cordelières FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee TWR, Brownlee KG, Conway SP, Denton M, Littlewood JM. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros. 2003;2:29–34. doi: 10.1016/S1569-1993(02)00141-8. [DOI] [PubMed] [Google Scholar]
- 9.Ortori CA, Dubern JF, Chhabra SR, Cámara M, Hardie K, Williams P, et al. Simultaneous quantitative profiling of N-acyl-L-homoserine lactone and 2-alkyl-4(1H)-quinolone families of quorum-sensing signaling molecules using LC-MS/MS. Anal Bioanal Chem. 2011;399:839–850. doi: 10.1007/s00216-010-4341-0. [DOI] [PubMed] [Google Scholar]
- 10.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan CH, Hahn S, McMahon D, Bonislawski D, Kennedy DW, Adappa ND, et al. Nitric oxide production is stimulated by bitter taste receptors ubiquitously expressed in the sinonasal cavity. Am J Rhinol Allergy. 2017;31:85–92. doi: 10.2500/ajra.2017.31.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Liu X, Yue G, Adamian M, Bulgakov O, Li T. Rootletin, a novel coiled-coil protein, is a structural component of the ciliary rootlet. J Cell Biol. 2002;159:431–440. doi: 10.1083/jcb.200207153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerem E, Viviani L, Zolin A, MacNeill S, Hatziagorou E, Ellemunter H, et al. ECFS Patient Registry Steering Group. Factors associated with FEV1 decline in cystic fibrosis: analysis of the ECFS patient registry. Eur Respir J. 2014;43:125–133. doi: 10.1183/09031936.00166412. [DOI] [PubMed] [Google Scholar]