Abstract
Background
Impairments in biomechanics and neural control can disrupt the timing and muscle pattern activation necessary for smooth gait. Gait is one of the most affected motor characteristics in Parkinson’s disease (PD), but its smoothness has not been well-studied. This work applies the recently proposed spectral arc length measure (SPARC) to study, for the first time, gait in patients with PD. We hypothesized that the gait of patients with PD would be less smooth than that of healthy controls, as reflected in the SPARC measures.
Methods
The gait of 101 PD patients and 39 healthy controls was assessed using an inertial sensor. Smoothness of gait was estimated with SPARC (respectively from acceleration and angular velocity signals, SPARC-Acc and SPARC-Gyro) and harmonic ratios. Correlations between SPARC, traditional gait measures and the motor part of the Unified Parkinson’s Disease Rating Scale (UPDRS) were evaluated. Measurements and analysis were conducted with and without anti-PD medication.
Results
SPARC measures were lower (less smooth) in PD than in controls (SPARC-Acc: PD: − 6.11 ± 0.74; CO: -5.17 ± 0.79; p < 0.001). When comparing PD to controls, SPARC-Acc differed more than other measures of gait (i.e., largest effect size, which was > 1). SPARC measures were correlated with UPDRS motor score (r = − 0.65), while they were independent of other measures of gait smoothness. PD gait in the on state was smoother than in the off state (p < 0.001).
Conclusions
SPARC calculated from trunk acceleration and angular velocity signals provide valid measures of walking smoothness in PD. SPARC is sensitive to Parkinson’s disease and PD medications and can be used of as another, complementary measure of the motor control of walking in PD.
Keywords: Accelerometers, Parkinson’s disease, Smoothness, Motor control
Background
Gait is a major component of daily physical activities and a key to functional independence [1]. Gait disturbances are one of the clinical hallmarks of Parkinson’s disease (PD). Among patients with PD, the gait pattern is characterized by reduced speed, short stride length, shuffling steps, and, occasionally, typically in more advanced patients, freezing of gait episodes [2]. These changes in gait lead to disability, restricted function and reduced health-related quality of life.
Although faster gait speeds have been associated with better function [3–5], some aspects of physical function and mobility are not represented by speed but by the quality of movement control [6, 7]; i.e. how hesitant, stable, abrupt or smooth the gait is. The present work addresses estimation of gait smoothness from inertial sensors. Smoothness of gait is a quality that reflects the continuousness or non-intermittency of walking. Measuring walking smoothness aims to quantify intermittency during gait; intermittent movements tend to have many accelerating and decelerating phases.
Several measures have been used to estimate gait smoothness [8–10]. Video motion analysis permit calculation of the smoothness of the limbs movements from the estimated jerk measured at the joints during gait [11, 12]. Accelerometers need a different approach. In [8, 10], gait smoothness was estimated from the mean and maximum anteroposterior acceleration of the lower back. In [9, 10], gait variability (i.e., stride-to-stride fluctuations) is considered a descriptor of smoothness of gait. Indeed, patients with PD have increased stride-to-stride variability [13, 14], compared to healthy controls. Previous studies have also shown that reduced gait stability may predispose individuals with PD to falls [15–20]. However, while gait variability measures stepping arrhythmicity, it is not necessarily a direct measure of the smoothness of gait. Moreover it is of importance to note that jerk [21], mean acceleration or gait variability computation are not totally independent of the movement amplitude or duration, thus compounding interpretation of comparisons between tasks and subjects. In contrast, smoothness can be independent of those factors.
Harmonic ratios are commonly used (HR, the ratios between the sum of the magnitudes of the even to the odd harmonics over a single stride) to measure smoothness of gait [22–24]. They have been shown to differ in PD and controls [25]. However, rather than smoothness, the harmonic ratios may be viewed more as a measure of the symmetry of the movement of the two legs during walking (i.e., gait can be symmetric and not smooth).
Recently, Balasubramanian et al. introduced a novel measure to characterize movement smoothness, based on the spectral arc length [26]. The spectral arc length (SPARC) measures the arc length of the Fourier magnitude spectrum within an adaptive frequency range. This approach addresses limitations of previously proposed measures of smoothness [26, 27]. Indeed, SPARC quantifies movement intermittencies but is independent of its amplitude or duration. The goal of the present work was to study, for the first time, SPARC in the context of gait in PD in order to assess whether it might provide useful information for objective characterization of the gait of patients with PD. Furthermore, the PD cohort was split into fallers and non-fallers to begin to investigate the association of this measure with fall risk. We hypothesized that gait would be less smooth in patients with PD, compared to healthy controls, and, further, that the gait of PD fallers would be less smooth than the PD non-fallers. To better understand this new measure of smoothness in PD, we also examined the effects of anti-parkinsonian medications and explored the correlation and differences between SPARC, harmonic ratios, gait speed, stride time variability, and clinical measures.
Methods
Participants and procedures
The present analyses were applied to a dataset previously collected to study white matter changes in PD [28]. Briefly, 101 patients with PD (mean age: 64.5 ± 9.3 yrs., 24 women) and 39 elderly control subjects (mean age: 77.8 ± 3.9 yrs., 25 women) participated in this study. Patients with PD were included if they were diagnosed with PD by a movement disorders specialist, if they were between 40 and 85 years of age, and if they were not demented based on the Mini Mental State Examination (MMSE) [29]. PD patients with more than 1 fall in the previous year were considered fallers, while non-fallers reported no falls in that period. Patients were excluded if they underwent brain surgery in the past, including implanted deep brain stimulation, or had significant co-morbidities likely to affect gait, e.g., acute illness, orthopedic disease, other neurologic disease, or history of stroke. All subjects provided informed written consent as approved by the local human studies committee. The gait task consisted of a 50 m walk with a turn in the middle. Subjects were fitted with a small, lightweight inertial measurement unit (Hybrid, McRoberts, The Hague, Netherlands) placed on the lower back (at the level of lumbar vertebrae 4–5) using an elastic belt. The device includes a triaxial accelerometer (sensor range and resolution: ±6 g and ± mg, respectively) and a triaxial gyroscope (sensor range and resolution: ±100°/second and ± 0.0069°/second, respectively). The signals acquired were 3 acceleration axes, vertical acceleration (V), mediolateral acceleration (ML), anterior posterior acceleration (AP), and 3 angular velocity axes, yaw, pitch, and roll. The signals were recorded on a Secure Digital (SD) card at a sample frequency of 100 Hz, and later transferred to a personal computer for further analysis. The PD subjects were first tested in the OFF medication state, at least 12 h after they took their anti-parkinsonian medications. The examination was repeated in the ON state, about 1 h after taking their first morning dose of their anti-parkinsonian drugs.
Data processing and spectral arc length
Steps were located from the vertical field of the signal (V) as peaks above an adaptive threshold. The threshold was based on the median of the signal. The algorithm was developed in the lab and tested against an instrumented mat (GAITRite®) and prove to be more accurate than [30]. Turns were identified from the yaw axis as described in [30] and removed from further analysis. We chose to compare SPARC with several of the most common metrics characterizing gait and smoothness of gait in Parkinson’s disease. A summary of the measures calculated can be seen in Table 1. In the original paper [26], SPARC was used to measure smoothness from the velocity profile of a movement. However, as the authors suggested, to measure gait smoothness, SPARC could also be applied to any semi-periodic signal, e.g., acceleration or gyroscope signals during walking. To determine the spectral arc length, the algorithm requires two threshold values. In order to avoid overfitting, the thresholds values were decided from investigation on different cohorts [31]. A grid search, using a logistic regression was performed to decide on the best couple of parameters for the SPARC estimation. A frequency upper threshold that should bound the “normal and abnormal” movement range; i.e., normal and disease altered gait needs to be inside the threshold boundaries. Normal gait in healthy older adults is typically less than 2 steps per second [32]. Therefore, the higher bound threshold selected was (i.e., 5 Hz). The second value is a lower bound threshold; it controls the trade-off between SPARC sensitivity and noise contamination. Here was chosen.
Table 1.
Summary of the gait measures
| Gait measures | Description |
|---|---|
| SPARC(-Acc, -V, -AP, −ML & -Gyro, -Yaw, −Roll, -Pitch) | Spectral arc length (unitless) |
| Harmonic Ratios (V, AP, ML) | Measure of step to step symmetry (unitless) |
| Mean Gait Speed | Mean gait speed (m/s) |
| Stride time mean | Mean stride time (s) |
| Stride time variability | Coefficient of variation of the stride time (%) |
Note: Acc acceleration, V vertical, AP anteroposterior, ML medio-lateral, Gyro gyroscope
In short, the calculation of SPARC is performed from the following steps (more details in [27]):
Step 1: Select the walking boots (i.e., remove turns).
Step 2: Compute the spectrum for each acceleration bout.
Step 3: Normalize each spectrum.
Step 4: Calculate SPARC for each walking bout.
Step 5: Average SPARC over all the walking bouts.
Where A(ω) is the Fourier magnitude spectrum of the acceleration signal a(t), is the normalized magnitude spectrum.
For SPARC-Acc, (detrending aims at removing drift from the signal); and similarly, from the gyroscope (SPARC-Gyro), but with no detrending. Direction-wise SPARC measures were also investigated. The same formula were used, but with the values in two axes set to 0; the absolute value was always taken.
Statistical analysis
Comparisons of subject characteristics were determined using Student t tests for age, height and weight and the Chi square test for categorical variables (i.e., gender). Statistical analyses of the gait measures were adjusted for age, gender, height and weight (using a logistic regression with the logit link function). Comparison between the PD fallers and non-fallers was also adjusted for part III of the UPDRS (logistic regression with the logit link function) [33]. The logistic regression method allows for adjustment for confounding variables while not assuming normality from the data. Fourteen measures (see Table 1) were investigated, therefore, a Bonferroni corrected statistical significance was set at p < 0.004, i.e., 0.05/14. In order to compare the degree to which measures differed across groups, effect sizes were used for each measure to quantify the size of the difference between the two groups. For that purpose, Cohen’s d was used:
Where d is the effect size, μi is the mean for group i; s is the pooled standard deviation.
Where ni and si are respectively the mean and standard deviation of group i.
For the comparison of healthy controls and PD, the analysis was repeated with age matched subgroups. The data was normally distributed so p-values were calculated using Student t-tests when the two populations had same variance; Welch’s t-tests were used otherwise. Correlations between the values from the PD patients in the OFF condition and the healthy controls were also investigated. The ability of the measures to discriminate PD and control subjects were investigated using Receiver Operating Characteristic (ROC) curves for support vector machine (SVM) analysis performed with 1000 bootstrap replicas. The positive and negative classes were respectively defined as the PD and the controls; the area under the curve (AUC) was used to quantify the discriminative ability of the measures. All the analyses were implemented in MATLAB® (R2015a; MathWorks, Natick, MA).
Results
Participant characteristics are summarized in Table 2. The control group tended to be older and had a higher percentage of women. Patients with PD were early to mild in the disease course (mean disease duration below 6 years).
Table 2.
Characteristics of the subjects
| Healthy Controls | Patients with Parkinson’s Disease | p-value | Non-fallers | Fallers | p-value | |
|---|---|---|---|---|---|---|
| # of subjects | 39 (19) | 101 (24) | 64 | 25 | ||
| Age (years) | 77.8 ± 3.9 (76.6 ± 1.4) | 64.5 ± 9.3 (75.1 ± 2.2) | < 0.001 0.147 | 63.7 ± 9.8 | 67.1 ± 9.2 | 0.14 |
| Gender (% women) | 64 (63) | 24 (25) | < 0.001 (0.011) | 20 | 0 | 0.44 |
| Height (cm) | 163.1 ± 6.5 (163.0 ± 6.3) | 169.4 ± 8.8 (167.1 ± 9.9) | < 0.001 (0.107) | 170.7 ± 8.9 | 168.2 ± 8.8 | 0.24 |
| Weight (kg) | 71.3 ± 13.2 (68.5 ± 12.6) | 76.5 ± 11.9 (77.0 ± 10.8) | 0.11 (0.030) | 75.9 ± 10.5 | 80.2 ± 14.2 | 0.12 |
| UPDRS Part III (motor) | 0 | 39.8 ± 12.9 (42.6 ± 11.9) | – | 39.8 ± 13.6 | 41.3 ± 10.6 | 0.97 |
| Disease duration (years) | 0 | 5.4 ± 3.3 (5.2 ± 2.3) | – | 5.1 ± 2.8 | 6.7 ± 4.4 | 0.02 |
| Levodopa equivalent dose | 0 | 520.3 ± 314.4 (499.0 ± 300.8) | – | 480.0 ± 271.6 | 582.3 ± 348.0 | 0.18 |
Note: Characteristics of the age matched subset of the PD cohort are presented in parenthesis
Comparison between controls and PD
The SPARC values for accelerometer and gyroscope of the PD patients were generally lower than the controls. Their frequency spectrum compared to controls, once normalized, presents more high amplitude peaks.
In the ON medication state, Table 3 shows that SPARC metrics from the accelerometer were significantly lower in PD than in controls (p < 0.001). Representative spectra used to compute SPARC are shown in Fig. 1. The harmonic ratio in the medio-lateral direction was also significantly lower in PD than in controls (p = 0.002). In the OFF medication state, differences between the PD and the control group increased for most measures (see the effect sizes in Table 3). Importantly, Table 3 and Figs. 2 and 3 show that smoothness quantified by SPARC-Acc has a higher ability for distinguishing controls from PD than harmonic ratios, variability measures or gait velocity; the effect size is largest for the SPARC-Acc measure. Gait speed, harmonic ratios, and SPARC were all significantly differentiate in PD ON versus PD OFF (p < 0.005).
Table 3.
Mean and standard deviation of the gait measures in the two groups
| Healthy Controls (HC) | PD ON | PD OFF | Effect Size & p-value | ||||
|---|---|---|---|---|---|---|---|
| HC vs PD ON | HC vs PD OFF | ON vs OFF | |||||
| SPARC | Acc | -5.17 ± 0.79 | −6.11 ± 0.74 | −6.74 ± 0.64 | 1.25 (< 0.001) | 2.29 (0.001) | < 0.001 |
| V | − 5.08 ± 0.74 | − 5.22 ± 0.78 | − 5.74 ± 0.88 | 0.17 (0.107) | 0.78 (0.001) | < 0.001 | |
| AP | −5.57 ± 0.74 | −5.80 ± 0.80 | − 6.44 ± 0.77 | 0.30 (0.190) | 1.14 (0.001) | < 0.001 | |
| ML | − 5.44 ± 0.32 | −5.60 ± 0.48 | −5.96 ± 0.50 | 0.35 (0.388) | 1.13 (0.005) | < 0.001 | |
| Gyr | −4.87 ± 0.55 | −5.00 ± 0.52 | −5.43 ± 0.53 | 0.25 (0.156) | 1.06 (< 0.001) | < 0.001 | |
| Yaw | −5.96 ± 0.81 | −5.92 ± 0.85 | −6.47 ± 0.82 | − 0.05 (0.037) | 0.63 (0.002) | < 0.001 | |
| Roll | − 5.80 ± 0.73 | −5.97 ± 0.60 | −6.51 ± 0.70 | 0.26 (0.229) | 1.01 (0.007) | < 0.001 | |
| Pitch | −6.28 ± 0.75 | −6.55 ± 0.86 | − 7.09 ± 0.91 | 0.32 (0.365) | 0.94 (0.002) | < 0.001 | |
| Harmonic ratios | V | 3.10 ± 0.90 | 2.75 ± 0.82 | 2.95 ± 0.85 | 0.42 (0.998) | 0.17 (0.092) | < 0.001 |
| AP | 2.08 ± 0.48 | 2.04 ± 0.74 | 2.54 ± 0.93 | 0.06 (0.800) | − 0.55 (0.240) | < 0.001 | |
| ML | 0.60 ± 0.11 | 0.56 ± 0.09 | 0.52 ± 0.08 | 0.33 (0.002) | 0.89 (< 0.001) | < 0.001 | |
| Mean Gait Speed | – | 1.18 ± 0.24 | 1.16 ± 0.20 | 1.13 ± 0.19 | 0.05 (0.028) | 0.21 (0.008) | 0.117 |
| Stride Time | Mean | 1.04 ± 0.06 | 1.09 ± 0.09 | 1.09 ± 0.09 | − 0.62 (0.048) | − 0.53 (0.365) | 0.550 |
| Variability | 3.43 ± 1.17 | 4.72 ± 3.05 | 5.84 ± 4.92 | − 0.48 (0.044) | − 0.57 (0.055) | 0.826 | |
Note: Significant results are displayed in bold
Analysis was also performed without controlling for weight which did not modify the outcomes
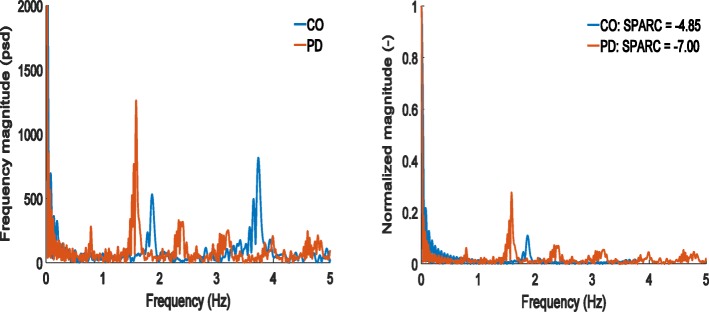
Fig. 1.
Spectra of a representative PD patient (age: 78 yrs.; disease duration: 7 yrs.; OFF medications) and a healthy control (age: 78). On the left, the un-normalized frequency spectrum is shown. The controls exhibit clear peaks around 1.8 Hz and 3.6 Hz, corresponding respectively to step and stride time. In the PD signal, only a clear peak around 1.5 Hz can be seen. In the normalized frequency spectrum, compared to the control, the spectrum of the PD patient has more peaks with larger amplitude, indicating a less smooth gait
Fig. 2.
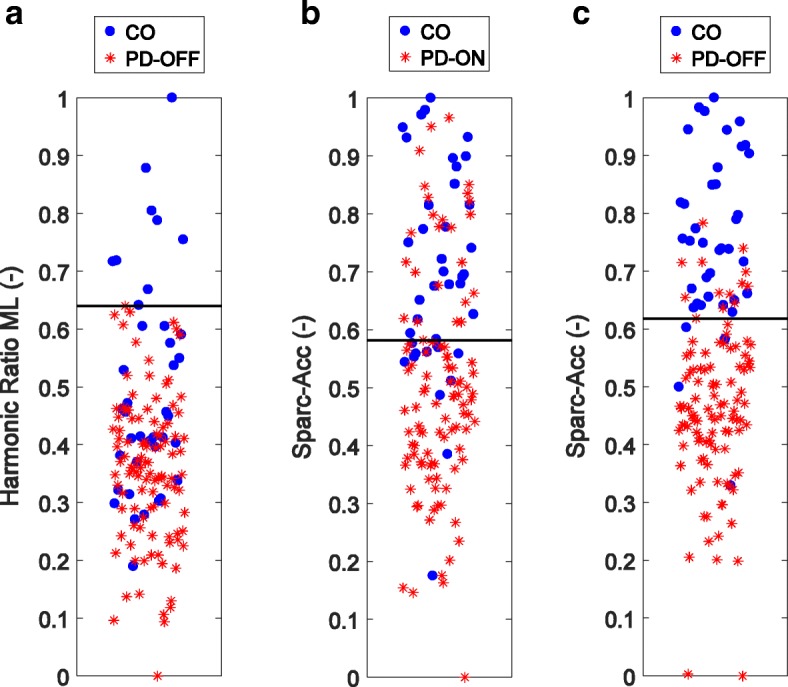
Separation between Controls and PD. a Separation from the harmonic ratio in the medio-lateral direction in the OFF state (accuracy: 79%). From SPARC, b PD in the ON medication state (accuracy: 74%); c PD in the OFF medication state (accuracy: 89%). Gait smoothness in PD decreases sharply in the OFF state. There is a large overlap between the two groups. The horizontal line on each plot is the threshold separating the two groups with the highest accuracy. Note that the horizontal jitter on each plot is meaningless and was added for better visualization
Fig. 3.
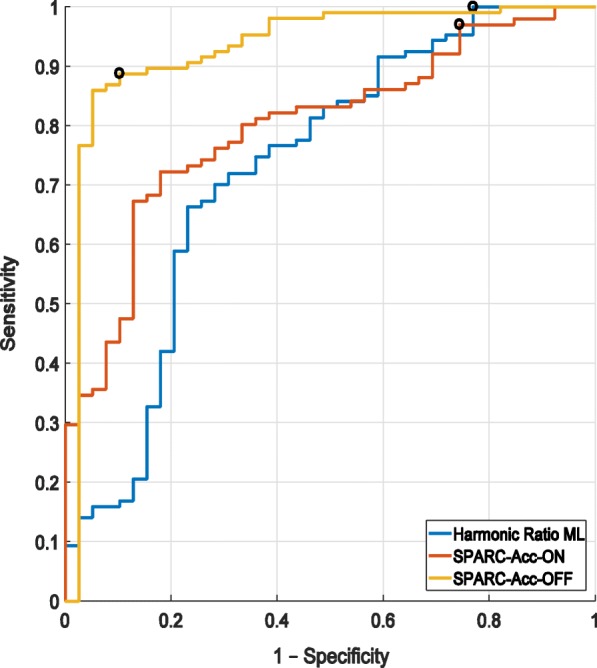
ROC analysis for harmonic ratios ML OFF (blue): AUC = 0.73, SPARC-Acc ON (red): AUC = 0.80 and OFF (yellow): AUC = 0.93. The positive class was defined as the PD. Hence the true positives are controls classified as such while the false positives are healthy controls classified as PD. Classification was done through SVM. Black circles mark optimal thresholds
As can be seen in Table 4, when the analysis was run on the age matched cohorts, the differences in smoothness, measured through SPARC or harmonic ratios, between groups tended to increase. On the other hand, the differences between the groups in gait speed, mean stride time and stride time variability tended to decrease.
Table 4.
Mean and standard deviation of the gait measures for the age matched groups
| Healthy Controls (HC) | PD ON | PD OFF | Effect Size & p-value | ||||
|---|---|---|---|---|---|---|---|
| HC vs PD ON | HC vs PD OFF | ON vs OFF | |||||
| SPARC | Acc | −5.08 ± 0.71 | −6.37 ± 0.64 | −6.88 ± 0.64 | 1.95 (< 0.001) | 2.69 (0.001) | 0.003 |
| V | −4.25 ± 0.14 | − 4.85 ± 0.61 | − 5.77 ± 0.76 | 1.36 (< 0.001) | 2.78 (0.001) | < 0.001 | |
| AP | −5.42 ± 0.70 | −5.83 ± 0.42 | − 6.69 ± 0.47 | 0.73 (0.033) | 2.18 (< 0.001) | < 0.001 | |
| ML | −5.69 ± 0.56 | −5.33 ± 0.43 | −5.38 ± 0.49 | − 0.73 (0.025) | − 0.61 (0.060) | 0.934 | |
| Gyr | − 5.01 ± 0.64 | −5.30 ± 0.56 | −5.83 ± 0.71 | 0.49 (0.120) | 1.23 (< 0.001) | 0.009 | |
| Yaw | −5.93 ± 0.82 | − 6.14 ± 0.84 | − 6.76 ± 0.86 | 0.26 (0.408) | 1.00 (0.002) | 0.030 | |
| Roll | − 5.91 ± 0.90 | − 6.25 ± 0.75 | −6.75 ± 0.68 | 0.41 (0.190) | 1.06 (0.002) | 0.013 | |
| Pitch | −6.30 ± 0.85 | −6.77 ± 0.88 | −7.61 ± 1.10 | 0.54 (0.087) | 1.33 (< 0.001) | 0.005 | |
| Harmonic ratios | V | 2.62 ± 0.65 | 2.53 ± 0.60 | 2.99 ± 0.78 | 0.14 (0.648) | − 0.52 (0.104) | 0.010 |
| AP | 1.02 ± 0.46 | 1.14 ± 0.55 | 1.98 ± 0.53 | − 0.25 (0.424) | − 0.52 (< 0.001) | < 0.001 | |
| ML | 0.63 ± 0.12 | 0.57 ± 0.09 | 0.52 ± 0.06 | 0.52 (0.100) | 1.18 (0.001) | 0.112 | |
| Mean Gait Speed | – | 1.20 ± 0.23 | 1.10 ± 0.20 | 1.04 ± 0.23 | 0.48 (0.127) | 0.72 (0.026) | 0.509 |
| Stride Time | Mean | 1.05 ± 0.04 | 1.12 ± 0.10 | 1.09 ± 0.12 | − 0.87 (0.004) | − 0.43 (0.143) | 0.536 |
| Variability | 3.50 ± 1.27 | 3.67 ± 1.56 | 5.82 ± 5.05 | − 0.12 (0.717) | − 0.066 (0.054) | 0.550 | |
Note: Significant results are displayed in bold
Comparison between PD non-fallers and PD fallers
In the ON state, no significant differences between the fallers and non-fallers could be seen although fairly large effect sizes were observed in the SPARC from the yaw axis (p = 0.033, effect size = 0.77) and stride time variability (p = 0.010, effect size = − 1.08). In the OFF state, gait performances deteriorated for both the fallers and non-fallers. Significant differences between the two groups could be seen in mean velocity (p = 0.001, effect size = 1.18); large effect sizes were observed in SPARC from the vertical axis (p = 0.008, effect size = 1.05), yaw (p = 0.012, effect size = 0.75) and stride time variability (p = 0.006, effect size = − 0.82). ROC analysis was also performed to differentiate the two groups, the best area under the curves was achieved with mean velocity in the off medication case (AUC = 0.80); SPARC from the vertical axis yielded a smaller area (AUC = 0.71).
Correlation between measures
Correlations between measures are presented in Table 4. SPARC-Acc correlated strongly with the UPDRS motor scores (r > 0.60), moderately with the harmonic ratio in the medio-lateral direction, the mean gait speed and the levodopa equivalent dose (0.30 < r < 0.50). Correlations between these measures and SPARC-Gyro were usually lower. On the other hand, neither SPARC-Acc nor SPARC-Gyro was significantly related to stride time variability.
Discussion
To our knowledge, this is the first study to apply the SPARC approach to measure smoothness in gait. Previous work suggested that SPARC was reliable in quantifying smoothness of a reaching task [27]; however, SPARC was not yet applied to quantify smoothness of walking. Our findings support the use of SPARC as a complementary and possibly alternative method to the harmonic ratios commonly used in the literature for quantifying smoothness of gait.
As expected, SPARC was lower in patients with PD than in healthy controls. Similar to previous findings, based on measures from gait variability [13, 34–36] or harmonic ratios [23, 37] the gait of PD subjects was worse than controls. However, SPARC appears to be more accurate in separating the groups. Furthermore, it was previously shown that medication improved patients’ gait [36, 38] and that was also reflected by SPARC which was lower in OFF than ON mediations. Further investigation of medication effects demonstrated that patients in need of larger dose of levodopa tended to have a less smooth gait (r = − 0.48), suggesting that these SPARC metrics reflect dopaminergic dysfunction. There was also a strong correlation of SPARC metrics (i.e., accelerometer vector magnitude) with mean gait speed (r > 0.5). This makes intuitive sense as an unsmooth gait is associated with and may, perhaps, contribute to slow and unsteady pace. Surprisingly, there was no correlation between SPARC and stride time variability, indicating no linear contribution of smoothness to the rhythm of gait and vice versa. Conversely, SPARC was correlated strongly with the UPDRS motor score (recall Table 5), indicating that smoothness not only is different between PD and controls, but may also provide a general measure of overall motor control ability and its changes across disease severity in PD, at least among ambulatory subjects. In a sense, these findings are parallel to what was shown in patients with stroke: improvements in smoothness in tasks trained during therapy also generalize to movements not explicitly trained as part of the therapy [32].
Table 5.
Correlations between the gait measures
| SPARC Acc | SPARC Acc | ||||||||||||||
| SPARC V | 0.77 (< 0.001) | SPARC V | |||||||||||||
| SPARC AP | 0.73 (< 0.001) | 0.67 (< 0.001) | SPARC AP | ||||||||||||
| SPARC ML | 0.70 (< 0.001) | 0.75 (< 0.001) | 0.63 (< 0.001) | SPARC ML | |||||||||||
| SPARC Gyr | 0.68 (< 0.001) | 0.31 (< 0.001) | 0.28 (< 0.001) | 0.31 (< 0.001) | SPARC Gyr | ||||||||||
| SPARC Yaw | 0.54 (< 0.001) | 0.38 (< 0.001) | 0.28(< 0.001) | 0.26 (< 0.001) | 0.46 (< 0.001) | SPARC Yaw | |||||||||
| SPARC Roll | 0.64 (< 0.001) | 0.49 (< 0.001) | 0.28 (< 0.001) | 0.42 (< 0.001) | 0.58 (< 0.001) | 0.32 (< 0.001) | SPARC Roll | ||||||||
| SPARC Pitch | 0.63 (< 0.001) | 0.65 (< 0.001) | 0.58 (< 0.001) | 0.57 (< 0.001) | 0.47 (< 0.001) | 0.30 (< 0.001) | 0.34 (< 0.001) | SPARC Pitch | |||||||
| HR V | 0.13 (0.033) | 0.31 (< 0.001) | 0.23 (0.001) | 0.25 (0.001) | −0.03 (0.041) | −0.07 (0.216) | 0.10 (0.006) | 0.21 (0.031) | HR V | ||||||
| HR AP | − 0.39 (< 0.001) | − 0.20 (0.011) | − 0.24 (0.001) | − 0.25 (< 0.001) | − 0.22 (< 0.001) | −0.06 (0.004) | − 0.22 (< 0.001) | −0.30 (< 0.001) | − 0.02 (0.810) | HR AP | |||||
| HR ML | 0.36 (< 0.001) | 0.32 (< 0.001) | 0.34 (< 0.001) | 0.20 (0.014) | 0.23 (< 0.001) | 0.38 (< 0.001) | 0.20 (< 0.001) | 0.20 (< 0.001) | −0.05 (0.663) | − 0.27 (0.002) | HR ML | ||||
| Mean velocity | 0.55 (< 0.001) | 0.77 (< 0.001) | 0.57 (< 0.001) | 0.59 (< 0.001) | 0.54 (< 0.001) | 0.66 (< 0.001) | 0.57 (< 0.001) | 0.46 (< 0.001) | 0.32 (< 0.001) | −0.18 (0.032) | 0.33 (< 0.001) | Mean velocity | |||
| Stride time Mean | −0.31 (< 0.001) | − 0.45 (< 0.001) | − 0.27 (0.001) | −0.29 (< 0.001) | 0.36 (< 0.001) | 0.28 (< 0.001) | −0.40 (< 0.001) | −0.23 (0.006) | − 0.09 (0.272) | −0.12 (0.138) | − 0.24 (0.004) | −0.25 (0.002) | Stride time Mean | ||
| Stride time Variability | −0.30 (< 0.001) | − 0.41 (< 0.001) | − 0.26 (0.001) | −0.37 (< 0.001) | −0.29 (< 0.001) | −0.28 (< 0.001) | −0.30 (< 0.001) | −0.20 (0.013) | − 0.22 (0.007) | −0.01 (0.895) | − 0.06 (0.499) | −0.28 (0.001) | 0.51 (< 0.001) | Stride time Variability | |
| UPDRS Motor | −0.65 (< 0.001) | − 0.35 (< 0.001) | −0.50 (< 0.001) | −0.42 (< 0.001) | −0.41 (< 0.001) | −0.29 (< 0.001) | −0.38 (< 0.001) | −0.42 (< 0.001) | 0.02 (0.796) | 0.48 (< 0.001) | − 0.29 (< 0.001) | − 0.22 (0.009) | 0.10 (0.240) | 0.24 (0.004) | UPDRS Motor |
| LED | −0.48 (< 0.001) | − 0.32 (< 0.001) | − 0.29 (< 0.001) | −0.32 (< 0.001) | − 0.32 (< 0.001) | −0.31 (< 0.001) | −0.34 (< 0.001) | −0.25 (0.002) | − 0.05 (0.531) | 0.33 (< 0.001) | − 0.29 (< 0.001) | −0.13 (0.120) | 0.18 (0.026) | 0.19 (0.023) | 0.58 (< 0.001) |
Notes: In parenthesis the p-values for the correlations. Absolute correlation |r| > 0.60 displayed in bold
People with PD are more likely to fall than other older adults without PD [16] and are at a greater risk to sustain an injurious fall. PD fallers had significantly decreased smoothness along the yaw direction, in both ON and OFF state, possibly due to bradykinesia and axial rigidity of the trunk [31, 39, 40]. Previous work emphasizes the importance of prospectively assess fall risk [30, 41] and upcoming studies should investigate decreased SPARC as a fall risk marker and further investigate the mechanism underlying the observed changes in this new measure of smoothness.
We demonstrated that gait smoothness could be easily estimated from inertial sensors, which are relatively inexpensive and unobtrusive. Furthermore, data collection is not circumscribed to the laboratory, and further work should explore gait smoothness in real-life settings. For this purpose, SPARC would be particularly suited. It is independent of the duration of the gait bout and, in contrast to stride time variability, does not necessitate identification of fiducial points.
Conclusions
These initial findings suggest that smoothness of walking is altered in patients with PD. Larger scale and longitudinal studies are needed to further assess the clinical utility of this new approach and to help to study changes in walking in response to specific treatments and interventions.
Acknowledgments
Funding
The work leading to this invention has received funding from the European Community’s Seventh Framework Programme FP7/2012 under Grant Agreement no. 316639.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
All authors contributed to conceptualization and design of the work, data interpretation, review and revision of the manuscript. All authors approved the final version of the manuscript. TH and MB performed data collection. YB performed data analysis and initial drafting of the manuscript.
Ethics approval and consent to participate
The study was approved by the human studies committee of the Tel Aviv Medical Center and the was carried out in accordance with the approved study protocol. After receiving a detailed explanation about the study, subjects provided informed written consent before participation.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yoav Beck, Email: yoavbeck@gmail.com.
Talia Herman, Email: talih@tlvmc.gov.il.
Marina Brozgol, Email: marinab@tlvmc.gov.il.
Nir Giladi, Email: nirg@tlvmc.gov.il.
Anat Mirelman, Email: anatmi@tlvmc.gov.il.
Jeffrey M. Hausdorff, Phone: +97236973081, Email: jhausdor@tlvmc.gov.il
References
- 1.Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry. 2000;69(3):308–12. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10945804. [DOI] [PMC free article] [PubMed]
- 2.Boonstra TA, van der Kooij H, Munneke M, Bloem BR. Gait disorders and balance disturbances in Parkinsonʼs disease: clinical update and pathophysiology. Curr Opin Neurol. 2008;24(4):461–471. doi: 10.1097/WCO.0b013e328305bdaf. [DOI] [PubMed] [Google Scholar]
- 3.Alexander N. Gait disorders in older adults. Clin Geriatr. 1999;7(3):73–81. [PubMed] [Google Scholar]
- 4.Perera S, Patel KV, Rosano C, Rubin SM, Satterfield S, Harris T, et al. Gait speed predicts incident disability: a pooled analysis. J Gerontol A Biol Sci Med Sci. 2015;71(1):63–71. doi: 10.1093/gerona/glv126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait Speed and Survival in Older Adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman T, Giladi N, Gurevich T, Hausdorff JM. Gait instability and fractal dynamics of older adults with a “cautious” gait: why do certain older adults walk fearfully? Gait Posture. 2005;21(2):178–185. doi: 10.1016/j.gaitpost.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Lord S, Galna B, Verghese J, Coleman S, Burn D, Rochester L. Independent domains of gait in older adults and associated motor and nonmotor attributes: validation of a factor analysis approach. J Gerontol A Biol Sci Med Sci. 2013;68(7):820–827. doi: 10.1093/gerona/gls255. [DOI] [PubMed] [Google Scholar]
- 8.Vienne A, Barrois RP, Buffat S, Ricard D, Vidal PP. Inertial sensors to assess gait quality in patients with neurological disorders: a systematic review of technical and analytical challenges. Front Psychol. 2017;8(MAY):1–12. doi: 10.3389/fpsyg.2017.00817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hausdorff JM. Gait variability: methods, modeling and meaning. J Neuroeng Rehabil. 2005;2:19. doi: 10.1186/1743-0003-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. J Gerontol Ser A Biol Sci Med Sci. 2009;64A(8):896–901. doi: 10.1093/gerona/glp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brodie MAD, Menz HB, Lord SR. Age-associated changes in head jerk while walking reveal altered dynamic stability in older people. Exp Brain Res. 2014;232(1):51–60. doi: 10.1007/s00221-013-3719-6. [DOI] [PubMed] [Google Scholar]
- 12.Castagna A, Frittoli S, Ferrarin M, Del Sorbo F, Romito LM, Elia AE, et al. Quantitative gait analysis in parkin disease: possible role of dystonia. Mov Disord. 2016;31(11):1720–1728. doi: 10.1002/mds.26672. [DOI] [PubMed] [Google Scholar]
- 13.Hausdorff JM, Cudkowicz ME, Firtion R, Wei JY, Goldberger AL. Gait variability and basal ganglia disorders: stride-to-stride variations of gait cycle timing in parkinson’s disease and Huntington’s disease. Mov Disord. 1998;13(3):428–437. doi: 10.1002/mds.870130310. [DOI] [PubMed] [Google Scholar]
- 14.Hausdorff JM, Schaafsma JD, Balash Y, Bartels AL, Gurevich T, Giladi N. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp Brain Res. 2003;149(2):187–194. doi: 10.1007/s00221-002-1354-8. [DOI] [PubMed] [Google Scholar]
- 15.Mirelman A, Maidan I, Herman T, Deutsch JE, Giladi N, Hausdorff JM. Virtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson’s disease? J Gerontol A Biol Sci Med Sci. 2011;66A(2):234–240. doi: 10.1093/gerona/glq201. [DOI] [PubMed] [Google Scholar]
- 16.Mirelman A, Rochester L, Maidan I, Del Din S, Alcock L, Nieuwhof F, et al. Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): a randomised controlled trial. Lancet. 2016;388(10050):1170–1182. doi: 10.1016/S0140-6736(16)31325-3. [DOI] [PubMed] [Google Scholar]
- 17.Hoskovcová M, Dušek P, Sieger T, Brožová H, Zárubová K, Bezdíček O, et al. Predicting falls in Parkinson disease: what is the value of instrumented testing in OFF medication state? Svenningsson P. PLoS One. 2015;10(10):e0139849. doi: 10.1371/journal.pone.0139849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson EJ, Lord SR, Brodie MA, Gaunt DM, Lawrence AD, Close JCT, et al. Rivastigmine for gait stability in patients with Parkinson’s disease (ReSPonD): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(3):249–258. doi: 10.1016/S1474-4422(15)00389-0. [DOI] [PubMed] [Google Scholar]
- 19.Mirelman A, Heman T, Yasinovsky K, Thaler A, Gurevich T, Marder K, et al. Fall risk and gait in Parkinson’s disease: The role of the LRRK2 G2019S mutation. Mov Disord. 2013;28(12):1683–1690. doi: 10.1002/mds.25587. [DOI] [PubMed] [Google Scholar]
- 20.König N, Singh NB, Baumann CR, Taylor WR. Can Gait Signatures Provide Quantitative Measures for Aiding Clinical Decision-Making? A Systematic Meta-Analysis of Gait Variability Behavior in Patients with Parkinson’s Disease. Front Hum Neurosci. 2016;10:319. Available from: http://journal.frontiersin.org/Article/10.3389/fnhum.2016.00319/abstract. [DOI] [PMC free article] [PubMed]
- 21.Hogan N, Sternad D. Sensitivity of smoothness measures to movement duration, amplitude, and arrests. J Mot Behav. 2009;41(6):529–534. doi: 10.3200/35-09-004-RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yack HJ, Berger RC. Dynamic stability in the elderly: identifying a possible measure. J Gerontol. 1993;48(5):M225–M230. doi: 10.1093/geronj/48.5.M225. [DOI] [PubMed] [Google Scholar]
- 23.Brach JS, McGurl D, Wert D, VanSwearingen JM, Perera S, Cham R, et al. Validation of a Measure of Smoothness of Walking. J Gerontol A Biol Sci Med Sci. 2011;66A(1):136–141. doi: 10.1093/gerona/glq170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buckley C, Galna B, Rochester L, Mazzà C. Attenuation of upper body accelerations during gait: piloting an innovative assessment tool for Parkinson’s disease. Biomed Res Int. 2015;2015:865873. doi: 10.1155/2015/865873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowry KA, Smiley-Oyen AL, Carrel AJ, Kerr JP. Walking stability using harmonic ratios in Parkinson’s disease. Mov Disord. 2009;24(2):261–267. doi: 10.1002/mds.22352. [DOI] [PubMed] [Google Scholar]
- 26.Balasubramanian S, Melendez-Calderon A, Roby-Brami A, Burdet E. On the analysis of movement smoothness. J Neuroeng Rehabil. 2015;12(1):112. doi: 10.1186/s12984-015-0090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balasubramanian S, Melendez-Calderon A, Burdet E. A robust and sensitive metric for quantifying movement smoothness. IEEE Trans Biomed Eng [Internet] 2012;59(8):2126–2136. doi: 10.1109/TBME.2011.2179545. [DOI] [PubMed] [Google Scholar]
- 28.Herman T, Rosenberg-Katz K, Jacob Y, Auriel E, Gurevich T, Giladi N, et al. White matter Hyperintensities in Parkinson’s disease: do they explain the disparity between the postural instability gait difficulty and tremor dominant subtypes? Toft M. PLoS One. 2013;8(1):e55193. doi: 10.1371/journal.pone.0055193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Weiss A, Herman T, Plotnik M, Brozgol M, Giladi N, Hausdorff JM. An instrumented timed up and go: the added value of an accelerometer for identifying fall risk in idiopathic fallers. Physiol Meas. 2011;32(12):2003–2018. doi: 10.1088/0967-3334/32/12/009. [DOI] [PubMed] [Google Scholar]
- 31.Mirelman A, Bernad-Elazari H, Thaler A, Giladi-Yacobi E, Gurevich T, Gana-Weisz M, et al. Arm swing as a potential new prodromal marker of Parkinson’s disease. Mov Disord. 2016;31(10):1527–1534. doi: 10.1002/mds.26720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burioka N, Imada A, Kiyohiro A, Sugitani F, Fujii T, Hosaka A, et al. Modified six-minute walk test: number of steps per second. Yonago Acta Med. 2014;57(1):61–63. [PMC free article] [PubMed] [Google Scholar]
- 33.Mds T, Goetz CG, Poewe W, Rascol O, Christina S. The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and recommendations. Mov Disord. 2003;18(7):738–750. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 34.Hausdorff JM. Gait dynamics in Parkinson’s disease: Common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos. 2009;19(2):26113. doi: 10.1063/1.3147408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirelman A, Gurevich T, Giladi N, Bar-Shira A, Orr-Urtreger A, Hausdorff JM. Gait alterations in healthy carriers of the LRRK2 G2019S mutation. Ann Neurol [Internet] 2011;69(1):193–197. doi: 10.1002/ana.22165. [DOI] [PubMed] [Google Scholar]
- 36.Curtze C, Nutt JG, Carlson-Kuhta P, Mancini M, Horak FB. Levodopa is a double-edged sword for balance and gait in people with Parkinson’s disease. Mov Disord. 2015;30(10):1361–1370. doi: 10.1002/mds.26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Latt MD, Menz HB, Fung VS, Lord SR. Acceleration patterns of the head and pelvis during gait in older people with Parkinson’s disease: a comparison of fallers and nonfallers. J Gerontol A Biol Sci Med Sci. 2009;64(6):700–706. doi: 10.1093/gerona/glp009. [DOI] [PubMed] [Google Scholar]
- 38.Schaafsma JD, Giladi N, Balash Y, Bartels AL, Gurevich T, Hausdorff JM. Gait dynamics in Parkinson’s disease: relationship to parkinsonian features, falls and response to levodopa. J Neurol Sci. 2003;212(1–2):47–53. doi: 10.1016/S0022-510X(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 39.REA VE, Wagenaar RC, Winogrodzka A, Wolters EC. Identification of axial rigidity during locomotion in parkinson disease. Arch Phys Med Rehabil. 1999;80(2):186–191. doi: 10.1016/S0003-9993(99)90119-3. [DOI] [PubMed] [Google Scholar]
- 40.Wright WG, Gurfinkel VS, Nutt J, Horak FB, Cordo PJ. Axial hypertonicity in Parkinson’s disease: direct measurements of trunk and hip torque. Exp Neurol. 2007;208(1):38–46. doi: 10.1016/j.expneurol.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laessoe U, Hoeck HC, Simonsen O, Sinkjaer T, Voigt M. Fall risk in an active elderly population – can it be assessed? J Negat Results Biomed. 2007;6(1):2. doi: 10.1186/1477-5751-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.