Abstract
Recently, the risk of viral infection has dramatically increased owing to changes in human ecology such as global warming and an increased geographical movement of people and goods. Howev-er, the efficacy of vaccines and remedies for infectious diseases is limited by the high mutation rates of viruses, especially, RNA viruses. Here, we comprehensively review the effectiveness of several probiot-ics and paraprobiotics (sterilized probiotics) for the prevention or treatment of virally-induced infectious diseases. We discuss the unique roles of these agents in modulating the cross-talk between commensal bacteria and the mucosal immune system. In addition, we provide an overview of the unique mechanism by which viruses are eliminated through the stimulation of type 1 interferon production by probiotics and paraprobiotics via the activation of dendritic cells. Although further detailed research is necessary in the future, probiotics and/or paraprobiotics are expected to be among the rational adjunctive options for the treatment of various viral diseases.
Keywords: Probiotics, paraprobiotics, virus infection, interferon, plasmacytoid dendritic cell, vaccines
1. Inroduction
Various strategies, such as those using vaccines and antibiotics, have been exploited for the prevention and treatment of infectious diseases, but infection control has not yet been achieved at a sufficient level. In addition to avian influenza [1], severe acute respiratory syndrome [2], and Ebola hemorrhagic fever [3], many problematic diseases of tropical origin remain poorly controlled, such as dengue fever and Zika virus infection [4].
As climate change including global warming and the increased geographical movement of people and goods have emerged, the numbers of pathogenic virus species and affected areas have increased [5]. Therefore, the risk of viral infection has now become a critical issue. A representative pathogenic virus, influenza virus, sometimes undergoes a process of discontinuous mutation. As a result, the efficacy of vaccines against influenza virus may become disrupted, and this phenomenon has sometimes caused pandemics [6]. In Japan, the first case of Zika virus infection was reported in 2016 [7]. Some cases infected with dengue virus were reported in 2014 after the virus had been absent for 70 years previously [8]. In addition, influenza and Norovirus infections often occur seasonally.
With the progress of recent immunological research, the innate immune response and the subsequently activated acquired immune response for the recognition and elimination of viruses have been unveiled [9]. It has been reported that the process of viral elimination largely depends on the induction of type 1 interferons (IFNs) [10], and that the regulation of inflammatory cytokines is mediated via pattern recognition receptors such as Toll-like receptors [9, 11] and retinoic-acid-inducible gene I [12]. Studies have begun to elucidate the immunostimulatory effects of lactic acid bacteria and have reported their capacity to contribute to the prevention of viral infections including influenza [13] as well as the treatment of Helicobacter pylori infection [14]. As discussed in the following sections, type 1 IFNs are considered to be pivotal in mediating the protective effect of lactic acid bacteria against these infections.
In this review, we first summarize the recent preventive and therapeutic strategies against infectious diseases with special emphasis on the viral infection and then outline the possible applications of several probiotics and paraprobiotics as prophylactics or therapies against (viral) infectious diseases on the basis of published clinical trial data. Since it is very difficult to cite all studies regarding the effects of paraprobiotics or probiotics on virus infectious disease, we showed representative studies below with two criteria; 1) Single probiotics or paraprobiotics study in human clinical trial (excluded mixture of strains and animal studies), 2) Evaluation of efficacy on (viral) infectious disease.
Finally, we introduce the unique topic of immune defense mechanisms against viral infections that are induced by probiotics and paraprobiotics.
2. Preventive and Therapeutic Strategies Against Viral infections
Infectious diseases, especially nosocomial infections (i.e. influenza, pneumonia, methicillin-resistant Staphylococcus aureus, Norovirus, severe acute respiratory syndrome corona virus, etc.), have periodically caused widespread outbreaks and are considered to represent serious threats to public health [15, 16]. In this review, we outline strategies for the prevention and treatment of infections of the upper respiratory and gastrointestinal tracts as representative infectious diseases. It is well known that influenza virus, rhinovirus, and respiratory syncytial virus play major roles in Upper Respiratory Tract Infections (URTIs), while norovirus and rotavirus are also important causes of gastrointestinal diseases.
It is generally acknowledged that the main prophylactic measures against these infectious diseases are vaccinations and everyday hygienic behaviors such as gargling and hand-washing. Nagatake et al. outlined that gargling with a povidone-iodine solution was effective to reduce the incidence of episodes of acute respiratory infections, since colonized bacteria were destroyed by gargling [17]. Heijne et al. reported that enhanced hygienic measures including proper hand cleaning using soap and disposable paper towels effectively limited the transmission of norovirus during an outbreak [18].
Although vaccines are promising prophylactics against influenza infection, their efficacy is limited by the frequent and fast mutation of RNA viruses including the influenza virus [19]. Popular influenza treatment regimens include neuraminidase inhibitors and M2 ion channel protein inhibitors, which are both actively prescribed in clinical settings [20]. Inhibitors of hemagglutinin, sialidase, nucleocapsid protein, or RNA-dependent RNA polymerase are also currently used [19, 21]. However, the development of acquired resistance against current anti-influenza drugs and emerging mutations of the influenza virus continue to present major challenges for the effective treatment of influenza.
Norovirus and rotavirus are representative gastrointestinal pathogens that are highly infectious and cause severe diarrhea [22]. In the present situation, without an effective vaccine or medicine, the prevention of viral transmission must rely mainly on basic measures including quarantine and thorough hand washing after physical contact. In particular, rotavirus is resistant to chlorine and alcohol, although infection can be prevented by wiping contaminated sites with a 79% ethanol solution containing 0.1% o-phenylphenol [23]. Vaccination against neonates is also being conducted and has shown a good efficacy [24]. Norovirus is also highly contagious and resistant to alcohol. For contaminated sites, it is recommended to wipe with bleach containing more than 500 ppm of chlorine [23]. Vaccines for norovirus are currently under development. There are currently no effective treatments for either of these viral infections. Thus, the treatment that is generally recommended is to prevent the dehydration caused by diarrhea and vomiting by maintaining a sufficient fluid intake and wait for the virus to be eliminated from the gastrointestinal tract.
3. Prophylaxis and Medical Treatment of Infectious Diseases by Probiotics and Paraprobiotics
With the progress of research on the relationship between the microbiota and diseases in recent years, commensal intestinal bacteria have been investigated for their ability to modulate the host immune system, not only in healthy individuals but also in those who are suffering from a wide range of diseases [25]. It has been revealed that commensal bacteria also regulate regulatory T cells, type 3 innate lymphoid cells, and T helper 17 cells through the recognition of the bacteria themselves or their metabolites/products by the immune cells and greatly affect mucosal immunity [25-29].
Probiotics act on both the innate and acquired immune systems and have the potency to decrease the severity of infections in the gastrointestinal [30] and upper respiratory tracts [13, 31]. Probiotics are defined as live microorganisms that have health benefits for the host [32], and they are generally consumed as a component of fermented foods such as yoghurt, cheese, and pickles or as supplements. Recently, “ghost probiotics” or paraprobiotics were reported to retain their immunomodulatory potency beyond their viability [33]. Probiotics contain immunostimulatory substances such as lipoteichoic acid, peptidoglycan, and nucleic acid, which are Toll-like receptor (TLR) ligands, and muramyl dipeptide, which is a Nod-like receptor ligand [34]. In the following sections, we focus on the effects of probiotics including paraprobiotics on viral infections. Particularly, we discuss representative probiotics that have shown unique preventive or therapeutic potencies against viral diseases in clinical studies (Table 1).
Table 1.
Clinical efficacy of various major lactic acid bacteria for infectious diseases.
| Strain | Efficacy | Refs. | ||
|---|---|---|---|---|
| Target Disease (Virus) | Subjects | Outcome | ||
| Lactobacillus casei (Yakult) | Upper respiratory tract infection Epstein–Barr virus (EBV) Cytomegalovirus (CMV) |
Healthy athletes | Reduced plasma CMV and EBV antibody titers | [34] |
| Upper respiratory tract infection | Elderly people | No significant difference in the incidence of respiratory symptoms and influenza-vaccination immune response | [35] | |
| Norovirus gastroenteritis | Elderly people | No significant difference in the incidence of Norovirus infection in elderly people | [36] | |
| Lactobacillus rhamnosus GG | Experimentally induced Rhinovirus infection | Healthy volunteers with intranasal inoculation of Rhinovirus (type 39) | Decrease in the occurrence and severity of cold symptoms and number of subjects with Rhinovirus infection, but not significant | [37] |
| Acute gastroenteritis (positive for Rotavirus or Cryptosporidium) | 6M to 5Y children with acute gastroenteritis positive for Rotavirus or Cryptosporidium | Significant decrease in repeated episodes of Rotavirus diarrhea. Improvement in intestinal function in children with rotavirus and cryptosporidial gastroenteritis | [38] | |
| Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 | Common cold symptoms | Elderly people (meta-analysis of two independent cohorts) | Significant increase of natural killer cell activity and reduced risk of catching the common cold | [39] |
| Lactobacillus paracasei ssp. paracasei, L. casei 431 | Response to influenza vaccination | Healthy adults with influenza vaccination | Significant reduction of the duration of upper respiratory symptoms No significant difference in immune responses to influenza vaccination and incidence or severity. |
[42] |
| Response to influenza vaccination | Healthy adults with influenza vaccination | Significant increases of vaccine-specific IgG, IgG1, and IgG3 in plasma as well as vaccine-specific secretory IgA in saliva in both probiotic-treated groups | [43] | |
| Lactobacillus paracasei MCC1849 (Morinaga) | Antibody response against vaccination | Eldery people with influenza vaccination | No significant effect of non-viable L. paracasei MCC1849 | [44] |
| Lactobacillus casei (DN-114 001) | Incidence of acute diarrhea | Children aged 6–24 months | Significant reduction in the incidence and frequency of diarrhea. | [45] |
| Incidence of common infectious diseases | Children aged 3–6 years | Significantly lower incidence rate of common infectious diseases in DN-114 group | [46] | |
| Lactobacillus plantarum L-137 | Upper respiratory tract infection | Healthy adults with high psycological stress | Significant decrease in the incidence of upper respiratory tract infections | [49] |
| Enterococcus faecalis FK-23 | Hepatitis C virus | Adult with anti-HCV antidodies positive | Significant decrease of ananie aminotransferase No significant change in viral load | [51] |
| Saccharomyces boulardii | Acute rotavirus diarrhea | Children (1-23 months) hospitalized for acute diarrhea by rotavirus | Significant decrease in duration period of diarrhea and fever | [52] |
| Bifidobacterium animalis (Bb12) | Intestinal antibody responses to polio- and rota-virus in infants | Healthy 6 week full-term infants (prospective study) | Bb12 significantly increased fecal anti-poliovirus specific IgA, and increased anti-rotavirus specific IgA. | [53] |
| Strain | Efficacy | Refs. | ||
| Target Disease (Virus) | Subjects | Outcome | ||
| Bifidobacterium lactis B94 | Acute rotavirus diarrhea | Children (5 months to 5 years) hospitalized for diarrhea by rotavirus | Sgnificantly decrease in duration period of diarrhea | [54] |
| Lactococcus lactis JCM5805 (L. lactis plasma) | pDCs acitivity among PBMCs and symptoms of common cold | Healthy adults | L. lactis JCM 5805 activated pDCs among PBMCs and significantly reduced the risk of morbidity from the common cold | [55] |
| Influenza-like illness and immunological response to influenza virus | Healthy adults | Significant decrease in the cumulative incidence days of “cough” and “feverishness”. Significant increase in IFN-α-inducible antiviral factor, interferon-stimulated gene 15 | [56] | |
| Influenza-like illness and immunological response to influenza virus | Healthy adults | Significant decrease in the cumulative incidence days of “sore throat” and “cough”. Significant increase in IFN-α mRNA in PBMCs |
[57] | |
| Anti-viral immune response and physical condition | Healthy adults | Significantly increased pDC activation and increased mRNA expression of ISG15 Significant decrease in the cumulative incidence days of cold-like symptoms |
[58] | |
| Influenza Infection | School children | Significant decreases in both the incidence rate and the cumulative incidence rate of influenza | [59] | |
| Anti-viral immune response to influenza virus | Healthy adults | Significant increase in secretary IgA in saliva Significant prevention of decrease in phagocytic activity of neutrophil during common cold season |
[60] | |
3.1. Lactobacillus casei Shirota (LcS)
LcS reduced plasma cytomegalovirus and Epstein–Barr virus antibody titers in highly physically active people (university athletes) [35]. However, there was no significant difference in the incidence of norovirus-induced gastroenteritis between the LcS and control groups in long-stay elderly people at a health service facility [36]. Although the effectiveness of LcS remains controversial, a potential mechanism was reported wherein LcS was found to modulate the activity of natural killer (NK) cells, which are one of the first-line defense mechanisms against viral infection [37].
3.2. Lactobacillus rhamnosus (LGG)
The preventive effects of LGG on experimental rhinovirus infections in healthy volunteers were evaluated. After the ingestion of LGG for 6 months, subjects were intranasally inoculated with rhinovirus. The infection rate, occurrence, and severity of cold symptoms were evaluated. The frequency and severity of cold symptoms and the number of subjects with rhinovirus infection in the LGG group were lower than those of the control group, although the difference between the groups did not reach significance [38]. When LGG was administered for 4 weeks to children with gastroenteritis who were positive for either rotavirus or Cryptosporidium species in stool, a significant increase in serum immunoglobulin (Ig)G levels post-intervention was observed in children with rotavirus-induced diarrhea who received LGG. Among the children with cryptosporidial diarrhea, those receiving LGG showed a significant improvement in intestinal permeability [39]. The mechanisms by which probiotics exert immunomodulatory effects are not completely understood. However, LGG was demonstrated to modulate the innate and adaptive immune responses, particularly those against gastrointestinal pathogens, resulting in increased levels of serum IgG and secretory IgA targeting enteric pathogens including rotavirus [39].
3.3. Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 (R-1)
The study demonstrated that the consumption of yoghurt fermented with R-1 augmented NK cell activity and reduced the risk of catching the common cold in elderly individuals [40]. Other studies showed that R-1 and its secreted polysaccharides improved immune system functions accompanied by the activation of NK cells. Thus, R-1 or its products might contribute to the prevention of respiratory infections caused by respiratory or influenza viruses [41, 42].
3.4. Lactobacillus paracasei ssp. paracasei (L. casei 431)
L. casei 431 was reported to have the potency to shorten the duration of upper respiratory symptoms, although it showed no effect on the immune response to influenza vaccination in healthy adults [43]. In another study, L. casei 431 was demonstrated to modulate the immune system using a vaccination model in healthy subjects. Increase from baseline in the titers of vaccine-specific IgG, IgG1, and IgG3 in plasma as well as that of vaccine-specific secretory IgA in saliva were significantly greater in both probiotic groups, as compared with the control group [44]. Although more detailed studies are needed to explore the comprehensive mechanisms underlying the immunomodulatory and anti-infectious effects of L. casei 431, it is considered that this probiotic stimulates the innate viral defense mechanisms and reduces inflammation in the host.
3.5. Lactobacillus paracasei MCC1849 (MCC1849)
Non-viable MCC1849 did not show significant effects on immune parameters involved in the response to influenza vaccination in elderly people with immunosenescence [45].
3.6. Lactobacillus casei strain DN-114 001 (DN-114)
Pedone et al. found that the administration of DN-114 reduced the incidence of acute diarrhea in healthy children aged 6–24 months. The incidence and frequency of diarrhea were significantly reduced by supplementation with DN-114 as compared with the control group [46]. Merenstein et al. reported that DN-114 001 could reduce the incidence of common infectious diseases including diarrhea in children aged 3–6 years who were attending daycare or school, although the detailed mechanism is still unclear [47].
3.7. Lactobacillus plantarum L-137 (HK L-137)
Previously the immunomodulatory effects of heat-killed HK L-137 were evaluated and the results showed that HK L-137 augmented the innate and acquired immune responses in mice and human subjects, especially in view of the production of type 1 IFNs and interleukin (IL)-12 [48, 49]. Hirose et al. assessed the effects of HK L-137 intake for 12 weeks on URTI symptoms and immune functions in human subjects who were experiencing high levels of psychological stress. The incidence of URTIs was significantly lower in the HK L-137-treated group than in the control group. In addition to the incidence of URTIs, the severity and duration of medication showed significant negative correlations with the duration of HK L-137 intake. The percentage change from baseline of the concanavalin A-induced proliferation of peripheral blood mononuclear cells (PBMCs) was significantly greater in the HK L-137-treated group than in the control group, although serum IFN-β production was not significantly different between these groups [50].
3.8. Enterococcus faecalis FK-23
Oo et al. reported that the paraprobiotic FK-23 (Enterococcus faecalis strain FK-23) significantly reduced alanine aminotransferase (ALT) levels in adult HCV-positive subjects but did not decrease viral load. Although detailed mechanism was still unclear, they suggested that FK-23 might change the microbiota in HCV patients then it played a role in decrease in ALT level [51].
3.9. Saccharomyces boulardii
Oral administration of Saccharomyces boulardii and rehydration significantly shortened duration of diarrhea in acute rotavirus gastroenteritis children in Bolivia, compared with control rehydration alone. Detailed mechanism was not available [52].
3.10. Bifidobacterium animalis Bb12
To investigate the effect of infant starter formula containing the probiotic Bifidobacterium animalis subspecies lactis (Bb12) on intestinal immunity and inflammation. Six-week-old healthy, full-term infants were enrolled in a prospective study (Control formula and Control + Bb12 (106CFU / g / head)) for 6 weeks. Anti-poliovirus-specific IgA and anti-rotavirus-specific IgA were assessed.
Bb12 significantly increased anti-poliovirus-specific IgA, but not anti-rotavirus-specific IgA, although it showed the tendency of increase (P =0.056) [53].
3.11. Bifidobacterium lactis B94
In Turkey, Erdoğan et al. reported that Bifidobacterium lactis B94 with oral rehydration treatment significantly shortened diarrheal period in acute rotavirus gastroenteritis children (5 months to 5 years old), compared with control oral rehydration alone [54].
3.12. Lactococcus lactis subsp. Lactis JCM 5805 (L. lactis JCM 5805)
Plasmacytoid dendritic cells (pDCs) play a crucial role in antiviral immunity through the production of large amounts of IFNs. L. lactis JCM5805 was found to activate human pDCs among PBMCs from healthy volunteers, especially in a subgroup of volunteers who originally showed a low pDCs activity, and it also significantly attenuated cumulative common cold symptoms [55].
The prophylactic effects of L. lactis JCM5805 on influenza-like illness in healthy volunteers during the winter season were reported based on the results of a double-blinded trial [56, 57]. The administration of L. lactis JCM5805 resulted in a significant decrease in the cumulative number of days of incidence of “cough” and “feverishness”, which were defined as the major symptoms of an influenza-like illness, as compared with the control group. Furthermore, when PBMCs from the volunteers treated with L. lactis JCM 5805 were cultured with inactivated human influenza virus A/H1N1 (A/PR/8/34), the expression of IFN-α showed a higher tendency and that of interferon-stimulated gene 15 (ISG15) was significantly elevated as compared with the control group. These results suggest that the intake of L. lactis JCM5805 can prevent the pathogenesis of an influenza-like illness via the enhancement of an IFN-α-mediated response to the influenza virus [56]. In two separate studies, the expression of IFN-α and the mRNA level of ISG15 in PBMCs were significantly higher in groups treated with L. lactis JCM 5805 as compared to the corresponding control groups [57, 58]. In addition, Sakata et al. reported that the intake of yogurt containing L. lactis JCM 5805 significantly reduced the cumulative incidence rate of influenza among schoolchildren in a rural area of Japan [59]. Finally Fujii et al. showed the effects of oral L. lactis JCM 5805 on systemic and mucosal immunological parameters of healthy volounteers in winter (common cold season in Japan). After 4 continuous weeks administration, L. lactis JCM 5805 significantly reduced cumulative days of symptom of sore throat, compared with control. In L. lactis JCM 5805 group, change in secretory IgA levels in saliva and phagocytic activity of neutrophil were significantly lower than those of initial level, but not in the placebo group, although they could not reach statistical differences between groups at endpoint [60]. The proposed mechanism of action for L. lactis JCM 5805 is discussed in detail in the next section.
4. Mechanism of L. lactis JCM 5805-mediated Inhibition of Viral Infections
4.1. Role of pDCs and Type 1 IFNs in Viral Infection
As dendritic cells (DCs) are well known to be a pivotal immune cell subset that links the innate and acquired immune responses by recognizing pathogenic and endogenous inflammatory signals [61]. Dendritic cells are subdivided into pDCs, myeloid DCs (mDCs), and CD8+ dendritic cells. Among them, pDCs are a rare and critical subset that acts as a “control tower” during viral infections [62, 63].
To detect the presence of bacteria and viruses, pDCs utilize certain TLRs. Especially, they use TLR9 for the recognition of microbial nucleic acids via detecting unmethylated CpG motifs of DNA and TLR7 for the recognition of microbial RNA or synthetic guanosine analogs. The activation of pDCs by TLR ligand binding leads to the production of type 1 IFNs [64]. The type 1 IFN family includes IFN-α and IFN-β, which serve as components of the first-line defense against infection by blocking viral replication [65]. The induction of type 1 IFNs is mostly associated with viral infections and it is well known that pathogenic bacteria stimulate IFN-α production [66]. However, nonpathogenic bacteria including probiotics used in food preparation have been less intensively studied regarding their ability to stimulate DC-mediated IFNs induction [67]. As described separately in detail, we previously screened various lactic acid bacteria for their ability to stimulate IFN-α production by pDCs and found that L. lactis JCM 5805 was the most potent stimulator of type 1 IFN production [67].
4.2. Direct Activation of pDC by L. Lactis JCM 5805
The stimulatory effects of L. lactis JCM 5805 on type 1 IFNs production by pDCs and mDCs were evaluated using DCs derived from Flt-3L-stimulated murine bone marrow obtained from several types of TLR-knockout mice. As a result of this direct activation of DCs by L. lactis JCM 5805, the major type 1 and type 3 IFNs (i.e., IFN-α, -β, and -λ) were found to be induced efficiently. However, IFN-α production was completely abolished in dendritic cells obtained from TLR9 or MyD88 knockout mice. Thus, these data strongly suggested that L. lactis JCM 5805 stimulated IFN-α production via TLR9/MyD88 signaling. Furthermore, we examined whether IFN-α production was induced by CpG DNA, which is a known TLR9 agonist, or DNA extracted from L. lactis JCM 5805. Both CpG DNA and the DNA extracted from L. lactis JCM 5805 strongly induced IFN production. In addition, L. lactis JCM 5805 was observed to be specifically taken up by pDC, suggesting that its phagocytosis played an important role in activating pDCs and consequently inducing the production of IFNs [67].
IFN-α production by pDCs was synergistically elevated by L. lactis JCM 5805 treatment when they were co-cultured with mDCs. Therefore, cross-talk or direct contact between mDCs and pDCs was considered to be necessary for the effective induction of IFN-α production by L. lactis JCM 5805. In addition, L. lactis JCM 5805 stimulated the expression of immunoregulatory receptors such as ICOS-L and PD-L1 on pDCs and accordingly reinforced the induction of CD4+CD25+FoxP3+ regulatory T cells [67].
4.3. The Mechanism of Action of L. Lactis JCM 5805 on the Immune System in Infectious Disease Models
When a lethal dose of parainfluenza virus (mPIV1) was inoculated intranasally to mice fed with L. lactis JCM5805, the survival rate was significantly higher than that of the control group (69% survival in L. lactis JCM 5805 and 0% survival in the control group on day 15 after inoculation). In the intestinal pDCs from the mice treated with L. lactis JCM 5805, type 1 IFN expression was significantly elevated, while a remarkable preventive effect against the infiltration of neutrophils into the lung tissue was observed. A significant increase in the expression of genes with antiviral activities including IFN-inducible mRNAs such as Isg15, Oasl2 (2'-5' oligoadenylate synthetase-like 2), and Rsad2 (radical S-adenosyl methionine domain containing 2) was observed upon treatment with L. lactis JCM 5805. Although lung is distantly located from the contact site (intestinal Peyer’s patches) and L. lactis JCM 5805 cannot stimulate local pDCs in the lung directly, oral L. lactis JCM 5805 treatment resulted in a strong resistance against parainfluenza virus infection in vivo [68].
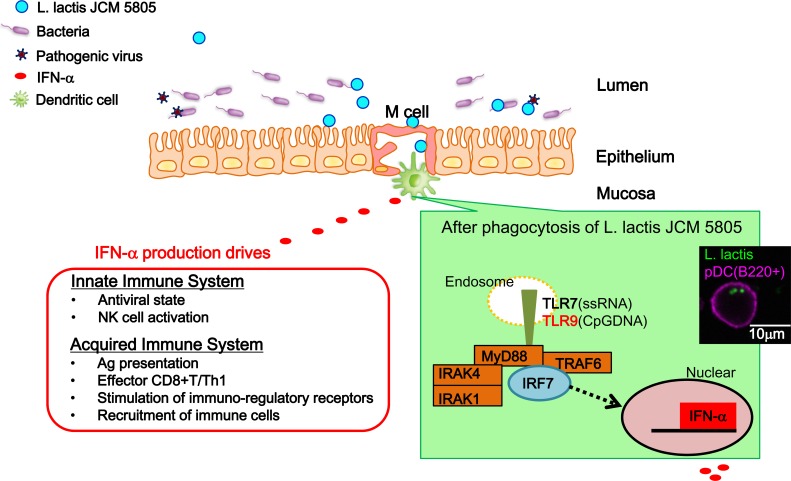
As is well known, IFN-α plays an important role in the antiviral immune response by inducing the cytotoxic activity of NK cells, which contributes to the host defense against viral infections [69, 70]. Indeed, Suzuki et al. reported that L. lactis JCM5805 activated NK cells both in vitro and in vivo [71]. Furthermore, the effect of L. lactis JCM5805 on NK cells was dependent upon the activity of dendritic cells [71]. Among a number of activation factors for NK cells including IFN-α, IL-2, IL-15, and IL-18, IFN-α is regarded as one of the most efficient NK cell activation factors [72]. In addition, it was reported that IFN-α production by virus-stimulated pDCs markedly increased the cytotoxic activity of NK cells [73] (Fig. 1).
Fig. (1).
Possible mechanism for the antiviral effects of L. lactis JCM 5805 via activation of plasmacytoid dendritic cells (pDCs).
Although we discussed the direct activation of pDCs by L. lactis JCM 5805 as the most plausible mechanism for the inhibition of viral infection, other underlying mechanisms have not been ruled out. Indeed, Salminen et al. reported that specific probiotic bacteria could bind and inactivate rotaviruses [74]. Besides this direct interaction with viruses, it is also possible that L. lactis JCM 5805 competes for viral receptors on the surface of target cells, produces antimicrobial and potentially antiviral substances, and stimulates host-cell immune defense systems. Thus, in the future, it is necessary to test whether L. lactis JCM 5805 can also directly bind and inactivate viruses, since the resulting data could provide a basis for novel approaches to inactivate viruses and reduce the risk of mucosa-associated viral infections.
To detect the presence of bacteria and viruses, pDCs utilize certain Toll-like receptor (TLR) families. Especially, they use TLR9 for the recognition of microbial nucleic acids by detecting unmethylated CpG motifs of DNA and TLR7 for the recognition of microbial RNA or synthetic guanosine analogs. The activation of pDCs by ligand binding to TLRs leads to the production of type 1 IFNs. L. lactis JCM 5805 was specifically taken up by pDCs and its DNA extracts strongly induced IFN production. These observations suggest that the phagocytosis of L. lactis JCM 5805 by pDCs plays an important role in activating pDCs and stimulating IFN production via TLR9/MyD88 signaling.
IFN-α plays an important role in mediating the antiviral immune response by inducing the cytotoxic activity of natural killer cells, which contributes to the host defense against viral infection.
Microscopic observations of human pDCs stimulated by L. lactis JCM5805 are also shown (Inset). The pDCs stained with anti-B220 (purple) are in the process of phagocytosing fluorescein isothiocyanate-stained L. lactis JCM5805 (green) [55]. (The color version of the figure is available in the electronic copy of the article).
Conclusion
We described the efficacy of probiotics and paraprobiotics for the prevention or treatment of infectious diseases, which have been increasing in incidence in recent years. Although the benefits of vaccines and antiviral drugs for the prevention and treatment of infectious diseases are obvious, their effectiveness is hindered by the large number of viral species and their subtypes as well as the high mutation rate of viruses. Since viruses evolve constantly and produce a serologically diverse viral population, it is challenging to establish an effective means of protecting humans from viral infections. There have been several clinical reports regarding the use of probiotics or paraprobiotics for the prophylaxis or treatment of infectious diseases. Here, we reviewed the literature regarding several probiotic or paraprobiotic agents based on the papers which described single paraprobiotics / probiotics in clinical trial to avoid the crosstalk or mutual interference between probiotics. Such agents are considered to be safe, affordable and easy to consume because of their long history of use in foods.
The state of knowledge regarding the immunomodulatory effects of probiotics has recently advanced and various studies have especially focused on the interactions between commensal bacteria and the mucosal immune system. Furthermore, the role of type 1 IFNs in the elimination of pathogenic viruses, which involves the concerted activities of the innate and acquired immune systems, has been widely studied.
In this review, we discussed the anti-influenza activity of L. lactis JCM 5805, which has the potency to directly stimulate pDCs via TLR9 and thereby promote viral control. Although further detailed research is necessary, probiotics and/or paraprobiotics are expected to be among the rational adjunctive options for the treatment and prophylaxis of viral infections.
Consent for Publication
Not applicable.
Acknowledgements
The authors deeply acknowledged Dr. Daisuke Fujiwara, Dr. Yuji Morita, Mr. Masaya Kanayama, and Mr. Ryohei Tsuji (Kirin company) for their constructive discussion.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Alan H., Ian B., Nancy C., et al. Improving the selection and development of influenza vaccine viruses - Report of a WHO informal consultation on improving influenza vaccine virus selection, Hong Kong SAR, China, 18-20 November 2015. Vaccine. 2017;35:1104–1109. doi: 10.1016/j.vaccine.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu S., Chan T.C., Chu Y.T., et al. Comparative Epidemiology of Human Infections with Middle East Respiratory Syndrome and Severe Acute Respiratory Syndrome Coronaviruses among Healthcare Personnel. PLoS One. 2016;11:e0149988. doi: 10.1371/journal.pone.0149988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El Sayed S.M., Abdelrahman A.A., Ozbak H.A., et al. Updates in diagnosis and management of Ebola hemorrhagic fever. J. Res. Med. Sci. 2016;21:84. doi: 10.4103/1735-1995.192500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakkas H., Economou V., Papadopoulou C. Zika virus infection: Past and present of another emerging vector-borne disease. J. Vector Borne Dis. 2016;53:305–311. [PubMed] [Google Scholar]
- 5.Franchini M., Mannucci P.M. Impact on human health of climate changes. Eur. J. Intern. Med. 2015;26:1–5. doi: 10.1016/j.ejim.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Vasin A.V., Temkina O.A., Egorov V.V., et al. Molecular mechanisms enhancing the proteome of influenza A viruses: an overview of recently discovered proteins. Virus Res. 2014;185:53–63. doi: 10.1016/j.virusres.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Taira M., Ogawa T., Nishijima H., et al. The first isolation of Zika virus from a Japanese patient who returned to Japan from Fiji in 2016. Jpn. J. Infect. Dis. 2017 doi: 10.7883/yoken.JJID.2017.042. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama E., Kotaki A., Tajima S., et al. Two different dengue virus strains in the Japanese epidemics of 2014. Virus Genes. 2016;52:722–726. doi: 10.1007/s11262-016-1356-4. [DOI] [PubMed] [Google Scholar]
- 9.Kawai T., Akira S. Innate immune recognition of viral infection. Nat. Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 10.Jung A., Kato H., Kumagai Y., et al. Lymphocytoid choriomeningitis virus activates plasmacytoid dendritic cells and induces a cytotoxic T-cell response via MyD88. J. Virol. 2008;82:196–206. doi: 10.1128/JVI.01640-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oviedo-Boyso J., Bravo-Patino A., Baizabal-Aguirre V.M. Collaborative action of Toll-like and NOD-like receptors as modulators of the inflammatory response to pathogenic bacteria. Mediators Inflamm. 2014;2014:432785. doi: 10.1155/2014/432785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meylan E., Tschopp J., Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 13.Lehtoranta L., Pitkaranta A., Korpela R. Probiotics in respiratory virus infections. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:1289–1302. doi: 10.1007/s10096-014-2086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homan M., Orel R. Are probiotics useful in Helicobacter pylori eradication? World J. Gastroenterol. 2015;21:10644–10653. doi: 10.3748/wjg.v21.i37.10644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Memoli M.J., Morens D.M., Taubenberger J.K. Pandemic and seasonal influenza: therapeutic challenges. Drug Discov. Today. 2008;13:590–595. doi: 10.1016/j.drudis.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindsay L., Wolter J., De Coster I., Van Damme P., Verstraeten T. A decade of norovirus disease risk among older adults in upper-middle and high income countries: a systematic review. BMC Infect. Dis. 2015;15:425. doi: 10.1186/s12879-015-1168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagatake T., Ahmed K., Oishi K. Prevention of respiratory infections by povidone-iodine gargle. Dermatology. 2002;204(Suppl. 1):32–36. doi: 10.1159/000057722. [DOI] [PubMed] [Google Scholar]
- 18.Heijne J.C., Teunis P., Morroy G., et al. Enhanced hygiene measures and norovirus transmission during an outbreak. Emerg. Infect. Dis. 2009;15:24–30. doi: 10.3201/1501.080299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barik S. New treatments for influenza. BMC Med. 2012;10:104. doi: 10.1186/1741-7015-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paules C, Subbarao K. 2017.
- 21.Wu X., Wu X., Sun Q., et al. Progress of small molecular inhibitors in the development of anti-influenza virus agents. Theranostics. 2017;7:826–845. doi: 10.7150/thno.17071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santosham M., Chandran A., Fitzwater S., et al. Progress and barriers for the control of diarrhoeal disease. Lancet. 2010;376:63–67. doi: 10.1016/S0140-6736(10)60356-X. [DOI] [PubMed] [Google Scholar]
- 23.Bobo L.D., Dubberke E.R. Recognition and prevention of hospital-associated enteric infections in the intensive care unit. Crit. Care Med. 2010;38:S324–S334. doi: 10.1097/CCM.0b013e3181e69f05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dennehy P.H. Effects of vaccine on rotavirus disease in the pediatric population. Curr. Opin. Pediatr. 2012;24:76–84. doi: 10.1097/MOP.0b013e32834ee594. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Lopez A., Behnsen J., Nuccio S.P., Raffatellu M. Mucosal immunity to pathogenic intestinal bacteria. Nat. Rev. Immunol. 2016;16:135–148. doi: 10.1038/nri.2015.17. [DOI] [PubMed] [Google Scholar]
- 26.Hall J.A., Bouladoux N., Sun C.M., et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–649. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Round J.L., Mazmanian S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atarashi K., Nishimura J., Shima T., et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 29.Goto Y., Uematsu S., Kiyono H. Epithelial glycosylation in gut homeostasis and inflammation. Nat. Immunol. 2016;17:1244–1251. doi: 10.1038/ni.3587. [DOI] [PubMed] [Google Scholar]
- 30.Guandalini S. Probiotics for prevention and treatment of diarrhea. J. Clin. Gastroenterol. 2011;45(Suppl.):S149–S153. doi: 10.1097/MCG.0b013e3182257e98. [DOI] [PubMed] [Google Scholar]
- 31.Park M.K., Ngo V., Kwon Y.M., et al. Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PLoS One. 2013;8:e75368. doi: 10.1371/journal.pone.0075368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill C., Guarner F., Reid G., et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 33.Taverniti V., Guglielmetti S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes Nutr. 2011;6:261–274. doi: 10.1007/s12263-011-0218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pimentel-Nunes P., Soares J.B., Roncon-Albuquerque R., Jr, Dinis-Ribeiro M., Leite-Moreira A.F. Toll-like receptors as therapeutic targets in gastrointestinal diseases. Expert Opin. Ther. Targets. 2010;14:347–368. doi: 10.1517/14728221003642027. [DOI] [PubMed] [Google Scholar]
- 35.Gleeson M., Bishop N.C., Struszczak L. Effects of Lactobacillus casei Shirota ingestion on common cold infection and herpes virus antibodies in endurance athletes: a placebo-controlled, randomized trial. Eur. J. Appl. Physiol. 2016;116:1555–1563. doi: 10.1007/s00421-016-3415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagata S., Asahara T., Ohta T., et al. Effect of the continuous intake of probiotic-fermented milk containing Lactobacillus casei strain Shirota on fever in a mass outbreak of norovirus gastroenteritis and the faecal microflora in a health service facility for the aged. Br. J. Nutr. 2011;106:549–556. doi: 10.1017/S000711451100064X. [DOI] [PubMed] [Google Scholar]
- 37.Van Puyenbroeck K., Hens N., Coenen S., et al. Efficacy of daily intake of Lactobacillus casei Shirota on respiratory symptoms and influenza vaccination immune response: a randomized, double-blind, placebo-controlled trial in healthy elderly nursing home residents. Am. J. Clin. Nutr. 2012;95:1165–1171. doi: 10.3945/ajcn.111.026831. [DOI] [PubMed] [Google Scholar]
- 38.Kumpu M., Kekkonen R.A., Korpela R., et al. Effect of live and inactivated Lactobacillus rhamnosus GG on experimentally induced rhinovirus colds: randomised, double blind, placebo-controlled pilot trial. Benef. Microbes. 2015;6:631–639. doi: 10.3920/BM2014.0164. [DOI] [PubMed] [Google Scholar]
- 39.Sindhu K.N., Sowmyanarayanan T.V., Paul A., et al. Immune response and intestinal permeability in children with acute gastroenteritis treated with Lactobacillus rhamnosus GG: a randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 2014;58:1107–1115. doi: 10.1093/cid/ciu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makino S., Ikegami S., Kume A., et al. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Br. J. Nutr. 2010;104:998–1006. doi: 10.1017/S000711451000173X. [DOI] [PubMed] [Google Scholar]
- 41.Nagai T., Makino S., Ikegami S., Itoh H., Yamada H. Effects of oral administration of yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 and its exopolysaccharides against influenza virus infection in mice. Int. Immunopharmacol. 2011;11:2246–2250. doi: 10.1016/j.intimp.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Makino S., Sato A., Goto A., et al. Enhanced natural killer cell activation by exopolysaccharides derived from yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. J. Dairy Sci. 2016;99:915–923. doi: 10.3168/jds.2015-10376. [DOI] [PubMed] [Google Scholar]
- 43.Jespersen L., Tarnow I., Eskesen D., et al. Effect of Lactobacillus paracasei subsp. paracasei, L. casei 431 on immune response to influenza vaccination and upper respiratory tract infections in healthy adult volunteers: a randomized, double-blind, placebo-controlled, parallel-group study. Am. J. Clin. Nutr. 2015;101:1188–1196. doi: 10.3945/ajcn.114.103531. [DOI] [PubMed] [Google Scholar]
- 44.Rizzardini G., Eskesen D., Calder P.C., et al. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12(R) and Lactobacillus paracasei ssp. paracasei, L. casei 431(R) in an influenza vaccination model: a randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2012;107:876–884. doi: 10.1017/S000711451100420X. [DOI] [PubMed] [Google Scholar]
- 45.Maruyama M., Abe R., Shimono T., et al. The effects of non-viable Lactobacillus on immune function in the elderly: a randomised, double-blind, placebo-controlled study. Int. J. Food Sci. Nutr. 2016;67:67–73. doi: 10.3109/09637486.2015.1126564. [DOI] [PubMed] [Google Scholar]
- 46.Pedone C.A., Arnaud C.C., Postaire E.R., Bouley C.F., Reinert P. Multicentric study of the effect of milk fermented by Lactobacillus casei on the incidence of diarrhoea. Int. J. Clin. Pract. 2000;54:568–571. [PubMed] [Google Scholar]
- 47.Merenstein D., Murphy M., Fokar A., et al. Use of a fermented dairy probiotic drink containing Lactobacillus casei (DN-114 001) to decrease the rate of illness in kids: the DRINK study. A patient-oriented, double-blind, cluster-randomized, placebo-controlled, clinical trial. Eur. J. Clin. Nutr. 2010;64:669–677. doi: 10.1038/ejcn.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arimori Y., Nakamura R., Hirose Y., et al. Daily intake of heat-killed Lactobacillus plantarum L-137 enhances type I interferon production in healthy humans and pigs. Immunopharmacol. Immunotoxicol. 2012;34:937–943. doi: 10.3109/08923973.2012.672425. [DOI] [PubMed] [Google Scholar]
- 49.Hirose Y., Murosaki S., Yamamoto Y., Yoshikai Y., Tsuru T. Daily intake of heat-killed Lactobacillus plantarum L-137 augments acquired immunity in healthy adults. J. Nutr. 2006;136:3069–3073. doi: 10.1093/jn/136.12.3069. [DOI] [PubMed] [Google Scholar]
- 50.Hirose Y., Yamamoto Y., Yoshikai Y., Murosaki S. Oral intake of heat-killed Lactobacillus plantarum L-137 decreases the incidence of upper respiratory tract infection in healthy subjects with high levels of psychological stress. J. Nutr. Sci. 2013;2:e39. doi: 10.1017/jns.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oo K.M., Lwin A.A., Kyaw Y.Y., et al. Safety and long-term effect of the probiotic FK-23 in patients with hepatitis C virus infection. Biosci. Microbiota Food Health. 2016;35:123–128. doi: 10.12938/bmfh.2015-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grandy G., Medina M., Soria R., Teran C.G., Araya M. Probiotics in the treatment of acute rotavirus diarrhoea. A randomized, double-blind, controlled trial using two different probiotic preparations in Bolivian children. BMC Infect. Dis. 2010;10:253. doi: 10.1186/1471-2334-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holscher H.D., Czerkies L.A., Cekola P., et al. Bifidobacterium lactis Bb12 enhances intestinal antibody response in formula-fed infants: a randomized, double-blind, controlled trial. JPEN J. Parenter. Enteral Nutr. 2012;36:106S–117S. doi: 10.1177/0148607111430817. [DOI] [PubMed] [Google Scholar]
- 54.Erdogan O., Tanyeri B., Torun E., et al. The comparition of the efficacy of two different probiotics in rotavirus gastroenteritis in children. J. Trop. Med. 2012;2012:787240. doi: 10.1155/2012/787240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugimura T., Jounai K., Ohshio K., et al. Immunomodulatory effect of Lactococcus lactis JCM5805 on human plasmacytoid dendritic cells. Clin. Immunol. 2013;149:509–518. doi: 10.1016/j.clim.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 56.Sugimura T., Takahashi H., Jounai K., et al. Effects of oral intake of plasmacytoid dendritic cells-stimulative lactic acid bacterial strain on pathogenesis of influenza-like illness and immunological response to influenza virus. Br. J. Nutr. 2015;114:727–733. doi: 10.1017/S0007114515002408. [DOI] [PubMed] [Google Scholar]
- 57.Shibata T., Kanayama M., Haida M., et al. Lactococcus lactis JCM5805 activates anti-viral immunity and reduces symptoms of common cold and influenza in healthy adults in a randomized controlled trial. J. Funct. Foods. 2016;24:492–500. [Google Scholar]
- 58.Suzuki H., Kanayama M., Fujii T., Fujiwara D., Sugimura H. Effects of the beverage containing Lactococcus lactis subsp. lactis JCM5805 on anti-viral immune responses and maintenance of physical conditions Jpn. Pharmacol. Ther. 2015;43:106–111. [Google Scholar]
- 59.Sakata K., Sasaki Y., Jounai K., Fujii T., Fujiwara D. Preventive Effect of Lactococcus lactis subsp. lactis JCM 5805 Yogurt Intake on Influenza Infection among Schoolchildren. Health. 2017;9:756–762. [Google Scholar]
- 60.Fujii T., Jounai K., Horie A., et al. Effects of heat-killed Lactococcus lactis subsp. lactis JCM 5805 on mucosal and systemic immune parameters, and antiviral reactions to influenza virus in healthy adults; a randomized controlled double-blind study. J. Funct. Foods. 2017;35:513–521. [Google Scholar]
- 61.Chow K.V., Sutherland R.M., Zhan Y., Lew A.M. Heterogeneity, functional specialization and differentiation of monocyte-derived dendritic cells. Immunol. Cell Biol. 2017;95:244–251. doi: 10.1038/icb.2016.104. [DOI] [PubMed] [Google Scholar]
- 62.Asselin-Paturel C., Boonstra A., Dalod M., et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 63.Hoene V., Peiser M., Wanner R. Human monocyte-derived dendritic cells express TLR9 and react directly to the CpG-A oligonucleotide D19. J. Leukoc. Biol. 2006;80:1328–1336. doi: 10.1189/jlb.0106011. [DOI] [PubMed] [Google Scholar]
- 64.Kawai T., Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 65.Swiecki M., Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 2015;15:471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haller O., Kochs G., Weber F. The interferon response circuit: induction and suppression by pathogenic viruses. Virology. 2006;344:119–130. doi: 10.1016/j.virol.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jounai K., Ikado K., Sugimura T., et al. Spherical lactic acid bacteria activate plasmacytoid dendritic cells immunomodulatory function via TLR9-dependent crosstalk with myeloid dendritic cells. PLoS One. 2012;7:e32588. doi: 10.1371/journal.pone.0032588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jounai K., Sugimura T., Ohshio K., Fujiwara D. Oral administration of Lactococcus lactis subsp. lactis JCM5805 enhances lung immune response resulting in protection from murine parainfluenza virus infection. PLoS One. 2015;10:e0119055. doi: 10.1371/journal.pone.0119055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trinchieri G., Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J. Exp. Med. 1978;147:1314–1333. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo Z., Tilburgs T., Wong B., Strominger J.L. Dysfunction of dendritic cells in aged C57BL/6 mice leads to failure of natural killer cell activation and of tumor eradication. Proc. Natl. Acad. Sci. USA. 2014;111:14199–14204. doi: 10.1073/pnas.1414780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suzuki H., Ohshio K., Fujiwara D. Lactococcus lactis subsp. lactis JCM 5805 activates natural killer cells via dendritic cells. Biosci. Biotechnol. Biochem. 2016;80:798–800. doi: 10.1080/09168451.2015.1116922. [DOI] [PubMed] [Google Scholar]
- 72.Lee C.K., Rao D.T., Gertner R., et al. Distinct requirements for IFNs and STAT1 in NK cell function. J. Immunol. 2000;165:3571–3577. doi: 10.4049/jimmunol.165.7.3571. [DOI] [PubMed] [Google Scholar]
- 73.Gerosa F., Gobbi A., Zorzi P., et al. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J. Immunol. 2005;174:727–734. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- 74.Salminen S., Nybom S., Meriluoto J., et al. Interaction of probiotics and pathogens--benefits to human health? Curr. Opin. Biotechnol. 2010;21:157–167. doi: 10.1016/j.copbio.2010.03.016. [DOI] [PubMed] [Google Scholar]