Abstract
Background
Human osteosarcoma (OS) is one of the most common primary bone sarcoma, because of early metastasis and few treatment strategies. It has been reported that the tumorigenicity and self-renewal capacity of side population (SP) cells play roles in human OS via regulating of target genes. This study aims to complement the differentially expressed genes (DEGs) that regulated between the SP cells and the non-SP cells from primary human OS and identify their functions and molecular pathways associated with OS.
Methods
The gene expression profile GSE63390 was downloaded, and bioinformatics analysis was made.
Results
One hundred forty-one DEGs totally were identified. Among them, 72 DEGs (51.06%) were overexpressed, and the remaining 69 DEGs (48.94%) were underexpressed. Gene ontology (GO) and pathway enrichment analysis of target genes were performed. We furthermore identified some relevant core genes using gene–gene interaction network analysis such as EIF4E, FAU, HSPD1, IL-6, and KISS1, which may have a relationship with the development process of OS. We also discovered that EIF4E/mTOR signaling pathway could be a potential research target for therapy and tumorigenesis of OS.
Conclusion
This analysis provides a comprehensive understanding of the roles of DEGs coming from SP cells in the development of OS. However, these predictions need further experimental validation in future studies.
Keywords: Osteosarcoma, Side population cells, Differentially expressed genes, Bioinformatics analysis
Background
Osteosarcoma (OS), which is produced by mesenchymal cells, is the most common primary malignant tumor originating from bone tissues. OS occurs mainly in children and adolescents and accounts for 8.9% of cancer-related diseases which lead to death [1, 2]. Although new therapies of neoadjuvant chemotherapy and surgery have contributed greatly to OS treatment, the 5-year survival rate of OS is difficult to exceed 60–65% [3, 4]. To sum up, the early diagnosis and effective treatment of OS are the breakthrough point of OS research and clinical application, and exploring the pathogenesis, development, and metastasis of OS is the key. Although a large number of studies have been made on OS at molecular and cellular levels, the mechanisms of OS formation and metastasis have not been fully elucidated.
Side population (SP) cells are a group of special cells, which were found when Hoechst and flow cytometry are used to separate hematopoietic stem cells and progenitor cells. SP cells are widely distributed in a variety of adult tissues, embryos, and some tumor cell lines [5]. They not only have self-renewal and multipotential differentiation potential, but also have unique phenotypic markers and biological characteristics of stem cells, whose characteristics are very similar to those of tumor stem cells [6, 7]. SP cells help maintain the tumorigenic potential of some tumor cell lines [8–10]. Ho et al. reported that SP cells were enriched in tumor-initiating capability compared with non-SP cells by nonobese diabetic/severe combined immunodeficiency xenograft experiments. Matrigel invasion assay showed that SP cells also have higher potential for invasiveness. Human telomerase reverse transcriptase expression was higher in the SP cells, suggesting that this fraction may represent a reservoir with unlimited proliferative potential for generating cancer cells [11]. Chiba et al. hold that a minority population of SP cells detected in hepatocellular carcinoma cells possessed extreme tumorigenic potential and provided heterogeneity to the cancer stem cell system characterized by distinct hierarchy [12]. Wang et al. observed a strong tumorigenesis ability of SP cells from HeLa cell line following in vivo transplantation into 5- to 6-week-old female Balb/c mice [13]. These findings indicate that SP cells is an enriched source of tumor-initiating cells with stem cell properties and may be an important target for effective therapy and a useful tool to investigate the tumorigenic process.
Interestingly, SP cells are present in primary mesenchymal neoplasms, including primary OS [14]. Here, we downloaded the gene expression profile GSE63390 from the Gene Expression Omnibus (GEO) database and made bioinformatics analysis to investigate differentially expressed genes (DEGs) that regulated between the SP cells and the non-SP cells from primary human OS. By doing this, we hope that the key target genes and pathways involved in the carcinogenesis and progression of human OS could be identified and existing molecular mechanisms could be revealed.
Methods
Gene expression microarray data
The gene expression profile GSE63390 was downloaded from the Gene Expression Omnibus (GEO, www.ncbi.nlm.nih.gov/geo/). GSE63390 was based on Illumina Human HT-12 V4.0 expression beadchip GPL10558 platform. The GSE63390 dataset contained three samples, including three SP cell samples and three non-SP cell samples.
DEGs in SP cells and non-SP cells
The raw data files used for the analysis included TXT files (Illumina platform). The analysis was carried out using GEO2R, which can perform comparisons on original submitter-supplied processed data tables using the GEO query and limma R packages from Bioconductor project. The P value < 0.05 and log fold change (FC) > 1.0 or log FC < − 1.0 were used as the cut-off criteria. The DEGs with statistical significance between the SP cells and non-SP cells were selected and identified.
GO and KEGG analysis of DEGs
Target genes list were submitted to the Cytoscape software version 3.4.0 (www.cytoscape.org) and ClueGO version 2.33 to identify overrepresented GO categories and pathway categories. Gene Ontology (GO) analysis was used to predict the potential functions of the DEGs in biological process (BP), molecular function (MF), and cellular component (CC). The Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.genome.jp/) is a knowledge base for systematic analysis of gene functions, linking genomic information with higher-level systemic functions. Finally, the overrepresented pathway categories with a P value < 0.05 were considered statistically significant using KEGG pathway enrichment analysis.
Gene interaction network construction
A large number of DEGs we obtained may be human OS-associated genes, and it is suggested that these DEGs in SP cells may participate in the progression of human OS. Firstly, DEGs list was submitted to the Search Tool for the Retrieval of Interacting Genes (STRING) database (http://www.string-db.org/) and an interaction network chart with a combined score > 0.4 was saved and exported. Subsequently, the interaction regulatory network of human OS-associated genes was visualized using Cytoscape software version 3.4.0. The distribution of core genes in the interaction network was made by NetworkAnalyzer in Cytoscape. Then, the plugin Molecular Complex Detection (MCODE) was applied to screen the modules of the gene interaction network in Cytoscape.
Results
Identification of DEGs
The gene expression profile GSE63390 was downloaded from the GEO, and the GEO2R method was used to identify DEGs in SP cells compared with non-SP cells. P value < 0.05, log FC > 1.0, or log FC < − 1.0 were used as the cut-off criteria. After analyzing, differentially expression gene profiles were obtained. Totally, 141 DEGs were identified including 72 upregulated DEGs and 69 downregulated DEGs screened in SP cells of human OS compared with non-SP cells. Parts of DEGs were listed in Table 1.
Table 1.
The top 10 regulated DEGs in OS SP cells with P value < 0.05
| ID | P value | logFC | Gene symbol |
|---|---|---|---|
| Upregulated | |||
| ILMN_2184250 | 6.45E−05 | 3.0817572 | SERPINB9 |
| ILMN_1713706 | 4.08E−02 | 3.08089766 | ZNF786 |
| ILMN_2260756 | 4.98E−02 | 2.76569527 | GSDMB |
| ILMN_2189870 | 3.65E−03 | 2.52175439 | FCF1 |
| ILMN_2078724 | 2.68E−02 | 2.46037207 | APOPT1 |
| ILMN_1681490 | 3.27E−02 | 2.34502196 | ZNF568 |
| ILMN_1809957 | 1.68E−03 | 2.32352121 | AP2S1 |
| ILMN_1812392 | 1.46E−02 | 2.28124068 | TMSB10 |
| ILMN_2246548 | 3.62E−02 | 2.24405561 | GSTTP2 |
| ILMN_2053178 | 0.96444 | 2.21210244 | ACTG1 |
| Downregulated | |||
| ILMN_1789196 | 2.84E−03 | − 2.95354379 | TPM2 |
| ILMN_1672496 | 2.45E−04 | − 2.53681489 | DNAJA1 |
| ILMN_1755733 | 7.92E−03 | − 2.32101519 | RPLP2 |
| ILMN_1690494 | 3.37E−03 | − 2.30094686 | RPL6 |
| ILMN_1686367 | 1.42E−02 | − 2.28782734 | HSPA8 |
| ILMN_1728870 | 6.65E−03 | − 2.24915587 | DDX3X |
| ILMN_2230624 | 2.93E−03 | − 2.22730708 | RPL18 |
| ILMN_1666385 | 5.79E−03 | − 2.18496943 | CALM3 |
| ILMN_2378868 | 3.56E−02 | − 2.17417408 | SRSF5 |
| ILMN_2139943 | 3.81E−03 | − 2.15657539 | RPS3A |
OS osteosarcoma, DEGs differentially expressed genes, SP side population, FC fold change
GO term enrichment analysis of DEGs
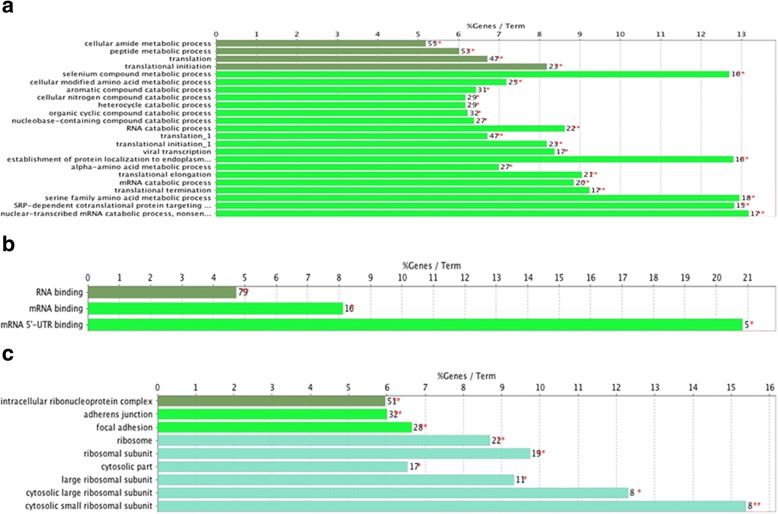
Functional annotation of the 141 DEGs was clarified using the Cytoscape software online tool. GO analysis indicated that these DEGs were significantly enriched in cellular amide metabolic process, peptide metabolic process, translation, translational initiation, selenium compound metabolic process, cellular modified amino acid metabolic process, aromatic compound catabolic process, cellular nitrogen compound catabolic process, heterocycle catabolic process, organic cyclic compound catabolic process, nucleobase-containing compound catabolic process, RNA catabolic process, viral transcription, establishment of protein, localization to endoplasmic reticulum, alpha-amino acid metabolic process, translational elongation, mRNA catabolic process, translational termination, serine family amino acid metabolic process, SRP-dependent cotranslational protein targeting to membrane, nuclear-transcribed mRNA catabolic process, nonsense-mediated decay, and other biological processes (Fig. 1). For MF, the DEGs were enriched in RNA binding, mRNA binding, mRNA 5′-UTR binding and others. In addition, GO CC analysis also showed that the DEGs were significantly enriched in intracellular ribonucleoprotein complex, adherens junction, focal adhesion, ribosome, ribosomal subunit, cytosolic part, large ribosomal subunit, cytosolic large ribosomal subunit, cytosolic small ribosomal subunit, and others.
Fig. 1.
Gene ontology (GO) enrichment analysis of biological processes (a), molecular functions (b), and cellular components (c). The red star in GO terms means term P value < 0.05, and the double red stars in GO terms mean term P value < 0.01
KEGG pathway analysis of DEGs
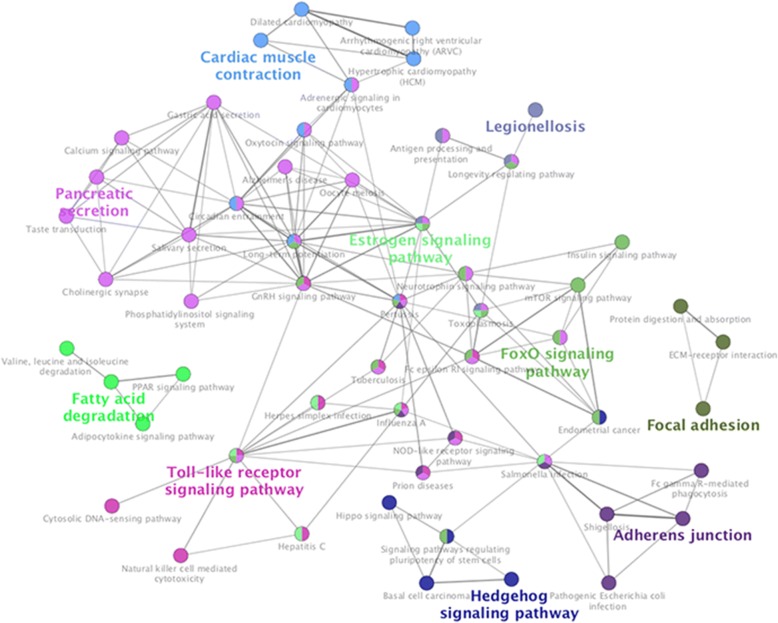
The result of KEGG pathway analysis revealed that target genes were enriched in estrogen signaling pathway, hippo signaling pathway, adherens junction, NOD-like receptor signaling pathway, apelin signaling pathway, ECM–receptor interaction, Toll-like receptor signaling pathway, mTOR signaling pathway, FoxO signaling pathway, cell adhesion molecules (CAMs) and hedgehog signaling pathway, and others. These key pathways were showed in Fig. 2. Fifty-five nodes and 163 edges could be discovered in this network. FoxO signaling pathway and mTOR signaling pathway clustered together. Estrogen signaling pathway and hippo signaling pathway clustered together. Besides, these core pathways and their associated genes found were summarized in Table 2. The first-ranking estrogen signaling pathway had the 6.12% associated genes, which included CALM3, CALML4, HSPA1L, HSPA8, ITPR3, and MAPK3. The second-placed hippo signaling pathway had the 5.84% associated genes, which included ACTG1, APC2, BIRC2, BMP2, FRMD6, LLGL2, RASSF6, SOX2, and WNT8A.
Fig. 2.
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of differentially expressed genes (DEGs). The different node colors mean different pathways, and the closer the colors are, the closer the function clustering of pathways are
Table 2.
Core pathways and their associated genes found
| GOID | GOTerm | Term P value | % associated genes | Associated genes found |
|---|---|---|---|---|
| GO:0004915 | Estrogen signaling pathway | 130.0E−3 | 6.12 | [CALM3, CALML4, HSPA1L, HSPA8, ITPR3, MAPK3] |
| GO:0004390 | Hippo signaling pathway | 62.0E−3 | 5.84 | [ACTG1, APC2, BIRC2, BMP2, FRMD6, LLGL2, RASSF6, SOX2, WNT8A] |
| GO:0004520 | Adherens junction | 290.0E−3 | 5.56 | [ACTG1, MAPK3, PTPRM, WAS] |
| GO:0004657 | IL-17 signaling pathway | 220.0E−3 | 5.38 | [IL6, MAPK15, MAPK3, S100A8, S100A9] |
| GO:0004621 | NOD-like receptor signaling pathway | 110.0E−3 | 5.29 | [BIRC2, ERBIN, IFNA1, IFNAR2, IL6, ITPR3, MAPK3, RNASEL, TP53BP1] |
| GO:0004210 | Apoptosis | 210.0E−3 | 5.07 | [ACTG1, BIRC2, ITPR3, LMNA, MAPK3, PDPK1, TUBA3D] |
| GO:0004371 | Apelin signaling pathway | 210.0E−3 | 5.07 | [CALM3, CALML4, GNG11, ITPR3, MAPK3, PRKAG2, RYR2] |
| GO:0004722 | Neurotrophin signaling pathway | 270.0E−3 | 5.04 | [CALM3, CALML4, IRAK2, MAGED1, MAPK3, PDPK1] |
| GO:0004020 | Calcium signaling pathway | 190.0E−3 | 4.95 | [CALM3, CALML4, CHRM3, ITPR3, P2RX2, PTGER3, RYR2, SLC25A4, STIM2] |
| GO:0004512 | ECM–receptor interaction | 330.0E−3 | 4.88 | [COL1A2, COL2A1, COL6A1, HSPG2] |
| GO:0004620 | Toll-like receptor signaling pathway | 380.0E−3 | 4.81 | [IFNA1, IFNAR2, IL6, MAP2K3, MAPK3] |
| GO:0004150 | mTOR signaling pathway | 340.0E−3 | 4.61 | [ATP6V1B2, DEPDC5, EIF4E, MAPK3, PDPK1, PRR5, WNT8A] |
| GO:0004068 | FoxO signaling pathway | 310.0E−3 | 4.55 | [BCL6, CCNG2, IL6, MAPK3, PDPK1, PRKAG2] |
| GO:0004510 | Focal adhesion | 290.0E−3 | 4.52 | [ACTG1, BCAR1, BIRC2, COL1A2, COL2A1, COL6A1, MAPK3, PARVB, PDPK1] |
| GO:0004514 | Cell adhesion molecules (CAMs) | 460.0E−3 | 4.14 | [CADM1, CLDN5, NCAM2, PTPRC, PTPRM, SELP] |
Interaction network of DEGs and core genes in the interaction network
Based on the information in the STRING database, the gene interaction network contained 542 nodes and 1163 edges. The nodes indicated the DEGs, and the edges indicated the interactions between the DEGs. NetworkAnalyzer in Cytoscape software was used to analysis these genes, and core genes were ranked according to the predicted scores. The top 10 high-degree hub nodes included glyceraldehyde-3-phosphate dehydrogenase (GAPDH), phosphoribosylglycinamide formyltransferase, phosphoribosylglycinamide synthetase, phosphoribosylaminoimidazole synthetase (GART), ubiquitin-like and ribosomal protein S30 fusion (FAU), heat shock protein family A member 8 (HSPA8), eukaryotic translation elongation factor 1 alpha 1 (EEF1A1), ribosomal protein S3A (RPS3A), eukaryotic translation initiation factor 4E (EIF4E), mitogen-activated protein kinase 3 (MAPK3), interleukin 6 (IL6), and ribosomal protein L6 (RPL6). Among these genes, GAPDH showed the highest node degree, which was 56. The core genes and their corresponding degree were shown in Table 3. The distribution of core genes in the interaction network was revealed in Fig. 3. The correlation between the data points and corresponding points on the line is approximately 0.932. The R-squared value is 0.846, giving a relatively high confidence that the underlying model is indeed linear. Then, we used MCODE to screen the modules of the gene interaction network, and 10 modules were showed in Fig. 4.
Table 3.
The core genes and their corresponding degree
| Gene | Degree | Gene | Degree | Gene | Degree | Gene | Degree |
|---|---|---|---|---|---|---|---|
| GAPDH | 56 | RPS3A | 32 | EIF3b | 28 | RPL18 | 25 |
| GART | 41 | EIF4E | 31 | DDX5 | 28 | CALM3 | 25 |
| FAU | 39 | MAPK3 | 31 | HSPD1 | 28 | ACTG1 | 25 |
| HSPA8 | 38 | IL6 | 29 | RPS29 | 26 | RPS27 | 24 |
| EEF1A1 | 36 | RPL6 | 28 | RPL18A | 26 | RPL32 | 24 |
Fig. 3.
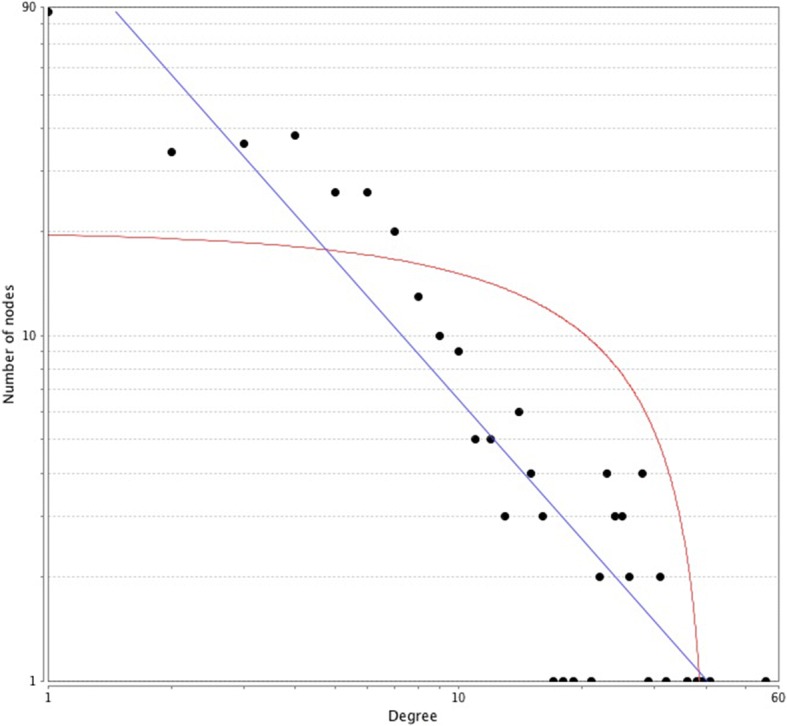
The distribution of core genes in the interaction network. The black node means the core gene. The red line means the fitted line, and the blue line means the power law. The correlation between the data points and corresponding points on the line is approximately 0.932. The R-squared value is 0.846, giving a relatively high confidence that the underlying model is indeed linear
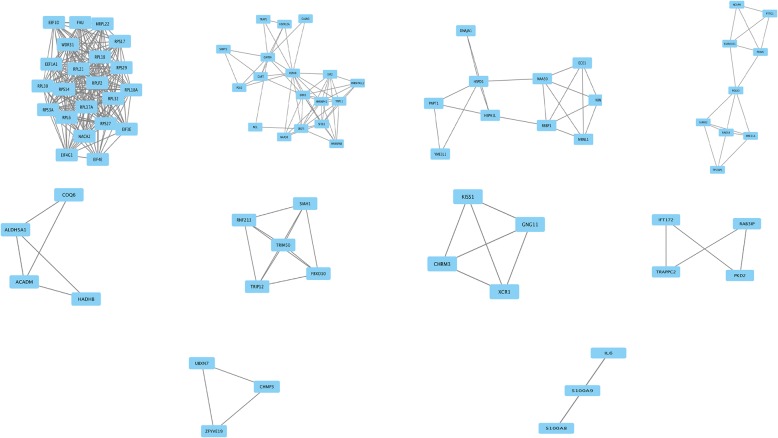
Fig. 4.
The top 10 modules from the gene–gene interaction network. The squares represent the differentially expressed genes (DEGs) in modules, and the lines show the interaction between the DEGs
The score of top 1 module including FAU and EIF4E was 19.81, which had 22 nodes and 208 edges. The score of top 2 module including GAPDH and GART was 6.824, which had 18 nodes and 58 edges. The score of top 3 module including FBXO10, RNF213, SIAH1, TRIM50, and TRIP12 was 5, which had 5 nodes and 10 edges. Lastly, the interaction network of the top 10 high-degree hub nodes (core genes) was made by STRING database in Fig. 5. GAPDH, GART, FAU, HSPA8, EEF1A1, RPS3A, EIF4E, MAPK3, IL6, and RPL6, which regulate 8, 4, 5, 6, 6, 6, 5, 3, 2, and 4 targets, respectively, showed the good connectivity.
Fig. 5.
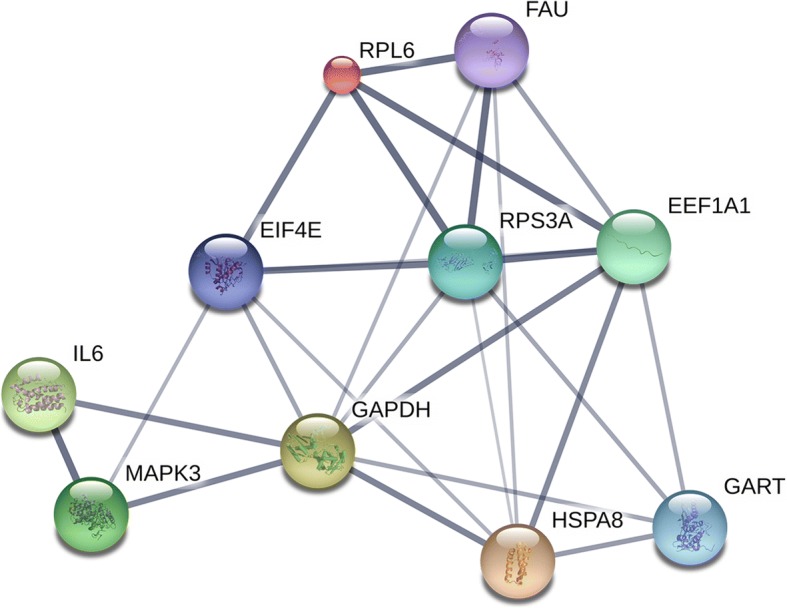
The interaction network of the top 10 core genes. The nodes indicated the top core genes, and the edges indicated the interactions between the core genes
Discussion
OS is the most common primary bone sarcoma [15–19]. Previous studies discovered that sarcomas contain a small subpopulation of tumor-propagating cells (TPCs) such as SP cells, characterized by enhanced tumorigenicity and self-renewal capacity [20–22]. Self-renewal is a defining characteristic of these cells and is associated with tumor recurrence [23, 24]. The inhibition of self-renewal in OS SP cells may offer valuable targets of therapy and tumorigenesis mechanisms. In the present study, the gene expression profile of GSE63390 was downloaded and a bioinformatics analysis was performed. The results showed that there were 141 DEGs in SP cells compared with non-SP cells of human OS. Furthermore, GO and KEGG pathway and gene–gene interaction network analysis were performed to obtain the biomarkers or the major genes related to cytogenetic pathways to OS tumorigenesis.
In order to disclose the underlying molecular mechanisms between SP cells and human OS, we characterized the possible GO functional terms and signaling pathways of DEGs. Considering the results of GO function analysis, we linked the DEGs with mRNA catabolic process and cellular modified amino acid metabolic process, which are probably very important for the development process of human OS. As previous articles reported, our KEGG pathway analysis showed that hippo signaling pathway, mTOR signaling pathway, hedgehog signaling pathway, and others were among the most relevant pathways for OS. Zhou et al. found that the correlation between the mTOR/p70S6K signal transduction pathway in human OS and patients’ prognosis, and the overexpression of mTOR and p70S6K, is well correlated with tumor metastasis pattern, which might be an important mechanism responsible for the survival and proliferation of OS cells [25]. Wang et al. identified that hippo/YAP signaling pathway not only is involved in tumorigenesis, but also hippo/YAP signaling pathway induces OS chemoresistance [26]. Chai et al. deemed that the oncogenic activities in OS are mediated by TED1 through hippo–YAP1 signaling [27]. Cheng et al. highlighted a new discovery that CNOT1–LMNA–Hedgehog signaling pathway axis exerts an oncogenic role in OS progression, which could be a potential target for gene therapy [28]. Emerging data suggested that interference with hedgehog signaling signal transduction by inhibitors may reduce OS cell proliferation and tumor growth, thereby preventing osteosarcomagenesis [29]. All these signaling pathways may play important roles in molecular mechanism of development process between SP cells and human OS.
Also of note is that there were numerous evidences for our DEGs of SP cells, which have proven to play important roles during OS tumorigenesis. The STRING database revealed top 20 high-degree hub nodes of DEGs including GAPDH, GART, FAU, HSPA8, EEF1A1, RPS3A, EIF4E, MAPK3, IL6, RPL6, eukaryotic translation initiation factor 3 beta (EIF3b), DEAD-box helicase 5 (DDX5), heat shock protein family D member 1 (HSPD1), ribosomal protein S29 (RPS29), ribosomal protein L18a (RPL18A), ribosomal protein L18 (RPL18), calmodulin 3 (CALM3), actin gamma 1 (ACTG1), ribosomal protein S27 (RPS27), and ribosomal protein L32 (RPL32). Furthermore, we analyzed the gene interaction network and top 10 modules using MCODE and found that ACTG1, eukaryotic translation initiation factor 3 subunit E (EIF3E), EIF4E, FAU, HSPD1, IL-6, KiSS-1 metastasis-suppressor (KISS1), PRIM1, pituitary tumor-transforming 1 (PTTG1), PRL32, S100 calcium-binding protein A8 (S100A8), S100 calcium-binding protein A9 (S100A9), serine hydroxymethyltransferase 1 (SHMT1), and TNF receptor-associated protein 1 (TRAP1) were the core interaction genes, which may be potential therapeutic targets for OS. Parts of them were in accord with STRING database results. Ajiro et al. found that serine/arginine-rich splicing factor 3 (SRSF3) regulates the expression of DDX5 in human OS U2OS cells [30]. By participating in the transcriptional regulation of ribosomal protein L34 (RPL34) which plays an important role in the proliferation of OS cells, MYC interacts with the subunits of EIF3 and probably involves the translational control of growth-promoting proteins [31]. EIF3, a multi-subunit complex, plays a critical role in translation initiation. Expression levels of EIF3 subunits are elevated or decreased in various cancers, suggesting a role for EIF3 in tumorigenesis [32]. Choi et al. confirmed that EIF3b silencing could completely suppress cell growth in multiple OS cell lines [33]. Osborne et al. also discovered that EIF4E is uniformly expressed in OS patient samples and it could be a relevant protein biomarker in OS [34]. Rossman et al. found that overexpressing FAU itself is able to transform human osteogenic sarcoma cells to anchorage independence and make them easy to proliferate [35]. Liang et al. proved that the expression of HSPD1 was high in OS tissues and cells; moreover, targeted inhibition of this gene could inhibit the proliferation of the tumor [36]. Zhang et al. indicated that the decreased expression of KISS1 is correlated with distant metastasis of OS, and KISS1 may function as a tumor suppressor in OS cells through inhibition of the MAPK pathway [37]. EEF1A1 is overexpressed in OS cell lines, and siRNA treatment against EEF1A1 produces a chemosensitization toward methotrexate, which showed that this gene is a potential therapeutic target of OS [38]. Through ASK1/p38/AP-1 signal pathway, IL-6 occurs, which in turn results in the activations of vascular endothelial growth factor (VEGF) expression and contributing the angiogenesis of human OS cells [39]. In addition, the ILK/Akt/AP-1 pathway is activated after IL-6 treatment, and IL-6 induces expression of ICAM-1 and migration activity of human OS cells [40]. Yotov et al. proposed that PRIM1 is a major target of 12q13 amplifications, playing an essential role in tumorigenesis of human OS [41]. PTTG1 siRNA markedly downregulates the expression of PTTGl protein in OS cells, leading to obvious inhibition of cell proliferation, alters cell cycle distribution, and reduces ability of invasion of OS cells [42]. Tsai et al. deemed that expression stability of RPL32 is high in OS samples, and this gene could be a potential target [43]. In Montesano’s study, the anti-apoptotic role of TRAP1 is confirmed in Saos-2 OS cells, which suggested that increased expression of this gene could make diethylmaleate-adapted and chemoresistant cells evade toxic effects of oxidants and anticancer drugs [44]. Endo-Munoz et al. proved downregulation of S100A8 between chemo-naive OS biopsies and non-malignant bone biopsies, highlighting their potential as therapeutic targets for OS [45]. Cheng et al. confirmed that through inactivating MAPK and NF-κB signaling pathways, downregulation of S100A9 could inhibit OS cell growth [46]. Besides, Both et al. concluded that some genes, including SHMT1, are candidate oncogenes in 17p11.2–p12 of importance in OS tumorigenesis [47]. Taken together, all these core genes discovered in OS SP cells by bioinformatics enrichment analysis and gene interaction network analysis may increase or decrease tumorigenicity and self-renewal capacity of OS SP cells; further, these changed SP cells could result in development process of human OS.
Some gene and pathway interaction relationship predicted in our study has been reported in previous researches. Oncogenic activation of mTOR signaling significantly contributes to the progression of different types of cancers including OS. EIF4E is one of the downstream effectors of mTOR. Activated mTOR contributes to OS cellular transformation and poor cancer prognosis via targeting the downstream effectors such as EIF4E [48]. In addition, our results of core pathways and their associated genes found also confirmed the relationship of EIF4E and mTOR signaling pathway. Therefore, EIF4E/mTOR signaling pathway could be a potential research target for therapy and tumorigenesis of OS.
Lastly, there are several limitations of this study. It is acknowledged that predicting key genes merely by means of bioinformatics is not sufficient, and further molecular biological experiments such as the use of gene transfection/knockdown and quantitative real-time polymerase chain reaction are needed to confirm these results.
Conclusion
In summary, 141 DEGs were identified including 72 upregulated DEGs and 69 downregulated DEGs screened in SP cells compared with non-SP cells. GO and KEGG pathway analysis provided a series of related key genes and pathways to contribute to the understanding of the molecular mechanisms between SP cells and human OS, thus yielding clues to speculate the EIF4E/mTOR signaling pathway is highly correlated with the development process of OS. Furthermore, these predictions need further experimental validation in future studies.
Acknowledgements
Yi-Ming Ren wants to thank, in particular, the continued supports received from Fiona Xue over 8 years.
Availability of data and materials
The data was freely downloaded from the public GEO database.
Abbreviations
- ACTG1
Actin gamma 1
- BP
Biological process
- CALM3
Calmodulin 3
- CAMs
Cell adhesion molecules
- CC
Cellular component
- DDX5
DEAD-box helicase 5
- DEG
Differentially expressed gene
- EEF
Eukaryotic translation elongation factor
- EIF3b
Eukaryotic translation initiation factor 3 beta
- FAU
Ubiquitin-like and ribosomal protein S30 fusion
- FC
Fold change
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GART
Phosphoribosylglycinamide formyltransferase, phosphoribosylglycinamide synthetase, phosphoribosylaminoimidazole synthetase
- GEO
Gene Expression Omnibus database
- GO
Gene Ontology
- HSPA8
Heat shock protein family A member 8
- HSPD1
Heat shock protein family D member 1
- IL6
Interleukin 6
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- KISS1
KiSS-1 metastasis-suppressor
- MAPK3
Mitogen-activated protein kinase 3
- MCODE
Molecular complex detection
- MF
Molecular function
- OS
Osteosarcoma
- PTTG1
Pituitary tumor-transforming 1
- RPL6
Ribosomal protein L6
- RPS
Ribosomal protein
- S100A8
S100 calcium-binding protein A8
- SHMT1
Serine hydroxymethyltransferase 1
- SP
Side population
- SRSF3
Serine/arginine-rich splicing factor 3
- STRING
Search Tool for the Retrieval of Interacting Genes
- TPC
Tumor-propagating cell
- TRAP1
TNF receptor associated protein 1
- VEGF
Vascular endothelial growth factor
Authors’ contributions
YMR, YHD, and YBS conceived the design of the study. TY, WJZ, DLZ, and ZWT performed and collected the data and contributed to the design of the study. TY and WJZ analyzed the data. YMR and MQT prepared and revised the manuscript. All authors read and approved the final content of the manuscript.
Ethics approval and consent to participate
Not applicable. This paper does not involve research on humans.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Yi-Ming Ren, Yuan-Hui Duan and Yun-Bo Sun contributed equally to this work.
Contributor Information
Yi-Ming Ren, Email: renyiming0404@126.com.
Yuan-Hui Duan, Email: dyhhui200182@126.com.
Yun-Bo Sun, Email: doctorsyb@163.com.
Tao Yang, Email: yangtao_07@126.com.
Wen-Jun Zhao, Email: dr_luoxuan@126.com.
Dong-Liang Zhang, Email: realqzff@126.com.
Zheng-Wei Tian, Email: 824584753@qq.com.
Meng-Qiang Tian, Email: tmqjoint@126.com.
References
- 1.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer. 2009;115(7):1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Damron T, Ward WA. Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National Cancer Data Base Report. Clin Orthop Related Res. 2007;459(459):40. doi: 10.1097/BLO.0b013e318059b8c9. [DOI] [PubMed] [Google Scholar]
- 3.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Pediatr Adolesc Osteosarcoma. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 4.Marina N, Gebhardt M, Teot L, et al. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9(4):422. doi: 10.1634/theoncologist.9-4-422. [DOI] [PubMed] [Google Scholar]
- 5.Folio C, Zalacain M, Zandueta C, et al. Cortactin (CTTN) overexpression in osteosarcoma correlates with advanced stage and reduced survival. Cancer Biomarkers. 2011;10(1):35. doi: 10.3233/CBM-2012-0227. [DOI] [PubMed] [Google Scholar]
- 6.Wu C, Alman BA. Side population cells in human cancers. Cancer Lett. 2008;268(1):1–9. doi: 10.1016/j.canlet.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 7.Patrawala L, Calhoun T, Schneiderbroussard R, et al. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 2005;65(14):6207. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 8.Mitsutake N, Iwao A, Nagai K, et al. Characterization of side population in thyroid cancer cell lines: cancer stemlike cells are enriched partly but not exclusively. Endocrinology. 2007;148(4):1797–1803. doi: 10.1210/en.2006-1553. [DOI] [PubMed] [Google Scholar]
- 9.Kondo T, Setoguchi T, Taga T, Kondo T, Setoguchi T. Taga TPersistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci U S A. 2004;101(3):781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haraguchi N, Utsunomiya T, Inoue H, et al. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24(3):506. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 11.Ho MM, Ng AV, Lam S, et al. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67(10):4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 12.Chiba T, Kita K, Zheng YW, et al. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44(1):240–251. doi: 10.1002/hep.21227. [DOI] [PubMed] [Google Scholar]
- 13.Wang K, Zeng J, Luo L, et al. Identification of cancer stem cell-like side population cells in human cervical carcinoma cell line HeLa[C]// The Tenth National Conference on obstetrics and Gynecology of China Medical Association. 2012. [Google Scholar]
- 14.Hirschmann-Jax C, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004;101(39):14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou W, Hao M, Du X, et al. Advances in targeted therapy for osteosarcoma. Discov Med. 2014;17(96):301. [PubMed] [Google Scholar]
- 16.Li S, Dong Y, Ke W, et al. Transcriptomic analyses reveal the underlying pro-malignant functions of PTHR1 for osteosarcoma via activation of Wnt and angiogenesis pathways. J Orthop Surg Res. 2017;12(1):168. doi: 10.1186/s13018-017-0664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fei Z, Hao P. LncRNA-ANCR regulates the cell growth of osteosarcoma by interacting with EZH2 and affecting the expression of p21 and p27. J Orthop Surg Res. 2017;12(1):103. doi: 10.1186/s13018-017-0599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng J, Lan R, Cai G, et al. Verification ofTREX1as a promising indicator of judging the prognosis of osteosarcoma. J Orthop Surg Res. 2016;11(1):150. doi: 10.1186/s13018-016-0487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang XR, Xiong Y, Duan H, et al. Identification of genes associated with methotrexate resistance in methotrexate-resistant osteosarcoma cell lines. J Orthop Surg Res. 2015;10(1):136. doi: 10.1186/s13018-015-0275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang CY, Wei Q, Han I, et al. Hedgehog and notch signaling regulate self-renewal of undifferentiated pleomorphic sarcomas. Cancer Res. 2012;72(4):1013–1022. doi: 10.1158/0008-5472.CAN-11-2531. [DOI] [PubMed] [Google Scholar]
- 21.Dela CFS. Cancer stem cells in pediatric sarcomas. Front Oncol. 2013;3:168. doi: 10.3389/fonc.2013.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu C, Wei Q, Utomo V, et al. Side population cells isolated from mesenchymal neoplasms have tumor initiating potential. Cancer Res. 2007;67(17):8216–8222. doi: 10.1158/0008-5472.CAN-07-0999. [DOI] [PubMed] [Google Scholar]
- 23.Kreso A, Van GP, Pedley NM, et al. Self-renewal as a therapeutic target in human colorectal cancer. Nat Med. 2014;20(1):29–36. doi: 10.1038/nm.3418. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Z, Khan MA, Weiler M, et al. Targeting self-renewal in high-grade brain tumors leads to loss of brain tumor stem cells and prolonged survival. Cell Stem Cell. 2014;15(2):185–198. doi: 10.1016/j.stem.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Q, Deng Z, Zhu Y, et al. mTOR/p70S6K signal transduction pathway contributes to osteosarcoma progression and patients’ prognosis. Med Oncol. 2010;27(4):1239–1245. doi: 10.1007/s12032-009-9365-y. [DOI] [PubMed] [Google Scholar]
- 26.Wang DY, Wu YN, Huang JQ, et al. Hippo/YAP signaling pathway is involved in osteosarcoma chemoresistance. Chin J Cancer. 2016;35(7):366–373. doi: 10.1186/s40880-016-0109-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chai J, Xu S, Guo F. TEAD1 mediates the oncogenic activities of hippo-YAP1 signaling in osteosarcoma. Biochem Biophys Res Commun. 2017;488(2):297–302. doi: 10.1016/j.bbrc.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 28.Cheng DD, Li J, Li SJ, et al. CNOT1 cooperates with LMNA to aggravate osteosarcoma tumorigenesis through the hedgehog signaling pathway. Mol Oncol. 2017;11(4):388–404. doi: 10.1002/1878-0261.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar RM, Fuchs B. Hedgehog signaling inhibitors as anti-cancer agents in osteosarcoma. Cancers. 2015;7(2):784–794. doi: 10.3390/cancers7020784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ajiro M, Jia R, Yang Y, et al. A genome landscape of SRSF3-regulated splicing events and gene expression in human osteosarcoma U2OS cells. Nucleic Acids Res. 2016;44(4):1854. doi: 10.1093/nar/gkv1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo S, Zhao J, Fowdur M, et al. Highly expressed ribosomal protein L34 indicates poor prognosis in osteosarcoma and its knockdown suppresses osteosarcoma proliferation probably through translational control. Sci Rep. 2016;6:37690. doi: 10.1038/srep37690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mamane Y, Petroulakis E, Rong L, et al. eIF4E from translation to transformation. Oncogene. 2004;23(18):3172–3179. doi: 10.1038/sj.onc.1207549. [DOI] [PubMed] [Google Scholar]
- 33.Choi YJ, Lee YS, Lee HW, et al. Silencing of translation initiation factor eIF3b promotes apoptosis in osteosarcoma cells. Bone Joint Res. 2017:186–93. [DOI] [PMC free article] [PubMed]
- 34.Osborne TS, Ren L, Healey JH, et al. Evaluation of eIF4E expression in an osteosarcoma-specific tissue microarray. J Pediatr Hematol Oncol. 2011;33(7):524–528. doi: 10.1097/MPH.0b013e318223d0c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossman TG, Visalli MA, Komissarova EV. Fau and its ubiquitin-like domain (FUBI) transforms human osteogenic sarcoma (HOS) cells to anchorage-independence. Oncogene. 2003;22(12):1817. doi: 10.1038/sj.onc.1206283. [DOI] [PubMed] [Google Scholar]
- 36.Liang W, Yang C, Peng J, et al. The expression of HSPD1, SCUBE3, CXCL14 and its relations with the prognosis in osteosarcoma. Cell Biochem Biophys. 2015;73(3):763–768. doi: 10.1007/s12013-015-0579-7. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Tang YJ, Li ZH, et al. KISS1 inhibits growth and invasion of osteosarcoma cells through inhibition of the MAPK pathway. Eur J Histochem Ejh. 2013;57(4):199–204. doi: 10.4081/ejh.2013.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selga E, Oleaga C, Ramírez S, et al. Networking of differentially expressed genes in human cancer cells resistant to methotrexate. Genome Med. 2009;1(9):83. doi: 10.1186/gm83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tzeng HE, Tsai CH, Chang ZL, et al. Interleukin-6 induces vascular endothelial growth factor expression and promotes angiogenesis through apoptosis signal-regulating kinase 1 in human osteosarcoma. Biochem Pharmacol. 2013;85(4):531–540. doi: 10.1016/j.bcp.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 40.Lin YM, Chang ZL, Liao YY, et al. IL-6 promotes ICAM-1 expression and cell motility in human osteosarcoma. Cancer Lett. 2013;328(1):135–143. doi: 10.1016/j.canlet.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 41.Yotov WV, Hamel H, Rivard GE, et al. Amplifications of DNA primase 1 (PRIM1) in human osteosarcoma. Genes Chromosomes Cancer. 1999;26(1):62. doi: 10.1002/(SICI)1098-2264(199909)26:1<62::AID-GCC9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 42.Wu D, Xia Y, Xu H, et al. Impact of PTTG1 downregulation on cell proliferation, cell cycle and cell invasion of osteosarcoma and related molecular mechanisms. Zhonghua Bing Li Xue Za Zhi. 2014;43(10):695. [PubMed] [Google Scholar]
- 43.Tsai PC, Breen M. Array-based comparative genomic hybridization-guided identification of reference genes for normalization of real-time quantitative polymerase chain reaction assay data for lymphomas, histiocytic sarcomas, and osteosarcomas of dogs. Am J Vet Res. 2012;73(9):1335. doi: 10.2460/ajvr.73.9.1335. [DOI] [PubMed] [Google Scholar]
- 44.Montesano GN, Chirico G, Pirozzi G, et al. Tumor necrosis factor-associated protein 1 (TRAP-1) protects cells from oxidative stress and apoptosis. Stress-Int J Biol Stress. 2007;10(4):342–350. doi: 10.1080/10253890701314863. [DOI] [PubMed] [Google Scholar]
- 45.Endo-Munoz L, Cumming A, Sommerville S, et al. Osteosarcoma is characterised by reduced expression of markers of osteoclastogenesis and antigen presentation compared with normal bone. Br J Cancer. 2010;103(1):73–81. doi: 10.1038/sj.bjc.6605723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng S, Zhang X, Huang N, et al. Down-regulation of S100A9 inhibits osteosarcoma cell growth through inactivating MAPK and NF-κB signaling pathways. BMC Cancer. 2016;16(1):1–12. doi: 10.1186/s12885-016-2294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Both J, et al. Identification of novel candidate oncogenes in chromosome region 17p11.2-p12 in human osteosarcoma. PLoS One. 2012;7(1):e30907. doi: 10.1371/journal.pone.0030907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu K, Dai HB, Qiu ZL. mTOR signaling in osteosarcoma: oncogenesis and therapeutic aspects (review) Oncol Rep. 2016;36(3):1219. doi: 10.3892/or.2016.4922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data was freely downloaded from the public GEO database.