Abstract
High circulatory insulin and leptin followed by underlying inflammation are often ascribed to the ectopic manifestations in non-alcoholic fatty liver disease (NAFLD) but the exact molecular pathways remain unclear. We have shown previously that CYP2E1-mediated oxidative stress and circulating leptin in NAFLD is associated with renal disease severity. Extending the studies, we hypothesized that high circulatory leptin in NAFLD causes renal mesangial cell activation and tubular inflammation via a NOX2 dependent pathway that upregulates proinflammatory miR21. High-fat diet (60% kcal) was used to induce fatty liver phenotype with parallel insulin and leptin resistance. The kidneys were probed for mesangial cell activation and tubular inflammation that showed accelerated NASH phenotype and oxidative stress in the liver. Results showed that NAFLD kidneys had significant increases in α-SMA, a marker of mesangial cell activation, miR21 levels, tyrosine nitration and renal inflammation while they were significantly decreased in leptin and p47 phox knockout mice. Micro RNA21 knockout mice showed decreased tubular immunotoxicity and proinflammatory mediator release. Mechanistically, use of NOX2 siRNA or apocynin,phenyl boronic acid (FBA), DMPO or miR21 antagomir inhibited leptin primed-miR21-mediated mesangial cell activation in vitro suggesting a direct role of leptin-mediated NOX-2 in miR21-mediated mesangial cell activation. Finally, JAK-STAT inhibitor completely abrogated the mesangial cell activation in leptin-primed cells suggesting that leptin signaling in the mesangial cells depended on the JAK-STAT pathway. Taken together the study reports a novel mechanistic pathway of leptin-mediated renal inflammation that is dependent on NOX-2-miR21 axis in ectopic manifestations underlying NAFLD-induced co-morbidities.
Keywords: Leptin, NOX-2, NADPH, Mesangial cells, miR21, Oxidative stress, NAFLD, JAK/STAT, siRNA
1. Introduction
Fatty liver is the most common cause of chronic liver injury [1]. Non-alcoholic fatty liver disease (NAFLD) is well-defined as the excessive accumulation of fat (> 5%) in the liver without excessive consumption of alcohol and often considered as a benign disease in the background of altered mediators of metabolic syndrome and pro-inflammatory immune response [1], [2]. If untreated, NAFLD can progress from simple steatosis to complex stage of nonalcoholic steatohepatitis (NASH), hepatic fibrosis and hepatocellular carcinoma [3] following a second or multiple hits from oxidative stress, underlying low grade sterile inflammation or environmental factors [4]. The contribution of NAFLD/NASH in several ectopic diseases and pathological conditions such as type 2 diabetes (T2DM), cardiovascular disease (CVD), Chronic Kidney Disease (CKD) has been discussed in previous studies [5], [6].
Recently, enormous interest has been generated following multiple studies dealing with NAFLD/NASH-associated CKD. [7]. We have shown previously that accelerated glomerular pathology follows a NASH phenotype [8]. Additionally, CKD represents a major health concern in adults over age 65 years and it covers 25% of the western populations [9]. Commonly, CKD is described as decreased estimated glomerular filtration (eGFR) and/or increased proteinuria. At the advanced stage, CKD patients develop the end-stage renal disease because of the high risk of cardiovascular disease. Further, NAFLD is characterized by metabolic disturbances including insulin resistance and leptin resistance and inflammatory response in the liver. Leptin is a cytokine mainly produced by adipocytes and plays a proinflammatory role in the liver [10], [11]. The primary function of leptin is to regulate satiety and control fat metabolism [12]. The increased levels of circulatory leptin (hyperleptinemia) found in NAFLD can influence inflammatory response in an ectopic organ such as the kidney in addition to other co-morbidities already reported in the clinics [7], [13], [14]. In the advanced stage of NAFLD/NASH, the common factor associated with NAFLD/NASH progression such as oxidative stress, activations of JAK/STAT, TGF-β signaling, renin-angiotensin system, TLR4 pathways and release of inflammatory cytokine may strongly associate with kidney disease [8], [15], [16], [17], [18], [19].
We and others have previously shown that oxidative stress is the key regulator in NASH via second hit/multiple hit theory [10], [20], [21], [22]. The redox stress generated by xenobiotic enzyme Cytochrome p450 2E1 (CYP2E1) via a free-radical mechanism generates reactive oxygen species (ROS) and reactive nitrative species (RNS) in the liver [23]. The oxidative stress resulted from reductive metabolism of CYP2E1, can accelerate metabolic disturbances, leptin release and trigger host innate immune response (ref). The cascade of redox signaling, presence of damage associated molecular patterns (DAMPS) and presence of high circulatory leptin can stimulate NADPH oxidase system in distal organs such as the kidney [10], [24], [25]. The NADPH oxidase (NOX) is commonly expressed in both phagocytic and non-phagocytic cells [26]. In Kidney, NADPH oxidases have divergent localization in the renal cells which include mesangial cells, tubular cells endothelial cells and podocytes [27]. Though NOX4 is predominant in the kidney, NOX2 is primarily expressed on mesangial cells and podocytes, but their functional significance remains unclear [28]. The NOX2 is primarily composed of several subunits mainly GP91 phox (membrane subunits) and P47 phox (cytosolic subunit)[26]. When the proper signal stimulates NOX2 activation it leads to the alignment of the cytosolic subunit (p47phox) to the membrane subunit (gp91phox). Localized NOX2 is involved in superoxide generation and can lead to the increased burden of oxidative stress and kidney inflammation [29]. NOX2 has also been shown to activate the TLR4 system which includes recruitment of TLR4 into lipid rafts and further receptor dimerization and activation in several inflammatory responses [16], [30]. Previously, we and others have shown that NOX2, when stimulated by leptin, can generate peroxynitrite in different disease model such as intestinal inflammation or inflammatory bowel disease (IBD), liver inflammation in NASH, [16], [25], [31]. Since evidence is scarce to explain the specific NOX2 mediator involved in TLR4 activation in the mesangial cell, we chose to investigate the role of peroxynitrite in NOX2 mediated TLR4 activation in kidney inflammation. Recently, it has been shown that the highly reactive oxidant, peroxynitrite mediates glomerular lesion by JAK/STAT signaling pathway [32]. In our previous studies, Leptin has been shown to stimulate epigenetic regulation and microRNA induction in experimental NASH [33]. The Non-coding microRNA (miR) negatively regulates target protein by degrading mRNA (transcript degradation) and/or interfering in the process of protein translation. miR21 has been significantly upregulated in several inflammatory responses such as experimental NASH, hematopoietic cells, and allergic response [33], [34], [35]. In both liver and kidney injury, miR21 plays a key role in fibroblast activation and is a prominent regulatory factor in Smad7/TGF-beta signaling [15], [36], [37]. Considering these facts, in the present study, we hypothesize that increased circulatory leptin due to CYP2E1-induced oxidative stress in NAFLD activates mesangial cells and renal inflammatory response. Further leptin, acting via JAK/STAT-mediated NOX2 activation in the glomerulus induces miR21-mediated proinflammatory pathway that affects the progression of kidney disease. This present study uses an established invivo mouse model of CYP2E1-primed NASH, leptin knockout (KO) mice, p47 phox KO mice and miR21 KO mice along with kidney mesangial cells to show the mechanism of NOX2-initiated renal inflammation.
2. Materials and methods
Bromodichloromethane (BDCM), a known substrate for CYP2E1-mediated oxidative stress in the liver and corn oil were purchased from Sigma-Aldrich (St. Louis, MO). Anti–α-SMA, anti–IL-1β, anti–TNF-α, anti-TLR-4, Anti-3-Nitrotyrosine (3-NT), anti-P47phox and anti-GP91phox primary antibodies were purchased from Abcam (Cambridge, MA). Species-specific biotinylated conjugated secondary antibody and streptavidin -HRP were purchased from Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA). Wild-type and gene-specific KO mice were purchased from The Jackson Laboratories (Bar Harbor, ME). NOX2-siRNA, RNAiMAX-Lipofectamine, Fluorescence-conjugated (Alexa fluor) secondary antibodies and ProLong Gold antifade mounting media with DAPI were bought from Thermo Fisher Scientific (Waltham, MA). Animal diets were purchased from Research Diets (New Brunswick, NJ). All other chemicals were purchased from Sigma Aldrich unless otherwise specified. Paraffinized tissue sections on slides were done by IRF, University of South Carolina School of Medicine and AML laboratories (Baltimore, MD).
2.1. Cell culture
Mouse kidney Mesangial cell line (CRL-1927) was purchased from ATCC (Manassas, VA) and maintained in Dulbecco's modified eagles medium, Corning (Tewksbury, MA). The media was supplemented with 10% fetal bovine serum, Atlanta Biologicals (Norcross, GA), 2 mM glutamine, 100 U/ml Penicillin, and 100 μg/ml streptomycin; Gibco (Grand Island, NY). Cells were serum-starved (DMEM with 0.25% FBS) and incubated overnight at 37 °C in a humidified atmosphere of 5% CO2 before any treatment. The cells were then treated with recombinant mouse Leptin 100 ng/ml, Biovision (Milpitas, CA), miR21 inhibitor 20 µM (Qiagen, Valencia, CA), JAK-STAT inhibitor (Ruxolitinib) 10 µM (Invivogen, San Diego, CA), Apocynin 100 µM as an inhibitor of NADPH oxidase activity, Phenyl Boronic Acid (FBA) 100 µM as an scavenger for peroxynitrite, Spin trap DMPO 100 µM (5,5 dimethyl-1- Pyrroline N- oxide) (Alexis biochemical, San Diego, CA) as an scavenger of free radicals via spin trapping used either separately (respective control groups) or in combination with Leptin (treated groups) for 24 h at 37 °C in a humidified atmosphere of 5% CO2. Upon completion of the experiment, cells were lysed in Trizol, Invitrogen (Grand Island, NY) for mRNA extraction. Another set of cells were plated on microscopic coverslips MatTek Corp, (Ashland, MA) and treated similarly. The adhered cells on coverslips were used for immune-fluorescence staining after completion of the treatment.
2.2. siRNA inhibition of NOX2
Actively growing mesangial cells were seeded into the 6 well plates at low density. At 60–70% confluency, the NOX2-siRNA transfection was done using Lipofectamine RNAiMAX transfection reagent in Opti-MEM media following manufacturer protocol (Invitrogen). Briefly, Lipofectamine RNAiMAX reagent was diluted in the Opti-MEM medium as recommended. Similarly, NOX2-siRNA (60 pmol) was diluted in Opti-MEM medium and it was added to the diluted lipofectamine RNAiMAX solution. Further, it was incubated at 37 °C incubator for 5 min. The siRNA-lipid complex formed was added to the cells in the final concentration of 25 pmol. The cells were then incubated for 48 h at 37 °C/5%CO2 incubator. The growth and health of cells were constantly monitored. After 48 h, culture media was changed and siRNA and lipofectamine complex was added to the cells to ensure continuous blocking of NOX2. The cells were then treated with recombinant mouse leptin (100 ng/ml) and incubated for 24 h at 37 °C/5%CO2 incubator. The control cells were treated with Lipofectamine-RNAiMAX solution without siRNA. Upon completion of the experiment, cells were lysed in Qiazol for mRNA and miRNA extraction. Another set of mesangial cells were plated on coverslips and treated with siRNA and leptin as mentioned. These cells on coverslips were used for immunofluorescence microscopic imaging.
2.3. Mouse models
All mice were housed one per cage at 23–24 °C with a 12-h/12-h light/dark cycle with ab-libitum access to food and water. The mice had been treated in strict accordance with the NIH guideline for Humane Care and Use of Laboratory Animals and local IACUC standards. All experiments have been approved by the University of South Carolina at Columbia. Pathogen-free, adult male mice were used in this study and all the wild-type and gene-specific knockout mice were from the C57BL/6J background. The wild-type mice and mice that contained the disrupted ob/ob gene (B6.Cg-Lepob/J) (Leptin KO), miRNA21 gene (B6;129S6-Mir21atm1Yoli/J)(miR21KO) and P47phox gene (alias Ncf1) (P47 phox KO; B6(Cg)-Ncf1m1J/J) were purchased from Jackson Laboratory (Bar Harbor, ME) and fed with high fat diet (60% kcal) from 6 weeks until 16 weeks and used as a diet-induced NAFLD model. Before and after the completion of BDCM exposure body weight of all mice of all study groups were measured (Supplementary material Table 1) and then sacrificed upon completion of exposure of bromodichloromethane (BDCM). Immediately after anesthesia, blood was drawn using cardiac puncture to collect serum for the experiments. The mice both kidneys were collected right after terminal surgery and a piece of kidney tissue was stored for further mRNA, miRNA and protein extraction and analysis. A piece of both kidneys was sliced and fixed with 10% formalin and then paraffin embedded for further histopathology or immunostaining.
2.4. Induction of CYP2E1-mediated redox stress in underlying NAFLD
We and others have shown previously that CYP2E1-mediated redox stress is a determining factor for progressive disease development in NAFLD. The high-fat diet fed wild-type mice (NAFLD model) and high-fat diet fed gene knockout mice at 16 weeks were administered BDCM (1 mmol/kg, diluted in corn oil) via intraperitoneal route, twice a week for 4 weeks to estimate the effects of chronic CYP2E1-mediated redox stress. A group of high-fat diet fed mice (NAFLD) that had simple steatosis and were not injected with BDCM and served as a non-redox primed control against high-fat diet fed wild-type mice injected with BDCM (NAFLD+BDCM). Upon completion of the experiment, the mouse liver slices were analyzed for NASH pathophysiology. An average NASH CRN score for all study groups mouse was listed in Supplementary material Table 2.
2.5. Laboratory analysis
2.5.1. Immunohistochemistry
The kidneys were collected from each mouse and fixed in 10% neutral buffered formalin (Sigma Aldrich, Missouri, USA) and paraffin embedded. These formalin-fixed, paraffin embedded tissues were cut into 5 µm thick sections. These sections were deparaffinized using a standard protocol. Briefly, sections were put in xylene twice for 3 min, then with xylene:ethanol (1:1) for 3 min, and rehydrated via a series of ethanol (twice with 100%, 95%, 70%, 50%) 3 min each, twice with distilled water, and finally rinsed twice with PBS (Sigma-Aldrich). Epitope retrieval of the sections was completed using epitope retrieval solution and steamer (IHC-World, Woodstock, MD) for 45 min. 3%H202 was used for 10 min to block the endogenous peroxidases. The primary antibodies like anti-αSMA, anti–IL1β, anti–TNFα, anti-TLR4 (Abcam, Cambridge, MA) were used overnight in recommended dilutions. Species-specific biotinylated conjugated secondary antibody and streptavidin conjugated with HRP were used to perform antigen-specific immunohistochemistry following manufacturer's protocols. 3,3′ Diaminobenzidine (Sigma-Aldrich, St. Louis, MO) was used as a chromogenic substrate. Kidney sections were counter-stained with Mayer's hematoxylin (Sigma-Aldrich). Phosphate buffer saline was used for washing three times between the steps. Sections were finally mounted in Simpo mount (GBI Laboratories, Mukilteo, WA) and observed under a 20×, 40× and 60× objectives using an Olympus BX51 microscope (Olympus, America). Morphometric analysis was done using CellSens Software from Olympus America (Center Valley, PA).
2.5.2. Immunofluorescence
For tissue: Formalin-fixed, paraffin-embedded tissues sections were deparaffinized using a standard protocol. Epitope retrieval of the deparaffinized sections was completed using epitope retrieval solution and steamer (IHC-World, Woodstock, MD) following the manufacturer's instructions. The primary antibody 3-NT (Abcam, Cambridge, MA) and anti-gp-91phox, anti-p47phox dual labeling were used at the recommended dilution. Species-specific anti-IgG secondary antibodies conjugated with Alexa Fluor 633 (Invitrogen, California, USA) was used against anit-3-NT and anti-gp-91phox, and Alexa Fluor 488 (Invitrogen) was used against anti-P-47phox. The sections were mounted in a ProLong gold antifade reagent with DAPI (Life technologies, EU, OR). Lastly, images were taken under 40× objectives using Olympus BX51 microscope.
Cultured cells: After completion of the treatments of the aforementioned cell culture section, cells attached on coverslips were fixed with 10% neutral buffered saline. After the cells were washed with PBS containing 0.1% Triton X (Sigma), they were blocked with 3% BSA, 0.2% Tween (Fisher), 10% FBS in PBS. Cells were incubated with primary antibodanti-3NT (Abcam), for immunofluorescence staining, followed by species-specific Alexa Fluor 633was used against anti- 3NT. The stained cells attached to the coverslips were mounted on slides with ProLong Gold antifade reagent with DAPI (Life Technologies) and viewed under 40× objectives with an Olympus BX51 microscope.
2.5.3. Quantitative real-time polymerase chain reaction
Gene expression levels in kidney tissue samples and Mesangial cells were measured by two-step qRT-PCR. Total RNA was isolated from kidney tissue and cells using TRIzol reagent (Invitrogen) according to the manufacturer's instructions and purified with the use of RNeasy mini columns (Qiagen, Valencia, CA). Purified RNA was converted to cDNA using iScript cDNA synthesis kit (Bio-Rad) following the manufacturer's standard protocol. qRT-PCR was performed with the gene-specific primers using SsoAdvanced SYBR Green Supermix (Bio-Rad) and CFX96 thermal cycler (Bio-Rad). Threshold Cycle (Ct) values for the selected genes were normalized against 18 S (internal control) in the same sample. The relative fold change was calculated by the 2 − ΔΔCt method. The sequences for the mouse-specific primers used for real-time PCR are provided in Table 1.
Table 1.
The sequences for mouse-specific real-time PCR primers.
| Gene | Primer sequencea |
|---|---|
| IL-1β | Sense: CCTCGGCCAAGACAGGTCGC |
| Antisense: TGCCCATCAGAGGCAAGGAGGA | |
| TNF-α | Sense: CAACGCCCTCCTGGCCAACG |
| Antisense: TCGGGGCAGCCTTGTCCCTT | |
| IFN-γ | Sense: TGCGGGGTTGTATCTGGGGGT |
| Antisense: GCGCTGGCCCGGAGTGTAG | |
| α-SMA | Sense: GGAGAAGCCCAGCCAGTCGC |
| Antisense: ACCATTGTCGCACACCAGGGC | |
| CD4 | Sense: CACACACCTGTGCAAGAAGC |
| Antisense: GCGTCTTCCCTTGAGTGACA | |
| CD8 | Sense: GCCCTTCTGCTGTCCTTGAT |
| Antisense: TAGTTGTAGCTTCCTGGCGG | |
| TLR4 | Sense: GGAGTGCCCCGCTTTCACCTC |
| Antisense: ACCTTCCGGCTCTTGTGGAAGC |
The primer sequences are given as 5′-3′ orientation; Sense: forward primer; Antisense: reverse primer.
2.5.4. miR21 expression
Total miRNA was isolated from kidney tissue and mesangial cells grown in a monolayer by homogenization in Qiazol reagent (Qiagen) following the manufacturer's instructions. The purification was done by using the miRNeasy mini kit (Qiagen). Purified miRNA (1000 ng) was converted to cDNA using miScript cDNA synthesis kit (Qiagen) following the manufacturer's protocol. qRT-PCR was performed with miRNA-21 specific primers (Qiagen) using miScript SYBR Green PCR master mix (Qiagen) and CFX96 thermal cycler (Bio-Rad). Ct values for the selected gene were normalized against RNU6-2 (internal miR expression control) values in the same sample.
2.5.5. Western blot
Western blot for serum leptin was performed by using the standard protocol as described by Seth et al. [23]. Briefly, 20 µg of denatured serum protein resolved on novex 4–12% bis-tris gradient gel. Resolved protein bands were transferred to nitrocellulose membrane using Trans-Blot Turbo transfer system. Further, blots were blocked with 5% non-fat milk solution for 1 h and then incubated with anti-leptin antibody for overnight at 4 °C. Species-specific anti-IgG secondary antibody conjugated with HRP was used to tag primary antibody. ECL western blotting substrate was used to develop the blot. Finally, the blot was imaged using G:Box Chemi XX6 and subjected to densitometry analysis using Image J software.
2.6. Statistical analysis
All in vivo experiments were repeated three times with at least 3 mice per group (n = 3; data from each group of three mice were pooled). All in vitro experiments and laboratory analysis experiments were repeated three times. The statistical analysis was carried out by unpaired t-test and analysis of variance (ANOVA) followed by Bonferroni post-hoc correction for intergroup comparisons. For all analysis P < 0.05 was considered statistically significant.
3. Results
3.1. Increased circulatory leptin in NASH causes mesangial cell activation in Kidney
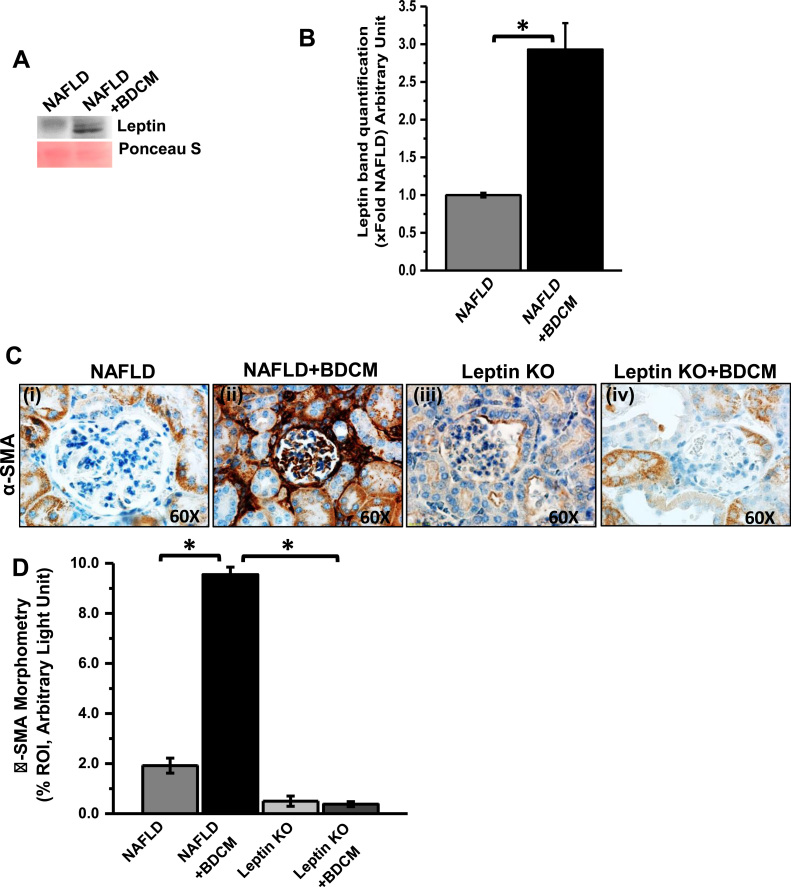
We have shown previously that NASH in an underlying condition of obesity causes an increase in hepatic CYP2E1 activity with concomitant free radical damage to lipids and proteins [23]. The adipokine, leptin is mainly produced by adipocytes in obesity and poses inflammatory response in the liver. In CYP2E1 priming progressive NASH, BDCM (Bromodichloromethane, or xenobiotics) act as a second hit and causes liver injury and activation of Hepatic stellate cells [11], [38]. Hepatocytes and Hepatic stellate cells produce endogenous leptin and further causes increase in circulatory leptin and leptin resistance [11], [24]. Our results in this study also showed that serum leptin was significantly increased in BDCM exposed group (NAFLD + BDCM) as compared to NAFLD alone group (control) (Fig. 1A and B) (p < 0.05). In a previously published study, it has been shown that circulatory leptin plays a significant role in chronic kidney disease (CKD). To study the involvement of increased circulatory leptin in CYP2E1-primed NAFLD in the progression of ectopic kidney disease; we tested the immunoreactivity and localization of α-SMA, a mesangial cell activation marker. The results showed that there was a significant increase in α-SMA immunoreactivity in glomerular lesions of the NAFLD + BDCM group as compared to NAFLD alone (Fig. 1C and D) (P < 0.05). Xenobiotic metabolism mediated mesangial cell activation was completely diminished in Leptin KO groups (in both untreated or treated with BDCM) (Fig. 1C and D) (P < 0.05). The results suggest that the increased circulatory leptin play a crucial role in mesangial cell activation and inflammatory response in the kidney. Although we cannot rule out the involvement of another kidney cell types at this stage, it appeared that mesangial cell activation and proliferation is an extensive event in NAFLD associated renal disease.
Fig. 1.
Increased circulatory leptin causes mesangial cell activation in the NAFLD-Kidney. A. Western blot analysis for serum leptin in NAFLD group and NAFLD group treated with BDCM. B. The morphometric analysis of the western blot normalized with ponceau S band. C. Immunoreactivity of alpha-smooth muscle actin (α- SMA, a marker for mesangial cell activation) as shown by immunohistochemistry in kidney slices from mice fed with high fat diet (60% kcal fat) NAFLD serve as a control, NAFLD mice exposed to BDCM (NAFLD + BDCM), Leptin gene-deficient mice fed with high fat diet (Leptin KO) and exposed to BDCM (Leptin KO+BDCM). Images were taken at 60×. D. Morphometric analysis of α- SMA immunoreactivity (mean data measured as arbitrary light units from three separate microscopic fields were plotted on y-axis) in NAFLD, NAFLD + BDCM, Leptin KO and Leptin KO+ BDCM groups (*P < 0.05).
3.2. Leptin causes NOX2 activation and increased peroxynitrite generation in kidney
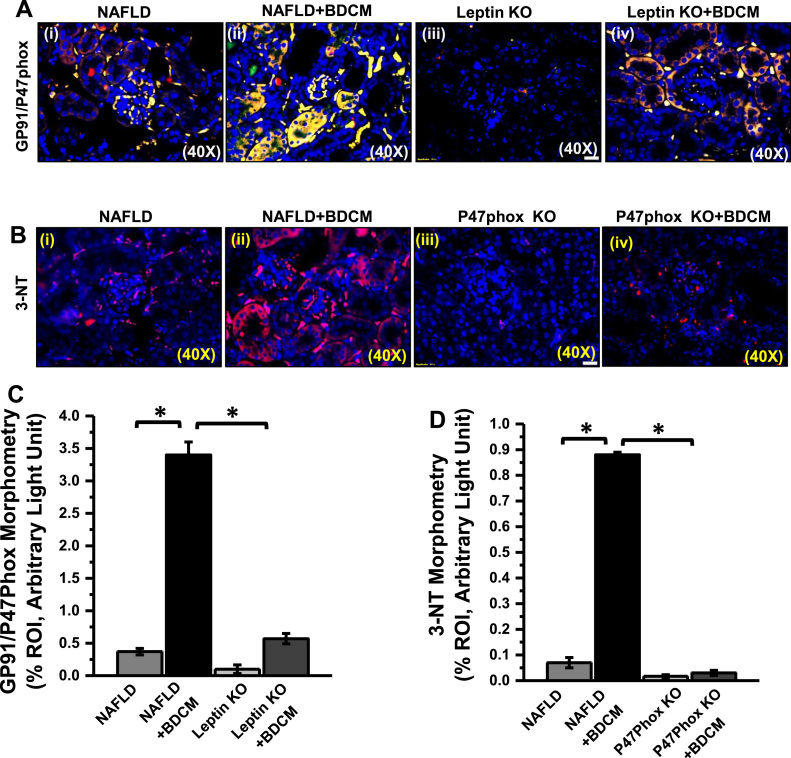
Though leptin involves in the regulation of food intake and energy metabolism hyperleptinemia found in obesity and NAFLD generates oxidative stress mediated by NADPH oxidase activation [39]. The NADPH oxidase isoform 2 (NOX2) is one of the several isoforms of the GP91 phox catalytic subunit of NADPH oxidase [40]. To see the activation of NADPH oxidase system dual-labeled (GP91 phox-Red and P47phox-green) immunofluorescence microscopy was carried out. The result showed that there was a significant increase in GP91 phox-P47phox co-localization (yellow) events in the glomerulus of BDCM exposed group as compared to NAFLD group alone (Fig. 2A and C) (p < 0.05). The leptin KO mice either exposed to BDCM or vehicle showed decreased co-localization of both subunits and suggested decreased NADPH oxidase activation in glomerulus in the absence of leptin (Fig. 2A and C). The activation of NADPH oxidase system is further associated with higher oxidative stress, as evident from the increased tyrosyl radical formation (3-nitrotyrosine immunoreactivity) in the glomerulus and other tubular structures [29], [41]. Further, the results showed that the mice exposed with BDCM had significantly increased 3-nitrotyrosine (3-NT) immunoreactivity as compared to NAFLD group alone that was significantly decreased in P47phox KO mice fed with a high-fat diet exposed to BDCM or vehicle (Fig. 2B and D) (p < 0.05). The data suggest that higher leptin-induced NOX2 assembly that was essential for activation of NADPH oxidase system and perpetuated nitrative stress generation in the kidney.
Fig. 2.
Leptin induces renal NOX2 activation and subsequent tyrosyl radical formation. A. Colocalization of GP91phox/P47phox (GP91/P47phox) as shown by immunofluorescence imaging in kidney slices from mice fed with high- fat diet (NAFLD), NAFLD mice exposed to BDCM, and Leptin gene-deficient mice fed with high-fat diet (Leptin KO) and exposed to BDCM (Leptin KO + BDCM). B Immunoreactivity of 3-nitrotyrosine (3-NT) as shown by immunofluorescence imaging in kidney slices from mice fed with high- fat diet (NAFLD), NAFLD mice exposed to BDCM, and P47phox gene-deficient mice fed with high-fat diet (P47phox KO) and exposed to BDCM (P47phox KO + BDCM). C Morphometric analysis of GP91/P47phox colocalized in NAFLD, NAFLD+BDCM, Leptin KO, Leptin KO + BDCM group of mice. All immunofluorescent Images were taken at 40× and mean colocalization events were measured as arbitrary light units from three separate microscopic fields(plotted on y-axis) (*P < 0.05). D Morphometric analysis of 3-NT immunoreactivity in NAFLD, NAFLD + BDCM, P47phox KO, and P47phox KO + BDCM group of mice. Morphometric analysis was performed as mean data (immunoreactivity measured as arbitrary light units) from three separate microscopic fields (plotted on y-axis) (*P < 0.05).
3.3. Leptin-NOX2 axis causes mesangial cell activation and renal inflammation
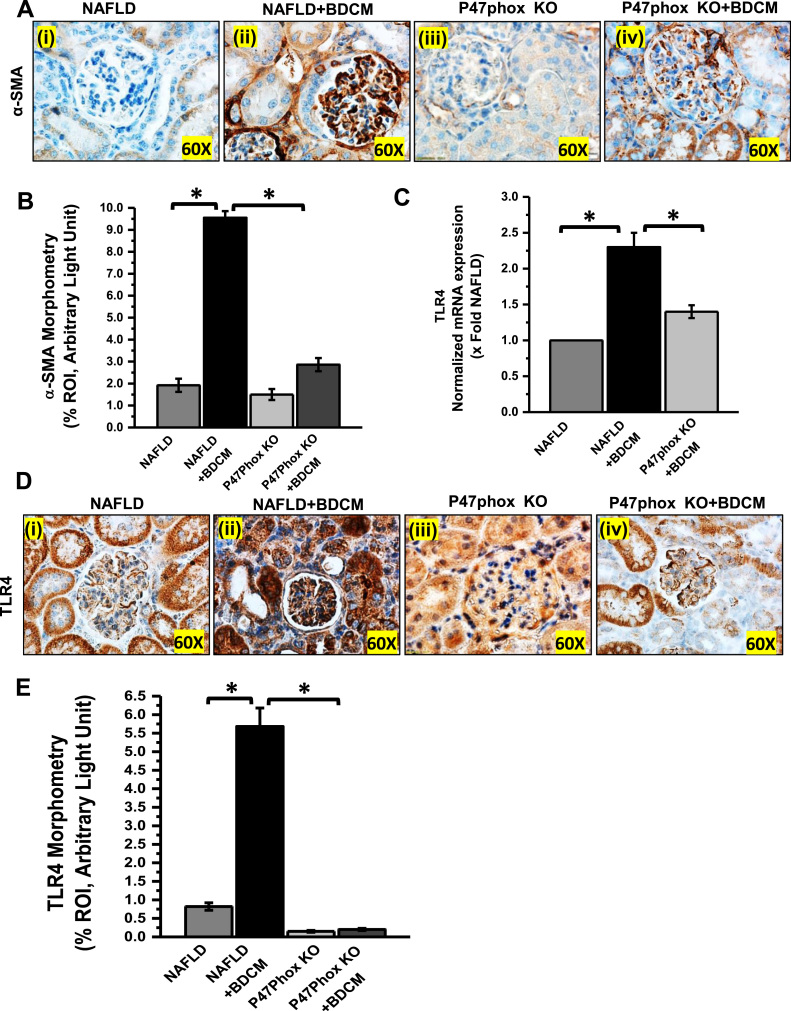
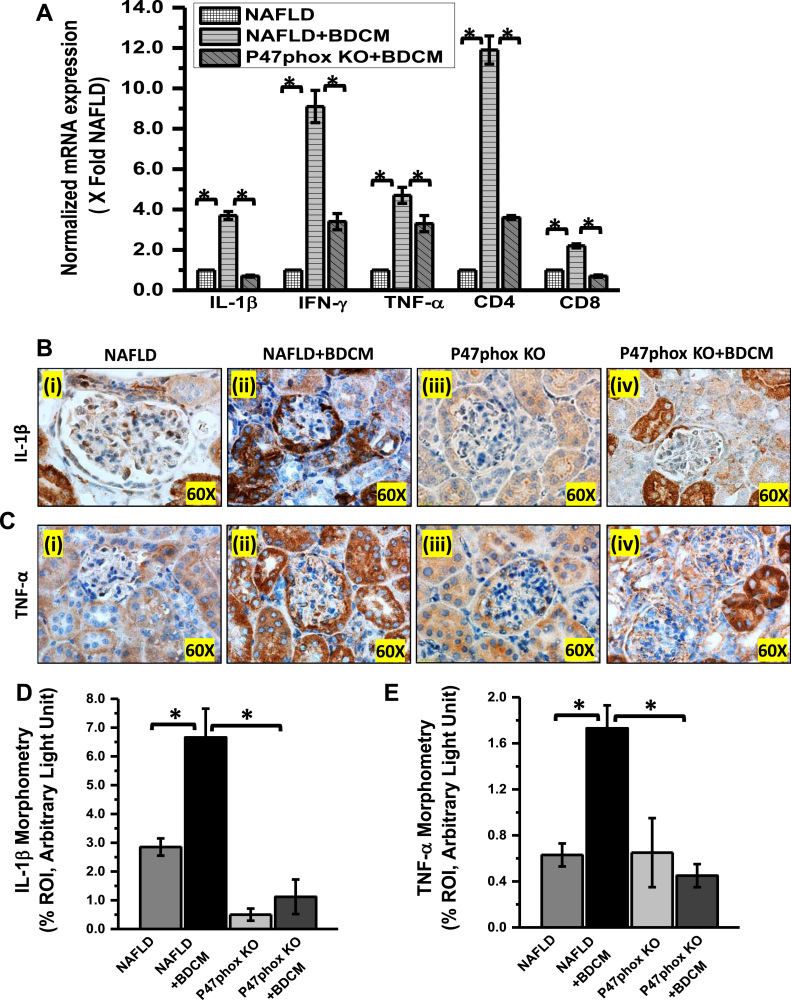
The role of NADPH oxidase has been demonstrated in several experimental models of renal injury such as Diabetic nephritis [42], a hHcys model of glomerulosclerosis [43]. To study the role of leptin-induced activation of NADPH system complex and peroxynitrite generation in mesangial cell injury, mouse kidney tissue was evaluated for α-Smooth Muscle Actin (α-SMA) immunoreactivity by immunohistochemistry. The result showed that mesangial cell activation marker, α-SMA was significantly increased in BDCM exposed NAFLD group as compared with NAFLD alone group (Fig. 3A and B) (p < 0.05). P47phox KO mice group exposed to BDCM or vehicle showed significantly decreased immunoreactivity for α-SMA (Fig. 3A and B) (p < 0.05). The data strongly suggested that mesangial cell activation was dependent on oxidative stress mediated by active NADPH oxidase assembly. The glomerular mesangial when activated imposes not only the thickening glomerular lining but also plays a crucial role in chronic renal inflammation [44]. NADPH oxidase-mediated 3-nitrotyrosine has also been studied in several inflammatory diseases [16], [41], [45], [46]. To study the role of NOX2 mediated renal inflammation in this study, qRTPCR, and immunoreactivity of TLR4 and inflammatory cytokines were carried out. Results showed that TLR4 expression was significantly increased in BDCM exposed NAFLD group (NAFLD + BDCM) as compared to NAFLD alone group, while the P47phox KO or P47phox KO+BDCM group showed a significantly decreased TLR4 expression (Fig. 3C–E) (p < 0.05). The qRTPCR analysis of inflammatory cytokines and markers of proinflammatory T cell subtypes showed significantly increased expression of IL-1β, IFN-γ, CD4, CD8 and TNF-α in BDCM exposed NAFLD kidney when compared with NAFLD alone while they were significantly decreased in P47phox KO mice group exposed with BDCM (Fig. 4A) (p < 0.05). A similar data was also obtained in the glomerular regions where immunoreactivity of IL-1β and TNF-α showed a significant increase in NAFLD mice exposed with BDCM (NAFLD + BDCM) as compared to NAFLD alone or P47phox KO alone groups of mice and a parallel significant decrease in the P47phox+BDCM group (Fig. 4B–E) (p < 0.05). Results also showed a significantly increased migration of CD4+ T-cells (12 folds) in comparison to CD8+ T-Cells (2.5 folds) in a NAFLD+BDCM group of mice as compared to NAFLD alone or P47phox KO alone and significantly decreased in P47phox KO+BDCM group suggesting a strong T cell inflammatory phenotype (Fig. 4A) (p < 0.05). The above data suggest that mesangial cell activation and inflammation in the kidney is dependent on activation of NADPH oxidase system.
Fig. 3.
NOX2 activation leads to mesangial cell priming and increased TLR4 expression in the renal tissue. A and B Immunoreactivity and morphometric analysis of α-SMA immunoreactivity as shown by immunohistochemistry in kidney slices from mice fed with high- fat diet (NAFLD), NAFLD mice exposed to BDCM (NAFLD + BDCM), and P47phox gene-deficient mice fed with high-fat diet (P47phox KO) and exposed to BDCM (P47phox KO + BDCM). All immunohistochemistry Images were taken at 60×. C. mRNA expression of TLR4 gene in kidney tissue of NAFLD, NAFLD + BDCM, P47phox KO + BDCM group of mice fed with high-fat diet. mRNA expression had been appraised by quantitative real-time PCR (qRTPCR) and expressions were normalized against NAFLD group (*P < 0.05). D and E Immunoreactivity and morphometric analysis of TLR4 as shown by immunohistochemistry in kidney slices from mice fed with high- fat diet (NAFLD), NAFLD mice exposed to BDCM (NAFLD + BDCM), and P47phox gene-deficient mice fed with high-fat diet (P47phox KO) and exposed to BDCM (P47phox KO + BDCM). All immunohistochemistry Images were taken at 60×. All morphometric analysis were carried out as mean data from three separate microscopic fields. (plotted on y-axis) in NAFLD, NAFLD + BDCM, P47phox KO, and P47phox KO + BDCM group of mice.
Fig. 4.
NOX2 activation leads to a surge of proinflammatory mediators in NAFLD-Kidney A. mRNA expression analysis of IL-1β, IFN-γ, TNF-α, CD4, and CD8 genes in kidney tissue of NAFLD, NAFLD + BDCM, and P47phox KO mice fed with high-fat diet. All mRNA expression had been assessed by quantitative real-time PCR (qRTPCR) and expressions were normalized against NAFLD group (*P < 0.05). B and C Kidney tissue slices were probed for IL-1β and TNF-α immunoreactivity as shown by immunohistochemistry in kidney slices from mice fed with high- fat diet NAFLD group (serve as a control), NAFLD+BDCM, and P47phox mice group. D and E Morphometric analysis of IL-1β and TNF-α immunoreactivity as observed in the region of interest (ROI) (*P < 0.05).
3.4. Leptin-mediated increased peroxynitrite generation in the mesangial cell is NOX2 dependent
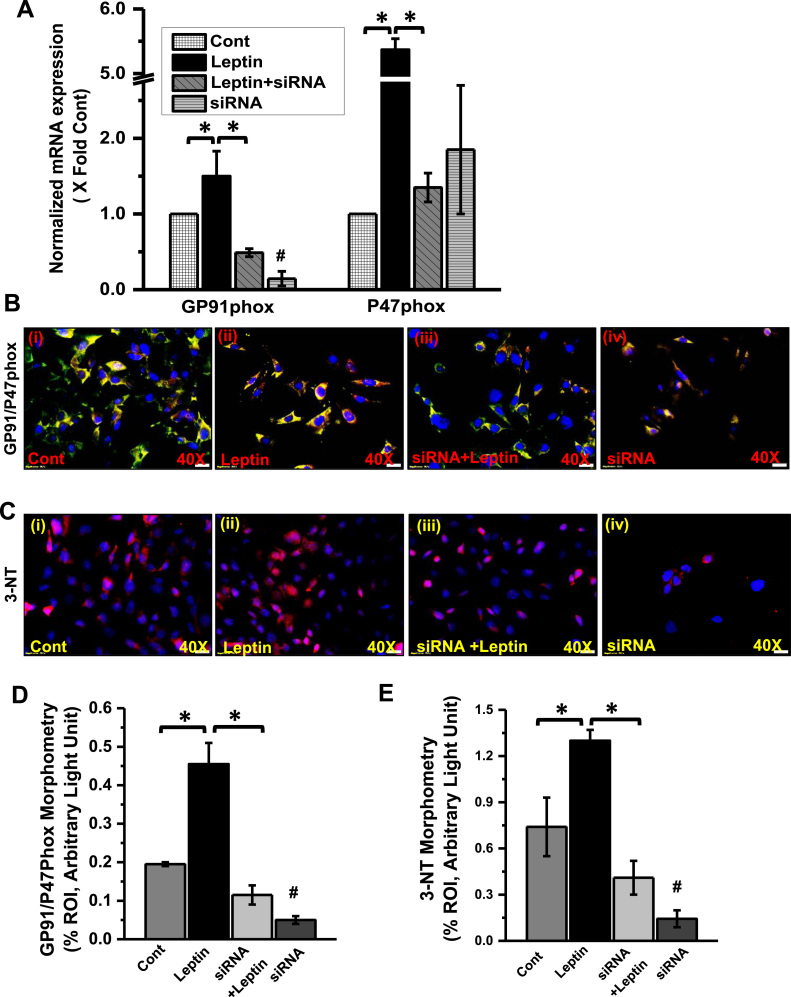
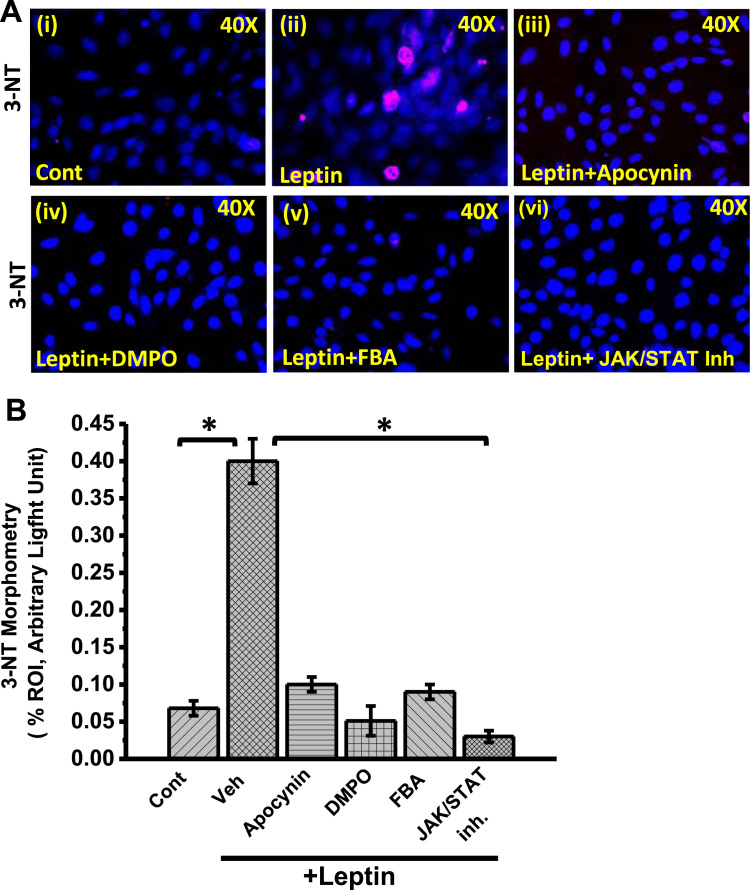
To study the mechanism of Leptin-induced NOX2 activation and subsequent peroxynitrite generation in mesangial cells of the kidney, experiments were performed with mesangial cell culture primed with adipocytokine leptin. To investigate the role of NOX2 in leptin-induced oxidative stress and disease pathogenesis, mesangial cells were exposed with NOX2-siRNA with or without leptin. NOX-2 siRNA specifically block the GP91phox mRNA expression by 85% but did not alter P47phox mRNA expression (Fig. 5A) as compared to vehicle treated control (Cont) groups of cells. However, leptin exposure to mesangial cells significantly upregulate mRNA expression of both GP91phox (1.5 fold) and P47phox (5.3 fold) and compared to the control cells (Fig. 5A). Further, to see the leptin-induced activation of NADPH oxidase system in mesangial cells, a dual-labeled (GP91phox-Red and P47phox-green) immunofluorescence microscopy was carried out. The result showed that there were a significant increase in GP91phox-P47phox co-localization (yellow) events in leptin exposed mesangial cells as compared to vehicle treated control groups (Fig. 5B and D) (p < 0.05). However, siRNA treated control groups or siRNA+leptin groups of cells showed a significant decrease in colocalization events as compared to leptin-treated groups (Fig. 5B and D) (p < 0.05). The activation of NADPH oxidase system is further associated with higher oxidative stress, as evident from the tyrosyl radical formation (3-nitrotyrosine). The immunofluorescence images (Red: 3-nitrotyrosine) of cultured mesangial cells showed that cells primed with leptin caused a significant increase in 3-nitrotyrosine (3-NT) immunoreactivity when compared with corresponding vehicle control (Fig. 5C and E) (p < 0.05). However, siRNA primed mesangial cells co-treated with Leptin (siRNA + Leptin) showed a significant decrease in 3-NT immunoreactivity. In another set of experiment, we used different approaches to block NADPH Oxidase activity or leptin pathway. The mesangial cells co-exposed with leptin and apocynin (NADPH oxidase nonspecific inhibitor), or DMPO (free radical spin trap) or FBA (peroxynitrite scavenger) showed a significant decrease in 3-nitrotyrosine immunoreactivity as compared to leptin-treated cells alone (Fig. 6A and B) (p < 0.05). The results suggest that the leptin-mediated generation of 3-Nitrotyrosine in the mesangial cell was NOX2 and peroxynitrite-dependent. Further to explore how leptin activates NOX2-induced peroxynitrite generation and tyrosyl radical formation, we used a JAK/STAT inhibitor to block leptin signaling. Results showed a significant decrease in 3-nitrotyrosine immunoreactivity in JAK/STAT inhibitor-treated cells primed with leptin when compared to cells primed with leptin alone (Fig. 6A and B) (p < 0.05). The data suggested that leptin activated NOX2 via a JAK/STAT pathway and ruled out an endogenous mesangial specific in situ activation independent of leptin.
Fig. 5.
Role of GP91phox in activation of leptin induced NADPH oxidase and oxidative stress. A. mRNA expression analysis of GP91phox and P47phox in mesangial cells exposed with leptin or siRNA or co-exposed with siRNA and Leptin. All mRNA expression have been assessed by quantitative real-time PCR (qRTPCR) and expressions were normalized against Control (Cont) group (*#P < 0.05). B and D. Colocalization and morphometric analysis of GP91phox/P47phox as shown by immunofluorescence imaging in mesangial cells exposed with leptin or siRNA or co-exposed with siRNA and Leptin.(*#P < 0.05). C and E. Immunoreactivity and morphometric analysis of 3-nitrotyrosine (3-NT, red) as shown by Immunofluorescence microscopy in kidney mesangial cells exposed with leptin or siRNA or co-exposed with siRNA and Leptin (*#P < 0.05).
Fig. 6.
NOX2-derived peroxynitrite causes generation of tyrosyl radicals in mesangial cells. A. Immunoreactivity of 3-nitrotyrosin (3-NT) as shown by Immunofluorescence microscopy (red) in mesangial cell line. Mesangial cells were treated with vehicle only served as control and other sets of Mesangial cells were treated with leptin, leptin + Apocynin, leptin + DMPO, leptin + FBA and JAK/STAT inhibitor. B Morphometric analysis of 3-NT immunoreactivity in control, leptin, leptin + Apocynin, leptin + DMPO, leptin + FBA, and leptin + JAK/STAT inhibitor groups of mesangial cells (*P < 0.05).
3.5. Leptin-NOX2-peroxynitrite-axis causes miR21 upregulation and mesangial cell activation
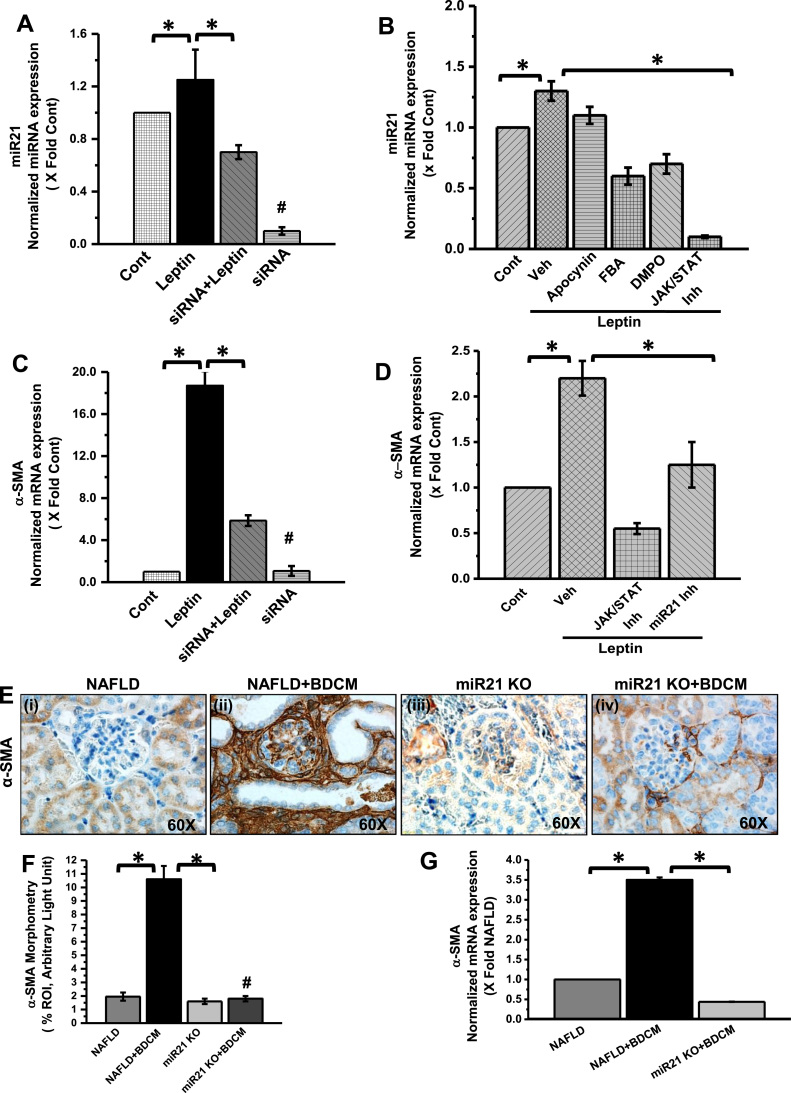
We and others have shown that miR21 plays a crucial role in modulating inflammation in liver disease [33], [47]. It has been demonstrated that the expression and function of miR21 are regulated by NADPH oxidase-dependent reactive oxygen species in prostate cancer [48]. In Kidney, the miR21 expression is associated with renal fibrosis and inflammation [49]. To study how leptin-mediated NOX2 induction and its downstream peroxynitrite caused miR21 upregulation, we used mesangial cell culture primed with leptin and co-treated with either NOX2-siRNA or apocynin or FBA or DMPO or a widely used JAK/STAT inhibitor. Results showed that miR21 expression in mesangial cells was significantly increased in leptin primed cells as compared to vehicle control group alone (Fig. 7A and B) (p < 0.05). Interestingly, miR21 expression was significantly decreased in leptin primed mesangial cells co-treated with siRNA (Fig. 7A) or NADPH oxidase inhibitor (Apocynin) or free radical scavenger (DMPO) or peroxynitrite scavenger (FBA) as compared to leptin primed mesangial cell alone (Fig. 7B) (p < 0.05). To confirm the role of leptin signaling in the increase of miR21 expression, we co-treated the leptin primed mesangial cells with JAK/STAT inhibitor. The results showed a significant decrease in miR21 expression as compared to leptin alone group (Fig. 7B). The data suggested that upregulation of miR21 in the mesangial cell is dependent on the leptin-mediated NOX2 pathway and its down-stream signaling mediators. Further, to explore Leptin-NOX2-peroxynitrite axis induced miR21 as a key event in mesangial cell activation, we co-treated leptin primed mesangial cells with siRNA or miR21 inhibitor or JAK/STAT inhibitor and tested for mesangial cell activation by qRTPCR for α-SMA, a mesangial cell activation marker. Results showed a significant decrease in α-SMA mRNA expression in siRNA or miR21 inhibitor or JAK/STAT inhibitor co-treated group as compared to Leptin primed mesangial cells (Fig. 7C and D) (p < 0.05). In parallel, to prove the results obtained from in vitro mesangial cell culture, BDCM was given intraperitoneally to miR21 KO mice. The invivo results showed that miR21 KO mice have significantly decreased α-SMA immunoreactivity as compared to a NAFLD+BDCM group of mice (Fig. 7E and F) (p < 0.05). Similarly, mRNA expression of α-SMA in miR21 KO treated with BDCM was significantly decreased as compared to NAFLD+BDCM mice or NAFLD mice alone (Fig. 7G) (p < 0.05). Taken together, Invitro and invivo results suggested that leptin-induced NOX2-peroxynitrite signaling causes mesangial cell activation via an increased expression of miR21.
Fig. 7.
Leptin-NOX2-Peroxynitrite axis causes mesangial cell activation via miR21. A and B. mRNA expression analysis of miRNA 21 (miR21) gene in control (mesangial cells line (CRL-1927) treated with vehicle only served as control), mesangial cells treated with leptin (leptin), siRNA, or co-exposed with leptin and siRNA (leptin + siRNA), leptin and Apocynin (leptin + Apocynin), leptin and FBA (leptin + FBA), leptin and DMPO (leptin + DMPO), and leptin and JAK/STAT inhibitor (Leptin + JAK/STAT inh). miRNA expression had been assessed by quantitative real-time PCR (qRTPCR) and expressions were normalized against control (*P < 0.05). C and D. mRNA expression analysis of α-SMA gene in mesangial cells in Control, Leptin, siRNA, Leptin + siRNA, Leptin + miRNA 21 inhibitor (miR21 inh) and leptin + JAK/STAT inhibitor. mRNA expression has been assessed by quantitative real-time PCR (qRTPCR) and expressions were normalized against mesangial cell control (*P < 0.05). E. Immunoreactivity of α-SMA as observed by immunohistochemistry in kidney slices from (NAFLD), NAFLD mice exposed to BDCM, and miRNA21 gene-deficient mice fed with high-fat diet (miR21 KO) and exposed to BDCM (miR21 KO + BDCM). All immunohistochemistry Images were taken at 60× oil. F. Morphometric analysis of α-SMA immunoreactivity in NAFLD, NAFLD + BDCM, miR21 KO, and miR21 KO + BDCM group of mice. All morphometric analysis was performed as mean data from three separate microscopic fields (designed on y-axis) (*P < 0.05). G. mRNA expression analysis of α-SMA gene in kidney tissue of NAFLD, NAFLD + BDCM, and miR21 KO + BDCM mice. mRNA expression has been assessed by qRTPCR and was normalized against NAFLD group (*P < 0.05).
3.6. Leptin-NOX2-peroxynitrite-axis induced miR21 upregulation causes an inflammatory response in the kidney
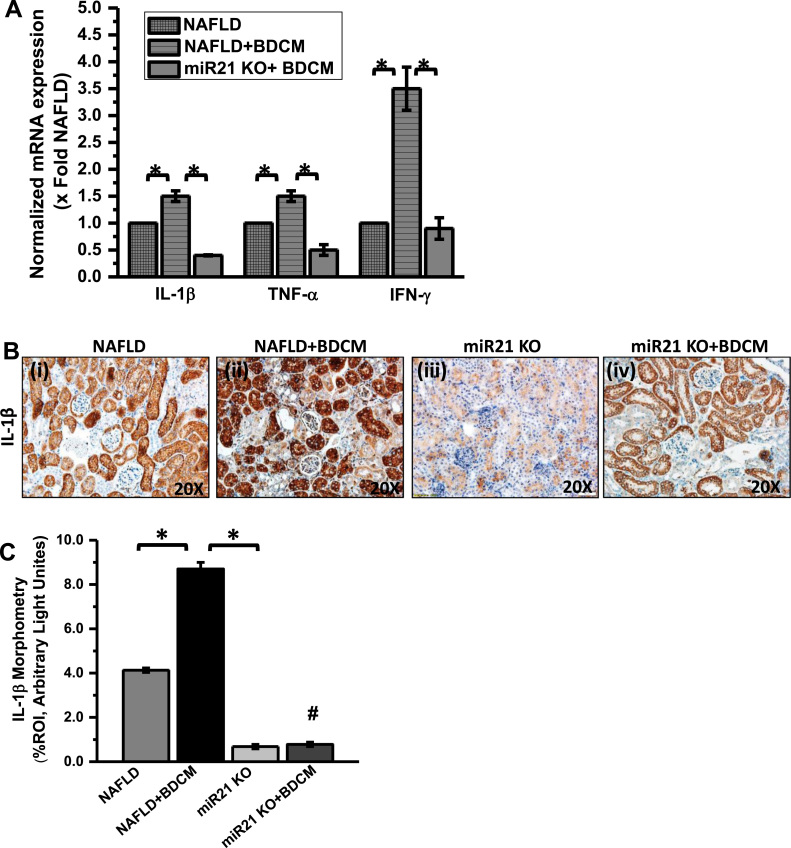
MiR21 induction has been reported in several inflammatory diseases [33], [47]. To study the role of elevated miR21 in the kidney due to NOX2 induced by leptin, we treated miR21 KO mice with BDCM and observed the inflammatory markers typical to glomerulonephritis and tubular disease. Results showed that the mRNA expression of pro-inflammatory cytokines IL-1β, TNF-α and IFN-γ were significantly elevated in BDCM treated NAFLD mice (NAFLD+BDCM) as compared with NAFLD group alone (Fig. 8A) (p < 0.05). However, miR21 gene deleted mice treated with BDCM (miR21 KO+BDCM) showed a significant decrease in mRNA expression of these pro-inflammatory markers when compared with a NAFLD+BDCM group of mice (Fig. 8A) (p < 0.05). Also, the protein levels of IL-1β showed significantly increased IL-1β immunoreactivity in BDCM treated NAFLD mice (NAFLD + BDCM) as compared with NAFLD mice alone (Fig. 8B and C) (p < 0.05). However, miR21 KO treated with BDCM showed significantly decreased IL-1β immunoreactivity as compared with a NAFLD + BDCM group of mice (Fig. 8B and C) (p < 0.05). The results suggested that miR21 plays a crucial role in kidney inflammation especially related to glomerulonephritis.
Fig. 8.
MiR21 increase causes kidney inflammation. A. mRNA expression of IL-1β, TNFα, and IFN-γ in kidney tissue from mice fed with high-fat diet. NAFLD serve as a control, NAFLD mice exposed to BDCM (NAFLD + BDCM), miRNA 21 gene-deficient mice exposed to BDCM (miR21 KO + BDCM) as assessed by quantitative real-time PCR, the expressions were normalized with 18S and compared to the NAFLD group (*P < 0.05). B. Immunoreactivity of IL-1β as shown by immunohistochemistry in kidney slices from. of NAFLD, NAFLD + BDCM, miR21 KO, and miR21 KO + BDCM group of mice Images were taken at 20×. C. Morphometric analysis of IL-1β immunoreactivity in NAFLD, NAFLD + BDCM, miR21 KO, and miR21 KO + BDCM group of mice (*P < 0.05).
4. Discussion
There is mounting evidence in the clinics of a higher prevalence of chronic kidney diseases in patients with advance stages of NASH [50], [51]. With NAFLD/NASH being considered a global pandemic, it is important that increased research emphasis is provided for an understanding of the pathology and ectopic mediators of NAFLD/NASH. Our present report builds logically from our previous findings that showed a strong link to glomerular inflammation in murine models of NAFLD [8]. We and others have shown that high circulatory leptin in NASH is proinflammatory and generates a high flux of reactive oxygen species generation primarily through the activation of NOX2 in the liver [10]. In the present study, we argued that high circulatory leptin that is a result of either leptin resistance or increased hepatic production of the protein might be a causal link to the inflammation observed in distal organ systems including the cardiac and renal organs. We found that NAFLD-induced high leptin was responsible for increased mesangial cell immunoreactivity for α-SMA, a marker for its activation. Interestingly both acute and chronic kidney disease show increased activation of the mesangial cells that lead to increased inflammation by the release of proinflammatory cytokines and a concomitant increase in TGF-beta signaling [8], [52]. Results in the present study also showed that absence of leptin abrogated the mesangial cell activation while deletion of p47phox in mice produced the same result. The activation of NOX2 in mesangial cells responsible for oxidative stress was significantly high in mice that had advanced form of NAFLD when primed with a CYP2E1 ligand, while the response was blunted in the absence of leptin. It appears from the data that higher circulatory leptin-induced an ectopic NADPH oxidase activation in the distal mesangial cells. The NOX2 activation caused a significant increase in proinflammatory mediators like IL-1β, TNF-α and IFN-γ in addition to higher CD4 and a CD8 expression suggesting an elicitation of a strong innate and adaptive immune response in the kidney. However, the use of whole-body systemic knockouts for leptin and p47 phox, do not clearly support the cause that underlines the role of leptin and NOX2 in the kidney alone. Underlying adiposity that is a hallmark of the present model can also produce low lying systemic inflammation that can augment mesangial cell NOX2 activation. The use of kidney-specific deletion of NOX2 might have answered the above limitation of the study. Interestingly our results using a mesangial cell line that is primed with identical concentrations of leptin that were found in the systemic circulation caused a similar NOX2 activation and tyrosine nitration suggesting that the lack of kidney-specific knockout did not alter our interpretations of an endocrine role of leptin in NAFLD/NASH. Our in vitro results further showed that a higher NOX2 activation led to an increased peroxynitrite generation that was abrogated by the use of either DMPO, a spin trap or FBA, a specific peroxynitrite scavenger or NOX2 specific siRNA. The results suggested that higher NOX2 activation was able to produce sufficient localized peroxynitrite generation that has been found to initiate redox signaling primarily through activation of the AKT pathway or rapidly nitrating enzymes like CPB1 thus rendering them inactive and allowing uninterrupted inflammation, though such an interpretation is speculative at this time [29]. Since glomerular inflammation has been shown to be associated with a higher miR21 levels, a mediator of inflammation, we sought to study the role of NOX2-derived peroxynitrite in modulating the levels of miR21 [53]. Results showed that peroxynitrite was instrumental in increasing miR21 levels in the mesangial cells since the use of FBA decreased its levels. However, it remains to be seen whether peroxynitrite might have directly induced miR21 levels or it has done so by increasing TLR4 activation. The chances of the latter in the present model is more likely since the use of P47phox KO mice decreased kidney TLR4 expression and use of FBA decreased TLR4 expression (data not shown) with a parallel decrease in miR21. We have shown previously that NOX2-derived peroxynitrite was instrumental in trafficking TLR4 to lipid rafts, an essential mechanistic event in TLR4 activation [16]. Thus, increased TLR4 expression coupled with its activation might be a likely cause of increased miR21 levels in the mesangial cells. This result is supported by the fact that miR21 coupled with other miRs are induced by TLR4 dependent mechanisms [34].
Having observed an increase in miR21 levels in the mesangial cells primarily driven by leptin-induced NOX2, we chose to study the effect of the inducible miR21 on the mesangial cell activation, release of proinflammatory meditators and glomerular inflammation. Results showed a significant increase in mesangial cell-mediated expression of α-SMA, that was inhibitable following the use of miR21 specific antagomir. Further, the data was supported by the corresponding use of miR21 KO mice that showed decreased mesangial cell activation and expression of proininflammatory mediators in the NAFLD-Kidney tissue suggesting that the above-cited miR was downstream of a NOX2-mediated peroxynitrite surge essentially caused by higher circulating leptin.
Thus, in summary, the present study addresses a novel and timely issue of NAFLD-induced ectopic manifestation of kidney disease and underlines a mechanistic role of NOX2 induced redox signaling that can be a future basis for therapeutic intervention in such pathology. The above mechanism can also be used to understand the pathways that exist in other comorbidities of NAFLD such as cardiovascular and neuronal complications. Further, use of miR21 as a clinical biomarker for kidney complications in NAFLD can be strengthened by the observations of this study and use of miR21 antagomirs can be used as future therapeutic tools.
Acknowledgment
The authors gratefully acknowledge the technical services of Benny Davidson at the IRF, University of South Carolina School of Medicine and AML Labs (Baltimore MD). We also thank the Instrumentation resource facility (IRF) at the University of South Carolina for equipment usage and consulting services.
Acknowledgments
Funding
This work has been supported by NIH Pathway to Independence Award, R00ES019875 and P01AT003961 to Saurabh Chatterjee, R01DK053792 to Anna Mae Diehl, P01AT003961, P20GM103641, R01AT006888, R01ES019313, R01MH094755 and VA Merit Award BX001357 to Mitzi Nagarkatti and Prakash S. Nagarkatti.
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.04.002.
Appendix A. Supplementary material
Supplementary material
References
- 1.Paschos P., Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 2009;13:9–19. [PMC free article] [PubMed] [Google Scholar]
- 2.Sundaram S.S., Zeitler P., Nadeau K. The metabolic syndrome and nonalcoholic fatty liver disease in children. Curr. Opin. Pediatr. 2009;21:529–535. doi: 10.1097/MOP.0b013e32832cb16f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kneeman J.M., Misdraji J., Corey K.E. Secondary causes of nonalcoholic fatty liver disease. Ther. Adv. Gastroenterol. 2012;5:199–207. doi: 10.1177/1756283X11430859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee S., Das S. P2X7 receptor as a key player in oxidative stress-driven cell fate in nonalcoholic steatohepatitis. Oxid. Med. Cell. Longev. 2015;2015:172493. doi: 10.1155/2015/172493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia L.S., Curzen N.P., Calder P.C., Byrne C.D. Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur. Heart J. 2012;33:1190–1200. doi: 10.1093/eurheartj/ehr453. [DOI] [PubMed] [Google Scholar]
- 6.Chalasani N., Younossi Z., Lavine J.E., Diehl A.M., Brunt E.M., Cusi K. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Marcuccilli M., Chonchol M. NAFLD and chronic kidney disease. Int. J. Mol. Sci. 2016;17:562. doi: 10.3390/ijms17040562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alhasson F., Dattaroy D., Das S., Chandrashekaran V., Seth R.K., Schnellmann R.G. NKT cell modulates NAFLD potentiation of metabolic oxidative stress-induced mesangial cell activation and proximal tubular toxicity. Am. J. Physiol. Ren. Physiol. 2016;310:F85–F101. doi: 10.1152/ajprenal.00243.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCullough K., Sharma P., Ali T., Khan I., Smith W.C., MacLeod A. Measuring the population burden of chronic kidney disease: a systematic literature review of the estimated prevalence of impaired kidney function. Nephrol. Dial. Transplant.: Off. Publ. Eur. Dial. Transplant. Assoc. – Eur. Ren. Assoc. 2012;27:1812–1821. doi: 10.1093/ndt/gfr547. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee S., Ganini D., Tokar E.J., Kumar A., Das S., Corbett J. Leptin is key to peroxynitrite-mediated oxidative stress and Kupffer cell activation in experimental nonalcoholic steatohepatitis. J. Hepatol. 2012 doi: 10.1016/j.jhep.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das S., Kumar A., Seth R.K., Tokar E.J., Kadiiska M.B., Waalkes M.P. Proinflammatory adipokine leptin mediates disinfection byproduct bromodichloromethane-induced early steatohepatitic injury in obesity. Toxicol. Appl. Pharmacol. 2013;269:297–306. doi: 10.1016/j.taap.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munzberg H. Leptin-signaling pathways and leptin resistance. Forum Nutr. 2010;63:123–132. doi: 10.1159/000264400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marra F., Aleffi S., Bertolani C., Petrai I., Vizzutti F. Adipokines and liver fibrosis. Eur. Rev. Med. Pharmacol. Sci. 2005;9:279–284. [PubMed] [Google Scholar]
- 14.Tilg H., Diehl A.M. NAFLD and extrahepatic cancers: have a look at the colon. Gut. 2011;60:745–746. doi: 10.1136/gut.2011.239392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dattaroy D., Pourhoseini S., Das S., Alhasson F., Seth R.K., Nagarkatti M. Micro-RNA 21 inhibition of SMAD7 enhances fibrogenesis via leptin-mediated NADPH oxidase in experimental and human nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;308:G298–G312. doi: 10.1152/ajpgi.00346.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das S., Alhasson F., Dattaroy D., Pourhoseini S., Seth R.K., Nagarkatti M. NADPH oxidase-derived peroxynitrite drives inflammation in mice and human nonalcoholic steatohepatitis via TLR4-lipid raft recruitment. Am. J. Pathol. 2015;185:1944–1957. doi: 10.1016/j.ajpath.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonora E., Targher G. Increased risk of cardiovascular disease and chronic kidney disease in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2012;9:372–381. doi: 10.1038/nrgastro.2012.79. [DOI] [PubMed] [Google Scholar]
- 18.Orlic L., Mikolasevic I., Bagic Z., Racki S., Stimac D., Milic S. Chronic kidney disease and nonalcoholic fatty liver disease-is there a link? Gastroenterol. Res. Pract. 2014;2014:847539. doi: 10.1155/2014/847539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Targher G., Chonchol M., Zoppini G., Abaterusso C., Bonora E. Risk of chronic kidney disease in patients with non-alcoholic fatty liver disease: is there a link? J. Hepatol. 2011;54:1020–1029. doi: 10.1016/j.jhep.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Day C.P., James O.F. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 21.Tilg H., Moschen A.R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 22.Sumida Y., Niki E., Naito Y., Yoshikawa T. Involvement of free radicals and oxidative stress in NAFLD/NASH. Free Radic. Res. 2013;47:869–880. doi: 10.3109/10715762.2013.837577. [DOI] [PubMed] [Google Scholar]
- 23.Seth R.K., Das S., Dattaroy D., Chandrashekaran V., Alhasson F., Michelotti G. TRPV4 activation of endothelial nitric oxide synthase resists nonalcoholic fatty liver disease by blocking CYP2E1-mediated redox toxicity. Free Radic. Biol. Med. 2016;102:260–273. doi: 10.1016/j.freeradbiomed.2016.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chatterjee S., Rana R., Corbett J., Kadiiska M.B., Goldstein J., Mason R.P. P2X7 receptor-NADPH oxidase axis mediates protein radical formation and Kupffer cell activation in carbon tetrachloride-mediated steatohepatitis in obese mice. Free Radic. Biol. Med. 2012;52:1666–1679. doi: 10.1016/j.freeradbiomed.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandrashekaran V., Seth R.K., Dattaroy D., Alhasson F., Ziolenka J., Carson J. HMGB1-RAGE pathway drives peroxynitrite signaling-induced IBD-like inflammation in murine nonalcoholic fatty liver disease. Redox Biol. 2017;13:8–19. doi: 10.1016/j.redox.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambeth J.D., Kawahara T., Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic. Biol. Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill P.S., Wilcox C.S. NADPH oxidases in the kidney. Antioxid. Redox Signal. 2006;8:1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 28.Sedeek M., Nasrallah R., Touyz R.M., Hebert R.L. NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. J. Am. Soc. Nephrol.: JASN. 2013;24:1512–1518. doi: 10.1681/ASN.2012111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chatterjee S., Lardinois O., Bonini M.G., Bhattacharjee S., Stadler K., Corbett J. Site-specific carboxypeptidase B1 tyrosine nitration and pathophysiological implications following its physical association with nitric oxide synthase-3 in experimental sepsis. J. Immunol. 2009;183:4055–4066. doi: 10.4049/jimmunol.0900593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science (New York) 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 31.Li J., Baud O., Vartanian T., Volpe J.J., Rosenberg P.A. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc. Natl. Acad. Sci. USA. 2005;102:9936–9941. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H., Li Y., Liu H., Liu S., Liu Q., Wang X.M. Peroxynitrite mediates glomerular lesion of diabetic rat via JAK/STAT signaling pathway. J. Endocrinol. Investig. 2009;32:844–851. doi: 10.1007/BF03345756. [DOI] [PubMed] [Google Scholar]
- 33.Pourhoseini S., Seth R.K., Das S., Dattaroy D., Kadiiska M.B., Xie G. Upregulation of miR21 and repression of Grhl3 by leptin mediates sinusoidal endothelial injury in experimental nonalcoholic steatohepatitis. PLoS One. 2015;10:e0116780. doi: 10.1371/journal.pone.0116780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheedy F.J. Turning 21: induction of miR-21 as a key switch in the inflammatory response. Front. Immunol. 2015;6:19. doi: 10.3389/fimmu.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawant D.V., Yao W., Wright Z., Sawyers C., Tepper R.S., Gupta S.K. Serum microRNA-21 as a biomarker for allergic inflammatory disease in children. MicroRNA. 2015;4:36–40. doi: 10.2174/2211536604666150220232507. [DOI] [PubMed] [Google Scholar]
- 36.Hennino M.F., Buob D., Van der Hauwaert C., Gnemmi V., Jomaa Z., Pottier N. miR-21-5p renal expression is associated with fibrosis and renal survival in patients with IgA nephropathy. Sci. Rep. 2016;6:27209. doi: 10.1038/srep27209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glowacki F., Savary G., Gnemmi V., Buob D., Van der Hauwaert C., Lo-Guidice J.M. Increased circulating miR-21 levels are associated with kidney fibrosis. PLoS One. 2013;8:e58014. doi: 10.1371/journal.pone.0058014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seth R.K., Kumar A., Das S., Kadiiska M.B., Michelotti G., Diehl A.M. Environmental toxin-linked nonalcoholic steatohepatitis and hepatic metabolic reprogramming in obese mice. Toxicol. Sci.: Off. J. Soc. Toxicol. 2013 doi: 10.1093/toxsci/kft104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blanca A.J., Ruiz-Armenta M.V., Zambrano S., Salsoso R., Miguel-Carrasco J.L., Fortuno A. Leptin induces oxidative stress through activation of NADPH oxidase in renal tubular cells: antioxidant effect of L-carnitine. J. Cell. Biochem. 2016;117:2281–2288. doi: 10.1002/jcb.25526. [DOI] [PubMed] [Google Scholar]
- 40.Braunersreuther V., Montecucco F., Asrih M., Pelli G., Galan K., Frias M. Role of NADPH oxidase isoforms NOX1, NOX2 and NOX4 in myocardial ischemia/reperfusion injury. J. Mol. Cell. Cardiol. 2013;64:99–107. doi: 10.1016/j.yjmcc.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Chatterjee S., Ganini D., Tokar E.J., Kumar A., Das S., Corbett J. Leptin is key to peroxynitrite-mediated oxidative stress and Kupffer cell activation in experimental non-alcoholic steatohepatitis. J. Hepatol. 2013;58:778–784. doi: 10.1016/j.jhep.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan C., Su H., Zhang C. Role of NADPH oxidase in metabolic disease-related renal injury: an update. Oxid. Med. Cell. Longev. 2016;2016:7813072. doi: 10.1155/2016/7813072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yi F., Xia M., Li N., Zhang C., Tang L., Li P.L. Contribution of guanine nucleotide exchange factor Vav2 to hyperhomocysteinemic glomerulosclerosis in rats. Hypertension. 2009;53:90–96. doi: 10.1161/HYPERTENSIONAHA.108.115675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radeke H.H., Resch K. The inflammatory function of renal glomerular mesangial cells and their interaction with the cellular immune system. Clin. Investig. 1992;70:825–842. doi: 10.1007/BF00180754. [DOI] [PubMed] [Google Scholar]
- 45.Murata M., Kawanishi S. Oxidative DNA damage induced by nitrotyrosine, a biomarker of inflammation. Biochem. Biophys. Res. Commun. 2004;316:123–128. doi: 10.1016/j.bbrc.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 46.Kaur H., Halliwell B. Evidence for nitric oxide-mediated oxidative damage in chronic inflammation. Nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS Lett. 1994;350:9–12. doi: 10.1016/0014-5793(94)00722-5. [DOI] [PubMed] [Google Scholar]
- 47.Quinn S.R., O'Neill L.A. A trio of microRNAs that control Toll-like receptor signalling. Int. Immunol. 2011;23:421–425. doi: 10.1093/intimm/dxr034. [DOI] [PubMed] [Google Scholar]
- 48.Jajoo S., Mukherjea D., Kaur T., Sheehan K.E., Sheth S., Borse V. Essential role of NADPH oxidase-dependent reactive oxygen species generation in regulating microRNA-21 expression and function in prostate cancer. Antioxid. Redox Signal. 2013;19:1863–1876. doi: 10.1089/ars.2012.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loboda A., Sobczak M., Jozkowicz A., Dulak J. TGF-beta1/smads and miR-21 in renal fibrosis and inflammation. Mediat. Inflamm. 2016;2016:8319283. doi: 10.1155/2016/8319283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Musso G., Cassader M., Cohney S., De Michieli F., Pinach S., Saba F. Fatty liver and chronic kidney disease: novel mechanistic insights and therapeutic opportunities. Diabetes Care. 2016;39:1830–1845. doi: 10.2337/dc15-1182. [DOI] [PubMed] [Google Scholar]
- 51.Musso G., Tabibian J.H., Charlton M. Chronic kidney disease (CKD) and NAFLD: time for awareness and screening. J. Hepatol. 2015;62:983–984. doi: 10.1016/j.jhep.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 52.Schnaper H.W., Hayashida T., Hubchak S.C., Poncelet A.-C. TGF-beta signal transduction and mesangial cell fibrogenesis. Am. J. Physiol. Ren. Physiol. 2003;284:F243–F252. doi: 10.1152/ajprenal.00300.2002. [DOI] [PubMed] [Google Scholar]
- 53.Li Y.F., Jing Y., Hao J., Frankfort N.C., Zhou X., Shen B. MicroRNA-21 in the pathogenesis of acute kidney injury. Protein Cell. 2013;4:813–819. doi: 10.1007/s13238-013-3085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material