Summary
Objectives
To evaluate obesity and overweight in children with congenital adrenal hyperplasia (CAH) and associations with glucocorticoids, fludrocortisone and disease control. Adjusting body mass index-for-height-age (BMIHA) percentile is proposed to correct misclassification of obese/overweight status in CAH children with advanced bone age and tall-for-age stature.
Design
Longitudinal.
Patients
One hundred and ninety-four children with CAH seen from 1970 to 2013: 124 salt wasting (SW); 70 simple virilizing (SV); 102 females.
Measurements
Body mass index (BMI) end-points were overweight (85–94 percentile) and obese (≥95 percentile).
Results
Approximately 50% of the children had at least one BMI measurement ≥95 percentile and about 70% had at least one ≥85 percentile. Using BMIHA percentiles, obesity incidence decreased slightly in SW children (47–43%) and markedly in SV children (50–33%); however, overweight status was not reduced. Only 6% of SW and 1% of SV children were persistently obese (≥3 clinic visits) when BMIHA was applied, whereas overweight status persisted in 35% of SW and 33% of SV children. Most obesity or overweight when using BMIHA occurred before age 10 and there was no association with hydrocortisone (HC) or fludrocortisone dosing. Adiposity rebound for SW children occurred by 3.3 years and in SV females by age 3.8 years, over a year earlier than the adiposity rebound for healthy children.
Conclusion
Children with CAH are at higher risk for early onset obesity and overweight with or without using BMIHA but rates of persistent obesity were lower than previously reported. Careful HC dosing during early childhood is needed to prevent increased weight gain and an early adiposity rebound.
Introduction
Congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency is characterized by impaired cortisol synthesis and adrenal androgen overproduction with a wide spectrum of clinical phenotypes based on the degree of the severity of the enzymatic defect. CAH is classified as either classic (severe phenotype) or nonclassic (NC; mild phenotype). The classic phenotype is further subdivided into simple virilizing (SV) and salt wasting (SW) based on whether there is adequate or deficient aldosterone production, respectively.
Standard treatment in children with CAH is cortisol (hydrocortisone [HC]) replacement therapy at a recommended dose in growing children of 10–15 mg/m2/day divided in three daily doses.1 HC has a short median elimination half-life of 58 min (range: 41–105 min) in children with CAH2; thus, most of the HC dose is eliminated from the body within 5–6 h resulting in alternating periods of hyper- and hypocortisolemia and intermittent hyperandrogenemia throughout each day. The shortcomings of HC therapy are evident as children with CAH still experience adverse outcomes in regard to their growth and pubertal development as well as weight and blood pressure control even on recommended HC dosing.3–5
Body composition of children with CAH is an emerging area of focus, and recent data indicate that body mass index (BMI) and/or fat mass is elevated in children and adults with CAH.6–13 In normal growth, BMI increases rapidly in infancy and peaks at around the first year of life. Afterwards, BMI decreases gradually then reaches a nadir on average at around 5 years of age and increases gradually through childhood to adulthood. The point at BMI nadir is defined as the adiposity rebound. Earlier adiposity rebound and childhood obesity are associated with higher BMI later in life, highlighting the importance of weight gain monitoring starting in infancy.14–16
A special consideration in children with CAH is adrenal androgen-driven growth (through aromatization to oestrogen) during childhood with significant bone age advancement and tall-for-age stature. In has been shown that delayed or accelerated growth in children can lead to invalid body composition estimates when using BMI-for-chronological-age.17,18 In a large cohort of healthy children, BMI-for-chronological-age clearly differed according to stature and was systemically higher in tall children compared to children with average height.17 In the absence of CAH-specific growth charts or BMI charts for children with accelerated growth, using BMI-for-height-age (BMIHA) adjustment is needed when comparing the BMI of children with CAH to the BMI of healthy children with normal growth patterns, as it has been shown to eliminate height-related BMI differences.17
Our report is the first longitudinal study to evaluate overweight (BMI percentile 85–94) and obesity (BMI percentile ≥95 overweight) rates using both BMI-for-age and BMIHA percentiles in a large cohort of children with CAH in order to control for the confounding effect of taller relative height on BMI and prevent over reporting of obesity in children with CAH. In this study, we report rates of obesity and overweight by CAH subtype and gender and assess relationships between glucocorticoids, fludrocortisone and disease control.
Methods
Patients
We performed a retrospective medical chart review of 247 patients with CAH born after 1970, seen at three paediatric medical institutions in Minnesota. This study was approved by the institutional review board at each participating site. CAH diagnosis and subtype were assigned by the treating paediatric endocrinologists based on hormonal data, clinical and biochemical presentation, and in some cases, molecular testing of the CYP21A2 gene using a common mutation panel or sequencing. Height and weight, HC and fludrocortisone dosage, 17α-hydroxyprogesterone (17-OHP) and plasma renin activity (PRA) levels were documented at clinic visits. We report on 194 patients (79%) who had simultaneous age, height and weight measurements documented at 2 years old or older. Subjects were divided into two eras: born during 1970–1994 or born during 1994–2013, shortly after newborn screening for CAH was initiated in Minnesota.
Most patients were treated with HC; 26 patients switched from HC to prednisone and 11 patients switched to dexamethasone before age 18 years. In the early decades of this study, seven patients were treated with cortisone acetate and five patients were initially on cortisone acetate before switching to HC. For this analysis, prednisone, dexamethasone and cortisone acetate doses were converted into equivalent HC doses in mg/m2/day using standard glucocorticoid equivalencies (20 mg of HC = 25 mg of cortisone acetate = 5 mg of prednisone = 0.4 mg dexamethasone).19,20 All SW children received fludrocortisone, but some SV children did not. Disease control was considered adequate if 17-OHP was between 400 and 999 ng/dl (12.12–30.3 nmol/l), undersuppressed if 17-OHP >1000 ng/dl (30.3 nmol/l) and oversuppressed if 17-OHP <400 ng/dl (12.12 nmol/l).1,21 Measurements of 17-OHP and PRA were performed at the Mayo Clinic, Esoterix and Quest Diagnostics laboratories.
Diagnosis of obesity and overweight using BMI-for-age percentile
BMI percentile and standardized height (height for age Z) were calculated from weight, height, sex and age in months using a program from the CDC.22 BMI categories were defined as: ‘normal’ for BMI-for-age percentile <85; ‘overweight’ for BMI-for-age percentile 85–94; and ‘obese’ for BMI-for-age percentile ≥95.
Obesity and overweight using BMIHA percentile
BMIHA percentile was calculated to remedy the potential misclassification of obese/overweight status of CAH children who are tall for their age. Rather than using their actual age, we used their height age when comparing their BMI to the population of healthy children. To find the height-age, each CAH child’s measured height was compared to the CDC 2000 height-forage tables, to identify the table in which the child’s height was at the 50th percentile, the age corresponding to this table being the child’s ‘height-age’. BMI percentile was then calculated from the CDC BMI distribution for this height-age, using the child’s measured weight and height to yield BMIHA percentile. In other words, BMIHA percentile was calculated using the CDC table for the child’s height-age instead of the table for their chronologic age. Children with BMIHA percentile at or above 95% were classified as ‘height-age-obese’, and those at or above 85% were ‘height-age-overweight’. CDC BMI percentiles are established starting at age 2, so BMIHA percentile could be determined only for children aged 2–18 in our sample.
Statistical methods
Patient characteristics were compared between diagnostic categories by analysis of variance for continuous characteristics and by chi-square test for categorical characteristics. Mean HC doses, fludrocortisone doses and PRA levels were calculated for each age interval as area under the curve (sum of the products of dose by time-interval) divided by total dosing interval in those children receiving the drug. Product-limit estimates of survival time free of height-age-overweight and height-age-obesity were calculated using children seen from diagnosis. Incidence rates per person were compared by chi-square tests. Incidence rates per 100-patient years were estimated and compared using Poisson’s regression models in which categories of medication dose were assumed to be constant between clinic measurements and each patient’s time at risk ended with the earlier of their 10th birthday or their final observation. Calculations were carried out in SAS 9.4 (SAS Institute, Cary NC, USA, 2012) and figures prepared in R (R Core Team, R Foundation for Statistical Computing, Vienna, Austria 2016).
Results
We report on 194 children with CAH (124 SW and 70 SV) born after 1970 (Table 1). Most (69%) were born in the first era, 1970–1994, before newborn screening. Children born in the second era, 1994–2013, had more frequent BMI measurements (Table 1). In both eras, children <6 years old received higher mean HC doses and higher relative fludrocortisone doses; PRA was lower in SW children <6 years old than in SW children aged 6–18. Oversuppression, indicated by at least 3 measurements with 17 OHP <400 ng/dl (12.12 nmol/l), affected 82% of SW children and 53% of SV children. In both 2- to 5-years and 6-to 18-years-age groups, 50% of SV children experienced height for age ≥95th percentile, five times higher than the rate for SW children.
Table 1.
Characteristics of patients born in 1970 or later by CAH type and era of birth
| Salt wasting | Simple virilizing | |
|---|---|---|
| Sample size (N = 194) | 124 | 70 |
| Males (%) | 48 | 46 |
| Birth era (%) | ||
| 1970–1994 | 62 | 80 |
| 1994–2013 | 38 | 20 |
| BMI measurements per year | ||
| Born 1970–1994 | 2.0 ± 1 | 2.1 ± 1 |
| Born 1995–2013 | 3.5 ± 7 | 2.6 ± 2 |
| Ever oversuppressed | 82* | 53* |
| (≥3 of 17 OHP | ||
| < 400 ng/dl)† (%) | ||
| Height for Age ≥95th percentile at least once (%) | ||
| 2–5 years old | 6* | 50* |
| 6–18 years old | 10* | 51* |
| Mean hydrocortisone dose‡ (mg/m2/day), born 1970–1994 | ||
| 0–5 years old | 15 ± 4** | 20 ± 4** |
| 6–18 years old | 14 ± 5 | 16 ± 5 |
| Mean hydrocortisone dose‡ /day), (mg/m2 born 1995–2013 | ||
| 0–5 years old | 12 ± 4** | 12 ± 4 |
| 6–18 years old | 9 ± 3 | 9 ± 4 |
| Mean fludrocortisone‡ (mg) | ||
| 0–5 years old | 0.09 ± 0.03 | 0.10 ± 0.05 |
| 6–18 years old | 0.09 ± 0.03 | 0.09 ± 0.03 |
| Mean relative fludrocortisone‡ (mcg/m2; 95% confidence interval) | ||
| 0–5 years old | 138 (128–148)** | 133 (106–166)** |
| 6–18 years old | 64 (60–68) | 62 (54–73) |
| Mean plasma renin activity (ng/ml/h; 95% confidence interval) | ||
| 0–5 years old | 1.7 (1–2)*** | 5.4 (3–9) |
| 6–18 years old | 2.8 (2–4) | 3.0 (2–4) |
Values are number (per cent of sample) or mean ± standard deviation.
Rates of SW and SV children were significantly different (P < 0.01).
Mean dose in children aged 0–5 significantly higher than in children aged 6–18 within same CAH subtype (P < 0.05).
Mean activity in SW children aged 0–5 significantly lower than in SW children aged 6–18 (P = 0.007).
Sample restricted to children with at least 3 measurements of 17-OHP; 400 ng/dl of 17OHP = 12.012 nmol/l. (ng/dl × 0.0303 = nmol/l.)
Mean dose for each child calculated for each age interval (0–5, 6–18 years) as area under the curve (sum of the products of dose by time-interval) divided by total dosing interval in those children receiving the drug.
Adiposity rebound
Smooth estimates of mean crude BMI in Fig. 1 suggest a nadir (a.k.a. adiposity rebound) for SW children by 3.3 years and SV females by age 3.8 years, over a year earlier than the BMI nadir for healthy children in the CDC growth curves14,15,23 The BMI nadir for SV males appears to occur before age 5 years (Fig. 1) but because SV males were diagnosed significantly later, we do not know whether they had an earlier nadir.
Fig. 1.
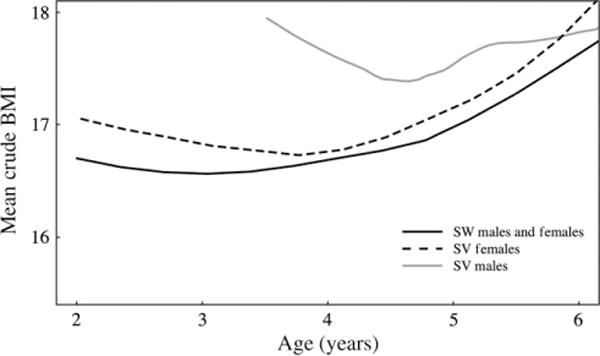
Smoothed estimates of mean crude BMI by age for salt wasting children, simple virilizing (SV) females and SV males.
Obesity using BMI-for-age and BMIHA percentile
Half the children had measured BMI percentile ≥95th percentile for their age and sex, the standard criterion for obesity, on at least one occasion (Table 2). However, when their BMI was compared to the percentile distribution using BMIHA percentile, fewer children were found to be height-age-obese. Incidence of height-age-obesity in SW children was 43%, down from 47% obese, and in SV children was 33%, down from 50% obese (Table 2).
Table 2.
Percent incidence rates of obesity (≥95th BMI-for-chronological-age percentile) and height-age-obesity (≥95th BMI-for-height-age percentile)
| Salt wasting | Simple virilizing | |
|---|---|---|
| Sample size (N = 194) | 124 | 70 |
| Obesity (%) | 47 | 50 |
| Height-age-obesity (%) | 43 | 33 |
| Height-age-obesity at 3 or more clinic visits (%) | 6 | 1 |
| Height-age-obesity by birth era (%) | ||
| 1970–1994 (n = 133) | 45 | 32 |
| 1994–2013 (n = 61) | 38 | 36 |
| Incidence of height-age-obesity among children observed in each age interval (%) | ||
| 2–5 years | 18 | 26 |
| 6–11 years | 26 | 11 |
| 12–18 years | 6 | 12 |
| Prevalence of obesity in US children (NHANES 2011–201233; %) | ||
| 2–5 years | 8 | |
| 6–11 years | 18 | |
| 12–19 years | 21 | |
| Incidence of height-age-obesity by hydrocortisone (HC) dose† | ||
| HC < 15 mg/m2/day | 2.3 (1–5) | 1.6 (0.2–12) |
| HC ≥ 15 mg/m2/day | 2.7 (1–7) | 1.3 (0.2–10) |
| Incidence of height-age-obesity by hydrocortisone (HC) and fludrocortisone (F) dose† | ||
| HC < 15 mg/m2/day, no F | 2.4 (0.3–18) | |
| HC ≥ 15 mg/m2/day, no F | 2.3 (0.3–18) | |
| HC < 15 mg/m2/day, with F | 2.1 (1.0–5) | |
| HC ≥ 15 mg/m2/day, with F | 2.2 (0.8–6) | |
| Age 2–5 years: Height-age-obesity by measurements of 17 OHP < 400 ng/dl‡ (%) | ||
| <3 | 14* | |
| 3 or more | 27* | |
| Age 6–11 years: Height-age-obesity by measurements of 17 OHP < 400 ng/dl‡ (%) | ||
| <3 | 19 | |
| 3 or more | 18 | |
Rates were compared between CAH subtypes, between hydrocortisone and fludrocortisone dose groups and between those with <3 and 3 or more measurements of 17 OHP <400 ng/dl; there were no significant differences at the 0.05 level.
Values are incidence rates per 100 patient years (95% confidence interval). Restricted to children aged 2–9 years old; all SW children received fludrocortisone.
Restricted to subjects with at least 3 measurements of 17-OHP; 400 ng/dl of 17OHP = 12.12 nmol/l. (ng/dl × 0.0303 = nmol/l).
Incidence in children with <3 such measurements trended lower (P = 0.064).
To illustrate using BMIHA percentile in evaluating obesity status in clinical practice, Fig. 2a–c shows longitudinal height, weight and BMI measurements against chronological age from a SV-CAH male who was diagnosed at age 4.9 years and presented with a bone age of 9 years. The patient had 21 BMI measurements classified as obese (Panel C) using chronological age. Figure 2d shows the relationship between chronological age and height-age at each measurement, and Fig. 2e–f shows the patient’s weight and BMI measurements against his height-age; only 4 BMI measurements were classified as obese (Panel F) which more closely represents his presentation over the years: the patient never appeared obese but rather tall for age, muscular and with consistently advanced bone age.
Fig. 2.
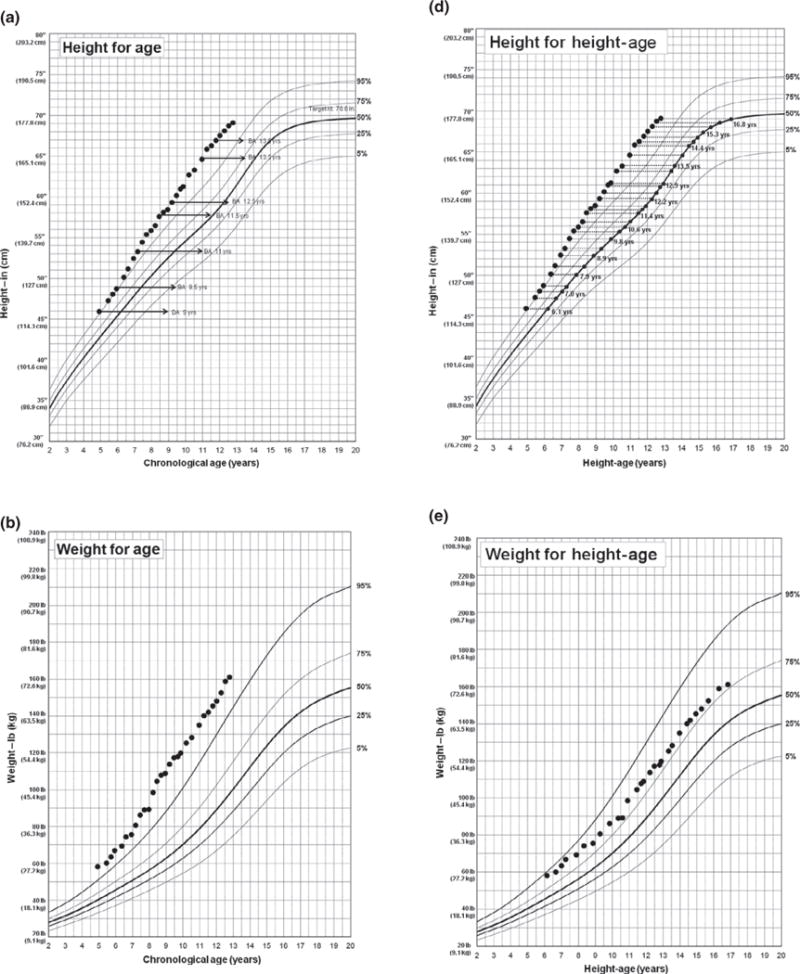
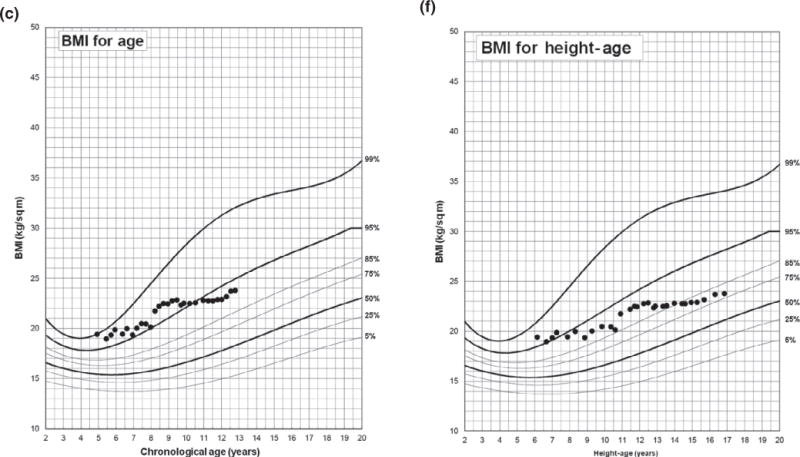
Longitudinal height, weight and BMI measurements of a patient diagnosed with simple virilizing congenital adrenal hyperplasia at 4.9 years of age using BMI-for-age percentile (a–c) and BMIHA percentile (d–f). BA, bone age.
Among children seen from diagnosis, survivor curves for age at first incidence of height-age-obesity were significantly different between SW and SV children (log-rank test, P = 0.012, Fig. 3); no difference between sexes was seen within either CAH subtype. Incidence of height-age-obesity in SV children occurred mostly before age 5, while incidence in SW children continued from birth through age 10. Longitudinal plots of individual BMIHA percentile by age for SW and SV children also show that most cases of height-age-obesity occurred before age 5 in SV children and age 10 in SW children, with only a few cases appearing in late adolescence (Figures S1–S2).
Fig. 3.
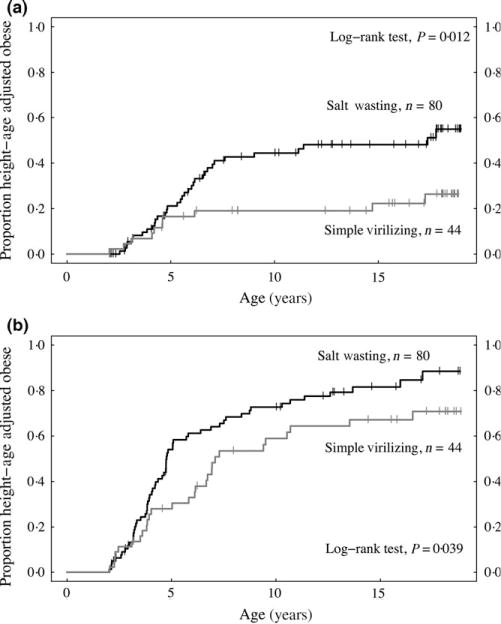
Kaplan–Meier estimates of height-age obesity-free survival (a) and overweight and obesity-free survival (b) for 124 children seen from diagnosis of congenital adrenal hyperplasia using BMIHA percentile.
Incidence of height-age-obesity did not differ between birth eras or between age groups (Table 2). Persistent height-age-obesity was rare: only 6% of SW and 1% of SV children were height-age-obese at three or more clinic visits. Although not directly comparable, this was in contrast to the trend of increasing obesity prevalence with age in US children (Table 2).
There was no apparent association between height-age-obesity and HC dose or fludrocortisone dose: incidence rates were statistically indistinguishable during the intervals when children received the combination of lower dose HC (<15 mg/m2/day) and no fludrocortisone, and intervals of higher dose HC (≥15 mg/m2/day) with fludrocortisone (Table 2). SW children all received fludrocortisone, and in those <10 years old, a slightly higher incidence of height-age-normed obesity was observed during intervals when children received a higher dose HC (≥15 mg/m2/day) but this was not statistically significant (P = 0.78, Table 2). In children aged 2–5 years, those who were oversuppressed (with 3 or more measurements of 17 OHP <400 ng/dl) tended to have higher incidence of height-age-obesity (P = 0.06, Table 2). Oversuppression was not related to incidence of height-age-obesity in older children.
Overweight using BMI-for-age and BMIHA percentile
About 70% of children had BMI ≥85th percentile for their age and sex on at least one occasion and these rates remained same even after using BMIHA percentile. Approximately one-third of the children were height-age-overweight at three or more clinic visits. Incidence of height-age-overweight did not differ between birth eras or between age groups, which differed from the trend of increasing prevalence of overweight with age reported for US children (Table 3). Among children seen from diagnosis, survivor curves for age at first incidence of height-age-overweight or height-age-obesity showed that a higher proportion of SW than SV children were affected at every age over 5 years (log-rank test, P = 0.039, Fig. 3).
Table 3.
Percent incidence rates overweight (≥85th BMI-for-chronological-age percentile) and height-age-overweight (≥85th BMI-for-height-age percentile)
| Salt wasting | Simple virilizing | |
|---|---|---|
| Sample size (N = 194) | 124 | 70 |
| Overweight (%) | 70 | 73 |
| Height-age-overweight (%) | 71 | 70 |
| Three or more height-age-overweight measurements (%) | 35 | 33 |
| Height-age-overweight by birth era (%) | ||
| 1970–1994 (n = 133) | 73 | 71 |
| 1994–2013 (n = 61) | 68 | 64 |
| Incidence of height-age-overweight among children observed in each age interval(%) | ||
| 2–5 years | 48 | 41 |
| 6–11 years | 59 | 47 |
| 12–18 years | 39 | 50 |
| Prevalence of overweight or obesity in US children (NHANES 2011–201233; %) | ||
| 2–5 years | 23 | |
| 6–11 years | 34 | |
| 12–19 years | 35 | |
| Incidence of height-age-overweight by hydrocortisone (HC) dose† | ||
| HC < 15 mg/m2/day | 11 (7–16) | 14 (6–33) |
| HC ≥ 15 mg/m2/day | 14 (9–23) | 8 (3–20) |
| Incidence of height-age-overweight by hydrocortisone (HC) and fludrocortisone (F) dose† | ||
| HC < 15 mg/m2/day, no F | 23 (10–51) | |
| HC ≥ 15 mg/m2/day, no F | 19 (9–40) | |
| HC < 15 mg/m2/day, with F | 10 (7–15) | |
| HC ≥ 15 mg/m2/day, with F | 10 (6–17) | |
| Age 2–5 years: Height-age-overweight by measurements of 17 OHP <400 ng/dl‡ (%) | ||
| <3 | 36* | |
| 3 or more | 56* | |
| Age 6–11 years: Height-age-overweight by measurements of 17 OHP <400 ng/dl‡ (%) | ||
| <3 | 54 | |
| 3 or more | 55 | |
Rates were compared between CAH subtypes, between hydrocortisone and fludrocortisone dose groups and between those with <3 and 3 or more measurements of 17 OHP <400 ng/dl.
Values are incidence rates per 100 patient years (95% confidence interval). Restricted to children aged 2–9 years old; all SW children received fludrocortisone.
Restricted to subjects with at least 3 measurements of 17-OHP; 400 ng/dl of 17OHP =12.12 nmol/l. (ng/dl × 0.0303 = nmol/l).
Incidence lower in children with <3 such measurements (P = 0.021).
There was no apparent association between height-age-overweight and HC dose or fludrocortisone dose: incidence rates were statistically indistinguishable during intervals when children received the combination of lower dose HC (<15 mg/m2/day) and no fludrocortisone, and during intervals of higher dose HC (≥15 mg/m2/day) with fludrocortisone (Table 3). In SW children <10 years old, a slightly higher incidence of height-age-overweight was observed during intervals when children received a higher dose HC (≥15 mg/m2/day) but this was not statistically significant (P = 0.225, Table 3). In children aged 2–5 years, oversuppressed children had higher incidence of height-age-overweight (P = 0.021, Table 3). Oversuppression was not related to height-age-overweight incidence in older children.
Discussion
Our longitudinal study’s findings of increased overweight and obesity in children with CAH is consistent with high BMI percentiles found by some prior cross-sectional studies9,24–30 but differs from others which did not find increased BMI percentiles.10,31 However, none of these studies examined differences in obesity status at different age intervals or used BMI-for height-age effects, independent of BMI-for-age ranking.
Adjusting the analysis for height-age reduced the overall obesity rate in our SV cohort CAH from 50% to 33%. A more marked decrease in the obesity rate was seen for SV children than for SW children, likely due to SV children being diagnosed with CAH at a later age than SW patients resulting in longer androgen-driven growth and taller-for-age stature. This suggests that the majority of children in the obese category are the tallest children and their obesity status is corrected using BMIHA percentile. BMIHA percentile did not change the rates of overweight in children with CAH, which may reflect the combination of effects of androgen-growth and glucocorticoid therapy. Height-age-overweight and height-age-obesity did not increase from childhood to adolescence as in the US population (Table 2).32
The expected relationship between overweight/obesity with HC dosing was not found in our study. However, the rate of height-age-overweight or height-age-obesity was higher in children aged 0–5 years who had more than three 17-OHP measurements <400 ng/dl (12.12 nmol/l) compared to those with fewer than three 17-OHP measurements (Tables 2 and 3). The effect of oversuppression was not seen in older children. This suggests that younger children may be more glucocorticoid sensitive and that careful titration of dosing and adrenal steroid control is needed to prevent increased weight gain and an earlier adiposity rebound in this age group.
As children with CAH have severe cortisol deficiency and are exposed to higher androgen levels prenatally and chronically even while on glucocorticoid therapy, they may be predisposed to being overweight compared to healthy norms. There is evidence of cross talk between androgens and glucocorticoids and that elevated androgen levels can be associated with increased abdominal adiposity in adult women and altered leptin axis in children with CAH.30,33,34 Chronically, intermittently elevated adrenal androgen levels due to shortcomings of current therapy in both males and females may be one of the reasons that there was no difference in obesity status between sexes within subtypes. A recent study reported higher visceral and subcutaneous fat measured by computed tomography imaging in 28 adolescents and adults with CAH compared to healthy controls; the study found no difference in visceral or subcutaneous fat between sexes and found no correlation with glucocorticoid dosing.11
Persistent height-age-overweight (noted at three or more clinic visits) was more common (50% of SW and 47% of SV children) than persistent height-age-obesity, (6% of SW and 1% of SV children). More aggressive monitoring and intervention, including HC dose adjustments, when children reached BMI >95% tile may explain the difference.
Several cross-sectional studies have investigated the rate of overweight, obesity and metabolic syndrome status in paediatric and adult CAH patients. Finkielstain et al.25 in a study of 170 children (≥8 years) and 74 adults (18–68 years) with CAH reported that one-third of paediatric and adult patients of all CAH subtypes including NC-CAH were obese and 18% of the adults had metabolic syndrome. In this study, obesity in CAH children was not associated with subtype, sex, age or glucocorticoid dose but rather with long acting glucocorticoids.25 Cornean et al.24 in a study of 22 children with SW-CAH reported an earlier adiposity rebound, as the ‘rebound’ in BMI percentile took place at the mean age 1.74 years (range: 0.71–4.57 years) compared with 5.5 years of adiposity rebound (range: 3.5–7.0 years) in the normal UK population. Because skeletal maturationin those 22 children was not advanced at 5 and 10 years of age and height z-score remained constant between these age groups, the authors suggested that the increased BMI was not due to taller stature but rather to a change in body weight.24 Similarly, we noted in our study that SW-CAH had an earlier rebound compared to health norms (Fig. 1). Isguven et al., examined 17 children with CAH (subtype was not reported) and found that BMI percentile, skin fold thickness and body fat ratio were all higher in CAH patients than in control patients.27 Matsubara, et al.,26 in a longitudinal retrospective study of 16 children with CAH (aged: 6.2–17.8 years) reported that adiposity rebound is earlier in CAH children compared to healthy controls and that BMI percentile and adiposity were significantly increased throughout childhood. SV patients tended to have lower BMI throughout the observation period of 15 years; no significant difference of height percentile between the SW and SV forms was found.26 Moreira, et al.,9 reported increased rates of both metabolic syndrome and obesity in 33 children and adolescents with CAH (aged: 6.2–17.8 years) compared to the general population and found no correlation between the BMI percentile, sex, CAH subtype, glucocorticoid dose or duration of therapy. Metabolic syndrome was present in 12% of the children with CAH, and metabolic profiles were worse in SW compared to SV children, which the authors attributed to their earlier exposure to glucocorticoid therapy and possible overtreatment during the first year of avoid salt-loss. A retrospective cross-sectional study of 107 British children with CAH (age range: 0.4–20.5 years) showed that there was not a significant correlation between BMI percentile and HC dose.28 In contrast to our study, they found a significant negative correlation between BMI percentile and fludrocortisone dose. Vokl et al.29 in a retrospective cross-sectional study of 89 CAH children (aged: 0.2–17.9 years) reported that BMI percentile did not differ within subtype and that BMI percentile was positively correlated with age and glucocorticoid dosing. In another study of 51 children with CAH (aged: 5.6–19.6 years), Vokl et al.30 showed that children with CAH had altered leptin axis with decreased levels of soluble leptin receptor (sOB-R), which they attributed to chronically elevated androgen levels in children with CAH. Hyperandrogenemia in women with PCOS has been shown to be associated with decreased levels of sOB-R.34
Our retrospective database, which covered five decades and three different paediatric institutions, did not document whether specific recommendations were given for lifestyle modifications (e.g. increasing exercise, decreasing food intake) to address obesity or overweight. An additional limitation is that laboratory tests were drawn at different times of day for each patient visit. Timing of blood draws in relation to the diurnal variation of ACTH secretion and in relation to the patient’s most recent glucocorticoid dose would impact 17-OHP levels. Androgens were not consistently recorded during clinic visits, so their effects could not be examined. Medication nonadherence was not assessed or recorded.
In conclusion, the findings from our longitudinal study represent the largest cohort of children with CAH (age 0–18 years) reported to date and the first attempt to control for tall-for-age stature effect on obesity/overweight classification. The BMIHA adjustment, which accounts for relative height, mainly reduced obesity rates in children with SV-CAH, with just a small decrease in SW-CAH; no decrease in overweight rates was seen in either subtype. The biggest impact of the BMIHA adjustment was showing that persistent (three or more clinic visits) obesity rates were not as common as previously reported.9,25,29,30 CAH children are at higher risk for earlier adiposity rebound, increased early onset obesity and overweight, regardless of subtype. Avoiding oversuppression and increasing BMI over the first 5 years of life is especially important as chronic exposure to glucocorticoids may increase the risk of earlier adiposity rebound and increased risk of obesity during adulthood.14,15 Longitudinal, prospective studies starting in infancy are needed to examine: (i) early adiposity rebound’s role in developing obesity and metabolic syndrome in adulthood; (ii) whether lower glucocorticoid doses during the first 5 years would reduce the risk of adiposity rebound and obesity; and (iii) whether a more physiologic and circadian delivery of HC that takes into account a child’s cortisol pharmacokinetics and pharmacodynamic response may lead to a decrease in obesity/overweight rates in children with CAH. Similarly to other conditions where clinical practice guidelines suggest that BMI should be expressed relative to height-age,35 BMIHA adjustment should be used for evaluation of obesity status in children with CAH to account for their accelerated growth.
Supplementary Material
Acknowledgments
Funding
This study had no external funding.
Footnotes
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher’s web site.
Financial disclosure
The authors have no financial relationships relevant to this article to disclose.
Conflict of interest
Authors have no conflict of interest to report.
References
- 1.Speiser PW, Azziz R, Baskin LS, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism. 2010;95:4133–4160. doi: 10.1210/jc.2009-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarafoglou K, Zimmerman CL, Gonzalez-Bolanos MT, et al. Interrelationships among cortisol, 17-hydroxyprogesterone, and androstenendione exposures in the management of children with congenital adrenal hyperplasia. Journal of Investigative Medicine. 2015;63:35–41. doi: 10.1097/JIM.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 3.Bomberg EM, Addo OY, Kyllo J, et al. The relation of peripubertal and pubertal growth to final adult height in children with classic congenital adrenal hyperplasia. Journal of Pediatrics. 2015;166:743–750. doi: 10.1016/j.jpeds.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 4.Maccabee-Ryaboy N, Thomas W, Kyllo J, et al. Hypertension in children with congenital adrenal hyperplasia. Clinical Endocrinology (Oxford) 2016;85:528–534. doi: 10.1111/cen.13086. [DOI] [PubMed] [Google Scholar]
- 5.Sarafoglou K, Addo OY, Turcotte L, et al. Impact of hydrocortisone on adult height in congenital adrenal hyperplasia-the Minnesota cohort. Journal of Pediatrics. 2014;164:1141–1146.e1141. doi: 10.1016/j.jpeds.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Arlt W, Willis DS, Wild SH, et al. Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. Journal of Clinical Endocrinology and Metabolism. 2010;95:5110–5121. doi: 10.1210/jc.2010-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goncalves EM, de Lemos-Marini SH, de Mello MP, et al. Impairment in anthropometric parameters and body composition in females with classical 21-hydroxylase deficiency. Journal of Pediatric Endocrinology and Metabolism. 2009;22:519–529. doi: 10.1515/jpem.2009.22.6.519. [DOI] [PubMed] [Google Scholar]
- 8.Mendes-Dos-Santos CT, Lemos-Marini SH, Baptista MT, et al. Normalization of height and excess body fat in children with salt-wasting 21-hydroxylase deficiency. Journal de Pediatria. 2011;87:263–268. doi: 10.2223/JPED.2095. [DOI] [PubMed] [Google Scholar]
- 9.Moreira RP, Villares SM, Madureira G, et al. Obesity and familial predisposition are significant determining factors of an adverse metabolic profile in young patients with congenital adrenal hyperplasia. Hormone Research in Paediatrics. 2013;80:111–118. doi: 10.1159/000353762. [DOI] [PubMed] [Google Scholar]
- 10.Williams RM, Deeb A, Ong KK, et al. Insulin sensitivity and body composition in children with classical and non-classical congenital adrenal hyperplasia. Clinical Endocrinology (Oxford) 2010;72:155–160. doi: 10.1111/j.1365-2265.2009.03587.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim MS, Ryabets-Lienhard A, Dao-Tran A, et al. Increased abdominal adiposity in adolescents and young adults with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Journal of Clinical Endocrinology and Metabolism. 2015;100:E1153–E1159. doi: 10.1210/jc.2014-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falhammar H, Frisen L, Hirschberg AL, et al. Increased cardiovascular and metabolic morbidity in patients with 21-hydroxylase deficiency: a Swedish Population-Based National Cohort Study. Journal of Clinical Endocrinology and Metabolism. 2015;100:3520–3528. doi: 10.1210/JC.2015-2093. [DOI] [PubMed] [Google Scholar]
- 13.Mooij CF, Kroese JM, Claahsen-van der Grinten HL, et al. Unfavourable trends in cardiovascular and metabolic risk in paediatric and adult patients with congenital adrenal hyperplasia? Clinical Endocrinology (Oxford) 2010;73:137–146. doi: 10.1111/j.1365-2265.2009.03690.x. [DOI] [PubMed] [Google Scholar]
- 14.Whitaker RC, Pepe MS, Wright JA, et al. Early adiposity rebound and the risk of adult obesity. Pediatrics. 1998;101:E5. doi: 10.1542/peds.101.3.e5. [DOI] [PubMed] [Google Scholar]
- 15.Peneau S, Gonzalez-Carrascosa R, Gusto G, et al. Age at adiposity rebound: determinants and association with nutritional status and the metabolic syndrome at adulthood. International Journal of Obesity. 2016;40:1150–1156. doi: 10.1038/ijo.2016.39. [DOI] [PubMed] [Google Scholar]
- 16.Koyama S, Ichikawa G, Kojima M, et al. Adiposity rebound and the development of metabolic syndrome. Pediatrics. 2014;133:e114–e119. doi: 10.1542/peds.2013-0966. [DOI] [PubMed] [Google Scholar]
- 17.Bonthuis M, Jager KJ, Abu-Hanna A, et al. Application of body mass index according to height-age in short and tall children. PLoS ONE. 2013;8:e72068. doi: 10.1371/journal.pone.0072068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodig NM, McDermott KC, Schneider MF, et al. Growth in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children Study. Pediatric Nephrology(Berlin, Germany) 2014;29:1987–1995. doi: 10.1007/s00467-014-2812-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller WL. Clinical review 54: genetics, diagnosis, and management of 21-hydroxylase deficiency. Journal of Clinical Endocrinology and Metabolism. 1994;78:241–246. doi: 10.1210/jcem.78.2.8106606. [DOI] [PubMed] [Google Scholar]
- 20.Hindmarsh PC. Management of the child with congenital adrenal hyperplasia. Best Practice & Research Clinical Endocrinology & Metabolism. 2009;23:193–208. doi: 10.1016/j.beem.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Wolters Kluwer Health. Drug Facts and Comparisons. 5th. Wolters Kluwer, St; Louis, MO: 1997. [Google Scholar]
- 22.Centers for Disease Control and Prevention. A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 y) 2014 http:// www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm.
- 23.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Statistics. 2002;11:1–190. [PubMed] [Google Scholar]
- 24.Cornean RE, Hindmarsh PC, Brook CG. Obesity in 21-hydroxylase deficient patients. Archives of Disease in Childhood. 1998;78:261–263. doi: 10.1136/adc.78.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finkielstain GP, Kim MS, Sinaii N, et al. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism. 2012;97:4429–4438. doi: 10.1210/jc.2012-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsubara Y, Ono M, Miyai K, et al. Longitudinal analysis of growth and body composition of Japanese 21-OHD patients in childhood. Endocrine Journal. 2013;60:149–154. doi: 10.1507/endocrj.ej12-0123. [DOI] [PubMed] [Google Scholar]
- 27.Isguven P, Arslanoglu I, Mesutoglu N, et al. Bioelectrical impedance analysis of body fatness in childhood congenital adrenal hyperplasia and its metabolic correlates. European Journal of Pediatrics. 2008;167:1263–1268. doi: 10.1007/s00431-007-0665-y. [DOI] [PubMed] [Google Scholar]
- 28.Subbarayan A, Dattani MT, Peters CJ, et al. Cardiovascular risk factors in children and adolescents with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clinical Endocrinology (Oxford) 2014;80:471–477. doi: 10.1111/cen.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volkl TM, Simm D, Beier C, et al. Obesity among children and adolescents with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics. 2006;117:e98–e105. doi: 10.1542/peds.2005-1005. [DOI] [PubMed] [Google Scholar]
- 30.Volkl TM, Simm D, Korner A, et al. Does an altered leptin axis play a role in obesity among children and adolescents with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency? European Journal of Endocrinology. 2009;160:239–247. doi: 10.1530/EJE-08-0770. [DOI] [PubMed] [Google Scholar]
- 31.Gussinye M, Carrascosa A, Potau N, et al. Bone mineral density in prepubertal and in adolescent and young adult patients with the salt-wasting form of congenital adrenal hyperplasia. Pediatrics. 1997;100:671–674. doi: 10.1542/peds.100.4.671. [DOI] [PubMed] [Google Scholar]
- 32.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasquali R, Vicennati V, Gambineri A, et al. Sex-dependent role of glucocorticoids and androgens in the pathophysiology of human obesity. International Journal of Obesity. 2008;32:1764–1779. doi: 10.1038/ijo.2008.129. [DOI] [PubMed] [Google Scholar]
- 34.Hahn S, Haselhorst U, Quadbeck B, et al. Decreased soluble leptin receptor levels in women with polycystic ovary syndrome. European Journal of Endocrinology. 2006;154:287–294. doi: 10.1530/eje.1.02078. [DOI] [PubMed] [Google Scholar]
- 35.KDOQI Work Group. KDOQI clinical practice guideline for nutrition in children with CKD: 2008 update. Executive summary. American Journal of Kidney Diseases. 2009;53:S11–S104. doi: 10.1053/j.ajkd.2008.11.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


