Abstract
Background:
Indirect pulp treatment (IPT) is a minimally invasive procedure based on the application of a protective liner on the affected dentin, thereby preserving the pulp vitality.
Aim:
This study aims to evaluate and compare the clinical and radiographic outcomes of IPT when a layer of calcium hydroxide (Dycal), mineral trioxide aggregate (MTA), or Biodentine was placed over the affected dentin in primary molars.
Materials and Methods:
A clinical trial with sample size of 45 primary molars between the age group of 4–9 years, of which 15 teeth were considered, each for Group I (Dycal), Group II (MTA), and Group III (Biodentine). Measurements on digitized radiographs were made at baseline, 3, and 6 months using Corel Draw software.
Results:
One-way ANOVA and post hoc tests indicated a statistically significant difference in dentin thickness (P < 0.05) in all the groups. Within Group I, the thickness of dentin was 0.066 ± 0.009 mm at 3 months and 0.099 ± 0.011 mm at 6 months. In Group II, 0.081 ± 0.010 mm at 3 months and 0.123 ± 0.016 mm at 6 months. In Group III, 0.102 ± 0.021 mm at 3 months and 0.154 ± 0.022 mm at 6 months.
Conclusions:
Clinically, 100% success rate was observed in all the groups whereas radiographically, Biodentine was superior to both the groups.
Keywords: Biodentine, Dycal, indirect pulp treatment, mineral trioxide aggregate, remaining dentine thickness
Introduction
Indirect pulp treatment (IPT) is a minimally invasive approach based on the maintenance of the inner portion of carious dentin, a vital tissue that contains intact collagen and is able to remineralize.[1] IPT is a form of a vital pulp therapy (VPT) which operates on the ground of maintenance of primary teeth until physiological exfoliation.[2] Historically, calcium hydroxide has served as a gold standard for IPT over the years. However, introduction of newer bioactive materials such as mineral trioxide aggregate (MTA) and Biodentine helped surpass the demerits of calcium hydroxide such as internal resorption, nonadherence to dentin, degradation over time, tunnel defects, and poor sealing ability.[3]
MTA is essentially composed of a mixture of tricalcium silicate, dicalcium silicate, tricalcium aluminate, and calcium oxide with an addition of bismuth oxide in a 4:1 ratio. This material appears to be successful because of its small particle size, sealing ability, alkaline pH when set, and slow release of calcium ions. It also induces pulp cell proliferation, cytokine release, and subsequent hard tissue formation with the synthesis of a mineralized dentin similar to that of biological hydroxyapatite.[4]
Biodentine, a new tricalcium silicate-based cement, has recently been commercialized and advertised as a bioactive material and pulp-capping agent. The main benefits of Biodentine over other products are reduced setting time, better mechanical properties, and great sealing ability. Its clinical application and physical properties have been widely described although so far few clinical studies have evaluated its efficacy as a pulp-capping agent.[5]
Therefore, the present study was undertaken to clinically and radiographically evaluate and compare the outcomes of Dycal, MTA, and Biodentine as IPT agents in primary molars.
Materials and Methods
This in vivo study was carried out in a sample of 45 primary molars selected from 22 healthy children aged 4–9 years from the Outpatient Department of Paedodontics and Preventive Dentistry, Himachal Institute of Dental Sciences, Paonta Sahib, District Sirmaur, Himachal Pradesh. These teeth were randomly divided into three groups (n = 15) depending on the type of material used [Figure 1].
Figure 1.
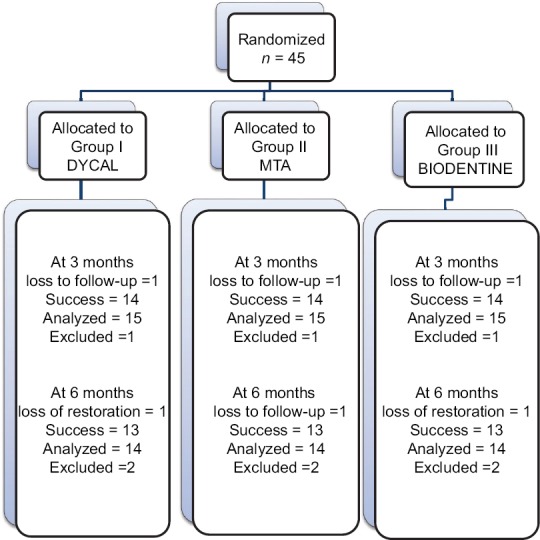
Sample collection and distribution
Inclusion criteria
Mild discomfort from chemical and thermal stimuli
Presence of active carious lesions involving either occlusal or proximal surfaces of primary molars
Extension of carious lesion such that complete caries removal would risk pulp exposure
Cooperative children and parents willing to follow the instructions and report for follow-up.
Exclusion criteria
History of spontaneous sharp, penetrating pain, or tenderness on percussion
Presence of abnormal tooth mobility, fistula, interrupted lamina dura, internal or external root resorption, interradicular or periapical pathosis, and widened periodontal ligament space
Presence of chronic systemic diseases such as congenital or rheumatic heart disease, hepatitis, and leukemia
On long-term medication such as corticosteroid therapy
Physically or mentally challenged.
The Institutional Ethical Clearance was obtained and patient's parents were informed about the procedure and a written consent was obtained both in English and Hindi.
Before the commencement of the procedure, a diagnostic preoperative IOPAR was obtained of the tooth in question. Initially, topical anesthetic was applied at the site of injection followed by nerve block for adequate anesthetization of selected tooth (2% lignocaine 1.5 ml). All the cases were treated under rubber dam isolation. The carious lesion was removed using a two-step procedure: (1) removal of the carious enamel and dentin from the lateral walls using a high-speed #330 round diamond bur and (2) manual dentin curettage using a sharp spoon excavator keeping in mind the removal of only infected dentin.
In Group I, Dycal was mixed for 10 s by extruding equal volumes of both base and catalyst paste on the mixing pad until a uniform color was achieved which was then applied to the floor of the cavity with a ball-ended condenser. In Group II, 1 sachet of MTA was mixed with 1 drop of distilled water for 30 s to a consistency similar to wet sand as per the manufacturer's instructions. The cement was carried in a sterilized amalgam carrier and applied to the site with a ball-ended condenser. In Group III, 5 drops of liquid from the single-dose container were poured into the capsule containing powder. The capsule was placed in a triturator for 30 s as per manufacturer's guidelines. The cement was carried in a sterilized amalgam carrier and applied to the site with a ball-ended condenser. The final restoration was done with Type II glass ionomer cement (GIC) in all the groups.
Immediately, a baseline digitized radiograph with grid was obtained. A metallic mesh grid with calibrations 1 mm × 1 mm was placed in contact with the RVG sensor during exposure, so as to obtain a radiographic image of known size. Radiographically, the criterion for measurement of baseline was recorded as the vertical distance between the highest point of the pulp horn (a) to the base of the restoration (b) [Figure 2].
Figure 2.
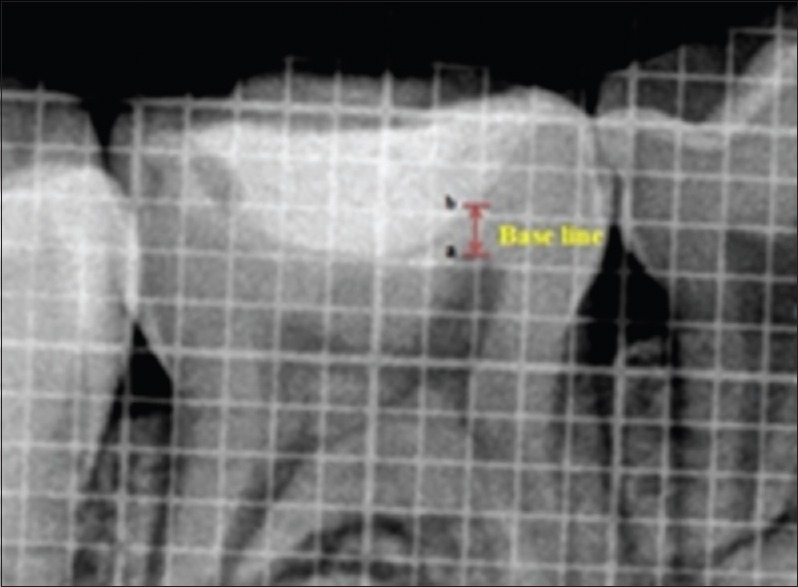
The baseline, i.e. the vertical distance between the highest point of the pulp horn (a) to the base of the restoration (b)
On recall visits, the patients were evaluated clinically for the presence of pain, tenderness, loss of restoration, abnormal tooth mobility, or presence of sinuses in relation to the tooth in question and recorded as yes or no at 3 and 6 months, respectively. Radiographically, the treatment was considered successful when no signs of periodontal space widening, interrupted lamina dura, interradicular radiolucency, and furcation involvement were seen. In cases showing no clinical and radiological signs of failure, the amount of dentine-bridge formed at the end of 3 and 6 months was evaluated radiographically.
To standardize the serial digitized radiographs obtained, the angulation of the X-ray tube and head position of the participant was kept constant. All the digitized radiographs were subsequently transferred to the computer for measurements of dentin thickness using CorelDraw software. The increment in the vertical distance was recorded as the amount of tertiary dentin deposited at 3 and 6 months, respectively, under a magnification of 200%. These measurements were performed by an investigator (PG student) who was blind to the clinical procedure and the nature of the pulp-capping material. The data obtained were statistically analyzed using one-way ANOVA and Tukey's post hoc test. Statistical significance was defined as P < 0.05.
Results
Out of 45 primary molars, six were excluded as four participants did not turn up for follow-up and two reported with a loss of final restoration on the recall visit. Hence, at the end of the study, 39 primary molars were available with complete follow-up. Clinically, 100% success rate was observed as none of the patients reported with a complaint of pain, tenderness, mobility, swelling, and sinus formation in relation to the teeth treated. Radiographically, a statistically significant difference in dentin thickness was observed in all the groups [Table 1]. Highest increment of dentin deposited was observed in Group III (Biodentine), at the end of 3 and 6 months, respectively. It was also observed that the increment of dentin deposited in the first 3 months was higher than at the second 3 months in all the groups [Table 2 and Figure 3].
Table 1.
Average tertiary dentin deposited in all the three groups
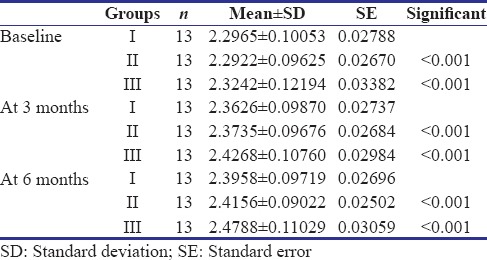
Table 2.
Intergroup increment in dentin deposited at the end of 3 months, 6 months, and between 3 and 6 months in all the groups, respectively
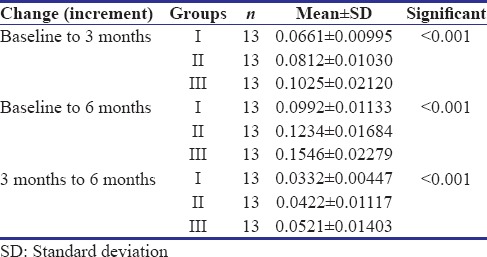
Figure 3.
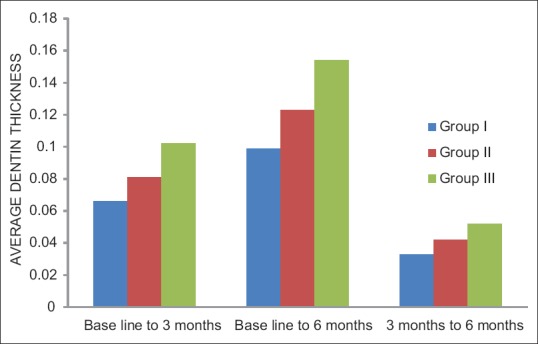
Intergroup increment in dentin deposited at the end of 3 months, 6 months, and between 3 and 6 months in all the groups, respectively
Discussion
The rationale for IPT is based on the observation that postmitotic odontoblasts can be induced to upregulate their synthetic and secretory activities in response to reduced infectious challenge. This results in deposition of a tertiary dentin matrix that has the effect of increasing the distance between the caries and the pulp that results in decreased dentin permeability.[6] IPC is credited to show a long-term success of 3–4 years over other alternatives of VPT such as pulpotomy in primary molars.[7,8]
Dycal is regarded as the gold standard for pulp-capping procedures as it possesses antibacterial properties, which can minimize or eliminate bacterial penetration into the pulp. In its pure form, the substance has a high pH that stimulates reparative dentinogenesis which is explained by the release of bioactive molecules.[9] Dycal is known to solubilize bone morphogenic protein and transforming growth factor-beta 1 from dentin, lending credence to the release of these bioactive molecules as a significant mediator in pulp repair following pulp capping.[10,11]
However, studies have indicated a declining rate of pulp survival over time after pulp capping with calcium hydroxide. The nonadhesive nature of the cement and its dissolution over time may lead to microleakage and entry of bacteria into the dentin–pulp complex.[12] In comparison to Dycal, MTA is known to have excellent sealing ability and no signs of solubility in water.[13] It is available as gray MTA (GMTA) and white MTA (WMTA). WMTA and GMTA differ mainly in their content of iron oxide.[14] Both types of GMTA and WMTA can be suggested as the material of choice for pulp capping.[15] The primary reaction product of MTA with water is calcium hydroxide, as a result, many of the advantages and potential mechanisms of action for MTA are similar to Dycal.[16]
In our study, a 100% clinical success rate was observed with Dycal and MTA as IPT agents. Radiographically, the average dentin thickness was found to be statistically significant in both the groups. However, the increment in dentin bridge was more for MTA than Dycal. Similar results were reported by Leye Benoist et al.,[17] Nair et al.,[18] and Aeinehchi et al.[19] MTA's success is likely because it serves as a reservoir for calcium hydroxide.[20] Takita et al.[21] showed that concentration increased approximately 0.3 mmol/L in MTA and essentially does not change with Dycal. However, MTA is reportedly difficult to use because of its long setting time, poor handling properties, and the discoloration potential.[22]
Based on the outstanding biological properties of Portland cements, new calcium silicate-based cement called Biodentine has been developed. The powder is mainly composed of tricalcium silicate, calcium carbonate, and zirconium oxide. The liquid contains water, calcium chloride, and modified polycarboxylate.
The main benefits of Biodentine over other products are reduced setting time (12 min), better mechanical properties, and great sealing ability. Arora et al.[23] stated that the sealing ability of Biodentine was similar to apatite crystals under scanning electron microscope; therefore, it can be used as a promising agent in VPT. Peng et al.[24] stated that stimulation of cell proliferation and differentiation might be enhanced due to the presence of silicon ions in Biodentine. In our study, a 100% clinical success rate was also observed in Biodentine group as well. However, the increment in dentin bridge formed was highest for Biodentine among all the groups.
It was also observed that the rate of dentin bridge formation was more in the first 3 months as compared to the last 3 months in all the groups. This may be explained on the basis that dentin is laid down fastest during the 1st month following IPT and the rate then reduces steadily with time.[25] Tran et al.[26] demonstrated that the percentage porosity of dentin bridge formed by Biodentine after 14 and 30 days were significantly comparable and better than Dycal. Nowicka et al.[22] also stated that Biodentine may be considered an interesting alternative to MTA during pulp capping as it had similar efficacy in the clinical setting.
The clinical application and physical properties of Biodentine have been widely described in permanent teeth, although so far few clinical studies have evaluated its efficacy as a pulp capping agent in primary teeth. Our study is the first one to confirm its usefulness as an IPT agent in primary teeth.
Another factor that influences the success of IPT is the final restorative material. The bacterial leakage through the final restoration is considered to be highly detrimental.[27] Nandini et al.[28] stated that the placement of GIC over MTA after 45 min did not affect its setting reaction and calcium salts may be formed in the interface of these two materials. Ashou et al.[29] demonstrated that GIC can be successfully applied over Biodentine as final restorative material due to its high shear bond strength (17–20 MPa).
Conclusions
Based on the results of our study, a 100% clinical success was recorded in all the groups. However, radiographically, the highest amount of tertiary dentin deposited was recorded with Biodentine. The amount of tertiary dentin deposited was more in the first 3 months than the last 3 months in all the groups. However, further research with larger samples and a longer follow-up is warranted. Additional histological investigations are needed to support these findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ribeiro CC, de Oliveira Lula EC, da Costa RC, Nunes AM. Rationale for the partial removal of carious tissue in primary teeth. Pediatr Dent. 2012;34:39–41. [PubMed] [Google Scholar]
- 2.Fuks AB. Current concepts in vital primary pulp therapy. Eur J Paediatr Dent. 2002;3:115–20. [PubMed] [Google Scholar]
- 3.Song M, Yu B, Kim S, Hayashi M, Smith C, Sohn S, et al. Clinical and molecular perspectives of reparative dentin formation: Lessons learned from pulp-capping materials and the emerging roles of calcium. Dent Clin North Am. 2017;61:93–110. doi: 10.1016/j.cden.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, Ford TR, et al. The constitution of mineral trioxide aggregate. Dent Mater. 2005;21:297–303. doi: 10.1016/j.dental.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Zanini M, Sautier JM, Berdal A, Simon S. Biodentine induces immortalized murine pulp cell differentiation into odontoblast-like cells and stimulates biomineralization. J Endod. 2012;38:1220–6. doi: 10.1016/j.joen.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Tziafas D, Smith AJ, Lesot H. Designing new treatment strategies in vital pulp therapy. J Dent. 2000;28:77–92. doi: 10.1016/s0300-5712(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 7.Farooq NS, Coll JA, Kuwabara A, Shelton P. Success rates of formocresol pulpotomy and indirect pulp therapy in the treatment of deep dentinal caries in primary teeth. Pediatr Dent. 2000;22:278–86. [PubMed] [Google Scholar]
- 8.Vij R, Coll JA, Shelton P, Farooq NS. Caries control and other variables associated with success of primary molar vital pulp therapy. Pediatr Dent. 2004;26:214–20. [PubMed] [Google Scholar]
- 9.Foreman PC, Barnes IE. Review of calcium hydroxide. Int Endod J. 1990;23:283–97. doi: 10.1111/j.1365-2591.1990.tb00108.x. [DOI] [PubMed] [Google Scholar]
- 10.Hilton TJ. Keys to clinical success with pulp capping: A review of the literature. Oper Dent. 2009;34:615–25. doi: 10.2341/09-132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham L, Cooper PR, Cassidy N, Nor JE, Sloan AJ, Smith AJ, et al. The effect of calcium hydroxide on solubilisation of bio-active dentine matrix components. Biomaterials. 2006;27:2865–73. doi: 10.1016/j.biomaterials.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Sangwan P, Sangwan A, Duhan J, Rohilla A. Tertiary dentinogenesis with calcium hydroxide: A review of proposed mechanisms. Int Endod J. 2013;46:3–19. doi: 10.1111/j.1365-2591.2012.02101.x. [DOI] [PubMed] [Google Scholar]
- 13.Ford TR, Torabinejad M, Abedi HR, Bakland LK, Kariyawasam SP. Using mineral trioxide aggregate as a pulp-capping material. J Am Dent Assoc. 1996;127:1491–4. doi: 10.14219/jada.archive.1996.0058. [DOI] [PubMed] [Google Scholar]
- 14.Asgary S, Parirokh M, Eghbal MJ, Brink F. Chemical differences between white and gray mineral trioxide aggregate. J Endod. 2005;31:101–3. doi: 10.1097/01.don.0000133156.85164.b2. [DOI] [PubMed] [Google Scholar]
- 15.Eskandarizadeh A, Shahpasandzadeh MH, Shahpasandzadeh M, Torabi M, Parirokh M. A comparative study on dental pulp response to calcium hydroxide, white and grey mineral trioxide aggregate as pulp capping agents. J Conserv Dent. 2011;14:351–5. doi: 10.4103/0972-0707.87196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fridland M, Rosado R. MTA solubility: A long term study. J Endod. 2005;31:376–9. doi: 10.1097/01.don.0000140566.97319.3e. [DOI] [PubMed] [Google Scholar]
- 17.Leye Benoist F, Gaye Ndiaye F, Kane AW, Benoist HM, Farge P. Evaluation of mineral trioxide aggregate (MTA) versus calcium hydroxide cement (Dycal (®)) in the formation of a dentine bridge: A randomised controlled trial. Int Dent J. 2012;62:33–9. doi: 10.1111/j.1875-595X.2011.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nair PN, Duncan HF, Pitt Ford TR, Luder HU. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: A randomized controlled trial. Int Endod J. 2008;41:128–50. doi: 10.1111/j.1365-2591.2007.01329.x. [DOI] [PubMed] [Google Scholar]
- 19.Aeinehchi M, Eslami B, Ghanbariha M, Saffar AS. Mineral trioxide aggregate (MTA) and calcium hydroxide as pulp-capping agents in human teeth: A preliminary report. Int Endod J. 2003;36:225–31. doi: 10.1046/j.1365-2591.2003.00652.x. [DOI] [PubMed] [Google Scholar]
- 20.Gandolfi MG, Siboni F, Prati C. Chemical-physical properties of theraCal, a novel light-curable MTA-like material for pulp capping. Int Endod J. 2012;45:571–9. doi: 10.1111/j.1365-2591.2012.02013.x. [DOI] [PubMed] [Google Scholar]
- 21.Takita T, Hayashi M, Takeichi O, Ogiso B, Suzuki N, Otsuka K, et al. Effect of mineral trioxide aggregate on proliferation of cultured human dental pulp cells. Int Endod J. 2006;39:415–22. doi: 10.1111/j.1365-2591.2006.01097.x. [DOI] [PubMed] [Google Scholar]
- 22.Nowicka A, Lipski M, Parafiniuk M, Sporniak-Tutak K, Lichota D, Kosierkiewicz A, et al. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J Endod. 2013;39:743–7. doi: 10.1016/j.joen.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Arora V, Nikhil V, Sharma N, Arora P. Bioactive dentin replacement. J Comput Eng J Dent Med Sci. 2013;12:51–7. [Google Scholar]
- 24.Peng W, Liu W, Zhai W, Jiang L, Li L, Chang J, et al. Effect of tricalcium silicate on the proliferation and odontogenic differentiation of human dental pulp cells. J Endod. 2011;37:1240–6. doi: 10.1016/j.joen.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 25.Cohen S, Burns RC. Pediatric endodontic treatment. In: Cohen S, Burns RC, editors. Pathways of the Pulp. 6th ed. St. Louis: Elsevier; 1994. pp. 633–71. [Google Scholar]
- 26.Tran XV, Gorin C, Willig C, Baroukh B, Pellat B, Decup F, et al. Effect of a calcium-silicate-based restorative cement on pulp repair. J Dent Res. 2012;91:1166–71. doi: 10.1177/0022034512460833. [DOI] [PubMed] [Google Scholar]
- 27.George V, Janardhanan SK, Varma B, Kumaran P, Xavier AM. Clinical and radiographic evaluation of indirect pulp treatment with MTA and calcium hydroxide in primary teeth (in-vivo study) J Indian Soc Pedod Prev Dent. 2015;33:104–10. doi: 10.4103/0970-4388.155118. [DOI] [PubMed] [Google Scholar]
- 28.Nandini S, Ballal S, Kandaswamy D. Influence of glass-ionomer cement on the interface and setting reaction of mineral trioxide aggregate when used as a furcal repair material using laser raman spectroscopic analysis. J Endod. 2007;33:167–72. doi: 10.1016/j.joen.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Ashou W, Nayif MM, Yahya MM. Shear bond strength of glass and resin based restorative materials to calcium based cement (biodentine) Int J Enhanced Res Sci Technol Eng. 2014;3:400–4. [Google Scholar]


