Abstract
There are various mechanisms underlying the resistance of EGFR-mutant lung adenocarcinoma to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs). We herein report a case of pulmonary adenocarcinoma with EGFR mutation (exon 19 deletion and T790M) that acquired resistance to osimertinib treatment because of transformation into small-cell lung carcinoma (SCLC). A 67-year-old ex-smoking woman was diagnosed with left upper lobe adenocarcinoma of clinical stage IIIA (cT2bN2M0). She was treated with chemoradiotherapy (cisplatin and vinorelbine plus radiation), gefitinib, cisplatin, and pemetrexed followed by pemetrexed maintenance therapy and erlotinib. Since a sample extracted from the metastatic lung tumor taken obtained via a transbronchial lung biopsy was found to be positive for the T790M mutation at the time of disease progression during erlotinib treatment, she received osimertinib treatment for 15 months until progressive disease. She developed resistance to osimertinib due to the histologic transformation to SCLC. Although the standard chemotherapy of carboplatin and etoposide for SCLC was administered, she died due to metastatic liver failure.
Keywords: Osimertinib, T790M, Acquired resistance, Small-cell carcinoma transformation, Non-small-cell carcinoma, Epidermal growth factor receptor
Introduction
Osimertinib is a third-generation epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) that shows great effectiveness against pulmonary adenocarcinoma with an EGFR T790M mutation, which induces acquired resistance to first- and second-generation EGFR-TKIs. Since about 50% of acquired resistance cases have the T790M mutation, examining the EGFR T790M status when the disease progresses during first- or second-generation EGFR-TKI treatment is essential for delivering osimertinib adequately.
However, re-examination of the EGFR status when patients acquire resistance to osimertinib treatment is controversial, as no EGFR-TKIs have yet been developed to overcome resistance to osimertinib induced by an EGFR mutation and/or other resistance mechanisms. Small-cell lung carcinoma (SCLC) transformation from adenocarcinoma during osimertinib treatment is rare but has been reported in cases of acquired resistance to first- and second-generation EGFR-TKIs. When SCLC transformation is confirmed in patients with acquired resistance to osimertinib treatment, we treat these patients with cytotoxic chemotherapy for SCLC. If the clinical features of the SCLC transformation cases after osimertinib treatment were examined, we might be able to decide on the indication and timing of a re-biopsy when the disease progresses during osimertinib treatment.
We herein report a patient with pulmonary adenocarcinoma who acquired resistance to a first-generation EGFR-TKI with a T790M mutation and then acquired resistance to osimertinib by transforming to SCLC without a T790M mutation.
Case Presentation
A 67-year-old woman visited our hospital due to a chest X-ray abnormality found on a routine screening. Chest computed tomography showed a mass in the left upper lobe that was later diagnosed as pulmonary adenocarcinoma harboring a deletion within exon 19 of the EGFR gene. According to positron emission tomography computed tomography and head magnetic resonance imaging results, her lung cancer was diagnosed as cT2bN2M0 stage IIIA. She received chemoradiotherapy, which consisted of three courses of cisplatin and vinorelbine, 32 Gy/16 fractions radiation and 42 Gy of proton beam therapy on the tumor.
Eighteen months later, the mediastinal lymph nodes on the right side were swollen, and progressive disease was confirmed. She received gefitinib for 19 months until progressive disease and then cisplatin and pemetrexed followed by pemetrexed monotherapy for 4 months and erlotinib for 9 months. At the time of progressive disease during erlotinib treatment, transbronchial lung biopsy of a pulmonary metastatic nodule (Fig. 1a) was performed to examine the status of the EGFR mutation. The DNA extracted from the tissue taken by the transbronchial lung biopsy showed the presence of EGFR T790M.
Fig. 1.
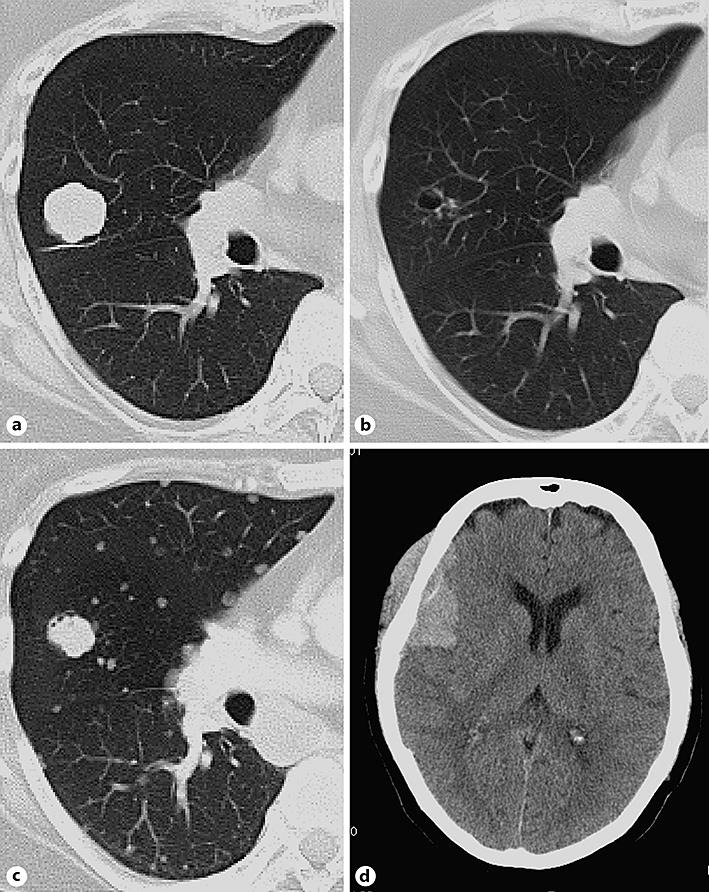
Chest computed tomography (a, b, c) and brain computed tomography (d) of our case. a T790M positivity at the diagnosis of EGFR mutation. b After 8 months of osimertinib treatment. c, d After 17 months of osimertinib treatment with disease progression.
The patient received osimertinib, and her cancer was well controlled for 13 months (Fig. 1b); however, a hematoma was noted on the right temporal part (Fig. 1d). A craniotomy procedure to verify the subdural hematoma showed that the hematoma was in fact a tumor. The tumor was partly resected and sent for pathologic examination. While she received additional radiotherapy (39 Gy/13 fractions) in the right temporal bone, the tissue was finally diagnosed as small-cell carcinoma (Fig. 2) morphologically showing poorly differentiated cells with a high nuclear-to-cytoplasmic ratio and stained with neuroendocrine markers (synaptophysin and NCAM). An EGFR mutation analysis showed that the exon 19 deletion was persistent in the small-cell carcinoma, but the T790M mutation had been lost, and C797S was not detected. Although we treated her with chemotherapy (carboplatin and etoposide), her liver function rapidly deteriorated due to the progression of her liver metastasis. She passed away 4 days after the initiation of therapy.
Fig. 2.
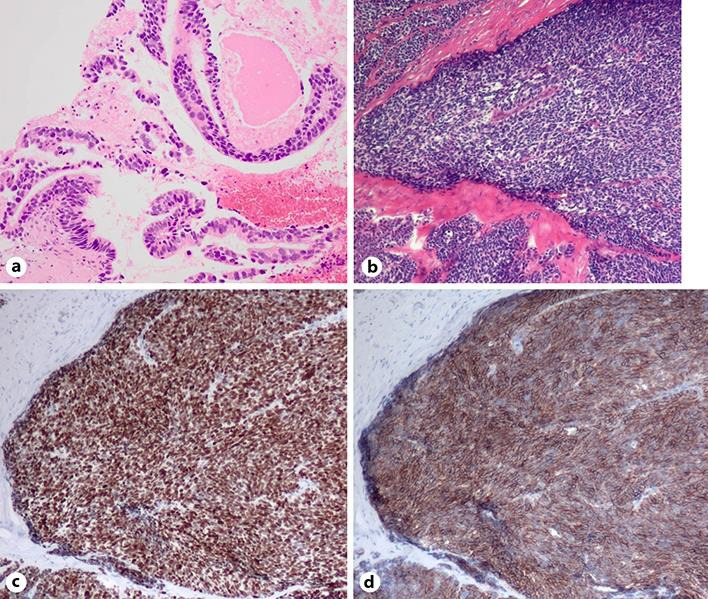
Small-cell lung carcinoma transformation of adenocarcinoma after osimertinib treatment. a Histology of the primary tumor at diagnosis. b–d Histology of the cranium and surrounding soft tissue, stained with hematoxylin and eosin (b), TTF-1 (c), and synaptophysin (d).
Discussion
Resistance to osimertinib is induced by tertiary EGFR mutations, such as C797S, pL7981, pL692V, and pL692V, and the T790M reduction or disappearance along with EGFR amplification and phenotype alterations, such as a histologic transformation to neuroendocrine morphology [1, 2]. Although histologic transformation to SCLC is observed in 3–14% of patients with EGFR-mutant non-SCLC as the acquired resistance to first- or second-generation EGFR-TKIs [1, 3], the transformation to SCLC after third-generation EGFR-TKI (osimertinib) treatment is rare, with only 7 cases reported to our knowledge [1, 4, 5]. Since all of the cases maintained the original EGFR mutations, it is unclear whether adenocarcinoma evolved into SCLC metachronously or developed from a common precursor.
The response rate to standard chemotherapy for usual SCLC is 70–90% in limited disease and 60–70% in extended disease. The overall survival is reported to be 14–20 months for limited disease and 9–11 months for extended disease [6]. SCLC transformation from adenocarcinoma after TKI therapy has a relatively poor prognosis, with an overall survival of 7.1 months. However, the response rate of combination therapy with platinum and etoposide can be as high as 83% [6]. As a result, a re-biopsy should be performed if the patient is young, a nonsmoker, or has an EGFR mutation or mixed histology, and NSE/proGRP and CEA should also be monitored accordingly so that the appropriate treatment can be administered.
In our case, proGRP was routinely monitored throughout the treatment course (Fig. 3). Since the proGRP levels remained the same and the CEA levels were elevated at the time of progression, except for the terminal phase, we were unable to predict the SCLC transformation in this case. Although inactivation of Rb1 and p53 is reported to be a predictor for transformation into SCLC [7], we did not examine the status of these genes in this case. As patients with transformation into SCLC tend to show a poor prognosis, a re-biopsy to confirm the SCLC transformation should be considered promptly when encountering unexpected rapid disease progression during EGFR-TKI treatment.
Fig. 3.
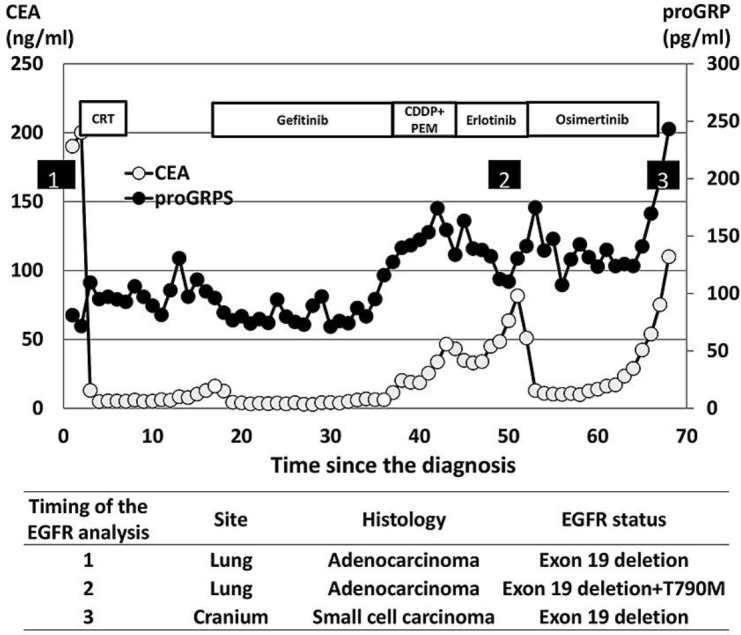
The clinical course and EGFR mutation status of our case. Numbers show the timing of the EGFR mutation analysis. CRT, chemoradiation; CDDP+PEM, cisplatin + pemetrexed.
Conclusion
This was a case of resistance to a third-generation EGFR-TKI due to SCLC transformation. A re-biopsy should be a priority when EGFR-mutant non-SCLC shows unexpected aggressive progression.
Statement of Ethics
The authors declare that they have no ethical conflicts to disclose.
Disclosure Statement
The authors have nothing to disclose.
References
- 1.Kim TM, Song A, Kim DW, Kim S, Ahn YO, Keam B, et al. Mechanisms of acquired resistance to AZD9291 a mutation-selective, irreversible EGFR inhibitor. J Thorac Oncol. 2015 Dec;10((12)):1736–44. doi: 10.1097/JTO.0000000000000688. [DOI] [PubMed] [Google Scholar]
- 2.Minari R, Bordi P, Tiseo M. Third-generation epidermal growth factor receptor-tyrosine kinase inhibitors in T790M-positive non-small cell lung cancer review on emerged mechanisms of resistance. Transl Lung Cancer Res. 2016 Dec;5((6)):695–708. doi: 10.21037/tlcr.2016.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu H, Chen B, Qin J, Xie F, Han N, Huang Z. Transformation to small-cell lung cancer following treatment with icotinib in a patient with lung adenocarcinoma. Oncol Lett. 2018 Apr;15((4)):5799–802. doi: 10.3892/ol.2018.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ham JS, Kim S, Kim HK, Byeon S, Sun JM, Lee SH, et al. Two cases of small cell lung cancer transformation from EGFR mutant adenocarcinoma during AZD9291 treatment. J Thorac Oncol. 2016 Jan;11((1)):e1–4. doi: 10.1016/j.jtho.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Minari R, Bordi P, Del Re M, Facchinetti F, Mazzoni F, Barbieri F, et al. Primary resistance to osimertinib due to SCLC transformation issue of T790M determination on liquid re-biopsy. Lung Cancer. 2018 Jan;115:21–7. doi: 10.1016/j.lungcan.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Jiang SY, Zhao J, Wang MZ, Huo Z, Zhang J, Zhong W, et al. Small-cell lung cancer transformation in patients with pulmonary adenocarcinoma A case report and review of literature. Medicine (Baltimore) 2016 Feb;95((6)):e2752. doi: 10.1097/MD.0000000000002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JK, Lee J, Kim S, Kim S, Youk J, Park S, et al. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J Clin Oncol. 2017 Sep;35((26)):3065–74. doi: 10.1200/JCO.2016.71.9096. [DOI] [PubMed] [Google Scholar]


