Abstract
Background:
Epidemiological studies have shown that individuals with chronic periodontitis have a significantly higher risk of developing cardiovascular complications, which might be attributed to the increased production of inflammatory cytokines initiated by the complex microbiota in dental biofilm.
Aim:
The study aims to evaluate the association between chronic periodontitis and C-reactive protein (CRP) levels in a group of hypertensive individuals in Nigeria.
Materials and Methods:
The investigator enrolled 50 hypertensive patients with chronic periodontitis into the study from the medical outpatient clinic of a teaching hospital in Lagos, Nigeria. Full-mouth periodontal examination was done to assess the participant's periodontal status, with probing depths and clinical attachment levels of six sites on all teeth. The investigator defined periodontitis as at least one interproximal site with probing depth ≥4 mm. Classification of participants into three groups was done based on their severity of periodontitis; mild (n = 16), moderate (n = 27), and severe (n = 7) periodontitis. Their CRP serum levels were measured, and the association with the severity of periodontitis was determined. P was found to be ≤ 0.05.
Results:
The median CRP levels were 1.0 (0.6, 2.2), 2.4 (1.1, 4.8), and 4.1 mg/L (3.3, 9.4) for mild, moderate, and severe chronic periodontitis, respectively. The association between the serum CRP levels and severity of periodontitis was statistically significant (P = 0.006).
Conclusion:
There was an association of elevated serum CRP level with increased severity of chronic periodontitis in hypertensive individuals. This preliminary finding among Nigerians suggests that chronic periodontal inflammation may contribute to systemic inflammatory burden in hypertensive patients.
Keywords: C-reactive protein, hypertension, Nigeria, severe chronic periodontitis
Introduction
Chronic periodontitis is an inflammatory disease of the supporting tissues, characterized by progressive attachment loss and alveolar bone loss.[1] Although plaque biofilm initiates the infection, the immune status of the host and the effectiveness of the host response are critical determinants of disease susceptibility, which influence the progression of periodontitis.[2]
This host response involves both innate and adaptive immunity.[3] While periodontitis is chronic, acute-phase elements such as C-reactive protein (CRP) are released as well as aspects of the natural resistance reflect the systemic burden of inflammation.[4] Previous studies have reported elevated CRP levels in chronic periodontitis following an acute-phase response.[5,6]
CRP is a plasma protein involved in the acute-phase response and is synthesized primarily by the liver in response to the presence of interleukin 1 and 6. It usually is present in minute quantities in human serum but may increase 100–1000-fold within 72 h of tissue injury.[7,8] Its several analytical properties make it attractive as a clinical marker.[9] These include its long half-life with no observable circadian rhythm,[10] rapid changes detected following the course of inflammation,[11] accessible measurement by blood tests, and commercially available high-sensitivity assays that provide similar results in fresh, stored, and frozen plasma.[12] The normal range for serum CRP is 0–10 mg/L.[13]
CRP was hypothesized to increase the risk of developing hypertension,[14] which has been corroborated by studies among individuals with hypertension.[15] Hypertension is the most prevalent risk factor for cardiovascular disease (CVD). Recent data conducted among multiple cohorts indicate that in individuals with normal blood pressure at baseline, CRP levels predicted the development of hypertension at follow-up.[16]
Periodontitis has been identified as a potential risk factor for systemic illnesses such as CVD, which is the leading cause of mortality in most developing nations and is predicted to continue till 2020.[17] In periodontitis, the bacterial products of dental biofilm trigger the production of circulating proinflammatory cytokines, interleukin 1, and tumor necrosis factor α. These cytokines typically mediate atherogenesis, thus contributing to the development of atherosclerosis and CVD among patients with periodontitis.[18,19,20]
CRP, been identified as an important marker of inflammation and an established independent predictor of CVD disease,[21,22] in view of this, the American Heart Association and Centers for Disease Control and Prevention (CDC) have recommended the clinical use of this marker to assess the risk of CVD.[23] Despite the body of evidence supporting an association between periodontitis and elevated CRP levels in people of different races and nationalities,[24] there is a shortage of literature in the Nigerian population. There is a need to investigate further this relationship among Nigerians considering the racial and genetic variations that exist.
The primary aim of this present study was to evaluate the serum CRP levels in individuals with hypertension and determine its relationship to the severity of periodontitis.
Materials and Methods
Study design and study population
This was a descriptive, cross-sectional study in which enrolment of hypertensive adults with chronic periodontitis was from the Cardiology Clinic. The investigator subsequently performed the periodontal assessment on them at the Periodontology Clinic of the Lagos University Teaching Hospital (LUTH). Recruitment of the participants was within a 6-month period. Ethical approval obtained from the Health Research and Ethics Committee (HREC) of the hospital (ADM/DCST/HREC/306). Each participant signed the written informed consent.
Hypertension determination was by persistent blood pressure measurement with systolic/diastolic blood pressure ≥140/90 mmHg. Inclusion criteria were patients above 18 years old, hypertension, patients on antihypertensive medications, diagnosis of chronic periodontitis, and presence of at least 20 natural teeth. Exclusion criteria included patients with systemic-modifying conditions for periodontitis (pregnancy, diabetes mellitus, chronic inflammatory or immunological conditions, i.e., arthritis, gastrointestinal disorder, skin conditions, bronchitis, or other chronic obstructive airway diseases). Also excluded were current smokers, patients who had mechanical/surgical therapy in the preceding 6 months, and those with prior use of gingival tissue modifying medications (oral contraceptives, cyclosporine, and phenytoin). Identification of the participants with chronic inflammatory conditions and concomitant infections were through detailed medical history, drug history, and clinical presentation.
Data collection
Consecutive hypertensive patients who attended the cardiology outpatient clinic of the hospital and met the inclusion criteria were initially selected using the community periodontal index probe, and those with periodontitis enrolled until the required sample size achieved. Interviewer-administered questionnaires were used to record the patient's sociodemographic data: age, gender, occupation, ethnicity, education, marital status, and duration of hypertension.
All the clinical examinations were carried out by a single investigator. The intraexaminer's reliability was 0.8 using kappa statistics. The periodontal clinical parameters utilized in this study were bleeding on probing (BOP), periodontal pocket depth, and clinical attachment level using the Williams periodontal probe.
Classification of periodontitis was performed according to the CDC and American Academy of Periodontology criteria. Moderate periodontitis was characterized by the presence of ≥2 interproximal sites with a clinical attachment loss of ≥4 mm occurring on ≥2 different teeth, or ≥2 interproximal sites with a probing depth of ≥5 mm, on different teeth.[25] Severe periodontitis was characterized by the presence of ≥2 interproximal sites with a clinical attachment loss of ≥6 mm, on different teeth, and the presence of one or more interproximal sites with a probing depth of ≥5 mm on different teeth. The investigator classified periodontitis that did not fit the first two categories into mild periodontitis.
Serum concentration of CRP was measured in milligrams per liter using the immunoturbidimetry method (Roche Hitachi C311 Autoanalyzer) and processed at the AIDS Prevention Initiative of Nigeria Laboratory, LUTH.
Statistical analysis
Data analysis was performed using the Statistical Package for Social Sciences (SPSS) version 20.0 (IBM SPSS for windows, Armonk, New York: IBM Corp).
Categorical variables reported such as frequencies and associations were determined using the Chi-square test. Continuous variables with normal distribution were described by mean ± standard deviation while medians and interquartile ranges were for continuous variables with skewed distribution. Kruskal–Wallis hypothesis testing was used to compare median CRP with the severity of periodontitis. All P < 0.05 was considered statistically significant at the 5% significance level (α = 0.05).
Results
Sociodemographics of the participants
The individuals eligible for the study were 50 comprising 11 (22.0%) males and 39 (78.0%) females, with male to female ratio 1:3.5. Mean age was 52.4 ± 6.96 years (range 37–73 years). No significant difference was observed between the mean age of females (52.6 ± 6.36 years) and males (51.8 ± 9.11 years). Nearly half (46%) of the study participants were in the 50–59 age group [Table 1].
Table 1.
Sociodemographic characteristics of the participants
The mean systolic and diastolic blood pressures for the participants were 147.4 ± 9.46 mmHg and 94.3 ± 14.66 mmHg, respectively, as shown in Figure 1.
Figure 1.
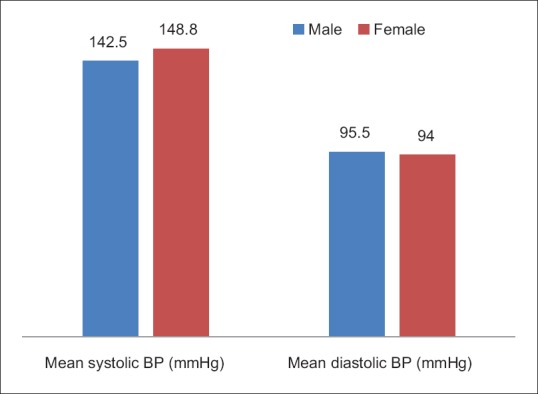
Blood pressure by gender of the participants
Distribution of chronic periodontitis among participants
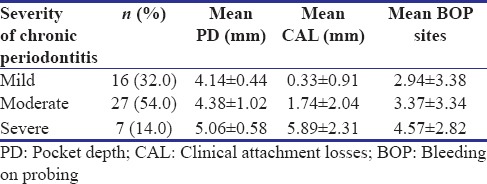
There was a steady increase in the mean pocket depth, mean clinical attachment level, and mean BOP sites, from the mild, moderate, to severe forms of chronic periodontitis, as shown in Table 2.
Table 2.
Severity of chronic periodontitis among participants
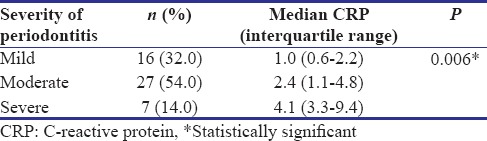
Relationship between C-reactive protein with severity of periodontitis
The median CRP levels of participants with mild, moderate, and severe chronic periodontitis were 1.0 (0.6, 2.2), 2.4 (1.1, 4.8), and 4.1 (3.3, 9.4), respectively. The difference between the three groups was statistically significant (P = 0.006) as shown in Table 3.
Table 3.
Comparison of C-reactive protein with severity of periodontitis
There was a definite correlation between the median CRP and BOP sites, as shown in Table 4.
Table 4.
Correlation between median C-reactive protein and mean bleeding on probing sites

Discussion
The study of the relationship between periodontitis and hypertension is poor among Nigerians. Reports from clinical and epidemiological studies have highlighted the role of periodontitis as a risk factor for CVDs.[26,27,28] However, the mechanisms by which periodontitis is related to CVD are unclear. Periodontitis-induced systemic inflammation through Gram-negative bacteria contributes to the development and maintenance of atherosclerosis through activation of a biochemical reaction cascade, which may, in turn, contribute to the initiation and development of plaque formation and injury to the endothelium.
Hypertensive individuals were the focus of this study because hypertension is no doubt one of the most prevalent CVDs in Nigeria[29] and is also a risk factor for other CVDs such as stroke, heart failure, and ischemic heart disease.[30] Furthermore, hypertension is associated with atherosclerosis which is considered a chronic inflammatory state.[31] Thus, inflammation may contribute to a rise in blood pressure by promoting changes in the endothelium.
In this study, the reported preponderance of female individuals was attributed to the higher prevalence of hypertension in females,[32] hence their presence at the clinic. Other studies have however reported no significant difference between males and females[33,34] or found a higher prevalence of hypertension in men compared with women.[35,36] The more elevated systolic and diastolic blood pressures observed among the female participants in this present study are also ascribed to the menopausal attainment at their age and the reduced effect of the anti-inflammatory (cardioprotective) effect of estrogen (which keeps the blood vessels flexible).[37] This present study also bolsters the prediction by Kearney et al.[38] of a higher female predominance with hypertension by the year 2025.
This predominant age group of 50–59 years noticed in the present study supports existing reports of hypertension in older age.[39] This finding may be due to age-related changes in hormone profile, thickening of the walls of arteries, as well as the decreased efficiency of the heart and kidneys.[40,41] The secondary level of education obtained by most of the individuals is consistent with the urban location of the hospital which serves as a referral center for other peripheral clinics.
There was a steady increase in the median CRP level from mild to severe periodontitis. This trend was significant, and the finding is in agreement with the previous studies,[42,43] but contrary to the past research by Baser et al.[44] We postulate that there may have been an increased CRP production from the liver in response to the enhanced cytokine production in the stage of severe periodontitis. Furthermore, there was a definite correlation between the number of sites that bled on probing and the CRP levels which signify the presence of an active infection. This finding is similar to a previous study by Salzberg et al.[45]
This study is limited however by the number of individuals, which was relatively small; hence, a prospective study using larger sample size is needed to demonstrate the true nature of these relationships.
Conclusion
There was a significant association between the severity of chronic periodontitis in the hypertensive individuals with elevated CRP levels. Thus, increased CRP levels in this category of hypertensive participants may place them at a higher risk for CVD, and this further highlights the need for better collaboration between physicians and dentists.
Recommendations
Hypertensive individuals should receive periodontal screening through regular dental visits or oral health screening at their medical outpatient clinics. CRP monitoring is essential in the management of hypertensive patients, especially those with chronic periodontitis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Mr. Tony Ani is acknowledged for his technical support for the CRP laboratory analysis. Dr. Opeyemi Obilade is acknowledged for his support for the statistical analysis.
References
- 1.Flemmig TF. Periodontitis. Ann Periodontol. 1999;4:32–8. doi: 10.1902/annals.1999.4.1.32. [DOI] [PubMed] [Google Scholar]
- 2.Masamatti S, Virdi M, Kumar A. Host modulation therapy: A novel approach in the treatment of periodontal diseases. Internet J Dent Sci. 2009;9:1–8. [Google Scholar]
- 3.Seymour GJ. Importance of the host response in the periodontium. J Clin Periodontol. 1991;18:421–6. doi: 10.1111/j.1600-051x.1991.tb02310.x. [DOI] [PubMed] [Google Scholar]
- 4.Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76:2106–15. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- 5.Buhlin K, Gustafsson A, Pockley AG, Frostegård J, Klinge B. Risk factors for cardiovascular disease in patients with periodontitis. Eur Heart J. 2003;24:2099–107. doi: 10.1016/j.ehj.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Craig RG, Yip JK, So MK, Boylan RJ, Socransky SS, Haffajee AD, et al. Relationship of destructive periodontal disease to the acute-phase response. J Periodontol. 2003;74:1007–16. doi: 10.1902/jop.2003.74.7.1007. [DOI] [PubMed] [Google Scholar]
- 7.Du Clos TW. Function of C-reactive protein. Ann Med. 2000;32:274–8. doi: 10.3109/07853890009011772. [DOI] [PubMed] [Google Scholar]
- 8.Claus DR, Osmand AP, Gewurz H. Radioimmunoassay of human C-reactive protein and levels in normal sera. J Lab Clin Med. 1976;87:120–8. [PubMed] [Google Scholar]
- 9.Libby P, Aikawa M. Stabilization of atherosclerotic plaques: New mechanisms and clinical targets. Nat Med. 2002;8:1257–62. doi: 10.1038/nm1102-1257. [DOI] [PubMed] [Google Scholar]
- 10.Meier-Ewert HK, Ridker PM, Rifai N, Price N, Dinges DF, Mullington JM, et al. Absence of diurnal variation of C-reactive protein concentrations in healthy human subjects. Clin Chem. 2001;47:426–30. [PubMed] [Google Scholar]
- 11.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 12.Rifai N, Tracy RP, Ridker PM. Clinical efficacy of an automated high-sensitivity C-reactive protein assay. Clin Chem. 1999;45:2136–41. [PubMed] [Google Scholar]
- 13.Ojo A, Jacob SJ, Ayolabi CI. C-reactive protein in tuberculosis and human immunodeficiency virus infection in Abeokuta, Nigeria. Bayero J Pure Appl Sci. 2011;4:91–6. [Google Scholar]
- 14.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM, et al. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290:2945–51. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 15.Lakoski SG, Cushman M, Palmas W, Blumenthal R, D’Agostino RB, Jr, Herrington DM, et al. The relationship between blood pressure and C-reactive protein in the multi-ethnic study of atherosclerosis (MESA) J Am Coll Cardiol. 2005;46:1869–74. doi: 10.1016/j.jacc.2005.07.050. [DOI] [PubMed] [Google Scholar]
- 16.Hage FG. C-reactive protein and hypertension. J Hum Hypertens. 2014;28:410–5. doi: 10.1038/jhh.2013.111. [DOI] [PubMed] [Google Scholar]
- 17.Celermajer DS, Chow CK, Marijon E, Anstey NM, Woo KS. Cardiovascular disease in the developing world: Prevalences, patterns, and the potential of early disease detection. J Am Coll Cardiol. 2012;60:1207–16. doi: 10.1016/j.jacc.2012.03.074. [DOI] [PubMed] [Google Scholar]
- 18.Tousoulis D, Charakida M, Stefanadis C. Endothelial function and inflammation in coronary artery disease. Heart. 2006;92:441–4. doi: 10.1136/hrt.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young JL, Libby P, Schönbeck U. Cytokines in the pathogenesis of atherosclerosis. Thromb Haemost. 2002;88:554–67. [PubMed] [Google Scholar]
- 20.Geerts SO, Nys M, De MP, Charpentier J, Albert A, Legrand V, et al. Systemic release of endotoxins induced by gentle mastication: Association with periodontitis severity. J Periodontol. 2002;73:73–8. doi: 10.1902/jop.2002.73.1.73. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 23.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the centers for disease control and prevention and the American heart association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 24.Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E, et al. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol. 2001;72:1221–7. doi: 10.1902/jop.2000.72.9.1221. [DOI] [PubMed] [Google Scholar]
- 25.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78:1387–99. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 26.Morrison HI, Ellison LF, Taylor GW. Periodontal disease and risk of fatal coronary heart and cerebrovascular diseases. J Cardiovasc Risk. 1999;6:7–11. doi: 10.1177/204748739900600102. [DOI] [PubMed] [Google Scholar]
- 27.Humphrey LL, Fu R, Buckley DI, Freeman M, Helfand M. Periodontal disease and coronary heart disease incidence: A systematic review and meta-analysis. J Gen Intern Med. 2008;23:2079–86. doi: 10.1007/s11606-008-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Academy of Periodontology. Periodontal Disease Linked to Cardiovascular Disease; April. 2012. [Last accessed on 2012 Dec 11]. Available from: http://www.perio.org/consumer/AHA-statement .
- 29.Geneva: World Health Organization; 2002. [Last accessed on 2009 Nov 11]. World Health Organization: World Health Report. Reducing Risks, Promoting Healthy Life. Available from: http://www.who.Int/whr/2002/en/index.html . [Google Scholar]
- 30.Ogah OS, Okpechi I, Chukwuonye II, Akinyemi JO, Onwubere BJ, Falase AO, et al. Blood pressure, prevalence of hypertension and hypertension related complications in Nigerian Africans: A review. World J Cardiol. 2012;4:327–40. doi: 10.4330/wjc.v4.i12.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 32.Adedoyin RA, Mbada CE, Balogun MO, Martins T, Adebayo RA, Akintomide A, et al. Prevalence and pattern of hypertension in a semiurban community in Nigeria. Eur J Cardiovasc Prev Rehabil. 2008;15:683–7. doi: 10.1097/HJR.0b013e32830edc32. [DOI] [PubMed] [Google Scholar]
- 33.Lawoyin TO, Asuzu MC, Kaufman J, Rotimi C, Owoaje E, Johnson L, et al. Prevalence of cardiovascular risk factors in an African, Urban inner city community. West Afr J Med. 2002;21:208–11. doi: 10.4314/wajm.v21i3.28031. [DOI] [PubMed] [Google Scholar]
- 34.Amole IO, OlaOlorun AD, Odeigah LO, Adesina SA. The prevalence of abdominal obesity and hypertension among adults in Ogbomosho, Nigeria. Afr J Prim Health Care Fam Med. 2011;3:188–92. [Google Scholar]
- 35.Sani MU, Wahab KW, Yusuf BO, Gbadamosi M, Johnson OV, Gbadamosi A, et al. Modifiable cardiovascular risk factors among apparently healthy adult Nigerian population – A cross sectional study. BMC Res Notes. 2010;3:11. doi: 10.1186/1756-0500-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahaneku GI, Osuji CU, Anisiuba BC, Ikeh VO, Oguejiofor OC, Ahaneku JE, et al. Evaluation of blood pressure and indices of obesity in a typical rural community in Eastern Nigeria. Ann Afr Med. 2011;10:120–6. doi: 10.4103/1596-3519.82076. [DOI] [PubMed] [Google Scholar]
- 37.Yang XP, Reckelhoff JF. Estrogen, hormonal replacement therapy and cardiovascular disease. Curr Opin Nephrol Hypertens. 2011;20:133–8. doi: 10.1097/MNH.0b013e3283431921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J, et al. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 39.Heitz-Mayfield LJ, Schätzle M, Löe H, Bürgin W, Anerud A, Boysen H, et al. Clinical course of chronic periodontitis. II. Incidence, characteristics and time of occurrence of the initial periodontal lesion. J Clin Periodontol. 2003;30:902–8. doi: 10.1034/j.1600-051x.2003.00399.x. [DOI] [PubMed] [Google Scholar]
- 40.Jani B, Rajkumar C. Ageing and vascular ageing. Postgrad Med J. 2006;82:357–62. doi: 10.1136/pgmj.2005.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kosugi T, Nakagawa T, Kamath D, Johnson RJ. Uric acid and hypertension: An age-related relationship? J Hum Hypertens. 2009;23:75–6. doi: 10.1038/jhh.2008.110. [DOI] [PubMed] [Google Scholar]
- 42.Pejcic A, Kesic L, Milasin J. Association between periodontopathogens and CRP levels in patients with periodontitis in Serbia. J Dent Res Dent Clin Dent Prospects. 2011;5:10–6. doi: 10.5681/joddd.2011.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slade GD, Ghezzi EM, Heiss G, Beck JD, Riche E, Offenbacher S, et al. Relationship between periodontal disease and C-reactive protein among adults in the atherosclerosis risk in communities study. Arch Intern Med. 2003;163:1172–9. doi: 10.1001/archinte.163.10.1172. [DOI] [PubMed] [Google Scholar]
- 44.Baser U, Oztekin G, Ademoglu E, Isik G, Yalcin F. Is the severity of periodontitis related to gingival crevicular fluid and serum high-sensitivity C-reactive protein concentrations? Clin Lab. 2014;60:1653–8. doi: 10.7754/clin.lab.2014.131217. [DOI] [PubMed] [Google Scholar]
- 45.Salzberg TN, Overstreet BT, Rogers JD, Califano JV, Best AM, Schenkein HA, et al. C-reactive protein levels in patients with aggressive periodontitis. J Periodontol. 2006;77:933–9. doi: 10.1902/jop.2006.050165. [DOI] [PubMed] [Google Scholar]


