Abstract
Trehalose 6'6-dimycolate (TDM) is the most abundant glycolipid on the cell wall of Mycobacterium tuberculosis (MTB). TDM is capable of inducing granulomatous pathology in mouse models that resembles those induced by MTB infection. Using the acute TDM model, this work investigates the effect of recombinant human and mouse lactoferrin to reduce granulomatous pathology. C57BL/6 mice were injected intravenously with TDM at a dose of 25 μg-mouse-1. At day 4 and 6, recombinant human or mouse lactoferrin (1 mg · (100 μL)−1 mouse−1) were delivered by gavage. At day 7 after TDM injection, mice were evaluated for lung pathology, cytokine production, and leukocyte populations. Mice given human or mouse lactoferrin had reduced production of IL-12p40 in their lungs. Mouse lactoferrin increased IL-6 and KC (CXCL1) in lung tissue. Increased numbers of macrophages were observed in TDM-injected mice given human or mouse lactoferrin. Granulomatous pathology, composed of mainly migrated leukocytes, was visually reduced in mice that received human or mouse lactoferrin. Quantitation of granulomatous pathology demonstrated a significant decrease in mice given human or mouse lactoferrin compared with TDM control mice. This report is the first to directly compare the immune modulatory effects of both heterologous recombinant human and homologous mouse lactoferrin on the development of TDM-induced granulomas.
Keywords: human lactoferrin, mouse lactoferrin, trehalose dimycolate
Keywords: lactoferrine humaine, lactoferrine de souris, tréhalose dimycolate
Résumé
Le tréhalose 6’6 dimycolate (TDM) est le glycolipide le plus abondant de la paroi cellulaire de Mycobacterium tuberculosis (MTB). Le TDM est capable d’induire des pathologies granulomateuses dans des modèles de souris qui ressemblent à celles qui sont induites par l’infection à MTB. Utilisant le modèle de TDM aigu, ce manuscrit examine l’effet de lactoferrine recombinante humaine et de souris pour réduire la pathologie granulomateuse. Des souris C57BL/6 ont reÇu par injection i.v. 25 µg souris−1 de TDM. Aux jours 4 et 6, de la lactoferrine recombinante humaine ou de souris (1 mg·(100 µL)−1·souris−1) a été administrée par gavage. Sept jours après l’injection de TDM, les souris ont été évaluées quant à la pathologie pulmonaire, à la production de cytokines et à la population de leucocytes. Les souris ayant reÇu de la lactoferrine humaine ou de souris présentaient une réduction de la production d’IL-12p40 dans le poumon. La lactoferrine de souris augmentait l’IL-6 et le KC (CXCL1) dans le tissu pulmonaire. Un accroissement de la population de macrophages était observé chez les souris injectées avec le TDM et ayant reÇu de la lactoferrine humaine ou de souris. La pathologie granulomateuse, composée principalement de leucocytes ayant migré, était visuellement réduite chez les souris ayant reÇu la lactoferrine humaine ou de souris. La quantification de la pathologie granulomateuse a démontré qu’elle était significativement diminuée chez les souris ayant reÇu de la lactoferrine humaine ou de souris comparativement aux souris contrôles ayant reÇu le TDM seul. Ce rapport est le premier qui compare directement les effets immuno-modulateurs tant de la lactoferrine humaine hétérologue que de la lactoferrine de souris homologue dans le développement des granulomes induits par le TDM. [Traduit par la Rédaction]
Introduction
Tuberculosis (TB) is an infectious lung disease caused by the intracellular bacterium Mycobacterium tuberculosis (MTB), which the World Health Organization (WHO) estimates to have infected a third of the world’s population. Despite the existence of a vaccine as well as antibiotic regimens, TB remains the leading cause of death due to a single infectious pathogen, and is the leading cause of death in HIV-AIDS patients. In 2014, WHO reported 9.6 million new cases of TB, resulting in 1.5 million deaths (WHO 2015). Several avenues of research are currently in progress to develop improved vaccines and (or) therapies to treat TB disease (Ahsan 2015).
The hallmark of TB disease is the interaction between MTB and the host immune responses, leading to a distinctive set of pathologies of which the granuloma plays a major role. The granuloma is made up of innate and adaptive leukocytes that have migrated to the site of MTB infection or MTB antigen deposition. Resident macrophages and (or) dendritic cells phagocytose MTB and (or) MTB antigens, initiating a local inflammatory response with increasing levels of TNF-α, IL-6, IL-1β, IL-12, and IFN-γ and production of chemokines. Innate and adaptive lymphocytes, and naïve monocytes/macrophages, continue to infiltrate, setting up a localized environment of immune stimulation, leading to formation of the granuloma (Korb et al. 2016).
The role of the granuloma is 2-fold. The granuloma acts to prevent spread of infection. But, as the size of the granuloma increases due to increasing accumulation of leukocytes at the site, the role of the granuloma becomes pathological, and acts to insulate the “trapped” MTB from being exposed to newly activated leukocytes. Additionally, the growing granuloma starts to overtake normal lung tissue and compromise normal lung functions, which is typically indicative of an unrestrictive inflammatory response (Welsh et al. 2013; Actor 2015). Thus, we observe that treatment with oral lactoferrin decreased the percentage of lung occupied by granulomas, indicating that lactoferrin was able to decrease the pathological aspect of the granuloma, allowing more functional space for normal lung actions. Thus, modulating the host immune response is a promising direction towards developing novel therapeutics that would improve current TB therapy. Lactoferrin is hypothesized to be one such promising immune modulator for TB disease.
Previous work on lactoferrin demonstrated that its immune modulatory effects depend on the state of activation of the targeted immune cells. In a highly activated inflammatory environment, such as under lipopolysaccharide (LPS) stimulation, the addition of lactoferrin actively decreased macrophage production of inflammatory cytokines TNF-α and IL-12 (Hwang et al. 2007a; Doursout et al. 2013). During low antigen stimulation, such as vaccination with the TB vaccine BCG (Bacillus Calmette Guerin), the addition of lactoferrin enhanced vaccine efficacy by increasing the antigen recall IFN-γ response (Hwang et al. 2005, 2007b, 2009a, 2009b, 2011a, 2011b; Hwang and Actor 2009), which is necessary for the control of MTB infection. Based on published data, it was hypothesized that oral lactoferrin could decrease the pathology of MTB-induced lung inflammation while preserving the cytokine environment required for the control of MTB organisms.
Trehalose 6'6-dimycolate (TDM) is the most abundant glycop- lipid on the cell wall surface of MTB (Hunter et al. 2009). TDM can induce the granuloma pathology often observed during MTB infection, including stimulation of the localized pulmonary inflammatory environment, resulting in increased production of TNF-α, IL-6, IL-1β, and IL-12 (Perez et al. 2000; Indrigo et al. 2002, 2003; Welsh et al. 2008, 2013). Previous preliminary studies indicated that the oral treatment of mice with bovine lactoferrin significantly reduced the granuloma pathology induced by TDM (Welsh et al. 2011). This article will explore the effect of our novel recombinant human (heterologous) and mouse (homologous) lactofer- rin to modulate TDM granulomatous pathology.
Materials and methods
Mice
Female C57BL/6 mice (aged 6 weeks; Jackson Laboratories) of ~20 grams initial body mass were used. All in-vivo experiments were conducted under the approved guidelines of the animal ethics committee at the University of Texas Health Science Center at Houston (HSC-AWC-14–0174).
TDM-induced lung pathology
Mycobacterium tuberculosis derived TDM (cord factor) was purchased from Enzo Life Sciences (Farmingdale, NewYork, USA). Drakeol 6VR, light mineral oil, was purchased from Penreco (Dickinson, Texas, USA).
TDM (25 μg#x00B7;mouse−1) was solubilized in hexane-ethanol (H:E; 9:1), the appropriate amount was aliquoted, and H:E was evaporated under a stream of air. The TDM oil-water emulsion was prepared as previously described (Welsh et al. 2008; Abbott et al. 2009). The evaporated TDM (25 μgmouse−1) was homogenized in Drakeol (2 μLmouse−1) for 1 min. To the TDM-Drakeol mixture, 48 μLmouse−1 of 1x DPBS (Dulbecco’s’ phosphate-buffered solution; Cellgro) with 0.2% Tween 80 (Mallinckrodt, Hazelwood, Missouri, USA), was added and homogenized for another 2 min. The TDM oil-water emulsions were injected intravenously (i.v.; 100 μL#x00B7;mouse−1).
Recombinant human and mouse lactoferrin
CHO-expressed recombinant human lactoferrin (endotoxin is <0.2 EU·mg−1) and mouse lactoferrin (endotoxin is <1.0 EUmg−1) were kindly provided as lyophilized powder by PharmaReview Corporation (Houston, Tex.) (Kruzel et al. 2013; Zimecki et al. 2013).
Both recombinant human and mouse lactoferrin were reconstituted in dH2O to a concentration of 10 mg·mL-1. At day 4 and 6 after TDM injection, mice were given 1 mg·(100 μL)−1·mouse−1 of human or mouse lactoferrin by gavage.
Lung weight index
All mice were sacrificed at day 7 after TDM injection. Upon sacrifice, lungs were perfused with 1 mmo·L−1 EDTA in DPBS, weighed, and sectioned to evaluate pathology, cytokine production, and immune cell typing by flow cytometry.
Lung weight index (LWI) (Actor et al. 2002; Guidry et al. 2004, 2006, 2007; Welsh et al. 2008; Abbott et al. 2009) was calculated as a rough measure of lung inflammation intensity using the following equation:
Lung cytokine analysis
A section of lung tissue was weighed and homogenized and placed into 2 mL Dulbecco’s modified Eagle’s medium containing 50 mg·L−1 L-arginine, 50 mg·L−1 HEPES, 10% fetal bovine serum, 100 μg.mL−1 penicillin, and 50 μg.mL−1 gentamycin (Sigma). Samples were incubated for 4 h at 37 °C with 5% CO2. The resulting supernatants were analyzed for levels of cytokines were measured by a sandwich ELISA, according to the manufacturer’s instructions (R&D Systems, Minneapolis, Minnesota, USA). Absorbance was read at 570 nm with background subtracted at 450 nm on a plate reader (Molecular Devices, Menlo Park, California, USA). The lower range limit for detection sensitivity was 16–32 pg.mL-1.
Identification of immune cells in lung tissue
Each section of lung tissue was processed into a single cell suspension. Briefly, each lung section was cut up into tiny pieces (<1 mm), digested in a solution of 1 mg mL−1 collagenase type II and 30 μg/mL DNase (Sigma) in 1x DPBS at 37 °C, 220 rpm, for 1 h.
After digesting, glass slides were used to breakup the tissue clumps. Once the bigger clumps settled to the bottom of the tube, the remaining cell suspension was pelleted at 400g for 5 min. The cell pellet was re-suspended in flow staining buffer (1% bovine serum albumin (BSA) in 1x DPBS).
The identification of surface molecule expression by flow cytometric analysis (average 40 000 eventssample−1) was conducted as previously described (Hwang and Actor 2009; Hwang et al. 2009b). Briefly, samples were blocked by incubating purified anti-CD16/32 (Fc block, BD Bioscience, San Diego, Calif.) on ice for 15 min. Then samples were stained with CD4-FITC, CD8-PE, CD3-APC, CD11b- PerCP-Cy5.5, Gr-1-Pacific Blue, and NK1.1-Cy7 (eBioscience, San Diego, Calif.) on ice for ~1 h. Stained samples were fixed with 4% paraformaldehyde on ice for 15 min. Stained and fixed samples were stored in 500 pL of staining buffer. Samples were read on a Gallios Flow Cytometer (Beckman-Coulter). Analysis was conducted with Kaluza flow cytometry analysis software (Beckman- Coulter).
Histological assessment
The large right lobe of the mouse lung was collected and fixed in 10% buffered formalin. For histologic analysis, the lung was sectioned (5 μm thick) and stained with hematoxylin and eosin (H&E) as per standard procedures.
ImageJ analysis
Quantitation of pathology was conducted using ImageJ 1.50b. The image was first adjusted to the color threshold, between blue and violet, which eliminated most of the pink staining in each lung section. The adjusted image was converted to an 8-bit image. The tissue section was outlined with the selection tool. The threshold on the grayscale image was readjusted until mostly only the interested areas were highlighted in black. The threshold was set and used for all of the subsequent images. The measurement on the selected lung section is reported as percent area. All data was graphed in GraphPad Prism 5.
Statistical analysis
Data obtained was compared across groups then analyzed using a paired Student t test or one-way ANOVA with a Tukey post-hoc test; differences between means were considered statistically significant at a value of p ≤ 0.05. Data are presented are a combination of 4–5 experimental repeats. Each experiment incorporated 4–5 mice.
Results
Mice injected with oil-water vehicle without TDM do not display any pathology in their lung tissue, and resemble naive controls. (Guidry et al. 2006, 2007; Welsh et al. 2010; McMullen et al. 2013; Donnachie et al. 2016). All cytokines analyzed for controls were at or below the level of detection for the assays and were no different from naive controls. Flow analysis of leukocyte populations from the control mice showed no changes in T cells or neutrophils in lung tissue. C57BL/6 mice injected with the oil-water vehicle demonstrated a slight increase in macrophages by comparison with the naïve controls, albeit approximately 2-fold lower compared with mice injected with TDM. All of the immune data for the control mice have been previously published (Donnachie et al. 2016).
Effect of recombinant human or mouse lactoferrin in reducing lung inflammation in the TDM-induced mouse granulomatous model
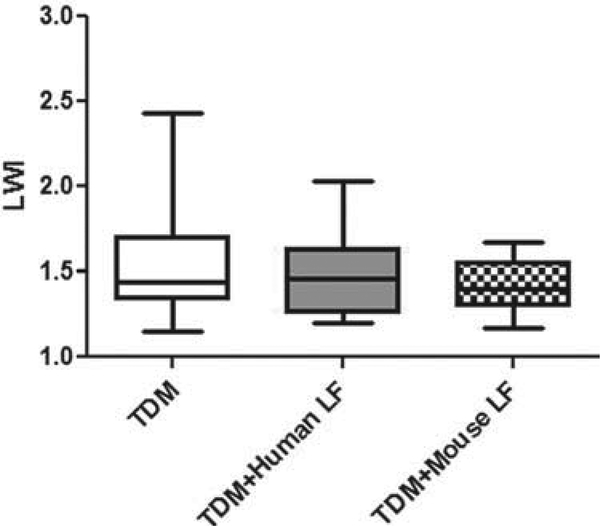
TDM-induced granuloma pathology is associated with an increase in overall lung inflammation. The first goal was to investigate whether the administration of human or mouse lactoferrin would have an overall anti-inflammatory effect on lung tissue. To quantitate lung inflammation, the LWI value was calculated by comparing the mass of the lung with the mass of the mouse. There were no significant changes in lung inflammation in mice given human or mouse lactoferrin (Fig. 1). However, there was less variability in the lactoferrin-treated groups by comparison with the control mice.
Fig. 1.
Effect of oral lactoferrin on lung weight index (LWI). C57BL/6 mice injected with trehalose 6'6-dimycolate (TDM) were treated with human or mouse lactoferrin (LF) on days 4 and 6 after injection. Mice were sacrificed on day 7. Whole lung mass was measured and compared with the mass of the mouse. Data are plotted as box and whiskers, marking the range, the 1st and 3rd quartiles, and the mean.
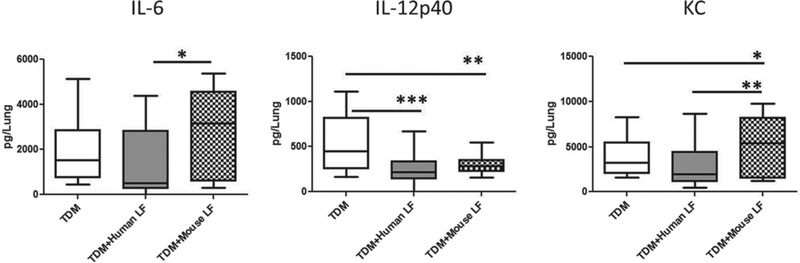
A second measure of TDM-induced inflammation is the production of cytokines and chemokines in the lung tissue. Sections of lung were homogenized and incubated in cell culture medium for 4 h. The resulting supernatant was collected and measured using ELISA. Inflammatory analytes examined were: T cell and innate lymphocyte cytokines (IFN-γ and IL-17), inflammatory cytokines (TNF-α, IL-6, and IL-1β), helper T cell modulating cytokines (IL-12p40 and IL-10), macrophage differentiation/growth factor (GM-CSF), and chemokine for neutrophil migration (KC).
No differences were observed among the TDM, TDM + human lactoferrin, and TDM + mouse lactoferrin treatment groups in lung production of IFN-γ, IL-17, TNF-α, IL-1β, IL-10, and GM-CSF (data not shown). Both the human and mouse lactoferrin treated TDM mice demonstrated decreased production of IL-12p40. Only mice treated with mouse lactoferrin displayed an increase in production of IL-6 and KC compared with the TDM and TDM + human lactoferrin treatment groups (Fig. 2).
Fig. 2.
Lung tissue cytokine environment. C57BL/6 mice injected with trehalose 6'6-dimycolate (TDM) were treated with human or mouse lactoferrin (LF) at days 4 and 6 after injection. Mice were sacrificed on day 7. A section of lung was collected, homogenized, and incubated with media for 4 h. Supernatants were analyzed by ELISA. Data are plotted as box and whiskers, marking range, the 1st and 3rd quartiles, and the mean; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Leukocyte populations in lung tissue treated with lactoferrin
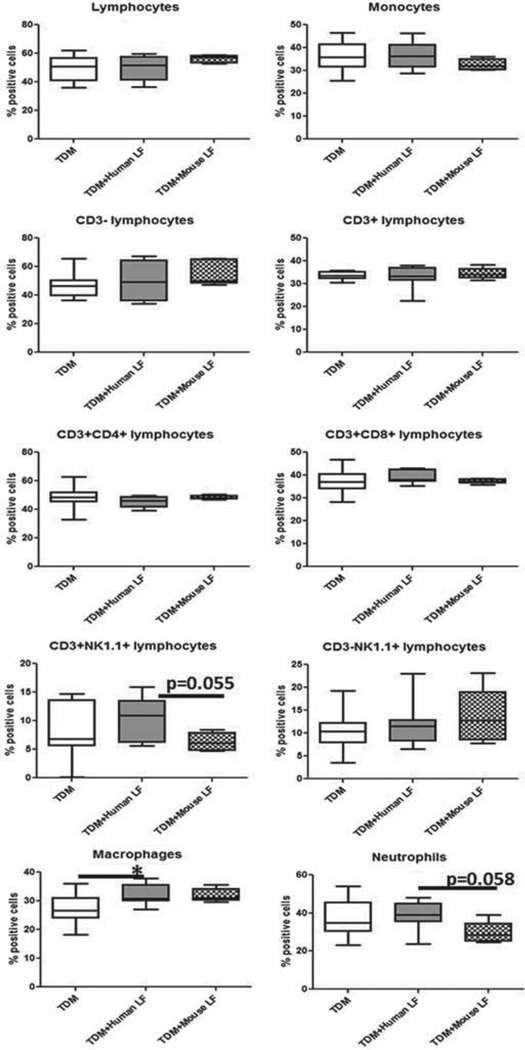
Analysis of lung inflammation demonstrated distinct differences in the cytokine environment between mice given human or mouse lactoferrin in the TDM mouse model. We used flow cytometry to determine whether the differences in cytokine environment reflected the population of leukocytes that migrated to the lung tissue.
Lymphocytes and monocytes were differentiated by size and granularity. Lymphocytes were further delineated into CD3+ and CD3- populations to determine T cells (CD3+) and innate lymphocytes and B cells (CD3--). T cells were than analyzed for the expression of CD4 and CD8, analyzing the percentage of T helper cells (CD4+) versus cytotoxic T cells (CD8+) in the lung tissue. Lymphocytes expressing NK1.1 were analyzed according to CD3 expression, separating natural killer cells (CD3-) and killer T cells (CD3+). The monocyte population was analyzed for macrophage and neutrophil populations by expression of CD11b+ GR-1- (macrophages) and CD11b+ GR-1+ (neutrophils).
The only differences observed in mice treated with lactoferrin, compared with those left untreated, were in the macrophage and neutrophil populations. Recombinant human lactoferrin treated mice had increased presence of macrophages in the lung tissue compared with the untreated TDM group. Mice treated with mouse lactoferrin demonstrated a nonsignificant increase in macrophages compared with the untreated TDM group, and a nonsignificant decrease in the presence of neutrophils compared with the human lactoferrin treatment group and the untreated groups (Fig. 3).
Fig. 3.
Leukocyte populations in lung tissue on day 7 after injection with trehalose 6'6-dimycolate (TDM). C57BL/6 mice injected with TDM were treated with human or mouse lactoferrin (LF) on days 4 and 6 after injection. Mice were sacrificed on day 7. A section of lung was collected and digested into single cell suspensions. Cells were stained for CD3, CD4, CD8, NK1.1, CD11b, and GR-1. Data are plotted as box and whiskers, marking range, the 1st and 3rd quartiles, and the mean; *, p < 0.05.
Oral lactoferrin decreased TDM-induced lung pathology
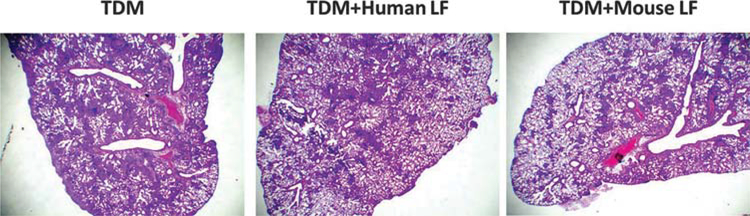
Oral treatment of TDM mice with human or mouse lactoferrin affected lung inflammation and migrated leukocyte populations in the lung tissue. To investigate whether orally administered lactoferrin affected overall TDM-induced lung pathology, histological analysis was conducted on H&E-stained lung sections.
Acute lung pathology in TDM has been reported consistently in previously published reports. Lung sections often demonstrate scattered granulomatous structures composed of clusters of lymphocytes and macrophages. TDM-treated C57BL/6 mice administered human or mouse lactoferrin showed lung sections with decreased size of granulomatous structures, leading to overall decreased inflammatory pathology. Additionally, TDM-treated mice administered human or mouse lactoferrin both showed a decrease in tissue edema, as demonstrated by increased areas of normal lung parenchyma with air-filled alveoli (Fig. 4).
Fig. 4.
Lung histology for trehalose 6'6-dimycolate (TDM) induced mice after treatment with lactoferrin. C57BL/6 mice injected with TDM were treated with human or mouse lactoferrin (LF) on days 4 and 6 after injection. Mice were sacrificed on day 7. A section of lung was collected, fixed in 10% formalin, and stained with H&E. Lung sections were visualized at 20x. Images are representative of 10–14 lung sections across 3 experimental repeats. [Colour online.]
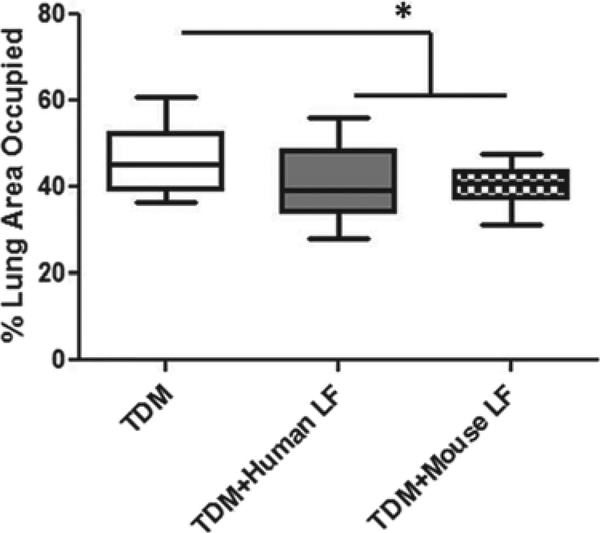
Quantitation of lung pathology was conducted using ImageJ, by limiting the color threshold to the hematoxylin and eosin stained infiltrating macrophages and lymphocytes (blue to dark violet). ImageJ calculated the percent area occupied by the granulomatous pathology compared with the total area of the lung section examined. The average percent area occupied by granulomatous structures in TDM-injected mice (46.09% ± 7.46%) was significantly higher compared with the mice treated with human (40.71% ± 7.87%) or mouse (40.6% ± 4.59%) lactoferrin (Fig. 5).
Fig. 5.
Quantitation of granulomatous structures in lung sections treated with or without lactoferrin. C57BL/6 mice injected with trehalose 6'6-dimycolate (TDM) were treated with human or mouse lactoferrin (LF) on days 4 and 6 after injection. Mice were sacrificed on day 7. A section of lung was collected, fixed in 10% formalin, and stained with H&E. Lung sections were visualized at 20x. Image analysis to determine the percentage of lung area occupied by granulomatous structures was conducted with ImageJ.
Discussion
The results presented here represent the first report that oral treatment with recombinant human or mouse lactoferrin can decrease granuloma pathology in the lung induced with the mycobacterium glycolipid TDM. The granuloma is an important part of the disease process of TB and involves major contribution from the host’s immune response. Owing to the anti-inflammatory activities of lactoferrin, it was hypothesized that oral delivery of lactoferrin would effectively reduce TDM-induced granuloma structures. This study clearly demonstrates that treatment with both recombinant human or mouse lactoferrin in the mouse TDM model effectively reduced granuloma pathology in lung. The immune mechanism affected with lactoferrin treatment varied slightly between the groups treated with human or mouse lacto- ferrin forms. This report provides in-vivo evidence that oral lacto- ferrin can control a mycobacterial induced pathology, and provides evidence that oral lactoferrin may be an effective adjunct therapy for the treatment of TB.
The acute TDM model has several limitations compared with events that occur during virulent MTB infection. The most critical is that development of pathology during MTB infection requires at least 4 weeks after the onset of adaptive immunity (Hwang et al. 2007b, 2009c, 2011a, 2011b). However, the TDM model more accurately represents initial innate responses to organisms, which define immediate responses leading to initiation of the granulomatous response (Welsh et al. 2008). During infection, the influx of CD4 and CD8 T cells into the lung tissue is most likely sensitized to MTB antigens, whereas in the acute TDM model, the CD4 and CD8 T cells that influx into the lung tissue are called in by the general inflammatory lung environment, and the T cells are not specific for the TDM antigen. There is a memory response to the TDM glycolipid, but it takes time for the adaptive immune response to develop (Guidry et al. 2004, 2006, 2007). Despite obvious differences in the immune response between the acute TDM and the MTB infection model, both models develop granulomatous lung pathology reflective of early events involved in establishment of pathology. More importantly, during both TDM- and MTB- induced lung pathology, treatment with lactoferrin leads to decreased granuloma occupation of normal lung tissue (Welsh et al. 2010, 2011).
Previous studies on the effect of lactoferrin to affect TDM- induced pathology used bovine lactoferrin, injected i.v., either at day 0 or day 1 after injection of TDM. Bovine lactoferrin, administered i.v., significantly reduced both granulomatous pathology and inflammatory cytokine production (TNF-α, IL-6, and IL-12p40) (Welsh et al. 2010). However, i.v. treatment is not an ideal clinical delivery method. To evaluate the effect of lactoferrin under a model that mimics clinical relevant events, this report examined delivered of lactoferrin by the oral route, with subsequent treatment beginning after TDM induced an inflammatory response (after day 3). With this new protocol, recombinant human and mouse lactoferrin demonstrated a similar decrease in lung cytokine production of IL-12p40.
IL-12p40 can form various heterodimers, leading to formations of IL-12p70 and IL-23, to name 2 cytokines important for differentiation of naive T cells, T cell helper type 1, and T cell helper type 17, respectively (Abdi and Singh 2015). While T cell helper type 1 is needed for protection against MTB infection and TB, the role of T cell helper type 17 is still being debated (O’Garra et al. 2013). The majority of IL-12p40 production is from activated macrophages and dendritic cells (Rossol et al. 2011). In the case of the acute inflammatory response, such as modeled in this reported TDM model, production of IL-12p40 is indicative of macrophage activation and increased inflammatory response. Interestingly, the decrease in IL-12p40 levels in lung tissue was associated with an increase in macrophage populations. This suggests that oral treatment with lactoferrin is capable of decreasing inflammatory cytokine production by macrophages, despite an increase in the macrophage population. Previous studies on LPS stimulated macrophages suggested that lactoferrin is a strong modulator of macrophage inflammatory activity (Hwang et al. 2005, 2007a), possibly without affecting other activity of the macrophage. These studies suggest that under a strong inflammatory stimulation, such as with LPS, lactoferrin acts to control inflammation, partly by decrease IL-12p40. In contrast, the effect of recombinant human and mouse lactoferrin on mouse bone marrow derived macrophages in an adjuvant model, under low antigen stimulation with BCG, is to enhance macrophage activation, partly by increasing the production of relevant inflammatory cytokines, such as IL-12p40 (O’Shea et al. 2015). This duel effect of lactoferrin on IL-12p40 production has been reported extensively using bovine lactoferrin (Wilk et al. 2007; Hwang et al. 2009b).
The increase in IL-6 and KC in lung tissue in mice treated with mouse lactoferrin can be produced from a variety of leukocytes, including macrophages and neutrophils. Neutrophils are one of the acute responders against pathogenic infection and plays essential roles in the initial host protective response (Netea et al. 2010; Blazek et al. 2015; Karmakar et al. 2015). Neutrophils express the receptors for TDM recognition and response via the MINCLE pathway (Lee et al. 2012). However, neutrophil population in the lung demonstrated a decrease in mice treated with mouse lacto- ferrin, despite the increase in the neutrophilic chemokine, KC. Owing to the variation between experiments and individual mice in this experimental model, the correlation between IL-6 and KC production and neutrophil influx into the lung tissue cannot be determined without further experiments.
Most investigations on lactoferrin are on the bovine milk derived form, which is easily obtained and abundant. There is approximately 30% difference in sequence homology among lactoferiins isolated from difference species: bovine, human, and mouse (Kim et al. 1998). Owing to these differences, concerns have been raised about the reliability of preclinical assessment of human lactofer-rin in the heterologous in vivo mouse model. Recently, both recombinant human and mouse lactoferrins were produced in sufficient quantities in the Chinese hamster ovary (CHO) cell line. Previous studies published on mouse-derived immune cells on CHO-derived recombinant human and mouse lactoferrins demonstrated similar immune modulatory effects (Hwang et al. 2015; O’Shea et al. 2015), suggesting that the mouse model can be used as a preclinical model for evaluating efficacy of recombinant human lactoferrin.
The most significant and consistent finding of this report is the effect of recombinant human and mouse lactoferrin on the reduction of granulomatous lung pathology. Previous reports on the effect of oral bovine lactoferrin in MTB-infected mice also showed a similar significant decrease in lung granulomatous pathology (Welsh et al. 2011). In combination with the data reported in this manuscript, oral lactoferrin is an effective therapy, working to reduce pathological host immunity response that leads to the development of deleterious lung pathology.
These studies present the first in-vivo evidence that oral recombinant human lactoferrin may be effective in decreasing TB- induced lung pathology, which is a critical step towards reducing disease progression. Further investigations should determine whether recombinant human lactoferrin can be employed as an effective adjunct treatment for TB. In addition, the similarity in the effects of recombinant human and mouse lactoferrin in the mouse model indicates that preclinical evaluation of recombinant human lactoferrin can be evaluated in the mouse model.
Acknowledgement
This work was supported by US National Institutes of Health, grant No. 1R41-AI117990–01.
Footnotes
This Article is part of a Special Issue from the XIIth Lactoferrin Conference.
Contributor Information
Shen-An Hwang, Department of Pathology and Laboratory Medicine, UTHealth McGovern Medical School, Houston, TX 77030, USA.
Jeffrey K. Actor, Department of Pathology and Laboratory Medicine, UTHealth McGovern Medical School, Houston, TX 77030, USA
Marian L. Kruzel, Department of Integrative Biology and Pharmacology, UTHealth McGovern Medical School, Houston, TX 77030, USA
References
- Abbott AN, Guidry TV, Welsh KJ, Thomas AM, Kling MA, Hunter RL, and Actor JK. 200911β-hydroxysteroid dehydrogenases are regulated during the pulmonary granulomatous response to the mycobacterial glycolipid trehalose-6,6’-dimycolate. NeuroImmunoModulation, 16(3): 147-154. doi:10.1159/000204227. PMID:. [DOI] [PubMed] [Google Scholar]
- Abdi K, and Singh NJ. 2015. Makingmany from few: IL-12p40 as a model for the combinatorial assembly of heterodimeric cytokines. Cytokine, 76(1): 53-57. doi:10.1016/j.cyto.2015.07.026. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Actor JK. 2015. Lactoferrin: a modulator for immunity against tuberculosis related granulomatous pathology. Mediators Inflammation, 2015: 409596. doi:10.1155/2015/409596. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Actor JK, Indrigo J, Beachdel CM, Olsen M, Wells A, Hunter RL, Jr., and Dasgupta A. 2002. Mycobacterial glycolipid cord factor trehalose 6,6’ - dimycolate causes a decrease in serum cortisol during the granulomatous response. NeuroImmunoModulation, 10(5): 270-282. doi:10.1159/000069971. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahsan MJ. 2015. Recent advances in the development of vaccines for tuberculosis. Ther. Adv. Vaccines, 3(3): 66-75. doi:10.1177/2051013615593891. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazek K, Eames HL, Weiss M, Byrne AJ, Perocheau D, Pease JE, et al. 2015. IFN-λ resolves inflammation via suppression of neutrophil infiltration and IL-1β production.J. Exp. Med. 212(6): 845-853. doi:10.1084/jem.20140995. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnachie E, Fedotova EP, and Hwang S-A. 2016. Trehalose 6,6-dimycolate from Mycobacterium tuberculosis induces hypercoagulation. Am. J. Pathol. 186(5): 1221-1233. doi:10.1016/j.ajpath.2015.12.019. PMID:. [DOI] [PubMed] [Google Scholar]
- Doursout M-F, Horton H, Hoang L, Liang Y, Hwang S-A, Boyd S, et al. 2013. Lactoferrin moderates LPS-induced hypotensive response and gut injury in rats. Int. Immunopharmacol. 15(2): 227-231. doi:10.1016/j.intimp.2012.12.009. PMID:. [DOI] [PubMed] [Google Scholar]
- Guidry TV, Hunter RL, Jr., and Actor JK. 2006. CD3+ cells transfer the hypersensitive granulomatous response to mycobacterial glycolipid trehalose 6,6’- dimycolate in mice. Microbiology, 152(Pt 12): 3765-3775. doi:10.1099/mic.0.29290-0. PMID:. [DOI] [PubMed] [Google Scholar]
- Guidry TV, Hunter RL, Jr., and Actor JK. 2007. Mycobacterial glycolipid trehalose 6,6’-dimycolate-induced hypersensitive granulomas: contribution of CD4+ lymphocytes. Microbiology, 153(Pt 10): 3360-3369. doi:10.1099/mic.0.2007/010850-0. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidry TV, Olsen M, Kil K-S, Hunter RL, Jr., Geng Y-J, and Actor JK. 2004. Failure of CD1D−/− mice to elicit hypersensitive granulomas to mycobacterial cord factor trehalose 6,6’-dimycolate. J. Interferon Cytokine Res. 24(6): 362371. doi:10.1089/107999004323142222. PMID:. [DOI] [PubMed] [Google Scholar]
- Hunter RL, Armitige L, Jagannath C, and Actor JK. 2009. TB research at UT-Houston-a review of cord factor: new approaches to drugs, vaccines and the pathogenesis of tuberculosis. Tuberculosis (Edinb), 89(Suppl. 1): S18-S25. doi:10.1016/S1472-9792(09)70007-1. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S-A, and Actor JK. 2009. Lactoferrin modulation of BCG-infected dendritic cell functions. Int. Immunol. 21(10): 1185-1197. doi:10.1093/intimm/dxp084. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S-A, Kruzel ML, and Actor JK. 2005. Lactoferrin augments BCG vaccine efficacy to generate T helper response and subsequent protection against challenge with virulent Mycobacterium tuberculosis. Int. Immunopharmacol. 5(3): 591-599. doi:10.1016/j.intimp.2004.11.006. PMID:. [DOI] [PubMed] [Google Scholar]
- Hwang S-A, Wilk KM, Bangale YA, Kruzel ML, and Actor JK. 2007a Lactoferrin modulation ofIL-12 and IL-10 response from activated murine leukocytes. Med. Microbiol. Immunol. 196(3): 171-180. doi:10.1007/s00430-007-0041-6. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S-A, Wilk KM, Budnicka M, Olsen M, Bangale YA, Hunter RL, et al. 2007b. Lactoferrin enhanced efficacy of the BCG vaccine to generate host protective responses against challenge with virulent Mycobacterium tuberculosis. Vaccine, 25(37–38): 6730-6743. doi:10.1016/j.vaccine.2007.07.005. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S-A, Arora R, Kruzel ML, and Actor JK. 2009a. Lactoferrin enhances efficacy of the BCG vaccine: comparison between two inbred mice strains (C57BL/6 and BALB/c). Tuberculosis (Edinb), 89(Suppl. 1): S49-S54. doi:10.1016/S1472-9792(09)70012-5. PMID:. [DOI] [PubMed] [Google Scholar]
- Hwang S-A, Kruzel ML, and Actor JK. 2009b. Influence ofbovine lactoferrin on expression of presentation molecules on BCG-infected bone marrow derived macrophages. Biochimie, 91(1): 76-85. doi:10.1016/j.biochi.2008.04.008. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S-A, Wilk K, Kruzel ML, and Actor JK. 2009c. A novel recombinant human lactoferrin augments the BCG vaccine and protects alveolar integrity upon infection with Mycobacterium tuberculosis in mice. Vaccine, 27(23): 30263034. doi:10.1016/j.vaccine.2009.03.036. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S-A, Welsh KJ, Boyd S, Kruzel ML, and Actor JK. 2011a. Comparing efficacy ofBCG/lactoferrin primary vaccination versus booster regimen. Tuberculosis (Edinb), 91(Suppl. 1): S90-S95. doi:10.1016/j.tube.2011.10.017. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S-A, Welsh KJ, Kruzel ML, and Actor JK. 2011b. Lactoferrin augmentation of the BCG vaccine leads to increased pulmonary integrity. Tuberc. Res. Treat. 2011: 835410. doi:10.1155/2011/835410. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S-A, Kruzel ML, and Actor JK. 2015. Effects ofCHO-expressed recombinant lactoferrins on mouse dendritic cell presentation and function. Innate Immun. 21(5): 553-561. doi:10.1177/1753425914564609. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indrigo J, Hunter RL, Jr., and Actor JK. 2002. Influence of trehalose 6,6’- dimycolate (TDM) during mycobacterial infection of bone marrow macrophages. Microbiology, 148(Pt 7): 1991-1998. doi:10.1099/00221287-148-7-1991. PMID:. [DOI] [PubMed] [Google Scholar]
- Indrigo J, Hunter RL, Jr., and Actor JK. 2003. Cord factor trehalose 6,6’- dimycolate (TDM) mediates trafficking events during mycobacterial infection ofmurine macrophages. Microbiology, 149(Pt 8): 2049-2059. doi:10.1099/mic.0.26226-0. PMID:. [DOI] [PubMed] [Google Scholar]
- Karmakar M, Katsnelson M, Malak HA, Greene NG, Howell SJ, Hise AG, et al. 2015. Neutrophil IL-1beta processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux. J. Immunol. 194(4): 1763-1775. doi:10.4049/jimmunol.1401624. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Yu DY, Pak KW, Jeong S, Kim SW, and Lee KK. 1998. Structure of the human lactoferrin gene and its chromosomal localization. Mol. Cells, 8(6): 663-668. PMID:. [PubMed] [Google Scholar]
- Korb VC, Chuturgoon AA, and Moodley D. 2016. Mycobacterium tuberculosis: manipulator of protective immunity. Int. J. Mol. Sci. 17(3): 131. doi:10.3390/ijms17030131. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzel ML, Actor JK, Zimecki M, Wise J, Ploszaj P, Mirza S, et al. 2013. Novel recombinant human lactoferrin: differential activation of oxidative stress related gene expression. J. Biotechnol. 168(4): 666-675. doi:10.1016/j.jbiotec.2013.09.011. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W-B, Kang J-S, Yan J-J, Lee MS, Jeon B-Y, Cho S-N, and Kim Y-J. 2012. Neutrophils promote mycobacterial trehalose dimycolate-induced lung inflammation via the Mincle pathway. PLoS Pathog. 8(4): e1002614. doi:10.1371/journal.ppat.1002614. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen AM, Hwang S-A, O’Shea K, Aliru ML, and Actor JK. 2013. Evidence for a unique species-specific hypersensitive epitope in Mycobacterium tuberculosis derived cord factor. Tuberculosis (Edinb), 93(Suppl.): S88-S93. doi:10.1016/S1472-9792(13)70017-9. [DOI] [PubMed] [Google Scholar]
- Netea MG, Simon A, van deVeerdonk F, Kullberg B-J,Van der Meer JWM, and Joosten LAB. 2010. IL-β processing in host defense: beyond the inflammasomes. PLoS Pathog. 6(2): e1000661. doi:10.1371/journal.ppat.1000661. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, and Berry MPR. 2013. The immune response in tuberculosis. Annu. Rev. Immunol. 31:475-527. doi:10.1146/annurev-immunol-032712-095939. PMID:. [DOI] [PubMed] [Google Scholar]
- O’Shea KM, Hwang S-A, andActor JK. 2015. Immune activity ofBCGinfected mouse macrophages treated with a novel recombinant mouse lactoferrin. Ann. Clin. Lab. Sci. 45(5): 487-494. PMID:. [PubMed] [Google Scholar]
- Perez RL, Roman J, Roser S, Little C, Olsen M, Indrigo J, et al. 2000. Cytokine message and protein expression duringlunggranuloma formation and resolution induced by the mycobacterial cord factor trehalose-6,6-dimycolate. J. Interferon Cytokine Res. 20(9): 795-804. doi:10.1089/10799900050151067. PMID: . [DOI] [PubMed] [Google Scholar]
- Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, and Hauschildt S. 2011. LPS-induced cytokine production in human monocytes and macrophages. Crit. Rev. Immunol. 31(5): 379-446. doi:10.1615/CritRevImmunol.v31.i5.20. PMID:. [DOI] [PubMed] [Google Scholar]
- Welsh KJ, Abbott AN, Hwang S-A, Indrigo J, Armitige LY, Blackburn MR, et al. 2008. A role for tumour necrosis factor-alpha, complement C5 and interleukin-6 in the initiation and developmentofthe mycobacterial cord factor trehalose 6,6’-dimycolate induced granulomatous response. Microbiology, 154(Pt 6): 1813-1824. doi:10.1099/mic.0.2008/016923-0. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh KJ, Hwang S-A, Hunter RL, Kruzel ML, and Actor JK. 2010. Lacto ferrin modulation of mycobacterial cord factor trehalose 6–6’-dimycolate induced granulomatous response. Transl. Res. 156(4): 207-215. doi:10.1016/j.trsl.2010.06.001. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh KJ, Hwang S-A, Boyd S, Kruzel ML, Hunter RL, and Actor JK. 2011. Influence of oral lactoferrin on Mycobacterium tuberculosis induced immuno- pathology. Tuberculosis (Edinb), 91(Suppl. 1): S105-S113. doi:10.1016/j.tube.2011.10.019. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh KJ, Hunter RL, and Actor JK. 2013. Trehalose 6,6’-dimycolate - a coat to regulate tuberculosis immunopathogenesis. Tuberculosis (Edinb), 93(Suppl.): S3-S9. doi:10.1016/S1472-9792(13)70003-9. [DOI] [PubMed] [Google Scholar]
- WHO 2015. Global tuberculosis report 2015 [online]. Available from http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf?ua=1 [accessed 16 June 2016].
- Wilk KM, Hwang S-A, and Actor JK. 2007. Lactoferrin modulation of antigen- presenting-cell response to BCG infection. Postepy Hig. Med. Dosw. (Online), 61: 277-282. PMID:. [PMC free article] [PubMed] [Google Scholar]
- Zimecki M, Artym J, Kocieba M, Kaleta-Kuratewicz K, Kuropka P, Kuryszko J, and Kruzel M. 2013. Homologous lactoferrin triggers mobilization of the myelocytic lineage of bone marrow in experimental mice. Stem Cells Dev. 22(24): 3261-3270. doi:10.1089/scd.2013.0242. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]