Abstract
Heterocyclic amines (HCAs) are primarily formed during cooking of meat at high temperature. HCAs have been extensively studied as mutagens and possible carcinogens. Emerging data suggest that HCAs are neurotoxic and may be relevant to Parkinson’s disease (PD) etiology. However, the majority of HCAs have not been evaluated for in vivo neurotoxicity. Here, we investigated acute in vivo neurotoxicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). PhIP is the most prevalent genotoxin in many meats. Adult, male Sprague-Dawley rats were subjected to acute, systemic PhIP at doses and time-points that have been extensively utilized in cancer studies (100 and 200 mg/kg for 8, 24 h) and evaluated for changes in dopaminergic, serotoninergic, GABAergic, and glutamatergic neurotransmission. PhIP exposure resulted in decreased striatal dopamine metabolite levels and dopamine turnover in the absence of changes to vesicular monoamine transporter 2 levels; other neurotransmitter systems were unaffected. Quantification of intracellular nitrotyrosine revealed higher levels of oxidative damage in dopaminergic neurons in the substantia nigra after PhIP exposure, while other neuronal populations were less sensitive. These changes occurred in the absence of an overt lesion to the nigrostriatal dopamine system. Collectively, our study suggests that acute PhIP treatment in vivo targets the nigrostriatal dopaminergic system and that PhIP should be further examined in chronic, low-dose studies for PD relevance.
Keywords: PhIP, heterocyclic amines, dopaminergic toxicity, Parkinson’s disease, oxidative stress
1. Introduction
Dietary heterocyclic amines (HCAs) are primarily produced in meat during high-temperature cooking through the Maillard reaction between amino acids, sugar and creatine, or pyrolysis of amino acids such as tryptophan (Matsumoto et al., 1981; Skog et al., 1998). Many HCAs are mutagenic (Felton et al., 1984). Potential links between HCAs and cancer have been extensively studied for the last three decades, where HCAs have been found to cause DNA damage in tissues such as the mammary gland and liver (Dietrich et al., 2011; Dobbernack et al., 2011) and induce oxidative stress (Li et al., 2013); resulting in tumor formation in several organ systems (Fujita et al., 1999; Ohgaki et al., 1987; Sugimura et al., 2004). Far less attention has been devoted to potential neurotoxic effects. However, early and recently emerging data do suggest that many HCAs should be evaluated as neurotoxins. β-carbolines, an HCA subclass, have been examined as possible Parkinson’s disease (PD) relevant neurotoxins due to structural similarity to known dopaminergic neurotoxicants (Matsubara et al., 1998). Levels of the β-carboline 1-methyl-9H-pyrido[3,4-b]indole (harmane) are elevated in blood and cerebrospinal fluid of patients with PD and essential tremor (Kuhn et al., 1996; Louis et al., 2011; Louis et al., 2013; Louis et al., 2014). In essential tremor, harmane accumulates in the brain compared to control showing that it crosses the blood-brain barrier (BBB) (Louis et al., 2013). With respect to aminoimidazoaazarenes (AIAs), another HCA subclass, early studies showed that 3-amino-1,4-dimethyl-5H-pyrido[4,3-b]indole (Trp-P-1) and 3-amino-1-methyl-5H-pyrido[4,3-b]indole (Trp-P-2) inhibit monoamine oxidase (MAO) activity, resulting in increased dopamine and decreased metabolite levels in striatum (Ichinose et al., 1988; Kojima et al., 1990; Maruyama et al., 1994). While two of these studies used PC12h rat pheochromocytoma cells and human brain synaptosomes, the third one performed unilateral infusion into the rat striatum. Although they provided valuable insight on MAO activity effects, the route of administration was not relevant to human health, and Trps are found in meat at very low levels compared to other HCAs; perhaps explaining why follow-up studies were not published (Skog et al., 1997). Taken together, these findings suggest that in vivo evaluation of HCA neurotoxicity should be a priority.
Of dietary HCAs, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) has been extensively studied, because PhIP is the most abundant AIA isolated from the crust of cooked meat, where levels may reach ~15 micrograms/kg uncooked meat (~75% of genotoxic material) (Felton et al., 1986a; Felton et al., 1986b) While PhIP has been extensively studied as a mutagen, effects on the nervous system have not yet been substantially evaluated. Recently, our group showed that PhIP is selectively toxic to dopaminergic neurons in primary rat midbrain cultures (Griggs et al., 2014). In that study, PhIP and its bioactive phase I metabolite, N-OH-PhIP induced loss of dopaminergic neurons and neurite retraction, whereas nondopaminergic neurons were spared. Further, we showed that PhIP induced oxidative damage in dopaminergic neurons, and intervention of this damage with anti-oxidants alleviated toxicity. Selective dopaminergic neurotoxicity and oxidative damage suggest potential relevance to PD, because these features are hallmarks of the disease (Cannon and Greenamyre, 2011). Given the devastating neurological symptoms of the disease, and that the causes of most cases are unknown, the identification of new etiological factors is critical. The prevalence of PhIP in the diet, the findings that it crosses the BBB, and selective toxicity in a primary culture system suggest that PhIP should be evaluated in vivo for effects on the nigrostriatal dopamine system, the primary neuropathological target in PD and PD models (Cannon and Greenamyre, 2010; Enokizono et al., 2008; Teunissen et al., 2010). To address whether PhIP might selectively target the nigrostriatal dopamine system, we evaluated effects on several neurotransmitter systems and oxidative damage after acute, systemic PhIP exposure. The goals of these first in vivo PhIP neurotoxicity studies were to use established doses from the genotoxicity literature to assess acute effects, and to determine whether time and cost intensive, chronic, long-term in vivo studies are justified and needed in the future to test PD relevance.
2. Materials and Methods
2.1. Animals
Wild type male Sprague Dawley rats (6–7 weeks old rats at 225–250 g) were purchased from Envigo (Indianapolis, IN). Rats were housed in a temperature-controlled facility with 12h light/dark cycle, and allowed to acclimate for at least 48 hours before treatments. During the entire experiment, animals received food and water ad libitum. All animal studies were approved by the Purdue Animal Care and Use Committee.
2.2 Dose Rationale and PhIP Treatment
To the best of our knowledge, the neurotoxic effects of PhIP have not been studied in mammalian systems. As the most relevant route of exposure, we chose to administer PhIP by oral gavage. The doses utilized in our study were chosen from extensive published cancer bioassays, where in vivo genotoxicity has been extensively studied as adverse outcomes. An extensive review of the literature (more than 70 studies examined; condensed citation list reported here), found that in rodents, the typical dose of PhIP administered via gavage in rats ranged from 5 to 200 mg/kg body weight (bw) daily, while the frequency might vary from a single dose to every other day for 10 weeks (Hikosaka et al., 2004; Inaguma et al., 2003; Khan et al., 2013; Naito et al., 2004). To examine acute toxicity of PhIP, we chose two doses: 100 and 200 mg/kg bw. Animals were sacrificed either 8 or 24 hours following the single oral gavage. These doses were chosen from the numerous reported regimens, because similar doses and/or time points have been shown to induce adduct formation, histopathological changes in gastric mucosa and oxidative stress in the stomach, and increase DNA adduct formation in prostate and colon (Inaguma et al., 2003; Li et al., 2013; Metry et al., 2009). It is worth noting that the doses used there are 2,000 – 40,000 times higher than the average human consumption (5 – 50 ng/kg daily). However, humans consume numerous HCAs (~30 known, likely many unknown) vs. PhIP alone, suggesting that considering dietary intake of a single HCA underestimates total HCA consumption (Augustsson et al., 1997; Roemer et al., 2016). Further, our previous studies found that of the two primary metabolic pathways, the N-hydroxylation product is far more neurotoxic than ring-hydroxylation (Griggs et al., 2014). N-hydroxylation is at least 13-fold less efficient in rodents vs. humans (rodent CYP1A2 converts far more PhIP to 4′-OH-PhIP than to the genotoxic and neurotoxic N-hydroxylated metabolite, N-OH-PhIP), suggesting that far higher doses may be required in rodents vs. humans to produce neurotoxic metabolites (Cheung et al., 2005; Griggs et al., 2014; Turesky et al., 1998). Lastly, PhIP food content can vary by >500-fold due to differences in meat types, cooking times, and temperatures (Augustsson et al., 1997; Byrne et al., 1998; Keating and Bogen, 2004; Knize et al., 1994; Layton et al., 1995; Zimmerli et al., 2001). Thus, rodents may require far higher doses of this single representative HCA to elicit neurotoxicity. Taken together, our doses in these initial acute neurotoxicity studies are higher than that which humans are exposed to, but are based on an extensive literature from cancer studies and justified by key differences between rodents and humans.
PhIP (TRC, A617000) was dissolved in corn oil (Sigma, C8237) at final concentration of 15 or 30 mg/ml for doses of 100 or 200 mg/kg bw, respectively. Suspensions were sonicated in an ultrasound water bath (Branson, 1800) at 37°C until a homogenous solution was formed with no precipitates. For oral gavage, 18-gauge feeding tubes at 3″ in length (Instech, FTP-18–75) attached to 3 ml syringes (BD Biosciences, 309657) were used. PhIP was administered only once, and animals were sacrificed after either 8 or 24 hours. Treatment groups were as following: 1) Vehicle-treated (8h and 24h pooled), 2) 100 mg PhIP/kg bw for 8h, 3) 100 mg PhIP/kg bw PhIP for 24h, 4) 200 mg PhIP/kg bw for 8h, 5) 200 mg PhIP/kg bw for 24h (n = 10 per treatment).
2.3. Tissue Collection and Preparation
After 8 or 24 hours following a single oral gavage, animals were placed in a medium size decapicone bag (BrainTree Sci, DC2000) and euthanized by decapitation. The brains were quickly removed from the skull and placed in pre-chilled PBS for 3 minutes. After brains were cut into half sagittally with the help of a sagittal brain matrix (BASi, RBM-4000S), one hemisphere was fixed in pre-chilled, 4% paraformaldehyde for histological examination. The other hemisphere was placed in a coronal brain matrix (BASi, RBM-4000C), a 2 mm section of striatum was extracted, and flash frozen in liquid nitrogen for neurochemistry.
2.4. High Performance Liquid Chromatography
Neurochemical analysis was performed as described in our previous studies (O’Neal et al., 2014; Wang et al., 2014; Wirbisky et al., 2015) using a high-performance liquid chromatography (HPLC) system that consisted of a Dionex Ultimate 3000 Model ISO-31000BM pump, a model WPS-3000TBSL autosampler, Coulochem III electrochemical detector and an ESA Coulochem data station (ThermoScientific, Waltham, MA). Samples were separated on a Waters XBridge reverse phase C18 column (150 × 3.0 mm, 3.5 μm particle size) (Waters Corp, Milford, MA). For monoamine separation, the mobile phase was: 80 mM NaH2PO4, 10% methanol, 2 mM octanesulfonic acid, 0.025 mM ethylenediaminetetraacetic acid and 0.2 mM trimethylamine, at pH 2.4. Monoamines in were detected by analytical cell set at E1 = −150mV and E2 = +350mV. For the quantification of GABA and glutamate, the mobile phase was: 0.1 M Na2HPO4, 22% methanol and 4% acetonitrile, at pH 6.75. Quantification of amino acid neurotransmitters requires derivatization. The samples were mixed with a derivatization agent (containing 0.2M o-phthalaldehyde (OPA), 0.05% 2-mercaptoethanol, 10% methanol in OPA diluent) prior to separation. Neurotransmitters in the sample was detected by analytical cell set at E1 = −150mV and E2 = +550mV. Levels of neurotransmitters tested here were calculated using area under the curve by comparison with a standard curve. Levels were normalized to total protein amount (ng neurotransmitter/mg protein).
2.5. Immunohistochemistry for Oxidative Damage and glial activation
For histological examination, tissues were prepared and processed as previously described (Wise and Cannon, 2016). For immunofluorescent staining, sections containing the substantia nigra (SN) or striatum were processed and stained as previously described (Lee et al., 2015; Wise and Cannon, 2016). The primary antibodies used here were sheep anti-tyrosine hydroxylase (TH; 1:2000; Millipore, AB152), rabbit anti-nitrotyrosine (NT; 1:500; Millipore, 06-284), mouse anti-microtubule-associated protein 2 (MAP2; 1:500; Millipore, MAB378). The secondary antibodies used were Alexa 488 anti-sheep (Jackson Immunoresearch, 713-545-147), Cy3 anti-rabbit (Jackson Immunoresearch, 711-165-152) and Alexa 647 anti-mouse (Jackson Immunoresearch, 715-605-151). High-resolution images of the stained sections showing individual neurons were captured in Nikon A1R confocal microscope (Nikon Instruments, USA) using 60X oil immersion objective.
For quantification of oxidative damage, regions of interest (ROIs) surrounding dopaminergic neurons with clear TH+ staining and visible nucleus were drawn. Mean NT intensity in each ROI was measured using the Nikon NIS Elements AR software, and normalized to the average of NT intensity of control group. Each ROI was taken into account as a single data point as previously described (Griggs et al., 2014; Horowitz et al., 2011; Wise and Cannon, 2016).
Glial activation was qualitatively assessed through stainings for astrocytes or microglial. Stainings were conducted similar to above, except the primary antibodies were: rabbit anti-Iba1 (1:500, Wako, WEE4506) or rabbit anti- glial fibrillary acidic protein (GFAP) (1:500, Dako, Z0334). Evidence of activated microglia was deemed to be cells exhibiting at a minimum: loss of processes and cell body hypertrophy will be scored as ‘activated’ (Switzer and Butt, 2011). Evidence of astrocyte activation was deemed to be increased GFAP staining intensity, increased process thickness, increased branching; often described as a ‘gnarly’ appearance (Switzer and Butt, 2011). Here, 1:12 sections (approximately 4–5/animal) were examined for evidence of glial activation.
2.6. Vesicular monoamine transporter 2 (VMAT2) and Striatal Terminal Density Quantification
Striatal tissue protein expression was assessed and quantified as described in our previous studies (Cannon et al., 2011; Lee et al., 2015). For VMAT2 or striatal terminal density quantification, 4–5 striatal sections (~1 in 12 evenly spaced sections) were stained for VMAT2 or TH, respectively. The primary antibodies used were: rabbit anti-VMAT2 (1:500, Millipore, AB1598P) or mouse anti-TH (1:2000; Millipore, MAB318) The secondary antibody used was: IRDDye 800CW anti-rabbit (1:750, LI-COR, 926-32213) or IRDye 800 anti-mouse (1:750; LI-COR, 926-32212). The striatal sections were imaged using Odyssey Infrared scanner (LI-COR, USA). Intensities of ROIs surrounding striatum were quantified and averaged for each animal (n = 4 – 5 sections/animal)
2.7. Statistical Analysis
Data from each endpoint were first subjected to the D’Agostino & Pearson normality test using GraphPad Prism 6 (GraphPad, La Jolla, CA). Datasets with a normal distribution were analyzed by one-way ANOVA followed by Tukey’s post-hoc test for multiple comparison. Data deemed not to be normally distributed were subjected to Kruskal–Wallis non-parametric test, followed by Dunn’spost-hoc test for multiple comparison. For all tests, p<0.05 was deemed significant. Data presented as mean ± standard error of mean (SEM).
3. Results
3.1. Acute PhIP treatment decreases striatal dopamine metabolites and dopamine turnover
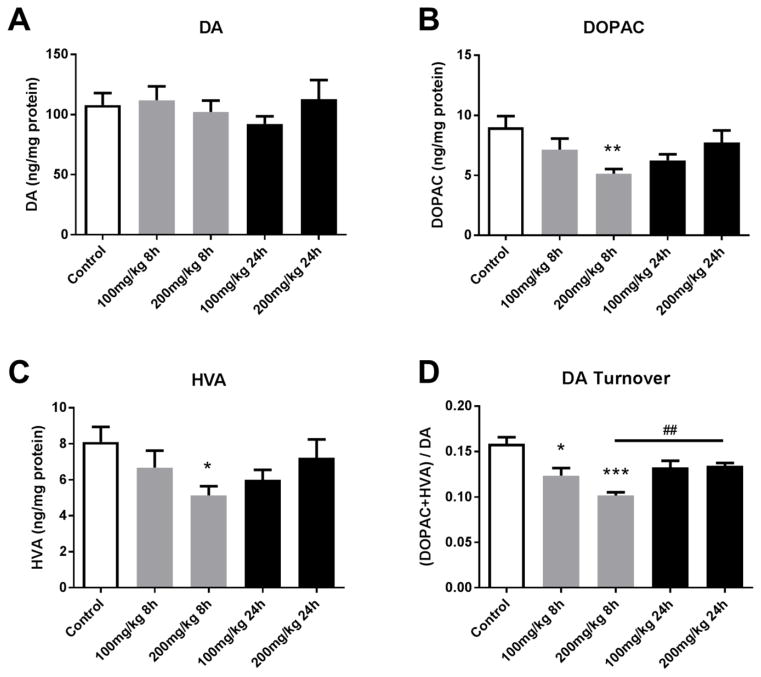
PhIP exposure alters dopaminergic neurotransmission. No significant changes were observed in striatal dopamine levels among groups (Figure 1A). However, metabolite levels were decreased. Striatal 3,4-dihydroxyphenylacetic acid (DOPAC) levels were significantly decreased at 200 mg/kg for the 8h group (9.009 ± 0.938 vs. 5.146 ± 0.383 ng/mg protein, control vs. 200 mg/kg for 8h, p<0.01) (Figure 1B). Similarly, rats treated with 200 mg/kg PhIP for 8h exhibited decreased striatal homovanillic acid (HVA) compared to control rats (8.108 ± 0.828 vs. 5.135 ± 0.510 ng/mg protein, p<0.05) (Figure 1C). We also calculated dopamine turnover, as the ratio of sum of metabolites to dopamine. Dopamine turnover was significantly decreased in all groups treated for 8h (both 100 and 200 mg/kg) compared to control groups (0.1585 ± 0.007 vs. 0.1235 ± 0.008, control vs. 100 mg/kg for 8h, p<0.05; 0.1585 ± 0.007 vs. 0.1018 ± 0.004, control vs. 200 mg/kg for 8h, p<0.001) (Figure 1D). Interestingly, striatal dopamine turnover rate in 200 mg/kg for 24h group was significantly higher than the same dose group with shorter exposure, and was not significantly different than control (0.1018 ± 0.004 vs. 0.1343 ± 0.003, 200 mg/kg for 8h vs. 24h, p<0.01), suggesting some recovery between 8 and 24 hours. These results suggest that acute PhIP exposure, especially at high doses for shorter period of time, disrupts dopamine metabolism, observed by decreases in dopamine metabolites and dopamine turnover.
Figure 1. Acute PhIP treatment decreases striatal dopamine metabolite levels and dopamine turnover.
Animals received a single oral dose of PhIP (100 or 200 mg/kg) and samples were obtained 8 or 24 h later. Striatal dopamine (DA) (A), 3,4-dihydroxyphenylacetic acid (DOPAC) (B) and homovanillic acid (HVA) (C) levels were measured using HPLC with electrochemical detection. Neurotransmitter levels were normalized to total tissue protein and expressed as ng neurotransmitter/mg protein. Dopamine turnover (D) was determined as (DOPAC + HVA)/DA. *p< 0.05, **p< 0.01, ***p<0.001 vs. control; ##p<0.01 8 h vs. 24 h (200 mg/kg); Kruskal-Wallis test, followed by Dunn’s multiple comparisons post hoc test. The data are presented as the mean ± SEM (n = 8–10/treatment).
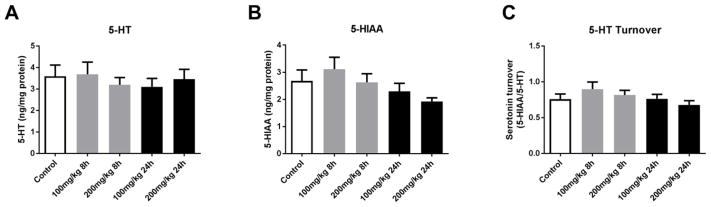
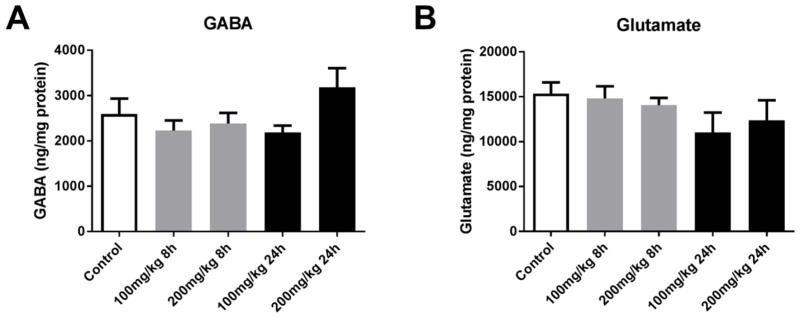
Quantification of other neurotransmitters including serotonin, the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA) and serotonin turnover; gamma-aminobutyric acid (GABA) and glutamate did not reveal significant alterations by acute PhIP treatment (Figures 2–3), further suggesting that PhIP selectively targets dopaminergic neurotransmission.
Figure 2. Acute PhIP treatment does not alter striatal serotonin or metabolite levels.
Animals received a single oral dose of PhIP (100 or 200 mg/kg) and samples were obtained 8 or 24 h later. Striatal serotonin (5-HT) (A) and 5-hydroxyindoleacetic acid (5-HIAA) (B) levels were measured using HPLC with electrochemical detection. Neurotransmitter levels were normalized to total tissue protein and expressed as ng neurotransmitter/mg protein. 5-HT turnover (C) was determined as 5-HIAA/5-HT. The data are presented as the mean ± SEM (n = 8–10/treatment).
Figure 3. Acute PhIP treatment does not alter amino acid neurotransmitter levels.
Animals received a single oral dose of PhIP (100 or 200 mg/kg) and samples were obtained 8 or 24 h later. Striatal gamma-aminobutyric acid (GABA) (A) and glutamate (B) levels were measured using HPLC with electrochemical detection. Neurotransmitter levels were normalized to total tissue protein and expressed as ng neurotransmitter/mg protein. The data are presented as the mean ± SEM (n = 8–10/treatment).
3.2. Acute PhIP treatment produces oxidative damage selectively in dopaminergic neurons in substantia nigra
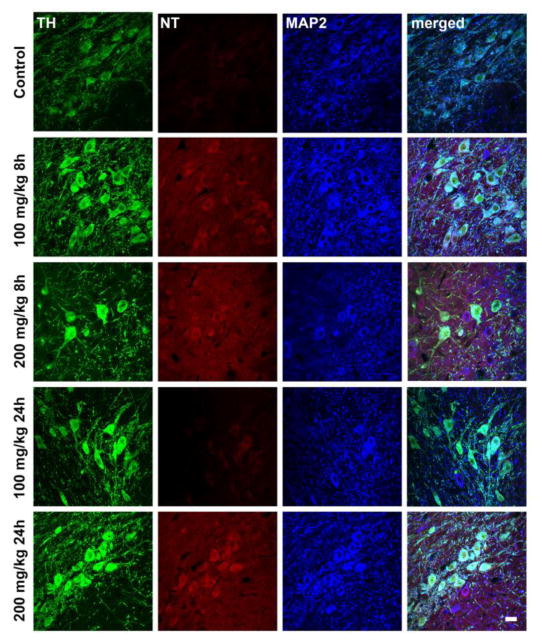
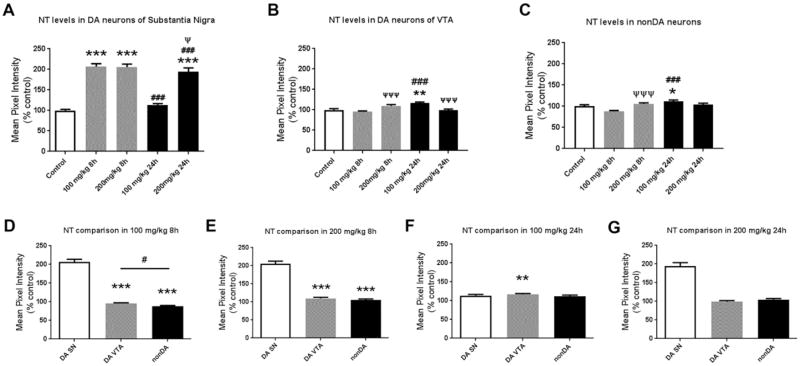
Histological analysis provides evidence of increased oxidative damage in nigral dopamine neurons after acute PhIP exposure (Figure 4). Quantification revealed statistically significant differences in NT levels (Figure 5). All PhIP-treated groups, except 100mg/kg for 24h, showed significant increases in oxidative damage in dopaminergic neurons of the SN (Fig. 5A). 100 mg/kg PhIP for 8h groups caused a two-fold increase in NT generation compared to control (100 ± 2.652 vs. 206.6 ± 7.256%, control vs. 100 mg/kg for 8h, p<0.001). A higher dose of PhIP for shorter time also caused a similar increase in NT quantifications (100 ± 2.652 vs. 205.6 ± 7.318%, control vs. 200 mg/kg for 8h, p<0.001). Interestingly, oxidative damage was recovered by time in animals treated with both lower (206.6 ± 7.256 vs. 113.4 ± 3.447%, 100 mg/kg for 8h vs. 24h, p<0.001) and higher dose of PhIP (205.6 ± 7.318 vs. 193.8 ± 9.91%, 200 mg/kg for 8h vs. 24h, p<0.001). At longer exposure (24h), only the high dose group exhibited significantly increased NT (100 ± 2.562 vs. 193.8 ± 9.91%, control vs. 200 mg/kg for 24h, p<0.001).
Figure 4. Acute PhIP treatment increases histological evidence of oxidative damage in dopaminergic neurons of substantia nigra.
Animals received a single oral dose of PhIP (100 or 200 mg/kg) and samples were obtained 8 or 24 h later. Coronal midbrain sections from control and treatment groups were stained for tyrosine hydroxylase (TH) (in green), nitrotyrosine (NT) (in red) and microtubule-associated protein 2 (MAP2) (in blue). Scale bars = 20 μm.
Figure 5. Quantitative analysis of cellular nitrotyrosine levels shows that PhIP treatment produces oxidative damage in dopaminergic neurons of substantia nigra relative to other cell populations.
To quantify oxidative damage, ROIs surrounding cell bodies of dopaminergic (DA) neurons (TH+) in substantia nigra (SN) were drawn and NT intensity in each ROI was quantified, and normalized to the mean of the control (A). The data are presented as the mean ± SEM (n = 390–671 cell bodies from 5 animals/treatment). NT levels were also quantified in DA neurons from the ventral tegmental area (VTA) (B) and non-dopaminergic (nonDA) neurons localized dorsally to the SN (C) (n = 162–287 bodies from 3 animals/treatment and n = 129–325 cell bodies from 3 animals/treatment, respectively). *p<0.05, **p<0.01, ***p<0.001 compared to control; ###p<0.001 compared to same-dose group; Ψp<0.05, ΨΨΨp<0.001 compared to same-time group; Kruskal-Wallis test followed by Dunn’s multiple comparisons post hoc test. To directly compare the magnitude of NT level changes for each of the three cell populations, normalized values were analyzed separately for each dose and time-point (D–G). **p<0.01, ***p<0.001 compared to DA SN; #p<0.05 compared to indicated group; Kruskal-Wallis test followed by Dunn’s multiple comparisons post hoc test.
We also quantified NT levels in other cell populations to determine if nigral dopamine neurons were more sensitive to PhIP-induced oxidative damage. In dopaminergic neurons of ventral tegmental area (VTA), only animals sacrificed 24h after 100 mg/kg PhIP showed increased levels of NT (100 ± 3.049 vs. 116.6 ± 2.399%, control vs. 100 mg/kg for 24h, p<0.01) (Figure 5B). NT intensity analysis in non-dopaminergic (nonDA) neurons localized in the dorsal side of SN exhibited increased oxidative damage only in 100 mg/kg for 24h group (100 ± 2.004 vs. 111.9 ± 3.11%, control vs. 100 mg/kg for 24h, p<0.05) similar to dopaminergic neurons of VTA (Figure 5C). In general, nigral dopaminergic neurons were affected at a greater magnitude than other populations analyzed (Figure 5D–G). Increases in NT intensity in dopaminergic neurons of the SN were significantly higher than the one in VTA dopaminergic neurons and nonDA neurons in animals treated with 100 mg/kg for 8h (206.6 ± 7.256 vs. 95.27 ± 1.991%, DA SN vs. DA VTA, p<0.001; 206.6 ± 7.256 vs. 87.97 ± 2.044%, DA SN vs. nonDA, p<0.001) (Figure 5D). Similar differences were observed in 200 mg/kg 8h group (205.6 ± 7.318 vs. 109.8 ± 2.953%, DA SN vs. DA VTA, p<0.001; 205.6 ± 7.318 vs. 105.7 ± 2.563%, DA SN vs. nonDA, p<0.001) (Figure 5E). Interestingly, in animals sacrificed 24h after 100 mg/kg PhIP treatment, dopaminergic neurons in VTA had significantly higher levels of NT compared to dopaminergic neurons in SN (113.4 ± 3.447 vs. 116.6 ± 2.399%, DA SN vs. DA VTA, p<0.01) (Figure 5F). Lastly, alterations in NT levels among three neuronal populations in the group of 200 mg/kg for 24h were not significantly different from each other (Figure 5G).
3.3. Striatal VMAT2 levels were unaffected by PhIP treatment
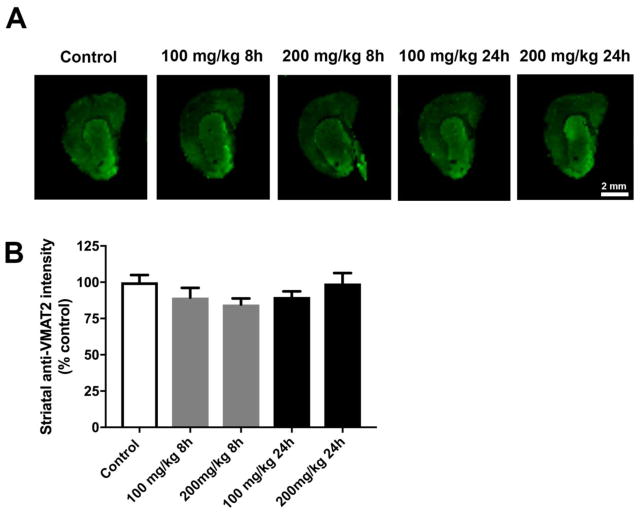
Given observed changes in dopamine metabolites, we conducted staining for VMAT2 to determine if PhIP effects dopamine packaging and release may underlie such changes. While a modest change was possibly evident, the ANOVA was not statistically significant (Figure 6). Thus, we were unable to conclude in this preliminary in vivo examination that dopamine packaging alterations are responsible for DOPAC and HVA decreases.
Figure 6. Acute PhIP treatment does alter striatal VMAT2 levels.
Coronal sections from the striatum from control and PhIP-treated animals were immunofluorescently stained for VMAT2 (A). Immunofluorescent intensity of ROIs surrounding the striatum was quantified using Image Studio version 3.1. All quantifications were normalized to average of control group (B). Data are presented as mean ± SEM (n = 4 or 5 sections/animal from 5 animals per treatment).
3.4. Acute PhIP exposure did not cause striatal terminal loss nor glial activation
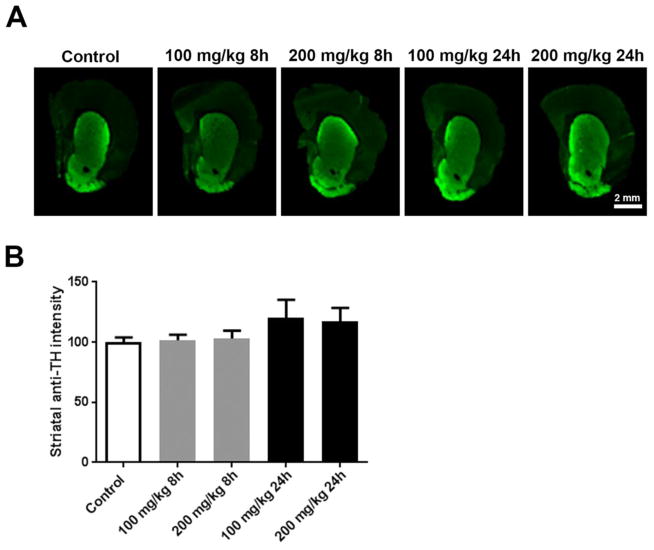
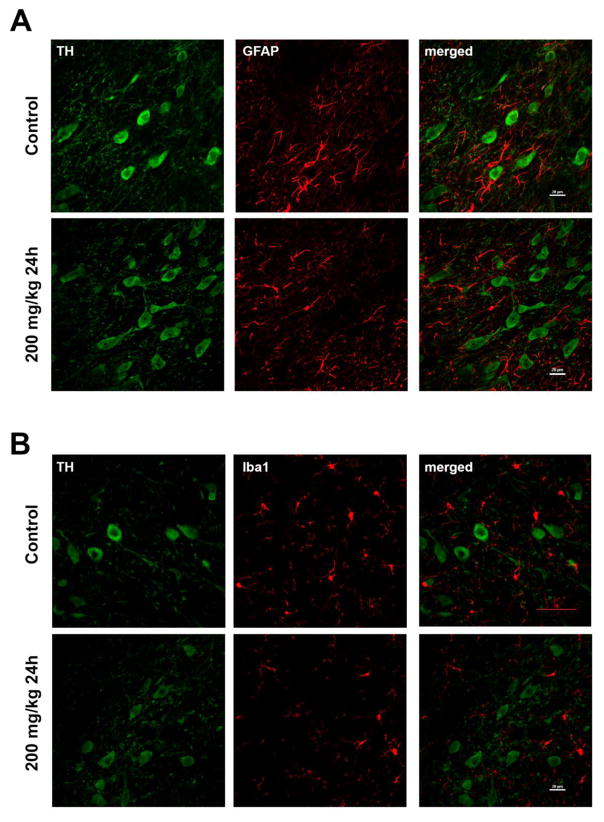
We did not anticipate an overt lesion to the nigrostriatal dopamine system in response to a single PhIP dose at acute time-points, but wanted to confirm that observed changes in neurotransmission and oxidative damage did indeed occur in the absence of neurodegeneration or overt neuroinflammation. No significant changes in striatal terminal density, as measured by striatal TH immunoreactivity were observed (Figure 7), nor was glial activation (Figure 8) suggesting the absence of an overt lesion.
Figure 7. Acute PhIP treatment does not cause striatal terminal loss.
Coronal sections from the striatum from control and PhIP-treated animals were immunofluorescently stained for tyrosine hydroxylase (TH) (A). Immunofluorescent intensity of ROIs surrounding the striatum was quantified using Image Studio version 3.1. All quantifications were normalized to average of control group (B). Data are presented as mean ± SEM (n = 4 or 5 sections/animal from 10 animals per treatment).
Figure 8. Acute PhIP exposure does not induce overt neuroinflammation in the substantia nigra.
Sections from the substantia nigra from control and 200 mg/kg for 24h groups were stained for TH (in green) and GFAP (in red) (A). The nigral sections from control and 200 mg/kg for 24h group were stained for TH (in green) and Iba1 (in red) (B). Scale bars represent 20 μm.
4. Discussion
PhIP is a prevalent dietary HCA that has been extensively investigated as a genotoxin. Here, we report that acute PhIP exposure selectively affects dopaminergic neurotransmission, where other neurotransmitter systems were spared; and PhIP produces oxidative damage in nigral dopamine neurons, which were more affected than other neuronal populations. Taken together with previous in vitro studies on PhIP-induced selective dopaminergic neurotoxicity, our results are expected to prompt chronic studies at human relevant doses to determine whether PhIP may be an important etiological factor in PD.
Decreases in DOPAC and HVA might be due to the disruption of dopamine metabolism, which is a hypothesis supported by early studies. Dopamine undergoes oxidative deamination by MAO (Cai, 2014; Meiser et al., 2013). The product is a potentially toxic intermediate 3,4-dihydroxyphenylacetaldehyde (DOPAL), which is quickly oxidized to DOPAC, primarily by aldehyde dehydrogenase (ALDH) (Elsworth and Roth, 1997). Previously, direct infusions of Trp-P-1 and Trp-P-2, have been shown to inhibit MAO, resulting in substantial decreases in DOPAC and HVA (Ichinose et al., 1988). Harmane and norharmane are also MAO inhibitors (Herraiz and Chaparro, 2006; Rommelspacher et al., 1994). A study by Manabe et al. confirmed MAO inhibition by Trp-P-1, Trp-P-2 and select β-carbolines, and showed that other HCAs tested (Glu-P-1, Glu-P-1, IQ and 4,8-DiMeIQx) do not inhibit MAO activity (Manabe et al., 1988). To, the best of our knowledge, MAO activity in response to PhIP or other abundant HCAs has not yet been explored. ALDH inhibition has been identified as a neurotoxic mechanism of action relevant to PD. Inhibition of ALDH by PD-linked pesticides such as benomyl resulted in accumulation of dopamine metabolite DOPAL (Fitzmaurice et al., 2014; Fitzmaurice et al., 2013). High levels of DOPAL also create toxicity in dopaminergic neurons due to its ability to be auto-oxidized into ortho-quinones and form protein adducts with alpha synuclein, TH and ALDH (Burke et al., 2008; Jinsmaa et al., 2011). HCAs have not yet been evaluated for effects on ALDH activity. However, considering that we found both DOPAC and HVA to be reduced, our data suggest effects of PhIP on all aspects of dopamine catabolism should be evaluated. It is worth noting that ALDH is also responsible for the production of the serotonergic metabolite, 5-HIAA, which was not altered in these experiments. Should HCAs affect ALDH, it is possible that neurotransmission alterations may differ between dopaminergic and serotonergic systems. For example, expression levels and resultant activity may differ between neuronal cell populations (Marchitti et al., 2008). Further, dopamine metabolism does seem to be more sensitive to ALDH alterations. In ALDH knockout mice, alterations in dopamine metabolites were evident as early as 5–8 months of age, whereas alterations in serotonin metabolism were not evident until 18–27 months (Wey et al., 2012). These observations further highlight the need for direct assays of the effects of HCAs on the activities of enzymes critical in neurotransmitter metabolism.
Given that metabolite levels were reduced, but not the parent neurotransmitter, whether dopamine release is affected was a logical mechanism to test. The majority of dopamine storage in dopaminergic neurons is by the vesicular monoamine transporter 2 (VMAT2). Dopamine is stored in vesicles where low-pH environment prevents auto-oxidation (Sulzer et al., 2000; Sulzer et al., 2016). Inhibition of VMAT2 impairs release of vesicle-bound dopamine, resulting in accumulation of cytosolic unpackaged dopamine, which is oxidized into toxic o-quinone aminochrome (Fuentes et al., 2007). Our preliminary examination of striatal tissue VMAT2 did not produce any significant differences. It is worth noting that our method of neurotransmitter analysis primarily measures intracellular levels. The use of microdialysis approaches may better elucidate PhIP effects on spontaneous and evoked release. Further, in vitro mechanistic studies may be better suited to examining potential PhIP-induced alterations in dopamine packaging, release, and reuptake.
Oxidative stress is critical to PD pathogenesis (Cannon and Greenamyre, 2011). In our previous study, we observed that PhIP induces oxidative damage in dopaminergic neurons in rat primary midbrain cultures (Griggs et al., 2014). In the present study, PhIP produced oxidative damage in dopamine neurons of the SN as evidenced by increased NT levels. NT formation may stem from NADPH-oxidase (especially from reactive microglia), iNOS, or mitochondrial activity and from a number of upstream reactive chemical species such as H2O2, NO•, and O2•− (Mohiuddin et al., 2006). Here, a dose-response relationship was not observed, which may have stemmed from utilization of a limited dose range. Other cell subpopulations typically spared in PD, were far less sensitive. Increased levels of reactive oxygen species (ROS) and subsequent oxidative damage are evident in most of neurotoxin-based models of PD (Cannon and Greenamyre, 2011). Dopaminergic neurons are more susceptible to oxidative stress-mediated cytotoxicity due to several reasons. For example: 1) Dopaminergic neurons have an increased burden of oxidative stress due to constant generation of ROS during dopamine metabolism. 2) Their long and unmyelinated processes forming multiple synapses cause higher energy demand increasing dependability to energy production mechanisms (Hastings and Zigmond, 1994; Matsuda et al., 2009). While these explain why dopaminergic neurons are more vulnerable compared to other neuronal populations, there are several differences between dopaminergic neurons of SN and those in VTA. Neuromelanin levels are higher in SN than VTA, resulting in enhanced accumulation of iron, of which oxidation via Fenton reaction generates high amount of ROS (Hirsch et al., 1988; Knorle, 2017). There is increased VMAT2 expression in dopaminergic neurons of VTA correlated with decreased accumulation of neuromelanin, contributing lower sensitivity to neurotoxic insults in the VTA (Liang et al., 2004). Finally, differential expressions of neurotrophic factors, dopamine transporter, and calcium and potassium channels in these two regions contribute the selective sensitivity differences between dopaminergic neurons in SN and VTA (Dragicevic et al., 2015; Smidt, 2009). With respect to the potential sources of the oxidative damage observed here, there are multiple possibilities worth considering: 1) Possible PhIP effects on enzymes critical to dopamine metabolism, which could lead to the accumulation of oxidized metabolites; 2) PhIP itself may be metabolized to reactive species such as nitroso-PhIP and N-acetoxy-PhIP (Wang et al., 2015). Here, it is worth noting that in vitro, both PhIP and a major metabolite N-OH-PhIP are selectively toxic to DA neurons (Griggs et al., 2014). N-OH-PhIP can directly react with DNA, or it can undergo conjugation reactions to form highly reactive metabolites that covalently bind to DNA (Turesky and Le Marchand, 2011). Nonetheless, given our in vitro and in vivo findings, it is possible that oxidative mechanisms are induced at lower levels and of greater importance relative to selective dopaminergic neurotoxicity.
Our data suggest that after the single PhIP dose, there is some recovery between 8 and 24 hours. We observed that dopamine metabolites and turnover decrease in animals sacrificed 8h after 200 mg/kg PhIP treatment, an effect not present after 24h. A similar phenomenon was observed in NT quantification: samples at 8h after 100 mg/kg PhIP had significantly elevated levels of NT in dopaminergic neurons of SN; after 24h, NT intensity in the same neuronal population reverted to comparable levels with control animals. These findings suggest acute but not persistent toxicity of PhIP in vivo, where there are several possible explanations for recovery. One possible explanation is that the activation of anti-oxidant response mechanisms in response to PhIP treatment might be acting to rescue the oxidative damage shown. Further, possible inhibition to dopamine metabolism enzymes or modulatory action on dopamine neurotransmission may be temporary and reversible. Another explanation for the reversion of acute PhIP toxicity is elimination of the compound short after the exposure. An early study from 1992 showed that 90% of the parent compound is excreted in urine and the rest via feces 96 h after administration in mice (Turteltaub et al., 1992). While most of the excretion of PhIP in urine happens in the first 6 hours after exposure, the levels in tissues tested, such as liver, kidney and stomach, are very low after 24h. It is worth noting that systemic PD models using environmentally relevant exposures (i.e. paraquat and rotenone) require repeated doses for several weeks or longer (Cannon and Greenamyre, 2010). Here, the goal of this first study being to investigate acute changes, neurochemical alterations occurred in the absence of an overt lesion to the nigrostriatal dopamine system as evidence by a lack of changes to striatal dopamine terminal density.
The main limitations of this study are the higher doses relative to human exposure and that evaluation was limited to acute time-points. The doses and time-points were chosen from an extensive literature and it is believed that these studies set the stage for chronic, low-dose studies with a full PD phenotype characterization that will involve behavioral, neurochemical, and neuropathological analysis. One final consideration is the future use of rodents humanized for CYP1A1/2, which potentially may require lower doses to achieve neurotoxicity (due to higher conversion to neurotoxic metabolites) (Cheung et al., 2005).
5. Conclusion
In summary, we have shown the effects of acute PhIP exposure on the nigrostriatal dopamine system through neurochemical alterations in the striatum, and oxidative damage in dopaminergic neurons in vivo. While PhIP decreased striatal dopamine metabolite levels and dopamine turnover, no alterations were observed in other neurotransmitters. Additionally, acute PhIP exposure increased oxidative damage measured by NT production in dopaminergic neurons in SN (more pronounced than VTA and non-dopaminergic neurons). Finally, the absence of dopaminergic terminal loss in the striatum and lack of glial activation in the substantia nigra suggest that alterations in dopamine neurotransmission and oxidative stress are early toxic events in response to PhIP treatment.
Highlights.
Acute PhIP exposure selectively affects dopamine neurotransmission.
Acute PhIP increases oxidative damage in nigral dopamine neurons.
Neuronal populations spared in PD are less sensitive to PhIP.
Acute PhIP exposure does not cause striatal dopaminergic terminal loss.
Acknowledgments
7. Funding
This work was supported by the National Institute of Environmental Health Sciences at the National Institutes of Health [R01ES025750 to J.R.C.].
We would like to thank Jennifer A. Hensel for her help with immunohistochemistry. We also thank Paola C. Montenegro Larrea for a critical reading of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Augustsson K, Skog K, Jagerstad M, Steineck G. Assessment of the human exposure to heterocyclic amines. Carcinogenesis. 1997;18(10):1931–5. doi: 10.1093/carcin/18.10.1931. [DOI] [PubMed] [Google Scholar]
- Burke WJ, et al. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol. 2008;115(2):193–203. doi: 10.1007/s00401-007-0303-9. [DOI] [PubMed] [Google Scholar]
- Byrne C, Sinha R, Platz EA, Giovannucci E, Colditz GA, Hunter DJ, Speizer FE, Willett WC. Predictors of dietary heterocyclic amine intake in three prospective cohorts. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1998;7(6):523–9. [PubMed] [Google Scholar]
- Cai Z. Monoamine oxidase inhibitors: promising therapeutic agents for Alzheimer’s disease (Review) Mol Med Rep. 2014;9(5):1533–41. doi: 10.3892/mmr.2014.2040. [DOI] [PubMed] [Google Scholar]
- Cannon JR, Greenamyre JT. Neurotoxic in vivo models of Parkinson’s disease recent advances. Prog Brain Res. 2010;184:17–33. doi: 10.1016/S0079-6123(10)84002-6. [DOI] [PubMed] [Google Scholar]
- Cannon JR, Greenamyre JT. The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicological sciences: an official journal of the Society of Toxicology. 2011;124(2):225–50. doi: 10.1093/toxsci/kfr239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C, Ma X, Krausz KW, Kimura S, Feigenbaum L, Dalton TP, Nebert DW, Idle JR, Gonzalez FJ. Differential metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in mice humanized for CYP1A1 and CYP1A2. Chemical research in toxicology. 2005;18(9):1471–8. doi: 10.1021/tx050136g. [DOI] [PubMed] [Google Scholar]
- Dietrich CG, Vehr AK, Martin IV, Gassler N, Rath T, Roeb E, Schmitt J, Trautwein C, Geier A. Downregulation of breast cancer resistance protein in colon adenomas reduces cellular xenobiotic resistance and leads to accumulation of a food-derived carcinogen. International journal of cancer. Journal international du cancer. 2011;129(3):546–52. doi: 10.1002/ijc.25958. [DOI] [PubMed] [Google Scholar]
- Dobbernack G, Meinl W, Schade N, Florian S, Wend K, Voigt I, Himmelbauer H, Gross M, Liehr T, Glatt H. Altered tissue distribution of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-DNA adducts in mice transgenic for human sulfotransferases 1A1 and 1A2. Carcinogenesis. 2011;32(11):1734–40. doi: 10.1093/carcin/bgr204. [DOI] [PubMed] [Google Scholar]
- Dragicevic E, Schiemann J, Liss B. Dopamine midbrain neurons in health and Parkinson’s disease: emerging roles of voltage-gated calcium channels and ATP-sensitive potassium channels. Neuroscience. 2015;284:798–814. doi: 10.1016/j.neuroscience.2014.10.037. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Roth RH. Dopamine synthesis, uptake, metabolism, and receptors: relevance to gene therapy of Parkinson’s disease. Experimental neurology. 1997;144(1):4–9. doi: 10.1006/exnr.1996.6379. [DOI] [PubMed] [Google Scholar]
- Enokizono J, Kusuhara H, Ose A, Schinkel AH, Sugiyama Y. Quantitative investigation of the role of breast cancer resistance protein (Bcrp/Abcg2) in limiting brain and testis penetration of xenobiotic compounds. Drug Metab Dispos. 2008;36(6):995–1002. doi: 10.1124/dmd.107.019257. [DOI] [PubMed] [Google Scholar]
- Felton JS, Knize MG, Shen NH, Andresen BD, Bjeldanes LF, Hatch FT. Identification of the mutagens in cooked beef. Environmental health perspectives. 1986a;67:17–24. doi: 10.1289/ehp.866717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton JS, Knize MG, Shen NH, Lewis PR, Andresen BD, Happe J, Hatch FT. The isolation and identification of a new mutagen from fried ground beef: 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Carcinogenesis. 1986b;7(7):1081–6. doi: 10.1093/carcin/7.7.1081. [DOI] [PubMed] [Google Scholar]
- Felton JS, Knize MG, Wood C, Wuebbles BJ, Healy SK, Stuermer DH, Bjeldanes LF, Kimble BJ, Hatch FT. Isolation and characterization of new mutagens from fried ground beef. Carcinogenesis. 1984;5(1):95–102. doi: 10.1093/carcin/5.1.95. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice AG, Rhodes SL, Cockburn M, Ritz B, Bronstein JM. Aldehyde dehydrogenase variation enhances effect of pesticides associated with Parkinson disease. Neurology. 2014;82(5):419–26. doi: 10.1212/WNL.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice AG, et al. Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease. Proc Natl Acad Sci U S A. 2013;110(2):636–41. doi: 10.1073/pnas.1220399110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes P, Paris I, Nassif M, Caviedes P, Segura-Aguilar J. Inhibition of VMAT-2 and DT-diaphorase induce cell death in a substantia nigra-derived cell line--an experimental cell model for dopamine toxicity studies. Chemical research in toxicology. 2007;20(5):776–83. doi: 10.1021/tx600325u. [DOI] [PubMed] [Google Scholar]
- Fujita H, Nagano K, Ochiai M, Ushijima T, Sugimura T, Nagao M, Matsushima T. Difference in target organs in carcinogenesis with a heterocyclic amine, 2-amino-3,4-dimethylimidazo[4,5-f]quinoline, in different strains of mice. Jpn J Cancer Res. 1999;90(11):1203–6. doi: 10.1111/j.1349-7006.1999.tb00696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs AM, Agim ZS, Mishra VR, Tambe MA, Director-Myska AE, Turteltaub KW, McCabe GP, Rochet JC, Cannon JR. 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) is selectively toxic to primary dopaminergic neurons in vitro. Toxicological sciences: an official journal of the Society of Toxicology. 2014;140(1):179–89. doi: 10.1093/toxsci/kfu060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings TG, Zigmond MJ. Identification of catechol-protein conjugates in neostriatal slices incubated with [3H]dopamine: impact of ascorbic acid and glutathione. Journal of neurochemistry. 1994;63(3):1126–32. doi: 10.1046/j.1471-4159.1994.63031126.x. [DOI] [PubMed] [Google Scholar]
- Herraiz T, Chaparro C. Human monoamine oxidase enzyme inhibition by coffee and beta-carbolines norharman and harman isolated from coffee. Life sciences. 2006;78(8):795–802. doi: 10.1016/j.lfs.2005.05.074. [DOI] [PubMed] [Google Scholar]
- Hikosaka A, Asamoto M, Hokaiwado N, Kato K, Kuzutani K, Kohri K, Shirai T. Inhibitory effects of soy isoflavones on rat prostate carcinogenesis induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Carcinogenesis. 2004;25(3):381–7. doi: 10.1093/carcin/bgh031. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature. 1988;334(6180):345–8. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- Horowitz MP, et al. Single-cell redox imaging demonstrates a distinctive response of dopaminergic neurons to oxidative insults. Antioxidants & redox signaling. 2011;15(4):855–71. doi: 10.1089/ars.2010.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose H, Ozaki N, Nakahara D, Naoi M, Wakabayashi K, Sugimura T, Nagatsu T. Effects of heterocyclic amines in food on dopamine metabolism in nigro-striatal dopaminergic neurons. Biochemical pharmacology. 1988;37(17):3289–95. doi: 10.1016/0006-2952(88)90641-7. [DOI] [PubMed] [Google Scholar]
- Inaguma S, Takahashi S, Ohnishi H, Suzuki S, Cho YM, Shirai T. High susceptibility of the ACI and spontaneously hypertensive rat (SHR) strains to 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) prostate carcinogenesis. Cancer science. 2003;94(11):974–9. doi: 10.1111/j.1349-7006.2003.tb01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinsmaa Y, Florang VR, Rees JN, Mexas LM, Eckert LL, Allen EM, Anderson DG, Doorn JA. Dopamine-derived biological reactive intermediates and protein modifications: Implications for Parkinson’s disease. Chem Biol Interact. 2011;192(1–2):118–21. doi: 10.1016/j.cbi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating GA, Bogen KT. Estimates of heterocyclic amine intake in the US population. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2004;802(1):127–33. doi: 10.1016/j.jchromb.2003.10.047. [DOI] [PubMed] [Google Scholar]
- Khan JA, Jalal JA, Ioanndes C, Moselhy SS. Impact of aqueous doash extract on urinary mutagenicity in rats exposed to heterocyclic amines. Toxicology and industrial health. 2013;29(2):142–8. doi: 10.1177/0748233711427053. [DOI] [PubMed] [Google Scholar]
- Knize MG, Dolbeare FA, Carroll KL, Moore DH, 2nd, Felton JS. Effect of cooking time and temperature on the heterocyclic amine content of fried beef patties. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 1994;32(7):595–603. doi: 10.1016/0278-6915(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Knorle R. Neuromelanin in Parkinson’s Disease: from Fenton Reaction to Calcium Signaling. Neurotox Res. 2017 doi: 10.1007/s12640-017-9804-z. [DOI] [PubMed] [Google Scholar]
- Kojima T, Naoi M, Wakabayashi K, Sugimura T, Nagatsu T. 3-amino-1-methyl-5H-pyrido[4,3-b]indole (Trp-P-2) and other heterocyclic amines as inhibitors of mitochondrial monoamine oxidases separated from human brain synaptosomes. Neurochemistry international. 1990;16(1):51–7. doi: 10.1016/0197-0186(90)90122-a. [DOI] [PubMed] [Google Scholar]
- Kuhn W, Muller T, Grosse H, Rommelspacher H. Elevated levels of harman and norharman in cerebrospinal fluid of parkinsonian patients. J Neural Transm (Vienna) 1996;103(12):1435–40. doi: 10.1007/BF01271257. [DOI] [PubMed] [Google Scholar]
- Layton DW, Bogen KT, Knize MG, Hatch FT, Johnson VM, Felton JS. Cancer risk of heterocyclic amines in cooked foods: an analysis and implications for research. Carcinogenesis. 1995;16(1):39–52. doi: 10.1093/carcin/16.1.39. [DOI] [PubMed] [Google Scholar]
- Lee JW, Tapias V, Di Maio R, Greenamyre JT, Cannon JR. Behavioral, neurochemical, and pathologic alterations in bacterial artificial chromosome transgenic G2019S leucine-rich repeated kinase 2 rats. Neurobiology of aging. 2015;36(1):505–18. doi: 10.1016/j.neurobiolaging.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Tian J, Li W, Xie J. Effects of 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP) on histopathology, oxidative stress, and expression of c-fos, c-jun and p16 in rat stomachs. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2013;55:182–91. doi: 10.1016/j.fct.2012.12.054. [DOI] [PubMed] [Google Scholar]
- Liang CL, Nelson O, Yazdani U, Pasbakhsh P, German DC. Inverse relationship between the contents of neuromelanin pigment and the vesicular monoamine transporter-2: human midbrain dopamine neurons. The Journal of comparative neurology. 2004;473(1):97–106. doi: 10.1002/cne.20098. [DOI] [PubMed] [Google Scholar]
- Louis ED, Factor-Litvak P, Gerbin M, Jiang W, Zheng W. Blood harmane concentrations in 497 individuals relative to coffee, cigarettes, and food consumption on the morning of testing. Journal of toxicology. 2011;2011:628151. doi: 10.1155/2011/628151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Factor-Litvak P, Liu X, Vonsattel JP, Galecki M, Jiang W, Zheng W. Elevated brain harmane (1-methyl-9H-pyrido[3,4-b]indole) in essential tremor cases vs. controls. Neurotoxicology. 2013;38:131–5. doi: 10.1016/j.neuro.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Michalec M, Jiang W, Factor-Litvak P, Zheng W. Elevated blood harmane (1-methyl-9H-pyrido[3,4-b]indole) concentrations in Parkinson’s disease. Neurotoxicology. 2014;40:52–6. doi: 10.1016/j.neuro.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe S, Kanai Y, Ishikawa S, Wada O. Carcinogenic tryptophan pyrolysis products potent inhibitors of type A monoamine oxidase and the platelet response to 5-hydroxytryptamine. J Clin Chem Clin Biochem. 1988;26(5):265–70. doi: 10.1515/cclm.1988.26.5.265. [DOI] [PubMed] [Google Scholar]
- Marchitti SA, Brocker C, Stagos D, Vasiliou V. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol. 2008;4(6):697–720. doi: 10.1517/17425255.4.6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama W, Ota A, Takahashi A, Nagatsu T, Naoi M. Food-derived heterocyclic amines, 3-amino-1,4-dimethyl-5H-pyrido[4,3-b]indole and related amines, as inhibitors of monoamine metabolism. Journal of neural transmission. Supplementum. 1994;41:327–33. doi: 10.1007/978-3-7091-9324-2_43. [DOI] [PubMed] [Google Scholar]
- Matsubara K, Gonda T, Sawada H, Uezono T, Kobayashi Y, Kawamura T, Ohtaki K, Kimura K, Akaike A. Endogenously occurring beta-carboline induces parkinsonism in nonprimate animals: a possible causative protoxin in idiopathic Parkinson’s disease. Journal of neurochemistry. 1998;70(2):727–35. doi: 10.1046/j.1471-4159.1998.70020727.x. [DOI] [PubMed] [Google Scholar]
- Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, Kaneko T. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29(2):444–53. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Yoshida D, Tomita H. Determination of mutagens, amino-alpha-carbolines in grilled foods and cigarette smoke condensate. Cancer letters. 1981;12(1–2):105–10. doi: 10.1016/0304-3835(81)90045-8. [DOI] [PubMed] [Google Scholar]
- Meiser J, Weindl D, Hiller K. Complexity of dopamine metabolism. Cell Commun Signal. 2013;11(1):34. doi: 10.1186/1478-811X-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metry KJ, Neale JR, Bendaly J, Smith NB, Pierce WM, Jr, Hein DW. Effect of N-acetyltransferase 2 polymorphism on tumor target tissue DNA adduct levels in rapid and slow acetylator congenic rats administered 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine or 2-amino-3,8-dimethylimidazo-[4,5-f]quinoxaline. Drug Metab Dispos. 2009;37(11):2123–6. doi: 10.1124/dmd.109.029512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohiuddin I, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C. Nitrotyrosine and chlorotyrosine: clinical significance and biological functions in the vascular system. J Surg Res. 2006;133(2):143–9. doi: 10.1016/j.jss.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Naito A, Suzuki A, Ueda S, Nomoto H, Toriyama-Baba H, Asamoto M, Tsuda H. Preferential mammary carcinogenic effects of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in human c-Ha-ras proto-oncogene transgenic rats. Cancer Sci. 2004;95(5):399–403. doi: 10.1111/j.1349-7006.2004.tb03222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neal SL, Lee JW, Zheng W, Cannon JR. Subacute manganese exposure in rats is a neurochemical model of early manganese toxicity. Neurotoxicology. 2014;44:303–13. doi: 10.1016/j.neuro.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgaki H, Hasegawa H, Suenaga M, Sato S, Takayama S, Sugimura T. Carcinogenicity in mice of a mutagenic compound, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) from cooked foods. Carcinogenesis. 1987;8(5):665–8. doi: 10.1093/carcin/8.5.665. [DOI] [PubMed] [Google Scholar]
- Roemer E, Meisgen T, Diekmann J, Conroy L, Stabbert R. Heterocyclic aromatic amines and their contribution to the bacterial mutagenicity of the particulate phase of cigarette smoke. Toxicology letters. 2016;243:40–7. doi: 10.1016/j.toxlet.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Rommelspacher H, May T, Salewski B. Harman (1-methyl-beta-carboline) is a natural inhibitor of monoamine oxidase type A in rats. European journal of pharmacology. 1994;252(1):51–9. doi: 10.1016/0014-2999(94)90574-6. [DOI] [PubMed] [Google Scholar]
- Skog K, Augustsson K, Steineck G, Stenberg M, Jagerstad M. Polar and non-polar heterocyclic amines in cooked fish and meat products and their corresponding pan residues. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 1997;35(6):555–65. doi: 10.1016/s0278-6915(97)00021-5. [DOI] [PubMed] [Google Scholar]
- Skog KI, Johansson MA, Jagerstad MI. Carcinogenic heterocyclic amines in model systems and cooked foods: a review on formation, occurrence and intake. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 1998;36(9–10):879–96. doi: 10.1016/s0278-6915(98)00061-1. [DOI] [PubMed] [Google Scholar]
- Smidt MP. Specific vulnerability of substantia nigra compacta neurons. Journal of neural transmission. Supplementum. 2009;(73):39–47. doi: 10.1007/978-3-211-92660-4_3. [DOI] [PubMed] [Google Scholar]
- Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer science. 2004;95(4):290–9. doi: 10.1111/j.1349-7006.2004.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, et al. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc Natl Acad Sci U S A. 2000;97(22):11869–74. doi: 10.1073/pnas.97.22.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Cragg SJ, Rice ME. Striatal dopamine neurotransmission: regulation of release and uptake. Basal Ganglia. 2016;6(3):123–148. doi: 10.1016/j.baga.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer RC, Butt MT. Histological markers of neurotoxicity (nonfluorescent) In: Bolon B, Butt MT, editors. Fundamental neuropathology for pathologists and toxicologists. Wiley; Hoboken: 2011. pp. 181–190. [Google Scholar]
- Teunissen SF, Vlaming ML, Rosing H, Schellens JH, Schinkel AH, Beijnen JH. Development and validation of a liquid chromatography-tandem mass spectrometry assay for the analysis of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and its metabolite 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine (N-OH-PhIP) in plasma, urine, bile, intestinal contents, faeces and eight selected tissues from mice. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2010;878(25):2353–62. doi: 10.1016/j.jchromb.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Turesky RJ, Garner RC, Welti DH, Richoz J, Leveson SH, Dingley KH, Turteltaub KW, Fay LB. Metabolism of the food-borne mutagen 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in humans. Chemical research in toxicology. 1998;11(3):217–25. doi: 10.1021/tx9701891. [DOI] [PubMed] [Google Scholar]
- Turesky RJ, Le Marchand L. Metabolism and biomarkers of heterocyclic aromatic amines in molecular epidemiology studies: lessons learned from aromatic amines. Chemical research in toxicology. 2011;24(8):1169–214. doi: 10.1021/tx200135s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turteltaub KW, Vogel JS, Frantz CE, Shen N. Fate and distribution of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in mice at a human dietary equivalent dose. Cancer Res. 1992;52(17):4682–7. [PubMed] [Google Scholar]
- Wang Y, Lee JW, Oh G, Grady SR, McIntosh JM, Brunzell DH, Cannon JR, Drenan RM. Enhanced synthesis and release of dopamine in transgenic mice with gain-of-function alpha6* nAChRs. Journal of neurochemistry. 2014;129(2):315–27. doi: 10.1111/jnc.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Peng L, Bellamri M, Langouet S, Turesky RJ. Mass Spectrometric Characterization of Human Serum Albumin Adducts Formed with N-Oxidized Metabolites of 2-Amino-1-methylphenylimidazo[4,5-b]pyridine in Human Plasma and Hepatocytes. Chemical research in toxicology. 2015;28(5):1045–59. doi: 10.1021/acs.chemrestox.5b00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wey MC, Fernandez E, Martinez PA, Sullivan P, Goldstein DS, Strong R. Neurodegeneration and motor dysfunction in mice lacking cytosolic and mitochondrial aldehyde dehydrogenases: implications for Parkinson’s disease. PloS one. 2012;7(2):e31522. doi: 10.1371/journal.pone.0031522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirbisky SE, Weber GJ, Sepulveda MS, Xiao C, Cannon JR, Freeman JL. Developmental origins of neurotransmitter and transcriptome alterations in adult female zebrafish exposed to atrazine during embryogenesis. Toxicology. 2015;333:156–167. doi: 10.1016/j.tox.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise JP, Jr, Cannon J. From the Cover: Alterations in Optineurin Expression and Localization in Pre-clinical Parkinson’s Disease Models. Toxicological sciences: an official journal of the Society of Toxicology. 2016;153(2):372–81. doi: 10.1093/toxsci/kfw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli B, Rhyn P, Zoller O, Schlatter J. Occurrence of heterocyclic aromatic amines in the Swiss diet: analytical method, exposure estimation and risk assessment. Food additives and contaminants. 2001;18(6):533–51. doi: 10.1080/02652030119545. [DOI] [PubMed] [Google Scholar]