Abstract
Octamer-binding transcription factor 4 (OCT4) is a transcription factor with a well-defined role in stem cell pluripotency. Two OCT4 isoforms, OCT4A and OCT4B, tend to be downregulated as normal cells differentiate. However, OCT4, particularly OCT4B, may become reactivated in cancer cells. Despite this observation, the exact function of OCT4B re-expression in cancer is unclear. In the present study, the role of OCT4 in breast cancer cells was determined. In particular, the ability of OCT4 to regulate key genes involved in cellular proliferation and apoptosis, two pathways that are frequently deregulated in cancer, was examined. The cyclin-dependent kinase inhibitor 2A locus encodes p16INK4a and p14ARF, two important cell cycle inhibitors. The tumor suppressor p53 also has well characterized roles in suppressing proliferation and promoting apoptosis. The present study demonstrated, via overexpression and genetic knockdown techniques, that OCT4B regulates the expression of several of these genes and ultimately regulates the rate of apoptosis of MCF-7 breast cancer cells. It was also observed that, while OCT4B and OCT4A regulate one another, it is OCT4B that serves a more prominent role in regulating the transcription of downstream genes. Taken together, the present results suggest that OCT4B is re-expressed in a number of breast cancer cell lines, where it affects both the transcription of cell cycle genes and the rate of apoptosis. These properties of OCT4B may depend on, at least in part, the co-function of OCT4A.
Keywords: apoptosis, breast cancer cells, cell cycle, OCT4
Introduction
Although stem cells comprise only a very small percentage of a tumor, they have a critical role in promoting disease pathogenesis (1). This is largely due to their self-renewal capabilities, which allow these cells to survive for a long period of time, during which, they may acquire mutations that confer a growth advantage (2). Cancer stem cells have also been shown to be associated with therapeutic resistance (3). Genes involved in stem cell biology during normal development, such as the transcription factor octamer-binding transcription factor 4 (OCT4), may also contribute to tumorigenesis (4).
OCT4 is a homeodomain transcription factor with an essential role in embryonic stem (ES) cell self-renewal (5,6). This was shown by genetic ablation of the OCT4 gene, since the loss of OCT4 suppressed a large number of the stem characteristics of an ES cell line (7). OCT4 has also been detected in adult cells possessing stem cell characteristics, such as mammary stem cells (8). In addition to its role in maintaining stem cell pluripotency, a role for OCT4 in cancer stem cells is also emerging (4). While the role of OCT4 in cancer is unknown, a previous study identified high expression of OCT4 in cluster of differentiation (CD)44+/CD24-breast cancer stem cells (4). Importantly, the authors determined that OCT4 was more highly expressed in tumor tissue than in normal adjacent tissue, and that individuals with high OCT4 expression had worse disease-free survival than those with low expression (4). The human OCT4 gene can generate three transcript variants (OCT4A, OCT4B and OCT4B1) and four protein isoforms via a combination of alternative splicing and use of multiple translation initiation sites (9,10). While, in ES cells, self-renewal is conferred by OCT4A, OCT4B appears to serve a role in responding to cellular stress (11–14). However, the exact roles of each of these OCT4 variants in normal physiology and in cancer remain unclear.
The deregulation of cell cycle progression and apoptosis may contribute to tumorigenesis. Two important cell cycle inhibitors, p16INK4a and p14ARF, are encoded by the cyclin-dependent kinase inhibitor 2A (CDKN2A) (INK4a/ARF) locus (15). p16INK4a regulates retinoblastoma protein-dependent G1 arrest (16). By contrast, p14ARF can be activated by oncogenic and hyperproliferative stimuli (including Myc, Ras and E1A), resulting in activation of the p53-p21 pathway and causing p53-dependent G1 cell cycle arrest (17–19). p53 is a tumor-suppressor protein with essential roles in apoptosis and cell cycle arrest (20). Activated p53 mediates apoptosis via regulating the expression of B-cell lymphoma (Bcl)-2 gene family members or by directly binding to anti-apoptotic Bcl-2 family proteins at the mitochondrial surface, thereby activating Bcl-2 associated X protein (Bax) and initiating the apoptosis cascade (21,22). Although numerous studies concerning p16INK4a, p14ARF and p53 have been published, little is known about the genes and networks that co-regulate the p16INK4a and p53 pathways.
Recent studies have started to elucidate the association between OCT4 and pathways regulating cell proliferation and apoptosis (23–25). These associations are important, since they aid to strengthen the link between OCT4 and cancer. One group recently demonstrated that OCT4B is upregulated by p53 under genotoxic stress, functioning in the stress response in ES cells (14). Another group recently published that OCT4 suppresses p53 expression (26). This raises the possibility that, in certain cellular contexts, these factors may be part of a negative regulatory loop. Despite these facts, the precise nature of the association between these proteins remains unclear.
Previous studies by the present authors confirmed the presence of OCT4B expression in MCF-7 cells, and it was speculated that the transcription of this gene in this cancer cell line may be driven by an independent promoter (27). In the present study, we build upon this initial finding by showing that OCT4B is widely expressed across a panel of human breast cancer cell lines. It was also observed that OCT4 regulates the expression of factors such as p53 and p16, which control essential cell regulatory pathways. Finally, the reciprocal regulation of OCT4A and OCT4B in cancer cells was explored. In conclusion, the present data will aid to elucidate the role of OCT4 in the regulation of proliferation and apoptosis of breast cancer cells. These findings could be clinically important for patients with breast tumors in which OCT4 becomes re-expressed.
Materials and methods
Cell culture
The human breast adenocarcinoma cell lines MCF-7, MDA-MB-231 (mm231) and MDA-MB-468 (mm468) were cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal calf serum (FCS; HyClone; GE Healthcare Life Sciences, Logan, UT, USA), 100 U/ml of penicillin and 100 µg/ml of streptomycin solution (HyClone; GE Healthcare Life Sciences) at 37°C and under 5% CO2. The T-47D (human ductal breast epithelial tumor cell line) cell line was cultured in RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FCS (HyClone; GE Healthcare Life Sciences), 100 U/ml of penicillin and 100 µg/ml of streptomycin solution (HyClone; GE Healthcare Life Sciences). All the cell lines were purchased from Peking Union Medical College (Beijing, China). Similar cell densities were maintained across different experiments. All experiments and protocols were reviewed by and approved by the Institutional Review Board of Peking Union Medical College.
Construction of overexpression vector and RNA interference (RNAi) vector
The open reading frames (ORFs) of OCT4A and OCT4B were amplified from the messenger RNA (mRNA) of MCF-7 cells, and were subcloned into the pDsRed-ex-C1 vector [containing Discosoma sp. red fluorescent protein (DsRed); Clontech Laboratories, Inc., Mountainview, CA, USA] or the pEGFP-N1 vector [containing enhanced green fluorescent protein (EGFP); Clontech Laboratories, Inc.] to generate the fusion expression vectors pDsRed-ex-C1-OCT4A and pEGFP-N1-OCT4B, respectively (which are referred to as pC1-4A and pN1-4B, respectively, throughout the present manuscript). An additional two OCT4 vectors lacking fluorescent protein fusions were also generated. For both OCT4A and OCT4B, these were generated by inserting the complementary DNAs (cDNAs) into pEGFP-N1, thus deleting EGFP. The resulting constructs were named pN1-4As and pN1-4Bs for OCT4A and OCT4B, respectively. The sequences of the primers used for subcloning the OCT4A ORF with a mutation at the stop codon were 5′-ACGTAAGCTTATGGCGGGACACCTGGCTTC-3′ (forward) and 5′-TTTTGAATTCCGGGCAGGCACCACAGTTT-3′ (reverse). The primers for subcloning the OCT4B ORF were 5′-ACTGAAGCTTATGCACTTCTACAGAC-3′ (forward) and 5′-TTTTGAATTCCGGGCAGGCACCACAGTTT-3′ (reverse). The primers used for OCT4A ORF with a stop codon were 5′-ACGTAAGCTTATGGCGGGACACCTGGCTTC-3′ (forward) and 5′-TTTTGAATTCCGGGCAGGCACCTCAGTTT-3′ (reverse). The primers for OCT4B ORF with a stop codon were 5′-ACTGAAGCTTATGCACTTCTACAGAC-3′ (forward) and 5′-TTTTGAATTCCGGGCAGGCACCTCAGTTT-3′ (reverse). RNAi expression vectors were generated by cloning small hairpin RNA (shRNA) oligonucleotides into the BbsI site of the pFIV-h1/U6-copGFP vector (Clontech Laboratories, Inc.). The sequences of the oligonucleotides used were 5′-AAAGAAGAGAAAGCGAACCAGTATTCCAGTCTATACTGGTTCGCTTTCTCT-3′ (sense) and 5′-AAAAAGAGAAAGCGAACCAGTATTAGCGATTATACTGGTTCGCTTTCTCTT-3′ (antisense). The sequence targets a common exon (exon 5) of OCT4A and OCT4B.
Transient expression and RNAi
Transfection of shRNA vectors was performed with VigoFect (Vigorous, Beijing, China), according to the manufacturer's recommendations. Cells grown to 50–60% confluence in 24-well plates were transfected with 4 µg of the small interfering RNA plasmid pFIV-4B. After 24 h, the cells were harvested for isolation of RNA and protein, as well as for flow cytometry analysis.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was prepared from cultured cells using the RNeasy Mini kit (Qiagen GmbH, Hilden, Germany) with on-column DNase (Promega Corporation, Madison, WI, USA) treatment. Next, cDNA was synthesized using the SuperScript® First-Strand Synthesis System (Promega Corporation). RT was performed in a 20-µl sample volume containing 2 µg RNA. The reaction included 60 min of RT at 42°C and 15 min of inactivation at 70°C. Negative controls were performed without reverse transcriptase. Amplification was performed in a 25-µl sample volume according to a standard procedure. The primer sets used in the experiment were as follows: OCT4A, 5′-ATGGCGGGACACCTGGCTTC-3′ (forward) and 5′-AAGGGCAGGCACCTCAGTTT-3′ (reverse); OCT4B, 5′-ATGCACTTCTACAGACTATTCCTTGGGGCC-3′ (forward) and 5′-AAGGGCAGGCACCTCAGTTT-3′ (reverse); p53, 5′-TCAGATCCGTGGGCGTGA-3′ (forward) and 5′-GTCAGTGGGGAACAAGAAG-3′ (reverse); p21, 5′-ACCATGTGGACCTGTCACTGTCTTG-3′ (forward) and 5′-AGGCTTCCTGTGGGCGGATTAG-3′ (reverse); p14ARF, 5′-TTTTCGTGGTTCACATCCC-3′ (forward) and 5′-TCTAAGTTTCCCGAGGTTTCT-3′ (reverse); p16INK4a, 5′-TGCCCAACGCACCGAATAGTTA-3′ (forward) and 5′-GTGCAGCACCACCAGCGTGTCC-3′ (reverse); Bcl-2, 5′-AGATTGATGGGATCGTTGCCTTAT-3′ (forward) and 5′-CCAATTCCTTTCGGATCTTTATTTC-3′ (reverse); Bax, 5′-GGGAGCGGCGGTGATGGA-3′ (forward) and 5′-GCAAAAGGGCCCCTGTCTTC-3′ (reverse); and β-actin, 5′-TCATCACTATTGGCAACGAGC-3′ (forward) and 5′-AACAGTCCGCCTAGAAGCAC-3 (reverse). PCR products were visualized and semiquantified by agarose gel electrophoresis and densitometry.
Immunocytochemistry detection of OCT4
Human breast cancer cells were cultured on coverslips and grown to 80% confluence. Cells were fixed in 4% paraformaldehyde for 30 min at room temperature and then incubated with 0.3% Triton X-100 in PBS at 4°C for 20 min. After blocking with 10% FCS in PBS, cells were incubated with an anti-OCT4 antibody that recognizes both OCT4A and OCT4B (1:50 dilution; sc-8629; Santa Cruz Biotechnology Inc., Dallas, TX, USA) for 1 h at room temperature. Fluorescein isothiocyanate (FITC)-conjugated anti-goat immunoglobulin G (1:100 dilution; sc-2489; Santa Cruz Biotechnology Inc.) was used as a secondary antibody. Propidium iodide (PI) staining of the nucleus was performed at room temperature for 5 min. Fluorescent images were photographed using a confocal laser scanning microscope (OLS3000; Nikon Corporation, Tokyo, Japan).
Western blotting
Western blotting followed by chemiluminescence detection was performed by a conventional method. A polyclonal antibody (ab19857; Abcam, Cambridge, UK) was used to detect the C-termini of both OCT4A and OCT4B. Detection of GAPDH with an anti-GAPDH antibody (1:200 dilution; 2251-1; Epitomics, Burlingame, CA, USA) served as a loading control.
RT-quantitative PCR (RT-qPCR)
RT-q PCR for quantification of cDNA was performed using SYBR Green II PCR Master Mix (DRR081S; Takara Biotechnology Co., Ltd., Dalian, China) and the ABI 7500 Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. RT-qPCR was performed in triplicate. The cycling conditions were 40 cycles of 95°C for 5 sec, 55°C for 10 sec and 72°C for 10 sec. The primer sets used in the study were as follows: OCT4B, 5′-CAGGGAATGGGTGAATGAC-3′ (forward) and 5′-CGTGTGGCCCCAAGGA-3′ (reverse); p53, 5′-TGCTTGCAATAGGTGTGCGTCA-3′ (forward) and 5′-AAACACCAGTGCAGGCCAACTT-3′ (reverse); p21, 5′-ATGTGTCCTGGTTCCCGTTTCT-3′ (forward) and 5′-TTGTGGGAGGAGCTGTGAAAGA-3′ (reverse); Bcl-2, 5′-TAATTGCCAAGCACCGCTTCGT-3′ (forward) and 5′-TGATCCGGCCAACAACATGGAA-3′ (reverse); p16INK4a, 5′-ACCCCGCTTTCGTAGTTTTC-3′ (forward) and 5′-GCAGAAGCGGTGTTTTTCTT-3′ (reverse); p14ARF, 5′-AGAACATGGTGCGCAGGTTCTTG-3′ (forward) and 5′-TAGACGCTGGCTCCTCAGTAGCAT-3′ (reverse); Bax, 5′-GGGAGCGGCGGTGATGGA-3′ (forward) and 5′-GCAAAAGGGCCCCTGTCTTC-3′ (reverse); and GAPDH, 5′-TCAAGAAGGTGGTGAAGCA-3′ (forward) and 5′-AAAGGTGGAGGAGTGGGT-3′ (reverse). Relative gene expression levels were quantified using the 2−ΔΔCq method (28).
Analysis of cell apoptosis
For each sample, ~105 cells were prepared for the apoptosis assay. An Annexin V-FITC and PI double staining kit (Vigorous) was used according to the manufacturer's protocol. Stained cells were observed using a confocal laser scanning microscope (OLS3000; Nikon Corporation) and analyzed by flow cytometry (FACSCalibur; BD Biosciences, San Jose, CA, USA).
Statistical analysis
Data were presented as means ± standard deviation, and statistical comparisons between data groups were conducted using the Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
OCT4 is expressed in a panel of breast cancer cell lines
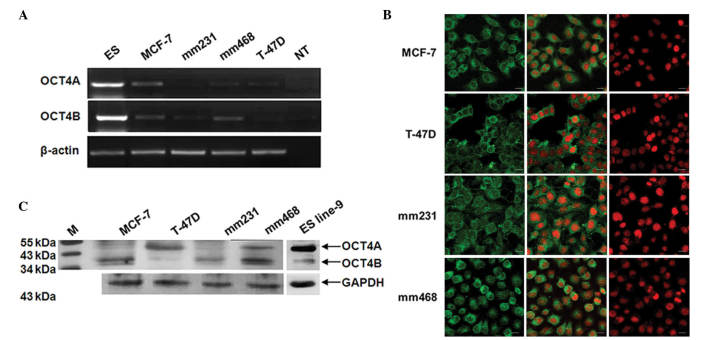
OCT4 gene expression was examined in a panel of four breast cancer cells, including two estrogen receptor (ER)-positive lines (MCF-7 and T-47D) and two ER-negative lines [MDA-MB-231 (mm231) and MDA-MB-468 (mm468)](Fig. 1). Semiquantitative RT-PCR was performed using primers capable of distinguishing OCT4A from OCT4B. PCR for each OCT4 variant produced a single band, which was then verified by cloning and sequencing. While transcripts for both OCT4A and OCT4B were detected in each breast cancer cell line, their levels of expression were significantly lower than their levels in ES cells (Fig. 1A). It should be noted that the transcript levels of OCT4A and OCT4B fluctuated with differences in cell density.
Figure 1.
Detection of endogenous OCT4 expression in human breast cancer cells. (A) Reverse transcription-polymerase chain reaction detection of OCT4 mRNA. The full open reading frames of both OCT4A and OCT4B mRNA were detected in all four breast cancer cell lines; comparisons were made against human ES cells. (B) Immunocytochemistry assay for OCT4 expression. Strong cytoplasmic staining (green) was observed in all four breast cancer cell lines. Nuclei were stained with propidium iodide (red). Scale bars, 20 µm. (C) Western blot analysis of OCT4 protein. OCT4B (35 kDa) was detected in all four breast cancer cell lines. OCT4A (45 kDa) was clearly detected in mm468 and T-47D cells. A total of 20 µg of protein from each cell extract was analyzed. GAPDH was used as loading control. ES, embryonic stem; NT, non-tumor; M, marker; OCT4, octamer-binding transcription factor 4; mRNA, messenger RNA.
Next, the protein expression of OCT4A and OCT4B was examined in breast cancer cell lines by western blotting and immunocytochemistry. By western blot analysis, it was observed that MCF-7 and mm231 cells express very low levels of OCT4A (45 kDa), and that the expression of OCT4A was higher in T-47D and mm468 cells (Fig. 1B). Differences in OCT4B (35 kDa) expression were more apparent among the four cell lines, with mm468 showing the highest level of OCT4B expression and T-47D showing the lowest (Fig. 1B). Immunocytochemistry for OCT4 was next performed using an antibody that fails to distinguish OCT4A from OCT4B. Prominent cytoplasmic staining of OCT4 was observed in each of the four breast cancer cell lines (Fig. 1C). Taken together, our data show that OCT4 is commonly re-expressed in multiple breast cancer cell lines and is predominantly located in the cytoplasm.
OCT4 regulates p53 and p16INK4a/p14ARF gene transcription
The possible role of OCT4 in breast cancer cells was examined by assessing its ability to influence the transcription of genes downstream of the p53 and p16 pathways. Since the MCF-7 and mm468 cell lines expressed the highest levels of OCT4B, these were selected for further study. Each of these two cell lines was transfected with OCT4-RNAi, which efficiently depleted the mRNA levels of OCT4 in both cell lines (Fig. 2A and B).
Figure 2.
OCT4 regulation of cell cycle and apoptotic genes. OCT4 expression was depleted in breast cancer cell lines via transfection of small hairpin RNA vectors. Following knockdown, gene expression changes were analyzed by reverse transcription-quantitative polymerase chain reaction. (A) Expression analysis of genes in MCF-7 cells. OCT4 was shown to negatively regulate the tumor-suppressor genes Bax, p14ARF and p16INK4a, and to induce p53 and the oncogene Bcl-2. Of note, Bax and Bcl-2 belong to the Bcl family of proteins. (B) Expression analysis of genes in mm468 cells. The expression changes in p53 and p16INK4a were similar to those observed in MCF-7 cells. Of note, cyclin-dependent kinase inhibitor 2A codes for two proteins: p16 (or p16INK4a) and p14ARF. Error bars represent the standard deviation of three replicates. *P<0.05. **P<0.01. OCT4, octamer-binding transcription factor 4; Bcl, B-cell lymphoma; Bax, Bcl-2-associated X protein.
Transcript levels of the cell cycle inhibitor genes p16INK4a and p14ARF were significantly increased in OCT4-RNAi cells compared with control cells transfected with empty pFIV vector (Fig. 2A). These results indicate that OCT4 modulates p16INK4a and p14ARF transcription, and may regulate cell cycle progression in MCF-7 cells.
Following OCT4 knockdown, mRNA expression of the anti-apoptotic gene Bcl-2 was significantly downregulated, while mRNA expression of the pro-apoptotic gene Bax was upregulated. These results indicate that OCT4B can influence the Bcl-2/Bax ratio and may regulate cell apoptosis.
Activated p53 is important in promoting apoptosis via its negative regulation of Bcl-2 and positive regulation of Bax (21,29). However, the results from our OCT4-RNAi cell experiments revealed that p53 was positively regulated by OCT4 (Fig. 2). Additionally, expression of p21, a downstream target gene of p53 functioning in cell cycle arrest (18), was unchanged following OCT4B interference.
The RT-qPCR results in MCF-7 cells (ER-positive), together with the results from flow cytometry (Fig. 3), suggest that OCT4 may induce p53-independent apoptosis. Furthermore, the similar transcriptional changes induced by OCT4 in both the ER-negative mammary epithelial cancer cell line mm468 (Fig. 2B) and the ER-positive line MCF-7 (Fig. 2A) suggest that OCT4 is a likely regulator of apoptosis in multiple breast cancer cell lines regardless of their ER status.
Figure 3.

OCT4 knockdown increases apoptosis in MCF-7 cells. Apoptosis analysis of MCF-7 cells by flow cytometry using Annexin V-fluorescein isothiocyanate and PI double staining following OCT4 knockdown. Apoptotic data were calculated as the percentage change compared with the control. Error bars represent the standard deviation of three replicates. *P<0.05. **P<0.01. LL, normal, live cells without staining; UL, dead cells with PI staining; UR, late apoptotic cells with both Annexin V and PI staining; LR, early apoptotic cells with Annexin V staining; OCT4, octamer-binding transcription factor 4; PI, propidium iodide.
OCT4 inhibits apoptosis in MCF-7 cells
The current study next explored whether OCT4 regulates cell cycle progression or apoptosis in MCF-7 cells. To do so, MCF-7 cells were transfected with OCT4 shRNA and then analyzed by flow cytometry. Transfection of empty vector served as control. Although no significant change in cell cycle distribution following OCT4 knockdown was observed (data not shown), a significant change in the rate of apoptosis was noticed. Cells transfected with OCT4 RNAi exhibited a greater degree of apoptosis (35.2%) than control cells (Fig. 3 and Table I). Notably, it was possible to distinguish early vs. late apoptotic cells in our analysis. These results suggest that endogenous OCT4 may be involved in preventing apoptosis in MCF-7 cells.
Table I.
Apoptosis analysis of MCF-7 cells by flow cytometry following OCT4 knockdown.
| Cells | MCF-7 | pFIV | OCT4 |
|---|---|---|---|
| UR (%) | 0.1 | 28.1 | 48.9 |
| LR (%) | 0.1 | 7.3 | 21.7 |
| Apoptosis (%) | 0.2 | 35.4 | 70.6 |
UR, late apoptotic cells with both Annexin V and propidium iodide staining; LR, early apoptotic cells with Annexin V staining; OCT4, octamer-binding transcription factor 4.
OCT4B and OCT4A regulate each other, but OCT4A is not involved in OCT4B function
The observation that a large number of the breast cancer cell lines examined in the present study, including MCF-7, express both OCT4A and OCT4B raises the possibility that these two OCT4 variants may cooperate at some level to regulate target gene expression, cell proliferation and apoptosis. To examine this possibility, overexpression vectors for both OCT4A and OCT4B were constructed. It was observed that OCT4B overexpression increased endogenous transcription of OCT4A; similarly, overexpression of OCT4A increased endogenous transcription of OCT4B (Fig. 4). Despite this mutual regulation, overexpression of each OCT4 variant had different effects on the expression of p16INK4a and p53 pathway genes. While overexpression of OCT4B significantly altered the levels of p53, p14ARF, p16INK4a, Bcl-2 and Bax, overexpression of OCT4A was unable to do so (Fig. 4C). These findings suggest that OCT4B may serve a dominant role compared with OCT4A, or that OCT4B may function via an independent pathway to regulate genes involved in cell cycle progression and apoptosis in MCF-7 cells.
Figure 4.
Comparison of OCT4B and OCT4A in the regulation of gene expression. (A) Reverse transcription-qPCR analysis of the mutual regulation between OCT4B and OCT4A in MCF-7 cells. Overexpression of OCT4A led to increased expression of OCT4B and vice versa. (B) Western blot analysis of the mutual regulation between OCT4B and OCT4A in MCF-7 cells. (C) qPCR transcriptional analysis of genes involved in cell cycle arrest and apoptosis following OCT4A and OCT4B overexpression in MCF-7 cells. Of note, cyclin-dependent kinase inhibitor 2A codes for two proteins: p16 (or p16INK4a) and p14ARF. Error bars represent the standard deviation from three replicates. *P<0.05. **P<0.01. OCT4, octamer-binding transcription factor 4; Bcl, B-cell lymphoma; Bax, Bcl-2-associated X protein; qPCR, quantitative polymerase chain reaction; N1, exogenous transcription of empty vector; N1-4B, overexpression vector for OCT4B; Red-4A, overexpression vector for OCT4A; NT, negative control; N1-4Bs, overexpression vector for OCT4B; N1-4As, overexpression vector for OCT4A.
Discussion
OCT4 is a key transcription factor for stem cell self-renewal (5). As cells differentiate, OCT4 is downregulated (6,30,31). However, it can become aberrantly re-expressed in several cancers, including breast cancer (8,32). It was observed that OCT4B is widely expressed across a panel of human breast cancer cell lines, and that OCT4 regulates the expression of factors such as p53 and p16, which control essential cell regulatory pathways. The present data suggest that re-expressed OCT4 may function as an oncogene in human breast cancer.
The expression of both OCT4A and OCT4B was examined in a panel of breast cancer cell lines, and evidence for re-expression of both variants was detected. However, the ratio of OCT4A to OCT4B was observed to vary among different cell lines. This could be an important factor for cancer cell biology, since the two variants may influence one another's function; the data provided in the present manuscript supports this claim, as does recent work from other groups (33). Although OCT4A and OCT4B may influence one another, the exact mechanisms governing this interaction and the biological consequence of this interaction require additional investigation. It should also be noted that OCT4 was predominantly located in the cytoplasm of the four breast cancer cell lines examined. This is of interest, given its canonical function as a transcription factor, and raises the possibility that OCT4 may have functions outside the nucleus as well.
The present study revealed that OCT4 regulates the expression of several factors, which control essential cell regulatory pathways such as proliferation and apoptosis. For example, RNAi-mediated knockdown of OCT4 in breast cancer cells results in upregulation of the cell cycle inhibitors p16INK4a and p14ARF. OCT4 knockdown also results in upregulation of the pro-apoptotic factor Bax and downregulation of the anti-apoptotic factor Bcl-2. Notably, the gene expression changes in apoptosis-related genes correlate with the biological outcome. That is, OCT4 knockdown cells exhibit increased rates of apoptosis. In contrast, despite transcriptional changes in the cell cycle regulators p16INK4a and p14ARF, no alteration was observed in cell cycle progression in breast cancer cells depleted of OCT4. This suggests that OCT4 may cooperate with other factors to control cell cycle and proliferation, and that this cooperation may exert a more robust effect on apoptosis than OCT4 alone. This influence on apoptosis may be p53-independent, since OCT4 knockdown results in decreased mRNA expression of p53. In addition, the p53-target gene p21 is unaltered following OCT4 knockdown. Although the exact mechanisms governing the influence of OCT4 on apoptosis require additional research, these findings suggest that it may occur in a p53-independent manner.
Additionally, the hyperactivity of mitogenic oncogenes, such as c-Myc, adenovirus E1A and Ras, triggers a common pathway of p53 accumulation via induction of the p14ARF tumor-suppressor gene (34). Thus, the inhibitory role of OCT4 in p14ARF transcription suggests that OCT4 can antagonize oncogene-induced apoptosis.
p16INK4a and p14ARF are both encoded by the CDKN2A locus but are under the control of independent promoters (15). Despite the fact that under certain circumstances each gene can be independently regulated (16), various authors have shown that p16INK4a and p14ARF can also be coordinately regulated in a process that largely depends on chromatin remodeling events (35). In the present study, concomitant transcriptional regulation of p16INK4a and p14ARF was detected in cells with OCT4B overexpression or silencing, suggesting that OCT4B may be involved in a chromatin remodeling event at this locus.
As aforementioned, OCT4A and OCT4B may cooperate at certain level to regulate target gene expression, cell proliferation and apoptosis. The present study demonstrated that OCT4B overexpression increases the endogenous transcription of OCT4A; similarly, overexpression of OCT4A increases the endogenous transcription of OCT4B. Despite this mutual regulation, overexpression of each OCT4 variant had markedly different effects on the expression of p16INK4a and p53 pathway genes. While overexpression of OCT4B significantly altered the levels of p53, p14ARF, p16INK4a, Bcl-2 and Bax, overexpression of OCT4A was unable to do so. These observations suggest that there is a certain threshold of OCT4B that must be attained to elicit these specific biological effects, and that this level is not achieved by OCT4A overexpression. Thus, although overexpression of OCT4A increases the levels of OCT4B, it does not cause a sufficient increase in OCT4B levels and subsequently does not affect the transcription of p16 or p53 target genes.
In conclusion, the OCT4B isoform serves a role in the regulation of p53 and p16 pathway genes in breast cancer cells. Although OCT4A and OCT4B may influence one another to regulate breast cancer cell biology, OCT4B appears to be the dominant player. OCT4B may serve as a tumor marker with potential diagnostic, prognostic and therapeutic value. In conclusion, the present results aid to elucidate a role for OCT4 in regulating the proliferation and apoptosis of breast cancer cells. These findings could be clinically important for patients with breast tumors in which OCT4 becomes re-expressed.
References
- 1.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions: Nature Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 2.Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, Bernard L, Viale G, Pelicci PG, Di Fiore PP. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140:62–73. doi: 10.1016/j.cell.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Liu R, Wang X, Chen GY, Dalerba P, Gurney A, Hoey T, Sherlock G, Lewicki J, Shedden K, Clarke MF. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356:217–226. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 4.Liu CG, Lu Y, Wang BB, Zhang YJ, Zhang RS, Lu Y, Chen B, Xu H, Jin F, Lu P. Clinical implications of stem cell gene Oct-4 expression in breast cancer. Ann Surg. 2011;253:1165–1171. doi: 10.1097/SLA.0b013e318214c54e. [DOI] [PubMed] [Google Scholar]
- 5.Niwa HJ, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 6.Okamoto K, Okazawa H, Okuda A, Sakai M, Muramatsu M, Hamada H. A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell. 1990;60:461–472. doi: 10.1016/0092-8674(90)90597-8. [DOI] [PubMed] [Google Scholar]
- 7.Monsef N, Soller M, Isaksson M, Abrahamsson PA, Panagopoulos I. The expression of pluripotency marker Oct 3/4 in prostate cancer and benign prostate hyperplasia. Prostate. 2009;69:909–916. doi: 10.1002/pros.20934. [DOI] [PubMed] [Google Scholar]
- 8.Atlasi Y, Mowla SJ, Ziaee SA, Bahrami AR. OCT-4, an embryonic stem cell marker, is highly expressed in bladder cancer. Int J Cancer. 2007;120:1598–1602. doi: 10.1002/ijc.22508. [DOI] [PubMed] [Google Scholar]
- 9.Atlasi Y, Mowla SJ, Ziaee SA, Gokhale PJ, Andrews PW. OCT4 spliced variants are differentially expressed in human pluripotent and nonpluripotent cells. Stem Cells. 2008;26:3068–3074. doi: 10.1634/stemcells.2008-0530. [DOI] [PubMed] [Google Scholar]
- 10.Papamichos SI, Kotoula V, Tarlatzis BC, Agorastos T, Papazisis K, Lambropoulos AF. OCT4B1 isoform: The novel OCT4 alternative spliced variant as a putative marker of stemness. Mol Hum Reprod. 2009;15:269–270. doi: 10.1093/molehr/gap018. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Dai J. Concise review: Isoforms of OCT4 contribute to the confusing diversity in stem cell biology. Stem Cells. 2010;28:885–893. doi: 10.1002/stem.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J, Kim HK, Rho JY, Han YM, Kim J. The human OCT-4 isoforms differ in their ability to confer self-renewal. J Biol Chem. 2006;281:33554–33565. doi: 10.1074/jbc.M603937200. [DOI] [PubMed] [Google Scholar]
- 13.Kotoula V, Papamichos SI, Lambropoulos AF. Revisiting OCT4 expression in peripheral blood mononuclear cells. Stem Cells. 2008;26:290–291. doi: 10.1634/stemcells.2007-0726. [DOI] [PubMed] [Google Scholar]
- 14.Gao Y, Wei J, Han J, Wang X, Su G, Zhao Y, Chen B, Xiao Z, Cao J, Dai J. The novel function of OCT4B isoform-265 in genotoxic stress. Stem Cells. 2012;30:665–672. doi: 10.1002/stem.1034. [DOI] [PubMed] [Google Scholar]
- 15.Xing EP, Nie Y, Song Y, Yang GY, Cai YC, Wang LD, Yang CS. Mechanisms of inactivation of p14ARF, p15INK4b, and p16INK4a genes in human esophageal squamous cell carcinoma. Clin Cancer Res. 1999;5:2704–2713. [PubMed] [Google Scholar]
- 16.Li J, Poi MJ, Tsai MD. Regulatory mechanisms of tumor suppressor P16(INK4A) and their relevance to cancer. Biochemistry. 2011;50:5566–5582. doi: 10.1021/bi200642e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherr CJ. Autophagy by ARF: A short story. Mol Cell. 2006;22:436–437. doi: 10.1016/j.molcel.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Sherr CJ. Divorcing ARF and p53: An unsettled case. Nat Rev Cancer. 2006;6:663–673. doi: 10.1038/nrc1954. [DOI] [PubMed] [Google Scholar]
- 19.Agrawal A, Yang J, Murphy RF, Agrawal DK. Regulation of the p14ARF-Mdm2-p53 pathway: An overview in breast cancer. Exp Mol Pathol. 2006;81:115–122. doi: 10.1016/j.yexmp.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Fisher DE. The p53 tumor suppressor: critical regulator of life & death in cancer. Apoptosis. 2001;6:7–15. doi: 10.1023/A:1009659708549. [DOI] [PubMed] [Google Scholar]
- 21.Haupt S, Berger M, Goldberg Z, Haupt Y. Apoptosis-the p53 network. J Cell Sci. 2003;116:4077–4085. doi: 10.1242/jcs.00739. [DOI] [PubMed] [Google Scholar]
- 22.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 23.Cao Y, Siegel D, Knöchel W. Xenopus POU factors of subclass V inhibit activin/nodal signaling during gastrulation. Mech Dev. 2006;123:614–625. doi: 10.1016/j.mod.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Cao Y, Knöchel S, Donow C, Miethe J, Kaufmann E, Knöchel W. The POU factor Oct-25 regulates the Xvent-2B gene and counteracts terminal differentiation in Xenopus embryos. J Biol Chem. 2004;279:43735–43743. doi: 10.1074/jbc.M407544200. [DOI] [PubMed] [Google Scholar]
- 25.Hinkley CS, Martin JF, Leibham D, Perry M. Sequential expression of multiple POU proteins during amphibian early development. Mol Cell Biol. 1992;12:638–649. doi: 10.1128/MCB.12.2.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng WL, Chen G, Wang M, Wang H, Story M, Shay JW, Zhang X, Wang J, Amin AR, Hu B, et al. OCT4 as a target of miR-34a stimulates p63 but inhibits p53 to promote human cell transformation. Cell Death Dis. 2014;5:e1024. doi: 10.1038/cddis.2013.563. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Wang Y, Meng L, Hu H, Zhang Y, Zhao C, Li Q, Shi F, Wang X, Lin A. Oct-4B isoform is differentially expressed in breast cancer cells: Hypermethylation of regulatory elements of Oct-4A suggests an alternative promoter and transcriptional start site for Oct-4B transcription. Biosci Rep. 2011;31:109–115. doi: 10.1042/BSR20100033. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal ML, Taylor WR, Chernov MV, Chernova OB, Stark GR. The p53 network. J Biol Chem. 1998;273:1–4. doi: 10.1074/jbc.273.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Pesce M, Gross MK, Schöler HR. In line with our ancestors: Oct-4 and the mammalian germ. Bioessays. 1998;20:722–732. doi: 10.1002/(SICI)1521-1878(199812)20:12<1056::AID-BIES15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 31.Schöler HR, Ruppert S, Suzuki N, Chowdhury K, Gruss P. New type of POU domain in germ line-specific protein Oct-4. Nature. 1990;344:435–439. doi: 10.1038/344435a0. [DOI] [PubMed] [Google Scholar]
- 32.Wang P, Branch DR, Bali M, Schultz GA, Goss PE, Jin T. The POU homeodomain protein OCT3 as a potential transcriptional activator for fibroblast growth factor-4 (FGF-4) in human breast cancer cells. Biochem J. 2003;375:199–205. doi: 10.1042/bj20030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li D, Yang ZK, Bu JY, Xu CY, Sun H, Tang JB, Lin P, Cheng W, Huang N, Cui RJ, et al. OCT4B modulates OCT4A expression as ceRNA in tumor cells. Oncol Rep. 2015;33:2622–2630. doi: 10.3892/or.2015.3862. [DOI] [PubMed] [Google Scholar]
- 34.Sherr CJ, Weber JD. The ARF/p53 pathway. Curr Opin Genet Dev. 2000;10:94–99. doi: 10.1016/S0959-437X(99)00038-6. [DOI] [PubMed] [Google Scholar]
- 35.Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]