Abstract
Background:
The prevalence, presentation, management, and prognosis of coronary heart disease differ according to sex. Greater understanding on the differences between men and women with acute aortic dissection (AAD) is needed. We aimed to investigate whether sex disparities are found in patients with AAD, and to study sex differences in complications, mortality in-hospital, and long-term.
Methods:
We included 884 patients enrolled in our institute between June 2002 and May 2016. Considering psychosocial factors, treatments, and the outcomes in men versus those in women with AAD, we explored the association of sex with psychosocial characteristics and mortality risk. For categorical variables, significant differences between groups were assessed with the Chi-square test or Fisher's exact test, and continuous parameters were assessed with Student's t-test. Univariate and stratified survival statistics were computed using Kaplan-Meier analysis.
Results:
A total of 884 patients (76.1% male, mean age 51.4 ± 11.8 years) were included in this study. There were fewer current smokers in female compared with male (17.5% vs. 67.2%, χ2 = 160.06, P < 0.05). The percentage of men who reported regular alcohol consumption was significantly higher than that in women (40.6% vs. 3.8%, χ2 = 100.18, P < 0.05). About 6.2% (55 of 884) of patients with AAD died before vascular or endovascular surgery was performed, 34.4% (304 of 884) of patients underwent surgical procedures, and 52.7% (466 of 884) and 12.8% (113 of 884) of patients received endovascular treatment and medication. Postoperative mortality similar (6.0% vs. 5.6%, respectively, χ2 = 0.03, P = 0.91) between men and women. Follow-up was completed in 653 of 829 patients (78.8%). Adjustment for age, history of coronary disease, hypertension, smoking and drinking, Type A and use of beta-blocker, angiotensin II receptor blockers, angiotensin converting enzyme (ACE) inhibitor, calcium-channel blockers and statins by multivariate logistic regression analysis suggested that age (odds ratios [ORs], 1.04; 95% confidence interval [CI], 1.01–1.07; P < 0.05), using of calcium-channel blockers (OR, 0.37; 95% CI, 0.18–0.74; P < 0.05), at discharge were independent predictors of late mortality, ACE inhibitors (OR, 1.91; 95% CI, 1.03–3.54; P = 0.04) was independent risk factor of late mortality.
Conclusions:
In Chinese with AAD, sex is not independently associated with long-term clinical outcomes. Age, the intake of calcium-channel blockers at discharge might help to improve long-term outcomes.
Keywords: Aortic Dissection, Prognosis, Sex
摘要
背景:
冠状动脉粥样硬化型疾病的发病率,临床表现,治疗措施及预后情况与性别相关。需要深入的了解主动脉夹层的性别 差异。本研究旨在探索急性主动脉夹层的性别差异,研究不同性别的在院并发症及随访期间并发症及死亡的发生情况。
方法:
自2002年6月至2016年5月共入选884例急性主动脉夹层患者。登记发病特点、治疗方式及治疗效果,进而研究性别与 发病特征、死亡风险的关联。计量资料以均数±标准差表示,正态分布资料的组间比较采用t检验。计数资料以率或百分数 表示,组间比较应用χ2 检验或 Fisher精确概率法,随访期间死亡率用Kaplan-Meier生存曲线表示。
结果:
884例患者,其中男性76.1%(673),平均年龄(51.4 ± 11.8)岁。男性吸烟及饮酒的比例均明显高于女性(67.2% vs. 17.5%, χ2 =160.06,P<0.05;40.6% vs. 3.8%, χ2 =100.18,P<0.05)。计6.2%(55/884)患者术前死亡,34.4% (304/884) 患者行外科手术治疗,52.7% (466/884)患者行主动脉腔内修复术治疗, 12.8%(113/884)患者保守治疗。围术期死亡率两组 无统计学差异 (6.0% vs. 5.6%, χ2 = 0.03,P = 0.91)。中位随访24个月,随访率为78.8%(653/829)。多因素回归分析显 示:年龄(OR,1.04;P = 0.003),钙离子拮抗剂的使用(OR, 0.37;P = 0.005)是长期预后的保护性因素, ACEI抑制剂的使用 (OR,1.91;P = 0.040)是影响死亡的独立危险因素。
结论:
急性主动脉夹层的中国患者,性别不是影响长期预后的独立危险因素,年少及钙离子通道阻滞剂的使用可能是远期不 良事件发生的保护性因素。
INTRODUCTION
Currently, acute aortic dissection (AAD) remains the most common catastrophic aortic condition.[1] AAD requires rapid diagnosis and appropriate treatment to improve survival and prevent potentially fatal complications.[2,3] Over the past two decades, understanding on the natural history, diagnosis, and management of AAD has improved considerably. Although several studies have focused on AAD, few data exist on sex-related differences in clinical presentation, diagnostic imaging, management, and outcomes in a large cohort.[4,5] Accordingly, the purpose of this investigation was to evaluate differences between male and female patients with AAD and to report both early and late outcomes over a 15-year period in our center.
METHODS
Ethical approval
The study was approved by the Ethics Committee of the General Hospital of the Shenyang Military Region.
Study population and data collection
We examined data on all patients with AAD admitted to the General Hospital of the Shenyang Military Region from June 2002 to May 2016 (n = 884). Multidetector computed tomography was performed in all cases.[6] Acute type A dissection was defined as any dissection that involved the ascending aorta and/or aortic arch. Acute type B was defined as that involving the descending aorta (without any tear in or involvement of the ascending aorta) appearing within 14 days of the onset of symptoms.[7,8,9] Patients were divided into two groups according to sex.
Management and follow-up protocol
While uncomplicated type B dissection can usually be managed with conservative treatment, type A dissection is a surgical emergency.[10] With improving technology and convincing long-term outcomes in favor of thoracic endovascular aortic repair (TEVAR), there is growing consensus for TEVAR to be used for both complicated and uncomplicated Type B aortic dissection.[8,11] After discharge, antihypertensive medicine was administered. For those requiring intervention, new deficits, not present before the operation, were considered postoperative outcomes. All cases were followed up clinically at 1, 6, 12 months, and annually thereafter.
Endpoints
The study's primary endpoint during the follow-up was the composite of aortic disease-related death, aortic dissection, paraplegia, myocardial ischemia, renal insufficiency, and other aortic complications.
Statistical analysis
Summary statistics of both groups (male and female) were presented as frequencies and percentages, median (Q1 and Q3), or mean ± standard deviation (SD). For categorical variables, significant differences between groups were assessed with the Chi-square test or Fisher's exact test, and continuous parameters were assessed with Student's t-test. Pre- and intra-operative variables were first analyzed using univariate analysis to determine whether any single factor was related to mortality in all patients. Many individual variables were examined using multivariate analysis by forward logistic regression to estimate the independent odds ratios (ORs) of factors related to mortality in patients. Univariate and stratified survival statistics were computed using Kaplan-Meier analysis. Subjects who did not return to the clinic and those we were unable to contact were considered as lost to follow-up. Data analysis was performed using Statistical Package for Social Sciences (SPSS) version 20.0 for Windows (SPSS Institute, Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
RESULTS
Sex-related differences in demographics, clinical characteristics, and imaging findings
A total of 884 patients (76.1% male, mean age 51.4 ± 11.8 years) were included in this study. Baseline characteristics by sex are presented in Table 1. There were fewer current smokers in female compared with male (17.5% vs. 67.2%, χ2 = 160.06, P < 0.05). The percentage of men who reported regular alcohol consumption was significantly higher than that in women (40.6% vs. 3.8%, χ2 = 100.18, P < 0.05). There were no significant differences in hypertension (79.8% vs. 77.3%, χ2 = 0.69, P = 0.41), diabetes mellitus (3.9% vs. 5.7%, χ2 = 1.30, P = 0.25), coronary heart disease (19.5% vs. 21.3%, χ2 = 0.35, P = 0.56), or type of dissection (38.3% vs. 46.0%, χ2 = 3.90, P = 0.05) between men and women. Proportions with classic presentation with chest pain (82.2% vs. 78.7%, χ2 = 1.29, P = 0.26), abdominal pain (16.0% vs. 10.9%, χ2 = 3.37, P = 0.07), and back pain (57.7% vs. 64.9%, χ2 = 3.53, P = 0.06) were similar. Combined pericardial effusion was seen in 16.6% of men and 22.3% of women, with a trend (χ2 = 3.46, P = 0.06) toward higher incidence in women. The mean systolic and diastolic blood pressure did not differ between the groups [Table 1].
Table 1.
Demographics and history of acute aortic dissection patients
| Characteristics | Men (n = 673) | Women (n = 211) | Statistical values | P |
|---|---|---|---|---|
| Age (years), mean ± SD | 51.4 ± 11.8 | 55.1 ± 12.5 | 2.71† | 0.10 |
| Smoking, n (%) | 452 (67.2) | 37 (17.5) | 160.06* | <0.05 |
| Drinking, n (%) | 273 (40.6) | 8 (3.8) | 100.18* | <0.05 |
| Hypertension, n (%) | 537 (79.8) | 163 (77.3) | 0.69* | 0.41 |
| Coronary heart disease, n (%) | 131 (19.5) | 45 (21.3) | 0.35* | 0.56 |
| Diabetes mellitus, n (%) | 26 (3.9) | 12 (5.7) | 1.30* | 0.25 |
| Type A dissection, n (%) | 258 (38.3) | 97 (46.0) | 3.90* | 0.05 |
| Chest pain, n (%) | 553 (82.2) | 166 (78.7) | 1.29* | 0.26 |
| Back pain, n (%) | 388 (57.7) | 137 (64.9) | 3.53* | 0.06 |
| Abdominal pain, n (%) | 108 (16.0) | 23 (10.9) | 3.37* | 0.07 |
| Limb ischemia, n (%) | 47 (7.0) | 7 (3.3) | 3.76* | 0.05 |
| Coma/alter consciousness, n (%) | 50 (7.4) | 23 (10.9) | 2.56* | 0.11 |
| Congestive heart failure, n (%) | 19 (2.8) | 7 (3.3) | 0.14* | 0.71 |
| Pleural effusion, n (%) | 171 (25.4) | 67 (31.8) | 3.29* | 0.07 |
| Pericardial effusion, n (%) | 112 (16.6) | 47 (22.3) | 3.46* | 0.06 |
| EF (%), mean ± SD | 64.0 ± 0.7 | 65.0 ± 0.5 | 4.82† | 0.35 |
| False lumen > true lumen, n (%) | 210 (31.2) | 62 (29.4) | 0.25* | 0.62 |
| SBP on admittance (mmHg), mean ± SD | 151.9 ± 29.7 | 147.8 ± 29.4 | 0.05† | 0.83 |
| DBP on admittance (mmHg), mean ± SD | 87.1 ± 19.0 | 80.6 ± 19.2 | 0.12† | 0.73 |
EF: Ejection fraction; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; SD: Standard deviation; 1 mmHg=0.133 kPa. *: Chi-square test; †: t-test.
Sex-related differences in inhospital management and complications
Overall, 6.2% (55 of 884) of patients with AAD died before vascular or endovascular surgery was performed, 34.4% (304 of 884) underwent surgical procedures, and 52.7% (466 of 884) and 12.8% (113 of 884) received endovascular treatment and medication. In surgically managed patients, hypothermic circulatory arrest, aortic cross-clamp time, and extracorporeal circulation time were equally distributed in groups. In patients who underwent endovascular surgery, the number of stent grafts implanted has no difference between men and women. There were no significant differences in medicine used [Table 2]. As shown in Table 3, inhospital complications including pulmonary complications, acute renal insufficiency (ARI), paraplegia, encephalopathy, myocardial ischemia, and length of stay occurred commonly in both men and women. Postoperative mortality was 6.0% (38 of 634) and 5.6% (11 of 195) between men and women, respectively [Table 3].
Table 2.
Inhospital treatments and surgical data of acute aortic dissection patients
| Characteristics | Men (n = 673) | Women (n = 211) | Statistical values | P |
|---|---|---|---|---|
| Surgery, n (%) | ||||
| Type A dissection | 220 (32.7) | 79 (37.4) | 1.62* | 0.20 |
| Type B dissection | 4 (0.6) | 1 (0.5) | 1.62* | 0.20 |
| Endovascular treatment, n (%) | ||||
| Type A dissection | 1 (0.1) | 2 (0.9) | 3.03* | 0.14 |
| Type B dissection | 370 (55.0) | 93 (44.1) | 7.65* | 0.01 |
| Medicine, n (%) | ||||
| Type A dissection | 37 (5.5) | 16 (7.6) | 1.24* | 0.27 |
| Type B dissection | 40 (5.9) | 20 (9.5) | 3.17* | 0.08 |
| Hypothermic circulatory arrest (min), median (interquartile range) | 37.0 (28.0,47.0) | 36.0 (29.0,46.5) | 0.01‡ | 0.95 |
| Aortic cross-clamp time (min), mean ± SD | 117.2 ± 35.1 | 114.5 ± 26.9 | 2.16† | 0.14 |
| Extracorporeal circulation time (min), mean ± SD | 200.3 ± 61.7 | 194.9 ± 45.2 | 0.83† | 0.36 |
| Number of stent grafts implanted, median (Q1, Q3) | 1 (1, 1) | 1 (1, 1) | 1.40‡ | 0.24 |
| Completely covering of the LSA, n (%) | 45 (6.7) | 10 (4.7) | 1.04* | 0.31 |
| Species of medical, median (Q1, Q3) | 4 (2, 5) | 3 (2, 5) | 0.47‡ | 0.88 |
| Sodium nitroprusside, n (%) | 500 (74.3) | 149 (70.6) | 1.11* | 0.29 |
| Beta-blocker, n (%) | 619 (92.0) | 192 (91.0) | 0.24* | 0.65 |
| Angiotensin II receptor blocker, n (%) | 156 (23.2) | 42 (19.9) | 0.99* | 0.32 |
| ACE inhibitor, n (%) | 461 (68.5) | 139 (65.9) | 0.51* | 0.48 |
| Calcium-channel blocker, n (%) | 563 (83.7) | 167 (79.1) | 2.27* | 0.13 |
| Aspirin, n (%) | 152 (22.6) | 43 (20.4) | 0.46* | 0.50 |
| Statin, n (%) | 161 (23.9) | 43 (20.4) | 1.14* | 0.29 |
LSA: left subclavian artery; SD: standard deviation; ACE: angiotensin converting enzyme. *Chi-square test; †t-test; ‡Mann-Whitney U-test.
Table 3.
Postoperative outcomes of acute aortic dissection patients
| Characteristics | Men (n = 634) | Women (n = 195) | Statistical values | P |
|---|---|---|---|---|
| Pulmonary, n (%) | 41 (6.5) | 12 (6.2) | 0.02* | 0.88 |
| ARI, n (%) | 78 (12.3) | 16 (8.2) | 2.49* | 0.11 |
| Paraplegia, n (%) | 3 (0.5) | 1 (0.5) | 0.01* | 1.00 |
| Encephalopathy, n (%) | 31 (4.9) | 10 (5.1) | 0.02* | 0.89 |
| Myocardial ischemia, n (%) | 46 (7.3) | 18 (9.2) | 0.82* | 0.37 |
| LOS (days), mean ± SD | 13.5 ± 2.3 | 13.6 ± 1.6 | 0.75† | 0.39 |
| Mortality, n (%) | 38 (6.0) | 11 (5.6) | 0.03* | 0.86 |
| Type A | 30 (4.7) | 10 (5.1) | 0.05* | 0.82 |
| Type B | 8 (1.3) | 1 (0.5) | 0.24* | 0.63 |
ARI: Acute renal insufficiency; LOS: Length of stay; SD: Standard deviation. *Chi-square test; †t-test.
Sex-related differences in mortality during follow-up
Follow-up was completed in 653 of 829 patients (78.8%). Figure 1 shows the survival of women versus men stratified by type of dissection. The follow-up ranged from 2 to 168 months (median, 24 months), there were 38 deaths (6.0%) in male patients and 11 deaths (5.6%) in female patients; there was no significant difference among these groups (χ2 = 0.03, P = 0.91). No significant sex-related difference was found in mortality between Type A and B dissections [Figure 1]. Adjustment for age, history of coronary disease, hypertension, smoking and drinking, Type A and use of beta-blocker, angiotensin II receptor blockers, angiotensin converting enzyme (ACE) inhibitor, calcium-channel blockers, and statins by multivariate logistic regression analysis suggested that age (OR, 1.04; 95% confidence interval [CI], 1.01–1.07; P < 0.05), using of calcium-channel blockers (OR, 0.37; 95% CI, 0.18–0.74; P < 0.05), at discharge were independent predictors of late mortality, ACE inhibitors (OR,1.91; 95% CI, 1.03–3.54; P = 0.04) was independent risk factor of late mortality [Table 4].
Figure 1.
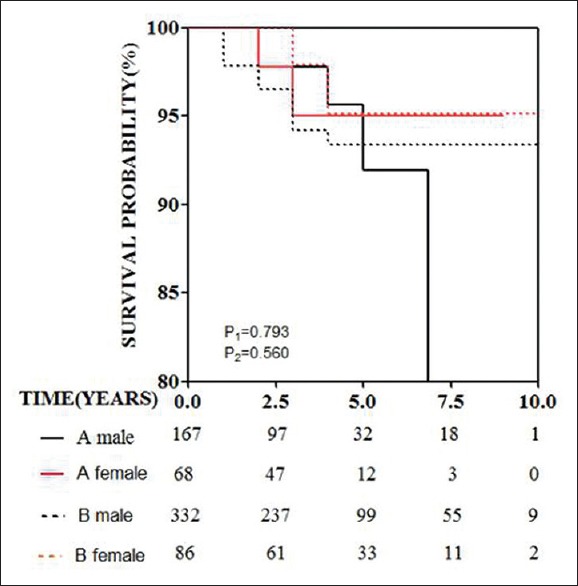
Survival curves for men and women with acute aortic dissection stratified by type of dissection (type A and type B). Log-rank test P1 = 0.793 for type A dissection and log-rank P2 = 0.560 for type B dissection in the two genders.
Table 4.
Independent effects on late mortality survival
| Characteristics | OR | 95% CI | P |
|---|---|---|---|
| Gender | 0.90 | 0.41–1.98 | 0.790 |
| Age | 1.04 | 1.01–1.07 | 0.003 |
| Smoking | 1.10 | 0.62–1.94 | 0.750 |
| Drinking | 1.18 | 0.61–2.26 | 0.620 |
| Coronary disease | 0.86 | 0.35–2.12 | 0.750 |
| Hypertension | 0.86 | 0.41–1.77 | 0.670 |
| Type A | 1.03 | 0.50–2.12 | 0.930 |
| Pleural effusion | 1.39 | 0.74–2.62 | 0.310 |
| Pericardial effusion | 1.20 | 0.54–2.71 | 0.650 |
| Beta-blocker at discharge | 0.57 | 0.28–1.14 | 0.110 |
| Angiotensin II receptor blocker at discharge | 0.69 | 0.22–2.14 | 0.520 |
| ACE inhibitor at discharge | 1.91 | 1.03–3.54 | 0.040 |
| Calcium-channel blocker at discharge | 0.37 | 0.18–0.74 | 0.005 |
| Statin at discharge | 0.43 | 0.17–1.08 | 0.070 |
OR: Odds ratio; CI: Confidence interval; ACE: Angiotensin converting enzyme.
DISCUSSION
Although sex-related differences in outcomes of acute coronary syndromes are well documented,[12] such information is not available for patients with aortic dissection. This study based on large-scale and long-term follow-up in the Chinese population that provides evidence on the incidence, evolution, follow-up, and multivariate predictors of aortic dissection. The principal findings of this analysis were as follows. First, during the follow-up, the use of ACE inhibitors was associated with a risk of long-term aortic events in patients with aortic dissection, which might contribute greatly to guiding clinical practice. Second, calcium-channel blockers were associated with improved survival in aortic dissection.
In 2004, Nienaber et al.[13] provided the first systematic registry analysis of a large series of patients with AAD. The study found a higher death rate in women with Type A dissection than that in men, including a 9% point higher rate of all-cause inhospital mortality (30.1% vs. 21.0%, P < 0.001). The study showed that the rates of inhospital complications were similar between men and women. Differences in phenotype, habits, customs, and diet between Chinese and Western populations might also affect treatment outcomes.
Several studies have shown that outcomes for women who underwent surgery for aortic dissection were worse than for men.[13,14,15] The present study showed that outcomes were similar in both sexes. This difference can explain as follows. First, other studies have reported that aortic dissection occurs on average 6 or 7 years later in women compared with in men and that 50% of women with aortic dissection were ≥70 years of age. In this cohort, there was no significant difference in age between men and women. Second, men seem more likely to experience more abrupt onset of pain,[13] and thus timely hospital presentation. Third, pleural and pericardial and other imaging findings suggestive of impending rupture were seen more often in women. Our results showed that complications with aortic dissection were similar between men and women.
In 2008, Takeshita et al.[16] reported that treatment with ACE inhibitors was associated with a significantly reduced risk of dissection-related aortic events in patients who survived the acute phase. Similarly, a study of 15,326 patients with a primary diagnosis of ruptured or intact abdominal aortic aneurysm, found an association of ACE inhibitors with a reduced risk of ruptured abdominal aortic aneurysm.[17] Suzuki, et al.[16] reported that use of angiotensin-converting enzyme (ACE) inhibitor was associated with a reduced risk of long-term aortic events in patients. Our results showed that ACE inhibitor therapy was an independent risk factor for late mortality. The potential mechanism remains unknown.
The use of calcium-channel blocker therapy was a reduced independent risk factor for late mortality. Some related reports pointed out that calcium-channel blockers are associated with reduced aortic expansion and improved survival in AAD.[18,19] Calcium antagonists generally blocked the slow calcium channels, decreasing the influx of extracellular calcium,[20] which resulted in suppression of the sinoatrial and atrioventricular nodes,[21] vascular dilation with decreased blood pressure, and reduced cardiac contractility. Adequate blood pressure regulation and subsequent decreased pressure on the aortic wall through calcium-channel blockade may theoretically decrease risks of aortic enlargement. The role of calcium-channel blockers in the management of aortic dissection, in general, remains poorly addressed.
Some trials have found statin therapy to be independently associated with better long-term survival of aortic aneurysms.[22,23,24] Possibly owing to reasons as follows: The pleiotropic effects of statins, which influence endothelial function, inflammatory response, thrombus formation, and plaque stability, could affect postoperative and long-term outcomes.[25,26] The other effect is thought to be related to downregulation of metalloproteinases.[27,28,29] This may be similar in principle to studies showing a minimal change of the coronary lumen but marked decreases in the numbers of deaths and myocardial infarctions in patients receiving statin therapy for coronary atherosclerosis.[30] Our multivariate logistic regression analysis demonstrated that statin therapy was not an independent risk factor for late mortality. The differences between the Eastern and Western suggest that race, habits, and customs might affect treatment strategies. Thus, data and evidence for AAD therapy must be obtained for the Chinese population.
The present study is a retrospective observational study with inherent limitations. First, due to the non-randomized design and low incidence of aortic dissection in the normal population, the study should be considered as a hypothesis-generating one. Second, we have no way to confirm by imaging techniques the exact time of AAD onset or evolution. Furthermore, large-scale randomized controlled trial studies are required.
In summary, our results show that early and late mortality are similar in AAD patients of both sexes. Treatment with ACE inhibitors, calcium-channel blockers, and statins at discharge might help to improve long-term outcomes.
Financial support and sponsorship
The study was supported by the grants from the National Key Project of Research and Development Plan during the Thirteenth Five-year Plan Period (No. 2016YFC1301300), and the Construction Program of National Clinical Priority Speciality.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Scott Wysong, ELS from Liwen Bianji, Edanz Editing China for English improving of this manuscript.
Footnotes
Edited by: Ning-Ning Wang
REFERENCES
- 1.He RX, Zhang L, Zhou TN, Yuan WJ, Liu YJ, Fu WX, et al. Safety and necessity of antiplatelet therapy on patients underwent endovascular aortic repair with both Stanford type B aortic dissection and coronary heart disease. Chin Med J. 2017;130:2321–5. doi: 10.4103/0366-6999.215330. doi: 10.4103/0366-6999.215330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bossone E, LaBounty TM, Eagle KA. Acute aortic syndromes: Diagnosis and management, an update. Eur Heart J. 2017;30:1–15. doi: 10.1093/eurheartj/ehx319. doi: 10.1093/eurheartj/ehx319. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Q, Chai XP, Fang ZF, Hu XQ, Tang L. Association of plasma pentraxin-3 levels on admission with in-hospital mortality in patients with acute type A aortic dissection. Chin Med J. 2016;129:2589–95. doi: 10.4103/0366-6999.192785. doi: 10.4103/0366-6999.192785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li D, Ye L, Yu J, Deng L, Liang L, Ma Y, et al. Significance of the thrombo-inflammatory status-based novel prognostic score as a useful predictor for in-hospital mortality of patients with type B acute aortic dissection. Oncotarget. 2017;8:79315–22. doi: 10.18632/oncotarget.18105. doi: 10.18632/oncotarget.18105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takagi H, Ando T, Umemoto T (All-Literature Investigation of Cardiovascular Evidence [ALICE] Group) Meta-analysis of circadian variation in the onset of acute aortic dissection. Am J Cardiol. 2017;120:1662–6. doi: 10.1016/j.amjcard.2017.07.067. doi: 10.1016/j.amjcard.2017.07.067. [DOI] [PubMed] [Google Scholar]
- 6.Bachet J. Prognostic significance of early aortic remodeling in acute uncomplicated type B aortic dissection and intramural hematoma: Tell us what to do. J Thorac Cardiovasc Surg. 2017;154:1171–2. doi: 10.1016/j.jtcvs.2017.05.033. doi: 10.1016/j.jtcvs.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 7.Guo J, Zhang G, Tang D, Zhang J. A case report of Takayasu arteritis with aortic dissection as initial presentation. Medicine (Baltimore) 2017;96:e8610. doi: 10.1097/MD.0000000000008610. doi: 10.1097/MD.0000000000008610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krol E, Panneton JM. Uncomplicated acute type B aortic dissection: Selection guidelines for TEVAR. Ann Vasc Dis. 2017;10 doi: 10.3400/avd.ra.17-00061. pii: ra 17-00061 doi: 103400/avdra17-00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsushita A, Hattori T, Tsunoda Y, Sato Y, Mihara W. Impact of initial aortic diameter and false-lumen area ratio on type B aortic dissection prognosis. Interact Cardiovasc Thorac Surg. 2017;26:176–82. doi: 10.1093/icvts/ivx286. doi: 10.1093/icvts/ivx286. [DOI] [PubMed] [Google Scholar]
- 10.Elsayed RS, Cohen RG, Fleischman F, Bowdish ME. Acute type A aortic dissection. Cardiol Clin. 2017;35:331–45. doi: 10.1016/j.ccl.2017.03.004. doi: 10.1016/j.ccl.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Zhu T, Si Y, Fang Y, Chen B, Yang J, Jiang J, et al. Early outcomes of the conformable stent graft for acute complicated and uncomplicated type B aortic dissection. J Vasc Surg. 2017;66:1644–52. doi: 10.1016/j.jvs.2017.04.050. doi: 10.1016/j.jvs. [DOI] [PubMed] [Google Scholar]
- 12.Sadowski M, Janion-Sadowska A, Gąsior M, Gierlotka M, Janion M, Poloński L, et al. Higher mortality in women after ST-segment elevation myocardial infarction in very young patients. Arch Med Sci. 2013;9:427–33. doi: 10.5114/aoms.2013.35324. doi: 10.5114/aoms.2013.35324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nienaber CA, Fattori R, Mehta RH, Richartz BM, Evangelista A, Petzsch M, et al. Gender-related differences in acute aortic dissection. Circulation. 2004;109:3014–21. doi: 10.1161/01.CIR.0000130644.78677.2C. doi: 10.1161/01.CIR.0000130644.78677.2C. [DOI] [PubMed] [Google Scholar]
- 14.Fukui T, Takanashi S. Gender differences in acute type a aortic dissection. J Thorac Cardiovasc Surg. 2016;151:1772–3. doi: 10.1016/j.jtcvs.2016.02.051. doi: 10.1016/j.jtcvs. [DOI] [PubMed] [Google Scholar]
- 15.Speir A. Gender differences in patients undergoing surgery for acute type A aortic dissection: Is there really a glass ceiling? J Thorac Cardiovasc Surg. 2015;150:587–8. doi: 10.1016/j.jtcvs.2015.06.038. doi: 10.1016/j.jtcvs.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 16.Takeshita S, Sakamoto S, Kitada S, Akutsu K, Hashimoto H. Angiotensin-converting enzyme inhibitors reduce long-term aortic events in patients with acute type B aortic dissection. Circ J. 2008;72:1758–61. doi: 10.1253/circj.cj-08-0466. doi: 10.1097/HJH.0b013e3283562e35. [DOI] [PubMed] [Google Scholar]
- 17.Hackam DG, Thiruchelvam D, Redelmeier DA. Angiotensin-converting enzyme inhibitors and aortic rupture: A population-based case-control study. Lancet. 2006;368:659–65. doi: 10.1016/S0140-6736(06)69250-7. doi: 10.1016/S0140-6736(06)69250-7. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki T, Isselbacher EM, Nienaber CA, Pyeritz RE, Eagle KA, Tsai TT, et al. Type-selective benefits of medications in treatment of acute aortic dissection (from the International Registry of Acute Aortic Dissection [IRAD]) Am J Cardiol. 2012;109:122–7. doi: 10.1016/j.amjcard.2011.08.012. doi: 10.1016/j.amjcard.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Kanai T, Nemoto T, Yanagita T, Maruta T, Satoh S, Yoshikawa N, et al. Nav1.7 sodium channel-induced Ca2+ influx decreases tau phosphorylation via glycogen synthase kinase-3beta in adrenal chromaffin cells. Neurochem Int. 2009;54:497–505. doi: 10.1016/j.neuint.2009.02.002. doi: 10.1016/j.neuint.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez L, Escudero C, Torralba A, Millán I. Electrophysiologic assessment of calcium channel blockers in transplanted hearts: An experimental study. J Electrocardiol. 1998;31:51–6. doi: 10.1016/s0022-0736(98)90007-3. doi: 10.1016/s0022-0736(98)90007-3. [DOI] [PubMed] [Google Scholar]
- 21.Mathisen SR, Abdelnoor M. Beneficial effect of statins on total mortality in abdominal aortic aneurysm (AAA) repair. Vasc Med. 2017;22:406–10. doi: 10.1177/1358863X17724221. doi: 10.1177/1358863X17724221. [DOI] [PubMed] [Google Scholar]
- 22.DeMartino RR, Huang Y, Mandrekar J, Goodney PP, Oderich GS, Kalra M, et al. External validation of a 5-year survival prediction model after elective abdominal aortic aneurysm repair. J Vasc Surg. 2017 doi: 10.1016/j.jvs.2017.05.104. pii: S0741-5214(17)31618-X doi: 10.1016/j.jvs.2017.05.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groeneveld ME, van der Reijden JJ, Tangelder GJ, Westin LC, Renwarin L, Musters RJP, et al. Peroxynitrite footprint in circulating neutrophils of abdominal aortic aneurysm patients is lower in statin than in non-statin users. Eur J Vasc Endovasc Surg. 2017;54:331–9. doi: 10.1016/j.ejvs.2017.06.003. doi: 10.1016/j.ejvs.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Leenders GJ, Smeets MB, van den Boomen M, Berben M, Nabben M, van Strijp D, et al. Statins promote cardiac infarct healing by modulating endothelial barrier function revealed by contrast-enhanced magnetic resonance imaging. Arterioscler Thromb Vasc Biol. 2017 doi: 10.1161/ATVBAHA.117.310339. pii: ATVBAHA117310339 doi: 10.1161/ATVBAHA.117.310339. [DOI] [PubMed] [Google Scholar]
- 25.Du R, Zhao XQ, Cai J, Cui B, Wu HM, Ye P, et al. Changes in carotid plaque tissue composition in subjects who continued and discontinued statin therapy. J Clin Lipidol. 2016;10:587–93. doi: 10.1016/j.jacl.2016.01.004. doi: 10.1016/j.jacl.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Ferretti G, Bacchetti T, Banach M, Simental-Mendía LE, Sahebkar A. Impact of statin therapy on plasma MMP-3, MMP-9, and TIMP-1 concentrations: A Systematic review and meta-analysis of randomized placebo-controlled trials. Angiology. 2017;68:850–62. doi: 10.1177/0003319716688301. doi: 10.1177/0003319716688301. [DOI] [PubMed] [Google Scholar]
- 27.Brinjikji W, Shahi V, Cloft HJ, Lanzino G, Kallmes DF, Kadirvel R, et al. Could statin use be associated with reduced recurrence rates following coiling in ruptured intracranial aneurysms? AJNR Am J Neuroradiol. 2015;36:2104–7. doi: 10.3174/ajnr.A4422. doi: 10.3174/ajnr.A4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piechota-Polanczyk A, Demyanets S, Mittlboeck M, Hofmann M, Domenig CM, Neumayer C, et al. The influence of simvastatin on NGAL, matrix metalloproteinases and their tissue inhibitors in human intraluminal thrombus and abdominal aortic aneurysm tissue. Eur J Vasc Endovasc Surg. 2015;49:549–55. doi: 10.1016/j.ejvs.2015.02.011. doi: 10.1016/j.ejvs.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Li DD, Pang HG, Song JN, Huang H, Zhang M, Zhao YL, et al. The rapid lipopolysaccharide-induced release of matrix metalloproteinases 9 is suppressed by simvastatin. Cell Biol Int. 2015;39:788–98. doi: 10.1002/cbin.10445. doi: 10.1002/cbin.10445. [DOI] [PubMed] [Google Scholar]
- 30.Ercan E. Statin treatment in dialysis patients after acute myocardial infarction improves overall mortality. Atherosclerosis. 2017;267:156–7. doi: 10.1016/j.atherosclerosis.2017.10.028. doi: 10.1016/j.atherosclerosis. [DOI] [PubMed] [Google Scholar]


