Abstract
Background
Leishmaniasis is an emerging disease in Thailand with an unknown incidence or prevalence. Although the number of properly characterized and clinically confirmed cases is about 20, it is suspected that this low number masks a potentially high prevalence, with clinical disease typically manifesting itself against an immunocompromised background, but with a substantial number of subclinical or cured cases of infection. To date leishmaniasis in Thailand has been mainly ascribed to two taxa within the recently erected subgenus Mundinia Shaw, Camargo & Teixeira, 2016, Leishmania (Mundinia) martiniquensis Desbois, Pratlong & Dedet, 2014 and a species that has not been formally described prior to this study.
Results
A case of simple cutaneous leishmaniasis was diagnosed in a patient from Nan Province, Thailand. Molecular analysis of parasites derived from a biopsy sample revealed this to be a new species of Leishmania Ross, 1908, which has been named as Leishmania (Mundinia) orientalis Bates & Jariyapan n. sp. A formal description is provided, and this new taxon supercedes some isolates from the invalid taxon “Leishmania siamensis”. A summary of all known cases of leishmaniasis with a corrected species identification is provided.
Conclusions
Three species of parasites are now known to cause leishmaniasis is Thailand, L. martiniquensis and L. orientalis n. sp. in the subgenus Mundinia, which contains the type-species Leishmania enriettii Muniz & Medina, 1948, and a single case of Leishmania infantum Nicolle, 1908. This study now enables epidemiological and other investigations into the biology of these unusual parasites to be conducted. It is recommended that the use of the taxonomically invalid name “L. siamensis” should be discontinued.
Electronic supplementary material
The online version of this article (10.1186/s13071-018-2908-3) contains supplementary material, which is available to authorized users.
Keywords: Leishmania orientalis, Mundinia, Thailand, Cutaneous leishmaniasis
Background
Leishmaniasis is an emerging disease in Thailand. The first confirmed autochthonous case was reported in 1999 [1], but since then there have been further cases described that undoubtedly reflect a much higher underlying prevalence of undiagnosed cases and asymptomatic infections, given that no active case detection study has been performed to date and there is little experience in diagnosis of leishmaniasis in Thailand. To date a total of 22 parasitologically confirmed cases have been reported, and of these 19 have been identified using various molecular methods. There has been one case of visceral leishmaniasis due to Leishmania infantum Nicolle, 1908 infection [2], but no subsequent reports.
The most frequent parasite found so far is L. martiniquensis Desbois, Pratlong & Dedet, 2014, accounting for 15 of the identified cases. This parasite is named after the Caribbean island of Martinique, where it was first isolated [3, 4]. In some of the early Thailand reports this is referred to as “L. siamensis”; however, there are two problems with this usage. The first problem is that “L. siamensis” has never been formally described, so the name is taxonomically invalid (a nomen nudum), without any type specimen for reference, and therefore technically should not have been used in any publications. The second problem is subsequent evidence that the group of isolates that have previously been called “L. siamensis” has been found not to be monophyletic, and includes two taxa [5, 6]. One of these includes the aforementioned parasites that appear identical to L. martiniquensis and, since this is a valid species, this name takes precedence for these particular isolates. However, in the second taxon there are two isolates of “L. siamensis” that are very similar to each other but different from L. martiniquensis based on molecular analysis, the PCM2 Trang strain from a patient in southern Thailand [7], which is phylogenetically distinct [6, 8], and a recent isolate from central Thailand [9]. These two isolates were both from patients that were HIV-infected and presented with disseminated cutaneous leishmaniasis, the former also with visceral disease.
Here we describe a case of simple cutaneous leishmaniasis from northern Thailand that was caused by a parasite apparently very similar to those responsible for these two previous cases, and we formally name this as Leishmania orientalis Bates & Jariyapan n. sp., thereby establishing a taxonomically valid name for these parasites, and simultaneously eliminating the need to use the unavailable name “L. siamensis”. We place this new species in the recently erected Leishmania subgenus Mundinia Shaw, Camargo & Teixeira, 2016 [10] and provide an updated identification of all known previous isolates from Thailand into L. infantum, L. martiniquensis and L. orientalis n. sp.
Results
Case report
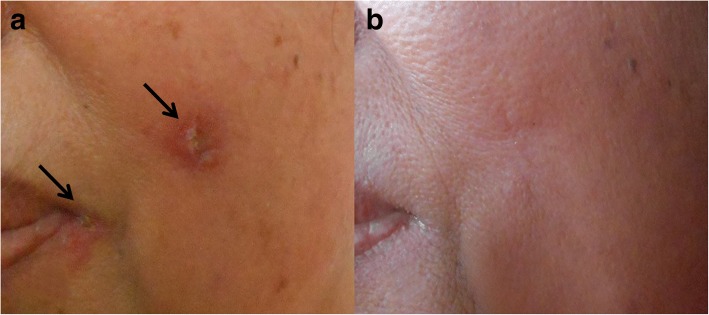
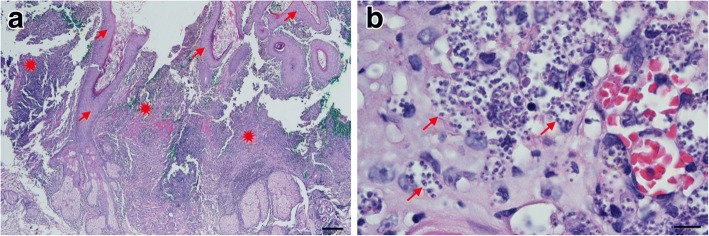
The patient was a 57-year-old woman who lives in Chiang Klang District, Nan Province, northern Thailand. She is a gardener and has never been abroad, only travelling to Phitsanulok and Phijit, provinces near Nan in Thailand. The patient presented in May 2014 at Chiang Klang Hospital with a single skin nodule on her left cheek (1.0 × 1.5 cm), and also with crusting at the left angle of the mouth (Fig. 1a). No skin nodules in other sites of the body were observed. Two pieces of formalin-fixed skin biopsy from the cheek nodule (0.6 × 0.5 × 0.4 cm3 and 0.5 × 0.5 × 0.2 cm3) were sent to the Department of Pathology, Faculty of Medicine, Chiang Mai University for histopathological investigation. Histopathological analyses revealed epidermal ulceration with a heavy, chronic inflammation of the dermis (Fig. 2a) and numerous intracellular small round or oval-shaped bodies, with the appearance of amastigotes (1–2 μm in width and 2–4 μm in length) of Leishmania spp. (Fig. 2b). A week later, a fresh skin biopsy from the nodule (0.4 × 0.5 × 0.3 cm3) was collected and sent to Department of Parasitology, Faculty of Medicine, Chiang Mai University for confirmation of diagnosis by parasite culture and species identification. The skin biopsy sample was cultured in Schneider’s insect medium supplemented with 20% foetal bovine serum (FBS) and 50 International Units penicillin/ml, 50 μg/ml streptomycin at 25°C. Motile promastigotes were first observed on day 3 of the culture. Therefore, the patient was confirmed as diagnosed with cutaneous leishmaniasis. She was treated with oral amphotericin B at 1 mg/kg/day for 1 day and fluconazole at 200 mg/day for 45 days. The skin lesion had disappeared completely by six months after treatment (Fig. 1b). Pre-treatment laboratory investigation showed only mild anaemia with a haemoglobin concentration of 10.9 g/dl, white blood count of 7700 cells/mm3, and platelet count of 483,000/mm3. There was no hepatosplenomegaly or palpable lymph nodes. Liver function was not investigated, renal function was within normal limits and HIV serology was negative. The patient declined a bone marrow biopsy for evaluation of visceral leishmaniasis. She did not report any other underlying disease, routine drug use, or any other symptoms, and in general was in a good state of health.
Fig. 1.
Clinical presentation of cutaneous leishmaniasis. a A nodule on the cheek and a crusted sore in the angle of the lips of the patient before treatment, both arrowed. b The same view of the patient’s skin after treatment
Fig. 2.
Histopathology of skin biopsy from a nodule on the left cheek (Giemsa stain). a Low power magnification photomicrograph showing pseudo-epitheliomatous hyperplasia of the epidermis (arrows) and heavy chronic inflammation of dermis (starbursts). b High power magnification photomicrograph showing numerous Leishmania amastigotes within the cytoplasm of macrophages and in the extracellular matrix (arrows). Scale-bars: a, 200 μm; b, 10 μm
Parasite characterization
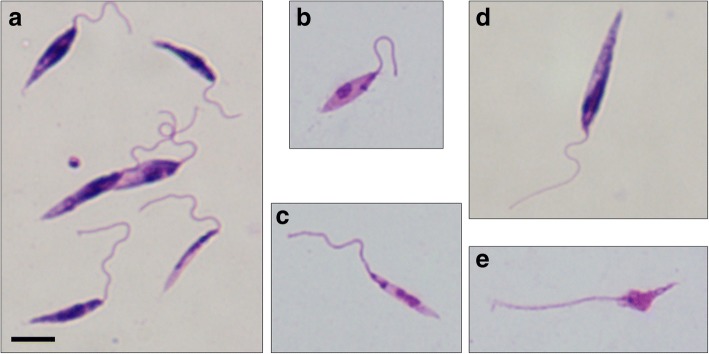
The patient isolate has the World Health Organisation strain designation MHOM/TH/2014/LSCM4, hereafter referred to as CM4. Promastigote cultures were established by a serial sub-passage from the initial culture using liquid media, and their morphology examined by light microscopy of Giemsa-stained slides (Fig. 3). The morphological forms observed were generally similar to those described for other Leishmania species, demonstrating a range of promastigote types, and including some similar to procyclic promastigotes, leptomonad promastigotes, nectomonad promastigotes and metacyclic promastigotes [11]. Although free-swimming individual promastigotes were readily observed, rosettes and large aggregates of promastigotes were prevalent in culture (Additional file 1: Video S1).
Fig. 3.
Morphology of Giemsa-stained promastigote forms from culture showing morphological variation of forms observed (a); procyclic-like promastigote (b); leptomonad-like promastigote (c); nectomonad-like promastigote (d); and metacyclic-like promastigote (e). All images are at the same magnification. Scale-bar: 5 μm
Additional file 1: Video S1. Live Leishmania orientalis promastigotes in vitro, illustrating both free-swimming and aggregations of promastigotes. (WMV 28731 kb)
Molecular analysis
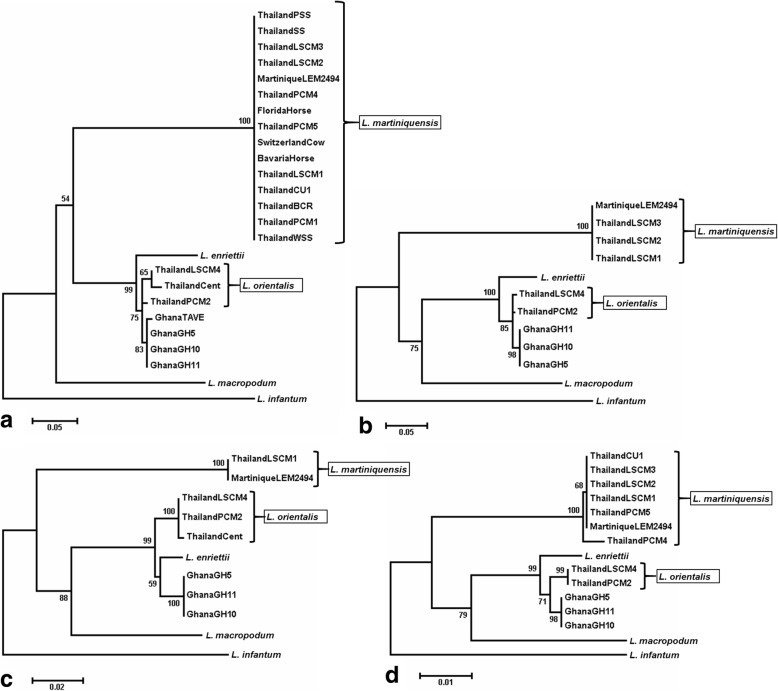
Four different sequences were analysed by polymerase chain reaction (PCR) and deoxyribonucleic acid (DNA) sequencing: the ribosomal ribonucleic acid (RNA) internal transcribed spacer 1 (ITS1) [12]; the ribosomal protein L23a intergenic sequence (RPL23a) [13]; the large subunit of RNA Polymerase II (RNA PolII) [14]; and heat-shock protein 70 (HSP70) [15]. The accession numbers for the new sequences generated in this study together with others used in the following analyses are given in Additional file 2: Table S1. Initial Basic Local Alignment Search Tool analysis revealed the CM4 sequences to be closest to PCM2 Trang [7] and/or the related isolate from central Thailand [9] for all four sequences examined, and then to other members of the subgenus Leishmania (Mundinia) (Fig. 4). In three of the analyses the CM4 clade was closest to that containing the recently described (but not yet named) parasites from Ghana [8], and in the RNA PolII tree appeared equidistant between these and L. enriettii. In all phylogenetic analyses the CM4 clade (labelled L. orientalis in Fig. 4) is clearly distinct from the L. martiniquensis clade that also contains several Thai isolates. The sequences of CM4 and PCM2 Trang were very similar to each other or identical in all cases (Table 1), whereas CM4 and a Thai isolate of L. martiniquensis called CM1 [6] were different (Table 2).
Fig. 4.
Phylogenetic analysis of DNA sequences from LSCM4 and other members of the subgenus Leishmania (Mundinia). Each panel shows a maximum likelihood tree with all currently available sequence data from members of the subgenus, using L. infantum as an outgroup, and with numbers at nodes derived by bootstrapping at 1000 replicates. a ITS1. b RPL23a. c RNA PolII. d HSP70. For accession numbers see Additional file 2: Table S1. The CM4 clade is labelled L. orientalis
Table 1.
Comparisons of LSCM4 DNA sequences with those from PCM2 Trang [7]. The lengths of the sequences given are the number of nucleotides, the analysis excludes PCR primers and comparisons were made using Clustal Omega
| Sequence | Length LSCM4 | Length PCM2 | % identitya | No. of identical nt | No. of differences |
|---|---|---|---|---|---|
| ITS-1 | 251 | 251 | 96.4 | 242 | 9 |
| RPL23a IGS | 468 | 468 | 99.4 | 465 | 3 |
| RNAPolII | 1206 | 1206 | 100 | 1206 | 0 |
| HSP70 | 1319 | 1319 | 100 | 1319 | 0 |
a% identity = no. of identical nucleotides/longest length, where sequence length differs
Table 2.
Comparisons of LSCM4 DNA sequences with those from L. martiniquensis LSCM1 [6]. The lengths of the sequences given are the number of nucleotides, the analysis excludes PCR primers and comparisons were made using Clustal Omega
| Sequence | Length LSCM4 | Length LSCM1 | % identitya | No. of identical nt | No. of differences |
|---|---|---|---|---|---|
| ITS1 | 251 | 259 | 59.1 | 153 | 106 |
| RPL23a IGS | 468 | 472 | 64.8 | 306 | 166 |
| RNAPolII | 1206 | 1206 | 91.5 | 1104 | 102 |
| HSP70 | 1319 | 1319 | 95.7 | 1262 | 57 |
a% identity = no. of identical nucleotides/longest length, where sequence length differs
Taxonomic description
Class Kinetoplastea Honigberg, 1963
Order Trypanosomatida Kent, 1880
Family Trypanosomatidae Doflein, 1951
Genus Leishmania Ross, 1903
Subgenus L. (Mundinia) Shaw, Camargo & Teixeira 2016
Leishmania ( Mundinia ) orientalis Bates & Jariyapan n. sp.
Type-host : Homo sapiens.
Type-locality: Chiang Klang District (19°17'30"N, 100°51'42"E), Nan Province, Thailand.
Type-material: Hapantotypes, cryopreserved promastigotes stored in liquid nitrogen at the Department of Parasitology, Chiang Mai University, Thailand (accession LSCM4) and the Division of Biomedical and Life Sciences, Lancaster University, UK (accession LV768).
Strain designation: MHOM/TH/2014/LSCM4.
Homologous strains: PCM2 Trang [7] and an unnamed isolate from central Thailand [9].
Vector: Unknown.
Reservoir(s): Unknown.
Representative DNA sequences: The RNA internal transcribed spacer 1, ribosomal protein L23a intergenic sequence, partial large subunit of RNA Polymerase II and partial heat-shock protein 70 sequences were deposited in the GenBank database under the accession numbers MG731227, MG731231, MG731232 and MG731233, respectively.
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN) [16], details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) of the article is urn:lsid:zoobank.org:pub:6C6CBD86-4C7A-437E-B4C7-B6229C9F6C67. The LSID for the new name Leishmania orientalis is urn:lsid:zoobank.org:act:C03ADE08-34BD-4252-A534-831D57A3A8D9.
Etymology: Species name refers to eastern origin since this parasite is unlikely to be confined to Thailand only.
Description (Figs. 2, 3)
Morphology. Amastigotes 1–2 μm in width and 2–4 μm in length. Promastigotes of various sizes with body length ranging between 5–15 μm and motile with a free anterior flagellum of variable length.
Growth in vitro. Isolated in Schneider’s Drosophila medium supplemented with 20% (v/v) FBS and maintained in the same medium or Medium 199 supplemented with 10% (v/v) FBS and Basal Medium Eagle (BME) vitamins. Relatively easy to grow compared to other Leishmania species by subpassage every 1–2 weeks.
Pathology. Variable; in the type-host presentation was cutaneous leishmaniasis with no known underlying immunodeficiency; in other strains presentation as disseminated cutaneous leishmaniasis, with possible visceral disease, both in the presence of human immunodeficiency virus (HIV) co-infection.
Remarks
The new species was characterized using molecular techniques, revealing a distinct parasite within the subgenus Mundinia. Two isolates, PCM2 Trang [7] and an unnamed isolate from central Thailand [9] are considered as being L. (M.) orientalis due to their high level of similarity with the type-strain LSCM4.
Discussion
Here we describe a new species of Leishmania causing human disease in Thailand, Leishmania orientalis. Together with L. martiniquensis the new species accounts for almost all of the known cases of leishmaniasis reported in Thailand (Table 3). The only exception is one case of L. infantum reported in 2008 [2], but in that case it was suspected the infection may have been acquired elsewhere.
Table 3.
Reports of leishmaniasis from Thailand and Myanmar ordered by year of isolation. Where done, identification was performed by sequencing one or more DNA targets. Those identified witha were originally or subsequently reported as “L. siamensis” but later identified to be L. martiniquensis by Pothirat et al. [6]. Those identified withb were originally reported as “L. siamensis” but shown in this study to be very similar to L. orientalis
| Year | Location | Age | Sex | HIV | Primary clinical presentation | Species identification | Reference |
|---|---|---|---|---|---|---|---|
| 1996 | Surat Thani, Thailand | 3 | Female | No | Visceral leishmaniasis | Unknown | Thisyakorn et al. (1999) [1] |
| 2005 | Nan, Thailand | 40 | Male | No | Visceral leishmaniasis | Unknown | Kongkaew et al. (2007) [35] |
| 2006 | Phang-Nga, Thailand | 55 | Male | No | Visceral leishmaniasis | L. martiniquensis a | Sukmee et al. (2008) [36] |
| 2007 | Bangkok, Thailand | 66 | Male | No | Visceral leishmaniasis | L. infantum | Maharom et al. (2008) [2] |
| 2009 | Chantaburi, Thailand | 37 | Male | Yes | Visceral leishmaniasis | L. martiniquensis a | Suankratay et al. (2010) [37] |
| 2010 | Trang, Thailand | 35 | Female | Yes | Disseminated cutaneous and visceral leishmaniasis | L. orientalis b | Bualert et al. (2012) [7] |
| 2010 | Satun, Thailand | 7 | Female | No | Visceral leishmaniasis | L. martiniquensis a | Osatakul et al. (2014) [38] |
| 2011 | Songkhla, Thailand | 46 | Male | Yes | Visceral and cutaneous leishmaniasis | L. martiniquensis a | Chusri et al. (2012) [39] |
| 2011 | Trang, Thailand | 30 | Male | Yes | Disseminated cutaneous leishmaniasis | L. martiniquensis a | Chusri et al. (2012) [39] |
| 2011 | Lop Buri, Thailand | 3 | Female | No | Cutaneous leishmaniasis | Unknown | Kattipathanapong et al. (2012) [40] |
| 2012 | Yangon, Myanmar | 22 | Female | No | Asymptomatic | L. martiniquensis a | Phumee et al. (2013) [41] |
| 2012 | Chiang Rai, Thailand | 45 | Male | Yes | Disseminated cutaneous leishmaniasis | L. martiniquensis a | Phumee et al. (2013) [41] |
| 2012 | Yangon, Myanmar | 34 | Male | Yes | Disseminated cutaneous leishmaniasis | L. martiniquensis a | Phumee et al. (2013) [41] |
| 2012 | Yangon, Myanmar | 60 | Male | No | Disseminated cutaneous leishmaniasis | L. martiniquensis a | Noppakun et al. (2014) [42] |
| 2012 | Ban Thi, Thailand | 52 | Male | No | Visceral leishmaniasis | L. martiniquensis | Pothirat et al. (2014) [6] |
| 2013 | Hang Dong, Thailand | 48 | Male | Yes | Disseminated cutaneous leishmaniasis | L. martiniquensis | Chiewchanvit et al. (2015) [33] |
| 2013 | Mae Tha, Thailand | 38 | Male | Yes | Disseminated cutaneous leishmaniasis | L. martiniquensis | Chiewchanvit et al. (2015) [33] |
| 2014 | Chiang Klang, Thailand | 57 | Female | No | Cutaneous leishmaniasis | L. orientalis | Present study |
| 2017 | Kanchanaburi, Thailand | 42 | Female | Yes | Disseminated cutaneous leishmaniasis | L. orientalis b | Supsrisunjai et al. (2017) [9] |
Both L. orientalis and L. martiniquensis are members of the recently described Leishmania subgenus Mundinia, with the type-species L. enriettii [10, 17]. Until recently, this was known as the L. enriettii species complex [6, 8, 18–20], but based largely on molecular data these species have emerged as a distinct monophyletic clade. Regarding human pathogenicity, the three other subgenera of Leishmania apart from Mundinia are relatively uniform, with L. (Leishmania) (type-species L. donovani Laveran & Mesnil, 1903) and L. (Viannia) (type-species L. braziliensis Vianna, 1911) containing mainly human-infective species (with one or two exceptions), whereas the L. (Sauroleishmania) have never been found in humans - the so-called lizard Leishmania. In that respect the known members of Mundinia, to which we now add L. orientalis n. sp., present a rather eclectic mixture: three species are human pathogens, L. martiniquensis, L. orientalis n. sp. and Leishmania species Ghana; and two species are non-pathogenic to humans, L. enriettii, known to infect domestic guinea pigs in Brazil [21, 22], and L. macropodum Barratt, Kaufer & Ellis, 2017, known to infect certain species of kangaroo and other macropods in Australia [23, 24]. The two other clades closest to L. orientalis n. sp. are L. enriettii and Leishmania species Ghana. From its known features L. enriettii is clearly different from L. orientalis, and also, given the differences in clinical presentation [8] and the results of the current and previous molecular analyses [8], Leishmania species Ghana also appears to be different from L. orientalis. Overall the diversity of hosts and variation in human pathogenicity of the species of Mundinia is most likely a reflection of their basal position in Leishmania phylogeny [8, 25]. This diversity also supports a supercontinental origin of the subgenus, in which the Mundinia appeared before the other subgenera and prior to the breakup of Gondwana. This explains its presence in different continents and mammalian orders.
The vectors of the species of Leishmania (Mundinia) are not known with certainty, including efforts to incriminate sand flies, the normal vectors of leishmaniasis, although Lutzomyia monticola has been suggested as a possible vector for L. enriettii [26]. The best evidence is for L. macropodum, where unusually the vector appears to be a day-biting midge of the genus Forcipomyia [13]. Crucially these midges supported the development of infections beyond the blood meal stage and produced material similar to promastigote secretory gel and metacyclic promastigotes (infective stages). In addition, L. macropodum and L. enriettii can both develop beyond the blood meal in the experimental midge host Culicoides sonorensis Wirth & Jones, 1957, whereas neither can establish in the normally permissive sand fly host Lutzomyia longipalpis Lutz & Neiva, 1912 [27]. In contrast, Leishmania species in other subgenera show full development in Lu. longipalpis [28]. On the other hand, in Thailand there have been reports of Leishmania DNA in Sergentomyia sand flies [29, 30], suggesting the possible involvement of a new sand fly genus in transmission to humans, which would usually be a species of Phlebotomus in Asia. However, dissections were not performed to investigate whether these flies supported infection beyond the blood meal stage, which is required for transmission [31].
The recent emergence of leishmaniasis in Thailand is a worrying development, as there is little experience amongst clinicians and public health professionals in diagnosis, treatment or disease control in the country. This is compounded by the nature of the parasites themselves, which are two novel and therefore poorly understood species, L. martiniquensis and L. orientalis n. sp. and preliminary evidence suggesting that they may have a different type of vector to that normally associated with transmission. The underlying rate of infection is almost certainly much higher than the number of case reports indicates, and could conceivably be a common but sub-clinical infection in most infected individuals, only manifesting when patients become immunocompromised in some way. In a recent study, the prevalence of Leishmania infection in a cohort of HIV patients from Trang Province, Thailand was 25.1% [32]. Whilst these data may not be representative of the whole country, they clearly demonstrate the potential for a high underlying rate of exposure to Leishmania spp. in the general population. Clearly more work is required both on the basic biology and vectors of these species, as well as epidemiological investigation of the prevalence and incidence of infection in the human population.
Methods
Parasite culture and morphology
Promastigote cultures were obtained by inoculation of patient biopsy material into 5 ml of Schneider’s Drosophila medium (Sigma-Aldrich, St Louis, MO, USA) supplemented with 20% (v/v) FBS (Thermo-Fisher, Waltham, MA, USA) in a 25-cm2 tissue culture flask and maintained at 26 °C (Pothirat et al. [6]). Subsequently, promastigotes were passaged in the same medium, and also into Medium 199 (Lonza, Basel, Switzerland) supplemented with 10% (v/v) FBS, BME vitamins (Sigma-Aldrich) and 25 μg/ml gentamicin sulphate (Sigma-Aldrich) for long term maintenance. Promastigotes were cryopreserved in 7.5% (v/v) glycerol, 50% (v/v) fetal bovine serum in culture medium and stored in liquid nitrogen. For morphological characterization, the culture parasites were smeared on microscope slides, air-dried, and fixed with absolute methanol. The samples were stained with Giemsa (1:10 in phosphate buffered distilled water, pH 6.8) for 30 min, washed in running water, and drained dry. All sample slides were observed for Leishmania parasites under a light microscope at 1000× magnification using an oil immersion lens.
Histology
Skin samples were biopsied from a nodule of the left cheek of the patient, subsequently fixed in 10% formaldehyde and then sent to the Department of Pathology, Faculty of Medicine, Chiang Mai University. After processing, paraffin-embedded tissue sections of 5 μm thickness were cut and stained with standard haematoxylin-eosin (H&E). All sample slides were observed for general features and Leishmania parasites under a light microscope at 400× (dry lens) and 1000× (oil immersion lens), respectively.
PCR and DNA sequencing
PCR amplification of the ribosomal spacer ITS1 was performed with LeF/LeR primers[12], the RPL23a gene with BN1/BN2 primers [33], the RNA PolII gene with S1/S2 and S3/S4 primers [6], and the HSP70 gene with HSP70sen/HSP70ant primers [15], as previously described. Control DNA was from L. infantum (MCAN/ES/98/LEM-935;JPC;M5), which was used as an outgroup in the phylogenetic analyses. Twelve new sequences were generated as indicated in Additional file 2: Table S1. Amplification was performed with proof-reading DNA polymerase (Qiagen HotStar HiFidelity Polymerase) and products directly sequenced using commercial services. Results were checked for quality using Chromas Lite 2.1.1 (http://technelysium.com.au/).
DNA sequence analysis
Initial alignments and analyses were performed using Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/). For phylogenetic analysis, alignment and tree building programmes in Molecular Evolutionary Genetics Analysis (MEGA) version 7 were used [34]. For ITS1 sequences the Kimura 2-parameter model (Invariant) gave the best-fitting model of sequence evolution and was used for tree construction using the maximum likelihood (ML) and neighbour-joining (NJ) methods. For the RPL23a sequences the Kimura 2-parameter model, for the large subunit of RNA polymerase II the Tamura-Nei (Gamma) model, and for the HSP70 the Hasegawa-Kishino-Yano (Gamma) model were used for tree construction using the ML and NJ methods. Bootstrapping was performed on all trees with 1000 replicates.
Additional files
Table S1. Accession numbers for sequences analysed in this study. New sequences are indicated by *. (DOCX 15 kb)
Acknowledgements
We thank Associate Professor Dr Nimit Morakote and Associate Professor Dr Atchariya Jitpakdi for technical help and Professor Dr Saovanee Leelayoova for providing us with genomic DNA of Leishmania PCM2 Trang strain.
Funding
This work was supported by the Diamond Research Grant, Faculty of Medicine, Chiang Mai University, Thailand and by the Faculty of Health and Medicine, Lancaster University, UK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The data supporting the conclusions of this article are included within the article and its additional files. The newly generated sequences were submitted to the GenBank/EBI/DDBJ under the accession numbers MG731227-MG731238. Leishmania orientalis type-material is available from the authors upon request. The type-material was deposited at the Department of Parasitology, Chiang Mai University, Thailand (accession LSCM4) and Division of Biomedical and Life Sciences, Lancaster University, UK (accession LV768).
Abbreviations
- BME
Basal Medium Eagle
- DNA
Deoxyribonucleic acid
- FBS
Foetal bovine serum
- H&E
Haematoxylin-eosin
- HIV
Human immunodeficiency virus
- HSP70
Heat-shock protein 70
- ITS1
Internal transcribed spacer 1
- MEGA
Molecular Evolutionary Genetics Analysis
- ML
Maximum likelihood
- NJ
Neighbour-joining
- PCR
Polymerase chain reaction
- RNA PolII
Large subunit of RNA Polymerase II
- RNA
Ribonucleic acid
- RPL23a
Ribosomal protein L23a intergenic sequence
Authors’ contributions
NJ and PAB conceived and designed the experiments. TD and KJ took care of the patient. NJ, WC, NI, SS and MDB performed the experiments. NJ, PSi, PSo and PAB analyzed the data. NJ, TD and PAB wrote the paper. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The patient described in this report was treated at Nan hospital, northern Thailand for a skin lesion. All biopsies and other clinical investigations on the patient were performed with consent as part of routine clinical investigations to determine the nature of the condition. No samples or procedures were undertaken for research purposes only. This report does not contain any information that could be used to compromise patient confidentiality.
Consent for publication
Consent to publish the details described in this report has been provided by the patient.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13071-018-2908-3) contains supplementary material, which is available to authorized users.
Contributor Information
Narissara Jariyapan, Email: njariyapan@gmail.com.
Teerada Daroontum, Email: mewteerada@gmail.com.
Krit Jaiwong, Email: kritjai1107@gmail.com.
Wetpisit Chanmol, Email: wetpisitchanmol@gmail.com.
Nuchpicha Intakhan, Email: nuchpichaintakhan@gmail.com.
Sriwatapron Sor-suwan, Email: sriwatapronsor9@gmail.com.
Padet Siriyasatien, Email: padetcu@gmail.com.
Pradya Somboon, Email: pradya.somboon@cmu.ac.th.
Michelle D. Bates, Email: m.bates@lancaster.ac.uk
Paul A. Bates, Email: p.bates@lancaster.ac.uk
References
- 1.Thisyakorn U, Jongwutiwes S, Vanichsetakul P, Lertsapcharoen P. Visceral leishmaniasis: the first indigenous case report in Thailand. Trans R Soc Trop Med Hyg. 1999;93:23–24. doi: 10.1016/S0035-9203(99)90166-9. [DOI] [PubMed] [Google Scholar]
- 2.Maharom P, Siripattanapipong S, Mungthin M, Naaglor T, Sukkawee R, Pudkorn R, et al. Visceral leishmaniasis caused by Leishmania infantum in Thailand. Southeast Asian J Trop Med Public Health. 2008;39:988–990. [PubMed] [Google Scholar]
- 3.Dedet JP, Roche B, Pratlong F, Cales-Quist D, Jouannelle J, Benichou JC, Huerre M. Diffuse cutaneous infection caused by a presumed monoxenous trypanosomatid in a patient infected with HIV. Trans R Soc Trop Med Hyg. 1995;89:644–646. doi: 10.1016/0035-9203(95)90427-1. [DOI] [PubMed] [Google Scholar]
- 4.Desbois N, Pratlong F, Quist D, Dedet JP. Leishmania (Leishmania) martiniquensis n. sp. (Kinetoplastida: Trypanosomatidae), description of the parasite responsible for cutaneous leishmaniasis in Martinique Island (French West Indies) Parasite. 2014;21:12. doi: 10.1051/parasite/2014011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leelayoova S, Siripattanapipong S, Hitakarun A, Kato H, Tan-ariya P, Siriyasatien P, et al. Multilocus characterization and phylogenetic analysis of Leishmania siamensis isolated from autochthonous visceral leishmaniasis cases, southern Thailand. BMC Microbiol. 2013;13:60. doi: 10.1186/1471-2180-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pothirat T, Tantiworawit A, Chaiwarith R, Jariyapan N, Wannasan A, Siriyasatien P, et al. First record of leishmaniasis in northern Thailand: case report, identification as Leishmania martiniquensis and phylogenetic position within the Leishmania enriettii complex. PLOS Negl Trop Dis. 2014;8:e3339. doi: 10.1371/journal.pntd.0003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bualert L, Charungkiattikul W, Thongsuksai P, Mungthin M, Siripattanapipong S, Khositnithikul R, et al. Autochthonous disseminated dermal and visceral leishmaniasis in an AIDS patient, southern Thailand, caused by Leishmania siamensis. Am J Trop Med Hyg. 2012;86:821–824. doi: 10.4269/ajtmh.2012.11-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwakye-Nuako G, Mosore MT, Duplessis C, Bates MD, Puplampu N, Mensah-Attipoe I, et al. First isolation of a new species of Leishmania responsible for human cutaneous leishmaniasis in Ghana and classification in the Leishmania enriettii complex. Int J Parasitol. 2015;45:679–684. doi: 10.1016/j.ijpara.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Supsrisunjai C, Kootiratrakarn T, Puangpet P, Bunnag T, Chaowalit P, Wessagowit V. Disseminated autochthonous dermal leishmaniasis caused by Leishmania siamensis (PCM2 Trang) in a patient from central Thailand infected with human immunodeficiency virus. Am J Trop Med Hyg. 2017;96:1160–1163. doi: 10.4269/ajtmh.16-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espinosa OA, Serrano MG, Camargo EP, Teixeira MM, Shaw JJ. An appraisal of the taxonomy and nomenclature of trypanosomatids presently classified as Leishmania and Endotrypanum. Parasitology. 2016;15:1–13. doi: 10.1017/S0031182016002092. [DOI] [PubMed] [Google Scholar]
- 11.Rogers ME, Chance ML, Bates PA. The role of promastigote secretory gel in the origin and transmission of the infective stage of Leishmania mexicana by the sand fly Lutzomyia longipalpis. Parasitology. 2002;124:495–508. doi: 10.1017/S0031182002001439. [DOI] [PubMed] [Google Scholar]
- 12.Spanakos G, Piperaki ET, Menounos PG, Tegos N, Flemetakis A, Vakalis NC. Detection and species identification of Old World Leishmania in clinical samples using a PCR-based method. Trans R Soc Trop Med Hyg. 2008;102:46–53. doi: 10.1016/j.trstmh.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Dougall AM, Alexander B, Holt DC, Harris T, Sultan AH, Bates PA, et al. Evidence incriminating midges (Diptera: Ceratopogonidae) as potential vectors of Leishmania in Australia. Int J Parasitol. 2011;41:571–579. doi: 10.1016/j.ijpara.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Croan DG, Morrison DA, Ellis JT. Evolution of the genus Leishmania revealed by comparison of DNA and RNA polymerase gene sequences. Mol Biochem Parasitol. 1997;89:149–159. doi: 10.1016/S0166-6851(97)00111-4. [DOI] [PubMed] [Google Scholar]
- 15.Garcia L, Kindt A, Bermudez H, Llanos-Cuentas A, De Doncker S, Arevalo J, et al. Culture-independent species typing of neotropical Leishmania for clinical validation of a PCR-based assay targeting heat shock protein 70 genes. J Clin Microbiol. 2004;42:2294–2297. doi: 10.1128/JCM.42.5.2294-2297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ICZN International Commission on Zoological Nomenclature: Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Bull Zool Nomencl. 2012;69:161–169. [Google Scholar]
- 17.Paranaiba LF, Pinheiro LJ, Macedo DH, Menezes-Neto A, Torrecilhas AC, Tafuri WL, Soares RP. An overview on Leishmania (Mundinia) enriettii: biology, immunopathology, LRV and extracellular vesicles during the host-parasite interaction. Parasitology. 2017;10:1–9. doi: 10.1017/S0031182017001810. [DOI] [PubMed] [Google Scholar]
- 18.Akhoundi M, Kuhls K, Cannet A, Votýpka J, Marty P, Delaunay P, Sereno D. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sand flies. PLoS Negl Trop Dis. 2016;10:e0004349. doi: 10.1371/journal.pntd.0004349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paranaiba LF, Pinheiro LJ, Torrecilhas AC, Macedo DH, Menezes-Neto A, Tafuri WL, Soares RP. Leishmania enriettii (Muniz & Medina, 1948): A highly diverse parasite is here to stay. PLoS Pathog. 2017;13:e1006303. doi: 10.1371/journal.ppat.1006303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cotton JA. The expanding world of human leishmaniasis. Trends Parasitol. 2017;33:341–344. doi: 10.1016/j.pt.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Muniz J, Medina H. Cutaneous leishmaniasis of the guinea pig, Leishmania enriettii n. sp. Hospital (Rio J) 1948;33:7–25. [PubMed] [Google Scholar]
- 22.Machado MI, Milder RV, Pacheco RS, Silva M, Braga RR, Lainson R. Naturally acquired infections with Leishmania enriettii Muniz and Medina 1948 in guinea-pigs from São Paulo. Brazil. Parasitology. 1994;109:135–138. doi: 10.1017/S0031182000076241. [DOI] [PubMed] [Google Scholar]
- 23.Dougall A, Shilton C, Low Choy J, Alexander B, Walton S. New reports of Australian cutaneous leishmaniasis in Northern Australian macropods. Epidemiol Infect. 2009;137:1516–1520. doi: 10.1017/S0950268809002313. [DOI] [PubMed] [Google Scholar]
- 24.Barratt J, Kaufer A, Peters B, Craig D, Lawrence A, Roberts T, et al. Isolation of novel trypanosomatid, Zelonia australiensis sp. nov. (Kinetoplastida: Trypanosomatidae) provides support for a Gondwanan origin of dixenous parasitism in the Leishmaniinae. PLoS Negl Trop Dis. 2017;11:e0005215. doi: 10.1371/journal.pntd.0005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harkins KM, Schwartz RS, Cartwright RA, Stone AC. Phylogenomic reconstruction supports supercontinent origins for Leishmania. Infect Genet Evol. 2016;38:101–109. doi: 10.1016/j.meegid.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 26.Luz E, Giovannoni M, Borba A. Infeccão de Lutzomyia monticola por Leishmania enriettii. An Fac Med Univ Fed Parana. 1967;9:121–128. [Google Scholar]
- 27.Šeblová V, Sádlová J, Vojtková B, Votýpka J, Carpenter S, Bates PA, Volf P. The biting midge Culicoides sonorensis (Diptera: Ceratopogonidae) is capable of developing late stage infections of Leishmania enriettii. PLoS Negl Trop Dis. 2015;9:e0004060. doi: 10.1371/journal.pntd.0004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dostálová A, Volf P. Leishmania development in sand flies: parasite-vector interactions overview. Parasit Vectors. 2012;5:276. doi: 10.1186/1756-3305-5-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanjanopas K, Siripattanapipong S, Ninsaeng U, Hitakarun A, Jitkaew S, Kaewtaphaya P, et al. Sergentomyia (Neophlebotomus) gemmea, a potential vector of Leishmania siamensis in southern Thailand. BMC Infect Dis. 2013;13:333. doi: 10.1186/1471-2334-13-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chusri S, Thammapalo S, Chusri S, Thammapalo S, Silpapojakul K, Siriyasatien P. Animal reservoirs and potential vectors of Leishmania siamensis in southern Thailand. Southeast Asian J Trop Med Public Health. 2014;45:13–19. [PubMed] [Google Scholar]
- 31.Ready P. Biology of phlebotomine sand flies as vectors of disease agents. Annu Rev Entomol. 2013;58:227–250. doi: 10.1146/annurev-ento-120811-153557. [DOI] [PubMed] [Google Scholar]
- 32.Manomat J, Leelayoova S, Bualert L, Tan-Ariya P, Siripattanapipong S, Mungthin M, et al. Prevalence and risk factors associated with Leishmania infection in Trang Province, southern Thailand. PLoS Negl Trop Dis. 2017;11:e0006095. doi: 10.1371/journal.pntd.0006095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kongkaew W, Siriarayaporn P, Leelayoova S, Supparatpinyo K, Areechokchai D, Duang-ngern P, et al. Autochthonous visceral leishmaniasis: a report of a second case in Thailand. Southeast Asian J Trop Med Public Health. 2007;38:8–12. [PubMed] [Google Scholar]
- 36.Sukmee T, Siripattanapipong S, Mungthin M, Worapong J, Rangsin R, Samung Y, et al. A suspected new species of Leishmania, the causative agent of visceral leishmaniasis in a Thai patient. Int J Parasitol. 2008;38:617–622. doi: 10.1016/j.ijpara.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Suankratay C, Suwanpimolkul G, Wilde H, Siriyasatien P. Autochthonous visceral leishmaniasis in a human immunodeficiency virus (HIV)-infected patient: the first in Thailand and review of the literature. Am J Trop Med Hyg. 2010;82:4–8. doi: 10.4269/ajtmh.2010.09-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osatakul S, Mungthin M, Siripattanapipong S, Hitakarun A, Kositnitikul R, Naaglor T, Leelayoova S. Recurrences of visceral leishmaniasis caused by Leishmania siamensis after treatment with amphotericin B in a seronegative child. Am J Trop Med Hyg. 2014;90:40–42. doi: 10.4269/ajtmh.13-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chusri S, Hortiwakul T, Silpapojakul K, Siriyasatien P. Consecutive cutaneous and visceral leishmaniasis manifestations involving a novel Leishmania species in two HIV patients in Thailand. Am J Trop Med Hyg. 2012;87:76–80. doi: 10.4269/ajtmh.2012.11-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kattipathanapong P, Akaraphanth R, Krudsood S, Riganti M, Viriyavejakul P. The first reported case of autochthonous cutaneous leishmaniasis in Thailand. Southeast Asian J Trop Med Public Health. 2012;43:17–20. [PubMed] [Google Scholar]
- 41.Phumee A, Kraivichian K, Chusri S, Noppakun N, Vibhagool A, Sanprasert V, et al. Detection of Leishmania siamensis DNA in saliva by polymerase chain reaction. Am J Trop Med Hyg. 2013;89:899–905. doi: 10.4269/ajtmh.12-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noppakun N, Kraivichian K, Siriyasatien P. Disseminated dermal leishmaniasis caused by Leishmania siamensis in a systemic steroid therapy patient. Am J Trop Med Hyg. 2014;91:869–870. doi: 10.4269/ajtmh.13-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiewchanvit S, Tovanabutra N, Jariyapan N, Bates MD, Mahanupab P, Chuamanochan M, et al. Chronic generalized fibrotic skin lesions from disseminated leishmaniasis caused by Leishmania martiniquensis in two HIV-infected patients from northern Thailand. British J Dermatol. 2015;173:663–670. doi: 10.1111/bjd.13812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Accession numbers for sequences analysed in this study. New sequences are indicated by *. (DOCX 15 kb)
Data Availability Statement
The data supporting the conclusions of this article are included within the article and its additional files. The newly generated sequences were submitted to the GenBank/EBI/DDBJ under the accession numbers MG731227-MG731238. Leishmania orientalis type-material is available from the authors upon request. The type-material was deposited at the Department of Parasitology, Chiang Mai University, Thailand (accession LSCM4) and Division of Biomedical and Life Sciences, Lancaster University, UK (accession LV768).