Abstract
In-line and traditional lunge exercises present differences in technique as lower limb positioning (anterioposterior), and medio-lateral (ML) balance may differentially affect primary and stabilizer muscles. The purposes of this study were to examine ML balance and muscle activation in anterior and posterior leg positions between in-line and traditional lunge exercises. Fifteen young, healthy, resistance-trained men (25 ± 5 years) performed 2 different lunge exercises (in-line and traditional) at their 10 repetition maximum in a randomized, counterbalanced fashion. Surface electromyography measured muscle activation of the vastus lateralis, biceps femoris, gluteus maximus, and gluteus medius. ML balance was measured with a Wii Fit Balance Board. The vastus lateralis activity was not significantly different between exercises or leg positions. The biceps femoris activity was not significantly different between exercises, however, it was significantly greater in the anterior compared to the posterior position for the in-line (p = 0.003), and traditional lunge (p < 0.001). The gluteus maximus activity was not significantly different between exercises, however, it was significantly greater in the anterior compared to posterior position for the in-line (p < 0.001) and traditional lunge (p < 0.001). ML balance was significantly greater in the in-line exercise in the anterior limb (p = 0.001). Thus, both in-line and traditional lunge exercises presented similar overall levels of muscle activation, yet the anterior limb generated the highest biceps femoral and gluteus maximus muscle activation when compared to the posterior limb. The in-line lunge presents greater ML balance when compared to the traditional lunge exercise.
Key words: electromyography, strength, performance
Introduction
The split squat or lunge is an exercise that increases hip and knee extensor muscle strength, which in turn can indirectly improve the quality of life in a non-athletic population, sports performance in athletic populations, and rehabilitation (Turner and Barker, 2014). There are several variations of the lunge exercise such as bilateral (in-line, traditional or spilt squat), unilateral (Bulgarian lunge, step-up), with leg movements (forward step lunge, walking lunge, reverse lunge, lateral lunge), and associated with jump tasks (jump lunge) (Haff and Triplett, 2016; Mcclellan and Bugg, 1999; Turner and Barker, 2014).
The lunge exercise may be considered as a multi-joint exercise, however, there are important differences in mechanical techniques such as the lower limb position (anterio-posterior, AP and medio-lateral, ML), balance, stability of the upper body, and core stability which may differentially affect prime and stabilizer muscles in both legs. The traditional and in-line lunge are variations in which the feet are positioned on the floor, and weight is distributed between the legs. The main difference between exercises is the ML distance of the feet, where in the traditional lunge the feet are positioned hip-width apart, while in the in-line the feet are positioned 50% hip-width apart. Additionally, during these exercises the legs perform different movements (e.g. range of movement) that may affect knee and hip muscle activation levels. Consequently, knee extensors and hip extensors are both considered prime movers, with other muscles, such as hip abductors (e.g. gluteus medius, GMd), acting as secondary or stabilizers (Caterisano et al., 2002; Marchetti et al., 2013; Schoenfeld, 2010). To date, there is little scientific information about differences in muscle activation between legs during the lunge exercise.
Another important peculiarity of the lunge exercise is the ML balance required to keep the center of gravity over the base of support (Jancová, 2008). Assuming that a narrower base of support is critical to maintain ML balance, the in-line exercise may present a more challenging balance task when compared to the traditional one. In this way, Jancová (2008) reported that motor responses in the ML direction were dominated by a hip strategy. Consequently, the hip adductors and abductors may be more stressed during the in-line lunge exercise.
The purposes of this study were to: (a) examine muscle activation in anterior and posterior leg positions and (b) evaluate ML balance between traditional and in-line lunge exercises, respectively. The main hypotheses of the present study were: (1) the AP lower limb position would affect muscle mechanics of the legs differently, and consequently, affect activation of the prime muscles; and (2) changes in foot position would affect ML balance and increase muscle activation of the stabilizers.
Methods
Participants
To establish the appropriate sample size, a pilot study (n = 5) was conducted on the peak sEMG amplitude of the root mean square (RMS) of the VL in the traditional lunge exercise. Based on a statistical power analysis derived from these data, it was determined that fourteen subjects would be necessary to achieve an alpha level of 0.05, and a power (1-β) of 0.80 (Eng, 2003). Therefore, fifteen young, healthy, resistance-trained men (age: 25 ± 5 years, body height: 175.7 ± 7.7 cm and body mass: 81 ± 8 kg, 10RM of the traditional lunge: 52.9 ± 14.4 kg; 5 ± 2 years of experience with resistance training) volunteered to participate in the study. Subjects had no previous lower back injuries, surgery on their lower extremities, no history of injury with residual symptoms (pain, “giving-away” sensations) in their lower limbs within the last year, and at least one year of resistance training experience with the lunge exercise. The study was approved by the Methodist University of Piracicaba research ethics committee and all subjects read and signed an informed consent document (#08/2015).
Procedures
Prior to data collection, subjects were asked to identify their preferred leg for kicking a ball, which was then considered their dominant leg (Maulder and Cronin, 2005). All subjects were right-leg dominant. Tests were randomized and counterbalanced across subjects and experimental conditions. Subjects reported to have refrained from performing any lower body exercise other than activities of daily living for at least 48 hours prior to testing. They attended two sessions in the laboratory. During the first session, they were instructed and familiarized in the proper execution of both the traditional and in-line lunge exercises.
For the traditional lunge (figure 1a), a barbell was positioned on and vertically aligned with the shoulders (high-bar position). The exercise was performed with the measured leg forward in a stride stance with the back knee fully extended, feet were hip-width apart and facing forward. The forward knee was flexed to 45 degrees, followed by a return to full extension while maintaining a neutral alignment over the second metatarsal. The rear knee remained in full extension throughout the exercise, and both heels remained in contact with the floor. For the in-line lunge, the same barbell and body positions were adopted, however, feet were 50% of hip-width apart (Figure 1b).
Figure 1.
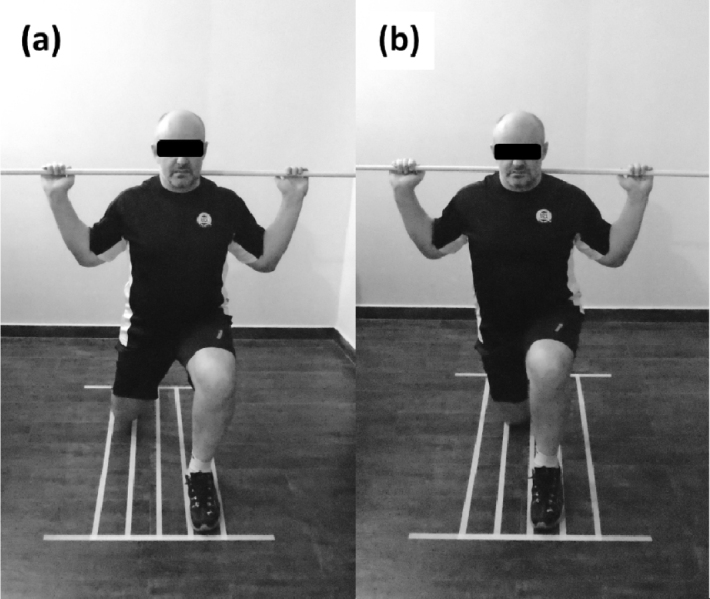
Feet width: (a) Traditional, and (b) In-line.
After a 5-min warm-up consisting of cycling at 70 rpm, subjects performed a ten repetition maximum (10RM) test of the traditional lunge (dominant leg in the front position) at a 60 beat cadence. If a 10RM was not achieved in the first attempt, the load was adjusted by 4–10 kg and a minimum of five-minute rest was given before the next attempt. Only three trials were allowed per testing session to avoid neuromuscular fatigue. Subjects received standard instructions regarding technique and exercise execution was monitored and corrected when necessary to ensure no stopping between eccentric and concentric actions for each test. Verbal encouragement was provided to facilitate maximal performance. The second session was conducted one week later, and all subjects reported to have refrained from performing any lower body exercise other than activities of daily living for at least 48 hours prior to testing. They warmed-up by cycling for 5-min at 70 rpm and then performed one set of 10RM for each lunge exercise, anterior and posterior leg positions. All measures (sEMG and ML balance) were collected on the dominant leg. In this way, four experimental conditions were performed in random order: (1) traditional lunge with the dominant leg in front; (2) traditional lunge with the dominant leg in back; (3) in-line lunge with the dominant leg in front; (4) in-line lunge with the dominant leg in back. A rest period of 30-min was provided between conditions. All conditions were performed between 9 and 12 AM, and measured by the same researcher.
Measures
Surface Electromyography (sEMG)
Subjects’ body hair was shaved at the site of electrode placement and the skin was cleaned with alcohol before affixing the sEMG electrode. Bipolar active disposable dual Ag/AgCl snap electrodes, 1-cm in diameter for each circular conductive area with 2-cm center-to-center spacing were used in all trials. Electrodes were placed on the dominant limb along the axes of the muscle fibers according to the SENIAM/ISEKI protocol (Hermens et al., 2000): VL at 2/3 of the distance between the anterior spina iliac and the superior aspect of the lateral side of the patella; BF at 50% on the line between the ischial tuberosity and the lateral epicondyle of the tibia; GM at 50% of the distance between the sacral vertebrae and the greater trochanter; GMd at 50% on the line from the iliac crest to the trochanter. All sEMG signals were recorded by an electromyographic acquisition system (EMG832C, EMG system Brasil, São José dos Campos, Brazil) with a sampling rate of 2000 Hz using commercially designed software (EMG system Brasil, São José dos Campos, Brazil). EMG activity was amplified (bi-polar differential amplifier, input impedance = 2MΩ, common mode rejection ratio > 100 dB min (60 Hz), gain x 20, noise > 5 μV), and converted from an analog to digital signal (12 bit). A ground electrode was placed on the right clavicle. sEMG signals for all conditions were normalized to a maximum voluntary isometric contraction (MVIC) against a fixed strap. Three trials of five-second MVICs were performed for each muscle with one-minute rest between actions for the dominant leg. The first MVIC was performed to familiarize the participant with the procedure. For VL and BF MVICs, subjects were seated with their knee flexed at 90o and the strap placed on the distal tibia. For GM MVIC, subjects laid prone with their knee flexed at 90o and the strap placed on the distal region of the thigh with the pelvis stabilized. For GMd MVIC, subjects laid prone with their knee extended and the strap placed on the distal region of both lower limbs in hip abduction. Verbal encouragement was given during all MVICs. Order of MVICs was counterbalanced to avoid any potential neuromuscular fatigue.
Range of Motion (ROM)
The ROM was measured by an electrogoniometer positioned on the knee joint of the dominant lower limb. Data were recorded by an acquisition system (EMG832C, EMG system Brasil, São José dos Campos, Brazil) with a sampling rate of 2000 Hz using commercially designed software (EMG system Brasil, São José dos Campos, Brazil).
Balance
For the assessment of ML displacement of the center of pressure a Wii Fit Balance Board (Nintendo, Nintendo Entertainment Analysis and Development, USA) was positioned under the anterior lower limb (dominant leg) in both exercises, with a sampling rate of 37 Hz. The Wii Fit was calibrated and adjusted with a calibrated weight of 10 kgf via custom Labview® 2013 software (ICC = 0.99) (Weaver et al., 2016).
sEMG data were analyzed with a customized Matlab routine (MathWorks Inc., Massachusetts, USA). All sEMG data were synced with the electrogoniometer, characterizing both the concentric and eccentric actions of each repetition. The first repetition was not used in order to ensure no body adjustments or changes in exercise cadence. The digitized sEMG data were band-pass filtered at 20-400 Hz using a fourth-order Butterworth filter with a zero lag. For muscle activation time domain analysis, RMS (200 ms moving window) was calculated for both the MVIC and sEMG data. The sEMG data were then normalized to the RMS average of the two peak MVICs for each amplitude and muscle. The integrated EMG (IEMG) analysis was calculated from the first three repetitions for each experimental condition and muscle. The ML balance data was filtered at 10 Hz using a fourth-order Butterworth filter. Then, balance displacement was calculated by subtracting the maximum and minimum values for each experimental condition.
Statistical Analysis
The normality and homogeneity of variances were confirmed by the Shapiro-Wilk and Levene’s tests, respectively. To test differences in muscle activity (sEMG), 2x2 repeated-measures ANOVAs (leg position x lunge technique) were used. To test differences in ML balance, a paired t-test was used. Cohen’s effect size (d) was calculated, and results were evaluated on the following criteria: <0.35 trivial; 0.35-0.80 small; 0.80-1.50 moderate; and >1.5 large, for recreationally trained subjects (Rhea, 2004). Intra-rater reliability was assessed for all muscles and experimental conditions. Reliability was operationalized using the following criteria: < 0.4 poor; 0.4 - < 0.75 satisfactory; ≥ 0.75 excellent. All ICCs ranged between 0.91 and 0.98 for all RMS data. An alpha level of 0.05 was used to determine statistical significance.
Results
Muscle Activity
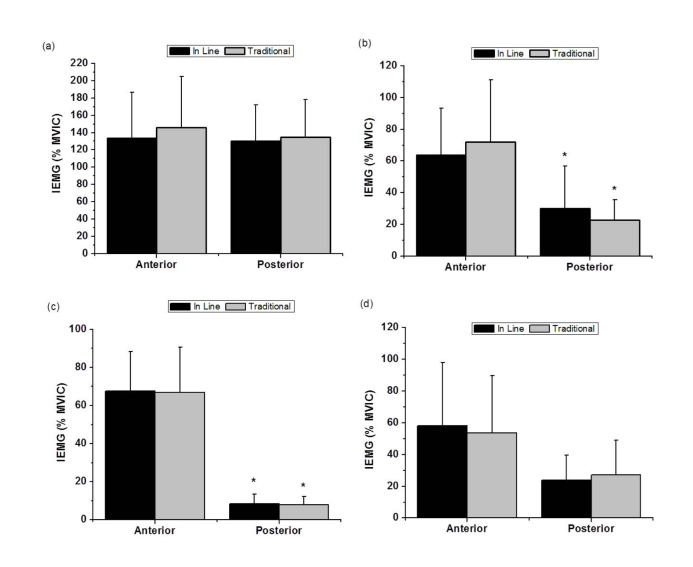
VL activity was not significantly (p > 0.05) different between exercises or leg positions (Figure 1a). BF activity was not significantly (p > 0.05) different between exercises, however, it was significantly greater in the anterior compared to the posterior position for both exercises: in-line (p = 0.003; d = 1.19; Δ% = 53), and traditional (p < 0.001; d = 1.69; Δ% = 68.3) (Figure 1b). GM activity was not significantly (p > 0.05) different between exercises, however, it was significantly greater in the anterior compared to the posterior position for both exercises: in-line (p < 0.001; d = 3.96; Δ% = 87.7) and traditional (p < 0.001; d = 3.45; Δ% = 88.3) (Figure 1c). GMd activity was not significantly (p > 0.05) different between exercises or lower limb positions (Figure 1d).
Figure 2.
Mean and standard deviation of IEMG from (a) Vastus Lateralis, (b) Bíceps Femoris, (c) Gluteus Maximus, and (d) Gluteus Medius, between lunge exercises and lower limb positions. *Significantly different between conditions, p < 0.05.
Balance
ML balance displacement was significantly greater in the in-line exercise in the anterior limb (in-line: 19.4 ± 4.1 cm, and traditional: 14.8 ± 2.9 cm; p = 0.001; d = 0.81; Δ% = 23.51).
Discussion
The purposes of this study were to examine muscle activation in anterior and posterior leg positions and to evaluate ML balance between traditional and in-line lunge exercises. The main findings were that both exercises presented a similar overall level of muscle activation, however, there were differences between the lower limb positions (anterior x posterior leg) for BF and GM. Additionally, greater ML balance was observed with the in-line when compared to the traditional lunge exercise.
The present study did not demonstrate differences in muscle activation between in-line and traditional lunge exercises when the legs were compared in the same position (anterior and posterior leg). This was probably due to similarities in lower limb mechanics in the sagittal plane, even with stance width differences. Therefore, differences in stance width did not affect either the prime (VL, BF, GM) or stabilizer (GMd) muscles’ activation. The lunge exercise simultaneously utilizes several muscles with different articulations (monoarticular and biarticular) in a manner that produces “muscle coordination” (Marchetti et al., 2016; Prilutsky, 2000). A multi-joint task to strengthen knee and hip extensors is more complex for the neuromuscular system as two joints work in concert to achieve a task (Robertson et al., 2008). However, the lunge exercise presents important mechanical characteristics such as positioning of the legs (AP). Consequently, each leg should be analyzed separately.
The movement of the anterior leg could be partially compared to the squat exercise, where the monoarticular muscles, including the VL and GM, contribute to movement (Schoenfeld, 2010). The present results demonstrate that muscle activation of the VL and GM did not differ between exercises, however, the highest muscle activation was observed in the VL (Δ% = ~53.4, for both exercises), as it is in the squat exercise (Contreras et al., 2016; Gorsuch et al., 2013; Marchetti et al., 2016). It is feasible to speculate that changes in muscle length of the GM modify muscle contractile abilities and, in turn, modify sEMG-force and sEMG-moment relationships (Prilutsky, 2000; Worrell et al., 2001). Alternatively, afferent signals from muscles could decrease motoneuronal firing frequency (i.e. Golgi tendon reflex) during muscle contractions when the fibers are in an elongated state (Gardiner, 2011). As in the squat exercise, the lunge presents similar muscle participation of the hamstrings (e.g. BF) (Schoenfeld, 2010). Biarticular muscles such as BF have intermediate activation when they are agonistic at one joint and antagonistic at the other joint. This is in contrast to high activation levels seen when a biarticular muscle works as an agonist for both joints simultaneously (Prilutsky, 2000). Lombard (1903) suggested that biarticular muscles of the lower extremities acted in a “paradoxical” fashion when the movement was constrained or controlled (named Lombard’s paradox), as observed in the BF. Hip and knee extension are the result of differential range of motion and moment arms of the two muscles at each joint. Quantitatively, the higher muscle activation of the VL compared to the BF may be explained by it acting as a joint stabilizer at the knee and a prime mover at the hip (Marchetti et al., 2016; Robertson et al., 2008). However, the GM presented lower muscle activity when compared to the BF (Δ% = ~6, for both exercises). Additionally, all muscles of the anterior limb during the lunge exercise may be affected by a sticking region. This is a poor mechanical force position in which the length and myofilament overlap of muscle fibers are less than optimal to produce maximal force, whereby the lifter experiences difficulty in exerting force against the barbell (Elliot et al., 1989; Tillaar and Saeterbakken, 2013; Van Den Tillaar and Sæterbakken, 2012; Van Den Tillaar et al., 2014; Van Den Tillaar, 2015).
In contrast, the movement of the posterior leg seems to be quite different to the anterior leg. To date, there is no scientific information about muscle activation of the posterior leg in the lunge exercise. It is probable that the position of the posterior leg may alter activation of the prime muscles. Results of the present study exhibited low BF and GM muscle activation compared to the anterior limb position, with no differences between exercises. This may be explained by the sEMG-force and sEMG-moment relationships (Prilutsky, 2000; Worrell et al., 2001) as both muscles were not optimally positioned to produce high activation. Interestingly, activation of the VL was similar between legs and exercises, which may be explained due to the ROM of the knee joint being similar in both leg positions (0-90 degrees), and the external load being the same. Finally, the in-line lunge showed greater ML balance when compared to the traditional exercise (Δ% = 23.51). Thus, changes in stance width resulting in a narrower base of support are critical to maintaining ML balance (frontal plane). This process consists of establishing active muscular constraints to minimise degrees of freedom within a joint or series of joints and results in stabilisation of and reduction in excessive mobility of external objects (Anderson and Behm, 2005). In this way, Jancová (2008) demonstrated that motor responses in the ML direction were dominated by a hip strategy. Previous studies have reported greater GMd activity to stabilize the lower limb in the single leg triple hop (Bley et al., 2014), and step-down tests (Bolgla et al., 2011). However, Nakagawa et al. (2012), and Aminaka et al. (2011) found lower activation of the GMd in a single-leg squat, partially supporting our results. This similar GMd activation between exercises may be explained because the GMd, acting primarily in the frontal plane of the hip joint (Aaberg, 1998), acts as a stabilizer during the lunge (sagittal plane). Alternately, the EMG was not sensitive enough to measure activation during small ML movements at the hip. In addition, Anderson and Behm (2005) showed that in very stable conditions, the requirements of stabilizing posture under the action of transient, motion-related perturbations are alleviated.
Our study was limited by the use of a goniometer instead of a motion analysis system. Unfortunately, it was not available in our laboratory but should be implemented in future studies. The study was also limited by the use of the Wii Fit Balance Board to analyze the ML balance.
Finally, the in-line and traditional lunge exercise demonstrated similar overall levels of muscle activation, yet the anterior limb generated the highest BF and GM muscle activation when compared to the posterior limb while the VL showed similar activation between legs and exercises. The in-line lunge showed greater ML balance when compared to the traditional lunge. Thus, both exercises are recommended for activating lower limb musculature. However, if balance or stability adaptations are the most important objectives, then the in-line lunge is recommended for challenging medio-lateral balance under load. This research may be useful for coaches and athletes as the lunge exercise can be used in a wide range of sports related to lower-body activities under unilateral conditions (i.e. tennis, squash, rugby, American football, etc.) or when the unilateral transfer of forces is required (i.e. change of direction, throwing, kicking, and striking).
References
- Aaberg E. Muscle Mechanics. United States: Human Kinetics; 1998. [Google Scholar]
- Aminaka N, Pietrosimone BG, Armstrong CW, Meszaros A, Gribble PA. Patellofemoral pain syndrome alters neuromuscular control and kinetics during stair ambulation. J Electromyogr Kinesiol. 2011;21:645–651. doi: 10.1016/j.jelekin.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Anderson K, Behm DG. The Impact of Instability Resistance Training on Balance and Stability. Sports Med. 2005;35(1):43–53. doi: 10.2165/00007256-200535010-00004. [DOI] [PubMed] [Google Scholar]
- Bley AS, Correa JCF, Reis AC, Rabelo NDA, Marchetti PH, Lucareli PRG. Propulsion Phase of the Single Leg Triple Hop Test in Women with Patellofemoral Pain Syndrome: A Biomechanical Study. PLOS ONE. 2014;9(5):1–7. doi: 10.1371/journal.pone.0097606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolgla LA, Malone TR, Umberger BR, Uhl TL. Comparison of hip and knee strength and neuromuscular activity in subjects with and without patellofemoral pain syndrome. Int J Sports Phys Ther. 2011;6:285–296. [PMC free article] [PubMed] [Google Scholar]
- Caterisano A, Moss RF, Pellinger TK, Woodruff K, Lewis VC, Booth W, Khadra T. The effect of back squat depht on the EMG activity of 4 superficial hip and thigh muscles. J Strength Cond Res. 2002;16(3):428–432. [PubMed] [Google Scholar]
- Contreras B, Vigotsky AD, Schoenfeld BJ, Beardsley C, Cronin J. A Comparison of Gluteus Maximus, Biceps Femoris, and Vastus Lateralis EMG Amplitude in the Parallel, Full, and Front Squat Variations in Resistance Trained Females. J Appl Biomech. 2016;32(1):16–22. doi: 10.1123/jab.2015-0113. [DOI] [PubMed] [Google Scholar]
- Gardiner PF. Advanced neuromuscular exercise physiology. United States: Human Kinetics; 2011. [Google Scholar]
- Gorsuch J, Long J, Miller K, Primeau K, Rutledge S, Sossong A, Durocher JJ. The effect of squat depth on multiarticular muscle activation in collegiate cross-country runners. J Strength Cond Res. 2013;27(9):2619–2625. doi: 10.1519/JSC.0b013e31828055d5. [DOI] [PubMed] [Google Scholar]
- Haff GG, Triplett NT. Essentials of strength training and conditioning. 4. United States: Human Kinetics; 2016. [Google Scholar]
- Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10(5):361–374. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- Jancová J.. Measuring the balance control system - Review. Acta Medica. 2008;51(3):129–137. [PubMed] [Google Scholar]
- Eng J. Sample Size Estimation: How many individuals should be studied? Radl. 2003;227(2):309–313. doi: 10.1148/radiol.2272012051. [DOI] [PubMed] [Google Scholar]
- Lombard WP. The action of two-joint muscles. Am J of Physics Education. 1903;9:141–145. [Google Scholar]
- Elliot BC, Wilson GJ, Kerr GK. A biomechanical analysis of the sticking region in the bench press. Med Sci Sports Exerc. 1989;21(4):450–462. [PubMed] [Google Scholar]
- Marchetti PH, Da Silva JJ, Schoenfeld BJ, Nardi PSM, Pecoraro SL, Greve JMD, Hartigan E. Muscle Activation Differs between Three Different Knee Joint-Angle Positions during a Maximal Isometric Back Squat Exercise. J Sports Med. 2016;2016:1–6. doi: 10.1155/2016/3846123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti PH, Gomes WA, Da Luz Junior DA, Giampaoli B, Amorim MA, Bastos HL, Ito DT, Vilela Junior GB, Lopes CR, Bley AS. Neuromechanical aspects of the squat exercise. CPAQV. J. 2013;5(2):1–16. [Google Scholar]
- Maulder P, Cronin J. Horizontal and vertical jump assessment: reliability, symmetry, discriminative and predictive ability. Phys Ther Sport. 2005;6(2):74–82. [Google Scholar]
- McClellan T, Bugg BS. Lunge variations to enhance specificity in tennis. Strength Cond J. 1999;21(6):18–24. [Google Scholar]
- Nakagawa TH, Moriya ET, Maciel CD, Serrao FV. Trunk, pelvis, hip, and knee kinematics, hip strength, and gluteal muscle activation during a single leg squat in males and females with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2012;42:491–501. doi: 10.2519/jospt.2012.3987. [DOI] [PubMed] [Google Scholar]
- Robertson DGE, Wilson J-MJ, St., Pierre TA. Lower Extremity Muscle Functions During Full Squats. J Appl Biomech. 2008;24:333–339. doi: 10.1123/jab.24.4.333. [DOI] [PubMed] [Google Scholar]
- Rhea MR. Determining the magnitude of treatment effects in strength training research through the use of the effect size. J Strength Cond Res. 2004;18(4):918–920. doi: 10.1519/14403.1. [DOI] [PubMed] [Google Scholar]
- Prilutsky BI. Coordination of Two- and One-Joint Muscles: Functional Consequences and Implications for Motor Control. M Control. 2000;4:1–44. doi: 10.1123/mcj.4.1.1. [DOI] [PubMed] [Google Scholar]
- Schoenfeld BJ. Squatting kinematics and kinetics and their application to exercise performance. J Strength Cond Res. 2010;24(12):3497–3506. doi: 10.1519/JSC.0b013e3181bac2d7. [DOI] [PubMed] [Google Scholar]
- Tillaar RVD, Saeterbakken AH. Fatigue effects upon sticking region and electromyography in a six-repetition maximum bench press. J Sports Sci. 2013;31(16):1823–1830. doi: 10.1080/02640414.2013.803593. [DOI] [PubMed] [Google Scholar]
- Turner G, Barker K. Exercise selection to develop optimal explosive lunge movements for World-Standard Squash. Strength Cond J. 2014;36(4):36–42. [Google Scholar]
- Van den Tillaar R, Sæterbakken A. The sticking region in three chest-press exercises with increasing degrees of freedom. J Strength Cond Res. 2012;26(11):2962–2969. doi: 10.1519/JSC.0b013e3182443430. [DOI] [PubMed] [Google Scholar]
- Van den Tillaar R, Andersen V, Saeterbakken A. The existence of a sticking region in free weight squats. J Hum Kinet. 2014;42:7–20. doi: 10.2478/hukin-2014-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Tillaar R. Kinematics and muscle activation around the sticking region in free weight barbell back squat. Kin Slov. 2015;21(1):15–25. [Google Scholar]
- Weaver TB, Ma C, Laing AC. Use of the Nintendo Wii Balance Board for Studying Standing Static Balance Control: Technical Considerations, Force-Plate Congruency, and the Effect of Battery Life. J Appl Biomech. 2016;13:1–22. doi: 10.1123/jab.2015-0295. [DOI] [PubMed] [Google Scholar]
- Worrell TM, Karst G, Adamczyk D, Moore R, Stanley C, Steimel B, Steimel S. Influence of joint position on electromyographic and torque generation during maximal voluntary isometric contractions of the hamstrings and gluteus maximus muscles. J Orthop Sports Phys Ther. 2001;31(12):730–40. doi: 10.2519/jospt.2001.31.12.730. [DOI] [PubMed] [Google Scholar]