Abstract
Preterm birth is a global health issue that can induce lifelong medical sequela. Presently, at least one in ten newborns are born prematurely. At birth, preterm newborns exhibit higher levels of oxidative stress (OS) due to the inability to face the oxygen rich environment in which they are born into. Moreover, their immature respiratory, digestive, immune and antioxidant defense systems, as well as the potential numerous medical interventions following a preterm birth, such as oxygen resuscitation, nutrition, phototherapy and blood transfusion further contribute to high levels of OS. Although the acute effects seem well established, little is known regarding the long-term effects of preterm birth on OS. This matter is especially important given that chronically elevated OS levels may persist into adulthood and consequently contribute to the development of numerous non-communicable diseases observed in people born preterm such as diabetes, hypertension or lung disorders. The purpose of this review is to summarize the current knowledge regarding the consequences of preterm birth on OS levels from newborn to adulthood. In addition, the effects of physical activity and hypoxia, both known to disrupt redox balance, on OS modulation in preterm individuals are also explored.
Keywords: Reactive oxygen species, Antioxidants, Prematurity, Physical exercise, Hypoxia
1. Introduction
The global incidence rate of preterm birth (PTB) is more than one in ten newborns [1] and PTB complications represent the leading cause of neonatal death worldwide [2]. A PTB is defined as a birth occurring before 37 weeks (259 days) of gestational age. PTB are generally classified into three types: medically induced (25% of all PTB), preterm premature rupture of membranes (25% of all PTB) and spontaneous preterm labor (50% of all PTB). There are also four degrees of prematurity: extremely preterm (before 28 weeks), very preterm (28–31 weeks), mild preterm (32–33 weeks) and moderate preterm (34–36 weeks) [3].
PTB is extremely physically challenging for neonates, as they must adapt to a new environment for which they are not sufficiently developed, especially in terms of oxygen consumption and use. Particularly, in preterm neonates, an imbalance between oxidant and antioxidant production can induce oxidative stress (OS) characterized by a high concentration of reactive oxygen species (ROS), which can therefore lead to oxidative damages. Oxygen resuscitation [4], [5], preterm nutrition [6], blood transfusions [7], phototherapy [8], inflammation and infection [9], high metabolic rate [10] and an immature antioxidant system [11] have been reported as potential causes for high OS in preterm newborns.
Additionally, with the improvements in medical care within the last three decades, the survival rate of neonates after a PTB has improved; in an English cohort study, a survival rate after a PTB improvement from 40% in 1995 to 53% in 2006 [12] was noted, and research by Liu et al. estimated a 2.1% reduction in PTB per year between 2000 and 2013 [2]. This improvement can therefore allow for a significant increase in the population of those who were born preterm being able to reach adulthood. Because of their higher susceptibility to OS, these adults may be prone to developing OS-associated diseases such as hypertension, metabolic syndrome or lung disorders. However, little is known regarding the long-term effects of high OS in this population. In this review, we differentiated acute and long term consequences by the arbitrary threshold of the first week of life. Furthermore, many external factors can also increase OS levels, such as acute physical exercise and hypoxic environments, however the OS response to these stimuli has not been studied in the prematurely born population.
Therefore, the purpose of this review is to discuss the causes and the pathophysiological consequences of OS in preterm individuals from birth to adulthood. In addition, the effects of physical activity and hypoxia, both known to disrupt redox balance, on OS modulation are also explored in preterm individuals.
2. Oxidative stress in preterm newborns
At birth, newborns undergo a strong increase in partial oxygen pressure; from 20 to 25 mmHg in utero to 100 mmHg in the extra uterine environment. This abrupt change leaves them highly susceptible to high OS levels and therefore increased oxidative damages. This effect is even more pronounced in newborns born prematurely. However, it is currently unclear whether OS is a cause and/or a consequence of PTB.
2.1. Preterm newborns exhibit higher oxidative stress level
At birth, preterm newborns exhibit higher levels of OS markers than those born full-term (Table 1). Indeed, the reported higher plasma F2-isoprostane [13], plasma malondialdehyde (MDA) [11], [14], [15], [16], [17], plasma MDA-hemoglobin [18], erythrocyte membrane hydroperoxide levels [19] and plasma lipid peroxidation [20] in preterm newborns clearly show higher levels of lipid peroxidation markers. Higher blood levels of 8-hydroxy-2-deoxyguanosine (8-OHdG) [16], protein carbonyl [11], [21] and desferrioxamine chelatable iron [13] show that preterm newborns also have higher levels of OS damaged DNA, proteins and erythrocytes than those born full-term. Compared to full-term birth, PTB also enhances plasma non-protein bound iron concentration [13], [22], which can lead to oxidative damages through the Fenton reaction. Some studies reported that OS levels are negatively correlated with gestational age [11], [13], [15], [16], [23].
Table 1.
Levels of various OS markers and antioxidants at birth in preterm newborns.
| Reference | Number of term/preterm newborns | GA of term newbornsa | GA of preterm newbornsa | Sample type | Sample timing | Available preterm newborns health status | Oxidative stress markers (vs. term newborns) | Antioxidant stress markers (vs. term newborns) | Additional findings in preterm newborns oxidative stress (vs. healthy PTB) |
|---|---|---|---|---|---|---|---|---|---|
| [19] | 10/10 | 40.4 (38–40.9) | 33.1 (32.9–35.7) | Erythrocytes / umbilical cord blood | at birth | No intensive care requirement and medical complications | ↑ hydroperoxides | ↓ vitamin E | |
| ↓ SOD | |||||||||
| ↓ GPx | |||||||||
| [11] | 116/124 | 38 (NA) | 34.7 (NA) | Umbilical cord blood | at birth | All were LBW and 51 were SGA | ↑ protein carbonyl | ↓ vitamin A | SGA PTB: ↑ protein carbonyl, ↑ MDA, ↓ vitamin A, ↓ vitamin C, ↓ vitamin E, ↓ TAS |
| No infection, hemolytic disease, hypertensive disorder, major malformations, history of difficult delivery, genetic disorder or fetal distress. | |||||||||
| ↓ vitamin C | |||||||||
| ↑ MDA | |||||||||
| ↓ vitamin E | |||||||||
| ↓ TAS | |||||||||
| [17] | 100/100 | 38 (37–40) | 31 (27–34) | Umbilical cord blood | at birth | No SGA newborns. | ↑ MDA | ↓ vitamin A | PTB with NEC: ↓vitamin A, ↑vitamin E, ↓CAT |
| 10 suffered from NEC, 20 from BPD, 24 from IVH and 28 did not survived. | |||||||||
| PTB with BPD: ↓vitaminA, ↑ vitamin E, ↓ TAS, ↓ CAT, ↑ MDA | |||||||||
| No gestational diabetes. No major congenital anomalies and no death within the first week. | |||||||||
| ↓ vitamin E | |||||||||
| ↓ TAS | |||||||||
| PTB with IVH: | |||||||||
| ↓ vitamin A, ↓ TAS, ↓ CAT, ↑ MDA | |||||||||
| ↓ CAT | |||||||||
| Parenteral nutrition begun for all within 24–48 h of life. | |||||||||
| PTB with death: | |||||||||
| ↑ MDA | |||||||||
| [21] | 179/21 | NA | NA | Umbilical cord blood | at birth | Healthy mothers without diabetes | ↑ protein carbonyl | ↓ vitamin A = vitamin E | |
| = 3 nitrotyrosine | |||||||||
| = MDA | |||||||||
| [31] | 25/33 | (38–42) | (24–36) | Umbilical cord blood | at birth | NA | ↑ vitamin C | ||
| [22] | 24/22 | 40.1 (NA) | 32.2 (NA) | Umbilical cord blood | at birth | No SGA. | ↑ NPBI | ↓ transferrin | |
| No hemolytic disease. | |||||||||
| 41% required respiratory support | |||||||||
| [14] | 32/32 | NA | NA | Umbilical cord blood | at birth | Born from mothers without history of diabetes mellitus, gestational diabetes or smoking. Fetal distress was not the cause of prematurity | ↑ MDA | ↓ SOD | |
| ↓ GSH | |||||||||
| [26] | 9/15 | 39.2 (38–40) | 32.3 (29–34) | Umbilical cord blood | at birth | Good condition, only one were not breathing spontaneously by | ↓ SOD | ||
| 5 min of age, all were breathing room air by 24 h, no supplementary | |||||||||
| Oxygen requirements, no evidence of perinatal | |||||||||
| Hypoxia or episodes of proven sepsis | |||||||||
| [16] | 24/55 | 38 (NA) | 34.2 (NA) | Umbilical cord blood | at birth | All were LBW. | ↑ MDA | ↓ TAS | MDA and 8-OHdG correlate negatively with birth weight |
| Mothers without eclampsia or hypertension. No intrauterine growth retardation, perinatal asphyxia, infection, hemolytic disease, major malformations, difficult delivery, genetic disorder or fetal distress. | |||||||||
| ↑ 8-OHdG | |||||||||
| [13] | 27/24 | 38.1 (37–41) | 33.7 (30–36) | Umbilical cord blood | at birth | NA | ↑ plasma F2-isoprostane | ||
| ↑ NPBI | |||||||||
| ↑ DCI | |||||||||
| ↑ placenta F2-isoprostane | |||||||||
| [15] | 91/47 | 39.2 (36–42) | 31.4 (23–36) | Umbilical cord blood | at birth | 32 required assisted ventilation, 22 suffered from respiratory distress syndrome and 10 from perinatal hypoxia | ↑ MDA | ||
| [18] | 29/31 | 39.4 (37.4–41.6) | 31.7 (28.1–35.7) | Umbilical cord blood | at birth | No SGA newborns. | ↑ MDA adduct to hemoglobin | ||
| No intensive care requirement and medical complications | |||||||||
| [25] | 25/25 | 38.04 (36–42) | 32.92 (28–35) | Umbilical cord blood | at birth | No congenital malformations or asphyxia. | = MDA | = TAS | |
| = CAT | |||||||||
| Healthy nonsmokers’ mothers. | ↓ SOD | ||||||||
| [20] | 30/40 | NA (37–42) | NA (24–36) | Umbilical cord blood | at birth | Healthy nonsmokers mothers | ↑ lipid peroxidation | ↑ vitamin C |
8-OHdG: 8-hydroxy-2-deoxy guanosine; BPD: bronchopulmonary dysplasia; CAT: catalase; DCI: erythrocyte chelatable iron; GA: gestational age; GPx: glutathione peroxidase; GSH: reduced glutathione; IVH: intraventricular hemorrhage; LBW: low birth weight (< 2500 g at birth); MDA: malondialdehyde; NA: not available; NEC: necrotizing enterocolitis; NPBI: Non-protein-bound iron; PTB: preterm birth; SGA: small for GA; SOD: superoxide dismutase; TAS: total antioxidant status; ↓ significantly decreased compared to term newborns; ↑ significantly increased compared to term newborns; = no significantly variation compared to term newborns. aexpressed in week as mean (minimum-maximum).
Additionally, previous research has shown that before labor MDA levels in maternal blood were higher in those who had preterm rather than full-term deliveries and were positively correlated with cord blood MDA levels in the newborns at birth [14]. This may suggest a link between high maternal levels of ROS and PTB. It could be also hypothesized that PTB contributes itself to the increased OS in mother and baby. Nevertheless, we should be precautious with this hypothesis since maternal blood MDA levels decrease between the second and third trimester [24]. Thus, higher MDA levels could be due to an earlier measurement in mothers who delivered prematurely. Moreover, while the blood protein carbonyl levels did not differ between the mothers of preterm and full-term newborns, higher protein carbonyl levels were found exclusively in those born preterm [21]. This strengthens the hypothesis that PTB itself can, at least partly, be responsible for higher OS in preterm newborns.
When compared to full-term birth, the antioxidant system after a PTB appears to be deficient. Indeed, at birth, superoxide dismutase (SOD) activity in both blood [14], [25] and erythrocytes [19], [26], catalase (CAT) activity in blood [17] as well as cytosolic glutathione peroxidase (GPx) in erythrocytes [19] are lower in preterm than in full-term newborns. This indicates lower antioxidant enzymatic activity in the former (Table 1). Preterm newborns also exhibit lower levels of non-enzymatic antioxidants such as erythrocyte vitamin E [19], blood vitamin E [11], [17], blood vitamin A [11], [17], [21], [27], [28], [29], blood vitamin C [11], erythrocyte reduced glutathione (GSH) [14], blood transferrin [22] and tracheal aspirates GSH [30] (Table 1). Two study however, reported higher blood vitamin C levels in preterm compared to full-term newborns [20], [31]. This can be explained by the abnormal pro-oxidant capacity of vitamin C through the Fenton reaction when free iron is available, as usually observed in preterm newborns as mentioned earlier. Thus, the blood of preterm newborns has a lower total antioxidant status (TAS), which may explain the higher OS observed when compared to those born at term [11], [16], [17] (Table 1).
2.2. Causes of oxidative stress in PTB
Many physiological mechanisms can induce the higher OS levels observed in preterm newborns. First of all, it is known that OS level in mothers vary during the pregnancy [24], [32] and might be due to an increased OS in the placenta [24]. OS in the placenta is likely necessary for its development by regulating the proliferation, the differentiation and the invasion of trophoblasts, promoting placental angiogenesis and regulating autophagy and apoptosis required for placentation [33]. However, a high level of systemic OS in mother has been shown to be associated with PTB [14], [34]. Consequently, high OS level in pregnant women could cause placental dysfunctions or other damages leading to PTB but could also be responsible for the high OS level observed in preterm newborns by direct blood exchange in the placenta.
Detrimentally, preterm newborns have an immature antioxidant system, as the last weeks of pregnancy corresponds to the maturation and upregulation of the fetuses antioxidant system [17], [27], [28] and the transfer of some antioxidants from the mother to the fetus [14], [27], [28]. This may explain why babies born early in their third trimester exhibit lower concentration of antioxidants. Further, because of their immature state, preterm newborns may require several medical interventions that can also induce ROS generation. Indeed, preterm neonates can have an immature digestive system and therefore cannot digest food in spite of their urgent need of nutrients. In this context, since parenteral nutrition contains peroxides, it can be a source OS damage [6], [35]. Preterm newborns usually require a formula feeding, since mothers have usually more difficulty to produce milk after a PTB. In preterm newborns, formula feeding induce higher OS than human milk feeding which is known to improve endogenous antioxidant activities and have exogenous antioxidants and anti-inflammatories properties [10], [23], [36]. In addition, the milk fortifiers used in the formula to improve the bone mineralization of preterm newborns have been shown to increase OS level [10].
The resuscitation with high fraction of inspired oxygen (≥ 0.9) required for preterm newborns who exhibit immature lungs, surfactant deficiency, an unstable thoracic cage and weak respiratory muscles induced elevated OS mainly explained the hyperoxia effects on ROS production [4], [5], [37].
To compensate their deficiencies in erythropoiesis inducing anemia, preterm newborns need blood transfusions that increase ROS production through an increase of free iron in blood [7].
A majority of preterm newborns suffer from hyperbilirubinemia and therefore need UV phototherapy as treatment. However, phototherapy increase ROS generation [8]. Moreover, preterm newborns are highly susceptible to infections as they are born with an immature immune system that may also contribute to increased OS [9]. As previously mentioned, these preterm newborns are also prone to the development of diseases such bronchopulmonary dysplasia, chronic lung disease, periventricular leukomalacia, intraventricular hemorrhage, retinopathy of prematurity and necrotizing enterocolitis that can increase ROS production. Another source of ROS production could be due to the high growth rate observed at birth in preterm newborns [38] and therefore high mitochondrial activity, resulting in higher metabolic rates [10] and positive association to mitochondrial ROS production. The higher metabolic rate could even be enhanced by the lack of heat production resulting from lower brown adipose tissue percentage in preterm newborns. However, further investigations are needed to test this hypothesis. Finally, term newborn infants with small for gestational age (SGA) have higher OS level and lower antioxidant defenses at birth than term newborn infants with normal weight for gestational age [26], [39]. Similarly, preterm newborns SGA exhibit higher OS level and lower antioxidant defenses than preterm newborns with appropriate weight (Table 1) [11], [16]. This suggests that the low birth weight could contribute, in part, to the higher OS level in preterm newborns.
As a result, lower antioxidant defenses associated with numerous sources of ROS overgeneration can disrupt the redox balance and lead to OS in preterm newborns.
2.3. Oxidative stress-associated diseases in preterm newborns
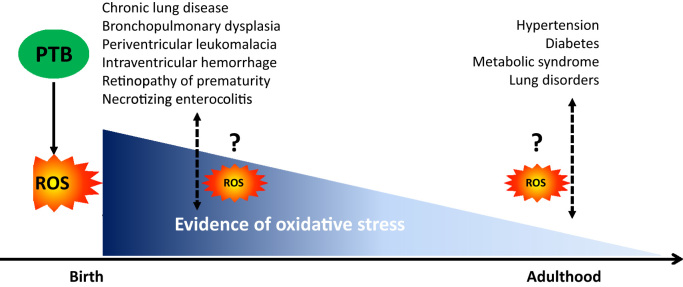
OS was suggested to be involved in several diseases associated with PTB, such as bronchopulmonary dysplasia or chronic lung disease, periventricular leukomalacia, intraventricular hemorrhage, retinopathy of prematurity and necrotizing enterocolitis [37], [40]. Indeed, although the causes of these pathologies are multifactorial, the levels of some antioxidants are lower in newborns developing bronchopulmonary dysplasia [17], [29], necrotizing enterocolitis and intraventricular hemorrhage [17], while the levels of OS markers are higher in newborns developing bronchopulmonary dysplasia [17], [37], [40], retinopathy of prematurity [37], [40], necrotizing enterocolitis, periventricular leukomalacia and intraventricular hemorrhage [40] than in newborns without these diseases. Moreover, OS levels correlate with the prevalence of these OS-associated diseases [40]. Therefore, as preterm newborns are exposed to high levels of OS and have impaired antioxidant defenses, their organs, in particular the retinas (retinopathy of prematurity), lungs (bronchopulmonary dysplasia), brain (periventricular leukomalacia, intraventricular hemorrhage) and intestines (necrotizing enterocolitis) are more exposed to OS damages. These high levels of OS, capable of inflicting damage on immature organs without sufficient antioxidant defenses, may contribute to the development of these diseases (Fig. 1). However, this assumption should be interpreted with caution, as the inflammation caused by these pathologies is also known to overproduce ROS and therefore the OS observed in those newborns developing diseases could also be a consequence of the diseases themselves. Nevertheless, whether these pathologies occurring at birth could have consequences during adulthood and may result in a higher susceptibility to the development of some disorders in which OS is involved are thus far unknown.
Fig. 1.
The chicken and egg paradigm of oxidative stress in preterm from birth to adulthood. PTB increases the risk to develop of several diseases at birth up to adulthood. The higher oxidative stress during the life of PTB could have a role in the pathogenesis of these diseases but could be induced by these diseases. PTB: preterm birth, ROS: reactive oxygen species.
3. Effects of PTB on oxidative stress after birth
3.1. Oxidative stress markers in individuals born preterm
Very few studies to-date have investigated the post-birth effects of PTB on OS and redox balance. A few days after birth, higher levels of OS markers have been reported in preterm compared to full-term newborns; higher levels of hydroperoxide was associated with lower levels of vitamin E as well as SOD and cytosolic GPx activity in the erythrocyte membrane at least 3 days post-birth [19], higher levels of MDA in cord blood at least 4 days post-birth [15] and higher levels of advanced oxidation protein products and total hydroperoxide in plasma at least 7 days post-birth [41]. Additionally, urine 8-OHdG levels can be elevated for up to 100 days after birth in preterm compared to full-term babies. This is also associated with lower glutathione peroxidase and SOD activity in erythrocytes of preterm babies [42]. Taken together these data confirm that, for a short time after their birth, OS levels remain higher in babies born preterm than in those born at term. Moreover, a study showed that blood vitamin A and E level is higher in preterm newborns at discharge than at birth [17]. Although there is little information about precise discharge time (between 7 and 49 days with a median of 20.5 days), the antioxidant system of preterm newborns is likely improved during the first days of life. This was confirmed by higher SOD activity in blood of preterm newborns at the expected date of delivery than at their PTB [26].
More interestingly, lipid peroxidation, measured by the levels of 8-isoprostane in exhaled breath condensate, was higher in adolescents (13–15 years) born very preterm (between 28 and 31 weeks of gestation) compared to those born at term, regardless of bronchopulmonary dysplasia history in those born prematurely. This suggests that OS is likely due to the PTB per se and not to the development of lung diseases or long-term oxygen exposure during resuscitation and therapy following birth [43].
Since excessive ROS concentration can reduce telomere length measuring changes in telomere length is another indirect way to assess OS levels [44], [45]. Telomere length was reported to be similar in the cord blood of preterm and full-term newborns at birth and in the saliva of adolescents born preterm and at term [44]. On the contrary, shorter telomeres were reported in leukocytes of preterm born young adults (18–24 years), when compared to their full-term born counterparts [45]. In addition, telomere length and lung disorders correlate only in extremely preterm born adolescents [44]. To date, this potential involvement of ROS in the reduction of telomere length in children and adults born prematurely is not fully elucidated.
3.2. Oxidative stress-associated pathologies in adults born preterm
Recent meta-analyses showed that adults born prematurely have higher risks of developing some diseases such as hypertension [46], [47] and as a consequence heart attack or stroke, type I and II diabetes [48], [49], metabolic syndrome [46] or lung disorders [50]. Moreover, although the causes of these diseases are multifactorial, OS is known to play an important role in their pathogeneses. Indeed, OS, by modifying DNA and proteins, can alter or change gene expression and proteins functions leading to the development and/or exacerbation of the majority of these non-communicable chronic diseases. In fact, ROS alter vascular development, mostly through the up-regulation of hypoxia-inducible factor-1α, involved in the regulation of the expression of genes (e.g. vascular endothelial growth factor). ROS can also cause endothelial dysfunction, inflammation and vascular remodeling mainly by inhibiting nitric oxide bioavailability. These pathological mechanisms alter vascular tone and disrupt the control of blood pressure by the brain [47], [51], leading to hypertension. ROS can also impair the proliferation and the development of ß pancreatic cells [48] and induce insulin resistance by causing mitochondrial dysfunction, decreasing glucose transporter type 4 expression, disturbing components of the insulin signaling pathway and inducing inflammation [52], which can all result in the development of type II diabete. Diabetes associated with cardiovascular diseases can contribute to metabolic syndrome development. Furthermore, ROS can induce inflammation in the lungs, disrupt proliferation and function of ciliated epithelial cells, enhance mucus production, activate airway smooth muscle contraction and damage lung extracellular matrix. These changes can lead to the development of asthma, chronic obstructive pulmonary diseases and/or acute lung injury [53].
Therefore, a large body of evidence indicates that adults born preterm are prone to develop some diseases in which OS is involved. It may be hypothesized that chronically elevated OS levels in adults born prematurely could increase the risk of developing these diseases. However, as these diseases are known to induce ROS overgeneration, the observed increases in OS may also be a result of the higher prevalence of these diseases in this population. Further investigations regarding the role of OS in the development of diseases in children, adolescents and adults born prematurely are required to improve the understanding of the relationship between PTB, OS and chronic diseases.
4. Oxidative stress modulation by external factors in individuals born preterm
4.1. Acute physical activity
While overall OS levels have been reported to be significantly higher in both newborns and adults born prematurely via endogenous/intrinsic mechanisms/pathways, other stimuli may also affect OS levels, particularly physical activity and hypoxia.
Indeed, acute physical activity is known to increase OS levels and antioxidant enzymatic activity in healthy populations [54], [55]. While the mechanisms involved in increased OS following exercise are multifactorial, the key factors include ROS overproduction within the mitochondria due to an increase in oxidative phosphorylation [56], [57] catecholamine release and ischemic reperfusion-induced activation of xanthine oxidase [58]. One may hypothesize that PTB could exacerbate the OS response to exercise compared to those born full-term. On the contrary, a PTB could also lead to a decreased OS response to exercise due to a long-term PTB preconditioning effect, thereby increasing the antioxidant defense to cellular redox disturbances. Currently, no study to-date has examined the effects of acute exercise on OS modulation in preterm born individuals. However, it has been recently suggested that exercise capacity is reduced in those born preterm throughout their lifetime as compared to full-term born individuals [59], [60], [61]. Therefore, the same absolute intensity exercise might induce higher relative intensities in preterm than born term individuals and thus potentially increase OS [54], [55], [56], [57]. Nevertheless, the effects of dietary habits and the use of medicaments during pregnancy and lactation, in respect to the health status of mothers and preterm newborns, that may modulate this exercise capacity, have not been investigated. Given the above, it seems integral to better understand the effects of acute physical activity on OS modulation in preterm individuals in taking into account the clinical and historical parameters of PTB (see paragraph “Causes of oxidative stress in PTB”). More specifically, it is important, in this population, to assess the methods in which physical exercise therapy can alleviate OS levels, as it has been shown to do under other pathologic conditions [62], [63].
4.2. Hypoxia
Hypoxia is another stimulus that can modulate OS levels [64], [65], [66], [67], [68], [69], [70] (see Table 2). Environmental hypoxia, resulting from a reduction of the barometric pressure (hypobaric hypoxia) or a reduction of the fraction of inspired oxygen (normobaric hypoxia), leads to a reduction of oxygen partial pressure in the ambient air, provokes ROS production and thereby increases OS [65], [66]. It is known that OS is regulated differently in hypobaric and normobaric hypoxia, the former leading to lower levels of stimulation [67], [71]. Hypoxia is known to activate xanthine oxidase pathway [72], to increase catecholamine production [73] and to increase the rate of electron leakage within the mitochondria [74]. All of these factors subsequently induce ROS overproduction and thereby modulate redox homeostasis increasing OS in general population [65], [66]. Currently, the effects of hypoxia exposure on OS in adults born preterm population has not been investigated. In addition to high altitude sojourns, preterm born individuals can also be exposed to hypoxia due to various respiratory and cardiorespiratory insufficiencies, pathologic condition commonly observed in both newborn and adult preterm cohorts [50], [75]. As mentioned above for the OS response to exercise, we can hypothesize that hypoxia exposure could exacerbate the redox balance and increase OS in preterm born individuals. Meanwhile PTB adults may have lower OS responses, as preterm individuals have been exposed to hypoxia early in life, they could be “preconditioned” and have developed some mechanisms to limit ROS overproduction in response to hypoxia. This potential lower OS may play a role in the lower tolerance to acute mountain sickness of preterm born individuals. Indeed, OS is known to stimulate ventilatory chemosensitivity to hypoxia [76] and a lower hypoxic ventilatory response is one of the risk factor of acute mountain sickness. Regardless of the over or under stimulating mechanism, further studies are required to know the vulnerability of PTB individuals to acute mountain sickness and the underlying role of OS in this response.
Table 2.
Studies investigating the acute and prolonged effects of environmental hypoxia on oxidative stress and antioxidant markers in humans.
| Reference | Study design | Oxidative stress markers | Antioxidant markers | Summary |
|---|---|---|---|---|
| [64] | 13-day HH @ 4300 m | ↑ LPO | ↑ α-tocopherol | Prolonged HH exposure augments oxidative stress. |
| Healthy active individuals (N = 18) | ↓ 8-OHdG | ↑ β-carotene | ||
| [65] | 4-h HH @ 5500 m | ↑ GSSG (%) | ↓ TGSH | Acute HH exposure augments oxidative stress. |
| Healthy active individuals (N=6) | ↑ TBARS | |||
| [66] | 10-min exercise in NH @ 4800 m & | ↑ MDA | ↓ FRAP (3000 m test only) | Even high antioxidant capacity of elite athletes does not counteract acute NH-induced oxidative stress. |
| 3-hour NH @ 3000 m | ||||
| Elite athletes (N=41) | ↑ AOPP | ↓ α-tocopherol | ||
| [67] | 24-h NH & HH @ 3000 m | ↑ AOPP (higher in HH) | ↑ SOD (only in HH) | HH induces higher oxidative stress level as compared to NH. |
| Healthy trained individuals (N=10) | ||||
| [68] | 10-day NH @ 4000 m | ↑AOPP | ↑ GPx | NH per se augments oxidative stress. |
| Healthy active individuals (N=6) | ↑ Nitrotyrosine | |||
| [69] | 10-h NH & HH @ 3450 m | ↑ AOPP | ↑ GPx (HH only) | HH provokes greater prooxidant/antioxidant imbalance than NH. |
| Healthy trained individuals (N=16) | ↑ SOD (HH only) | |||
| ↓ FRAP | ||||
| ↓ UA | ||||
| [70] | 10-day NH exposure @ 4000 m during bed rest | ↑ AOPP | ↑ Catalase | NH exposure augments inactivity-related oxidative stress. |
| ↓ GPx | ||||
| Healthy active females (N=12) |
HH: Hypobaric hypoxia; NH: Normobaric hypoxia; ↓: significantly decreased; ↑: significantly increased; LPO: Lipid hydroperoxides; 8-OHdG: 8-hydroxydeoxyguanosine; GSSG (%): oxidized glutathione percentage, TBARS: Thiobarbituric acid reactive substances, TGSH: total glutathione content; MDA: malondialdehyde; AOPP: advanced oxidation protein products; FRAP: ferric-reducing antioxidant power; SOD: superoxide dismutase; GPx: glutathione peroxidase; UA: Uric acid.
Moreover, the investigations regarding the additive or combined effects of hypoxia and exercise on OS modulation in born preterm individuals are lacking. Only one previous study has shown that exercise capacity was similar under hypoxia between adults born at term and those born preterm while exercise capacity is reduced in adults born preterm under normoxia [61]. The authors of this study hypothesized that cardiac or muscular adaptions in response to a PTB could precondition preterm individuals to hypoxic environment. Therefore, future well-controlled studies are warranted to assess both the detrimental or beneficial effects of environmental hypoxia on redox balance modulation in preterm population.
5. Conclusion
PTB is a global problem, however due to development and progression of the medical world, there are an increasing number of preterm newborns reaching adulthood. Since preterm newborns are too immature to withstand the environmental stress, they experience higher OS levels. This is mostly underlined by the fact that preterm newborns have immature defense systems against ROS, immature organs and need medical treatments that increase ROS production. However, little is known regarding the long-term effects of enhanced OS in preterm born individuals. It was shown that OS persists after a PTB and that subsequent redox imbalance could result in a “preconditioned” state. These adaptations could be involved in the pathogenesis of several non-communicable chronic diseases during the adulthood. Targeting OS in people born preterm early in their life, administrating antioxidants or introducing regular physical exercise for instance could be a strategy to limit the development of these diseases. However, since ROS are essential to maintain cellular function, artificial manipulation of ROS levels could also lead to adverse outcomes. This raises the question of specific OS-mediated responses to exercise and hypoxia in preterm born individuals. However, further studies are required to better understand the underlying mechanisms leading to this persistent OS and its involvement in response to exercise and hypoxia exposures.
Acknowledgments
We thank Dr. Déborah Prévot for her help and advice concerning this review and Sofia Correa, Marie-Anaïs Locquet and Julia Mattioni for their comments and Gilles Christoph for help with proofreading this manuscript.
References
- 1.Blencowe H., Cousens S., Oestergaard M.Z., Chou D., Moller A.-B., Narwal R., Adler A., Vera Garcia C., Rohde S., Say L., Lawn J.E. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Liu L., Oza S., Hogan D., Perin J., Rudan I., Lawn J.E., Cousens S., Mathers C., Black R.E. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 3.Moutquin J.-M. Classification and heterogeneity of preterm birth. BJOG: Int. J. Obstet. Gynaecol. 2003;110:30–33. doi: 10.1016/s1470-0328(03)00021-1. [DOI] [PubMed] [Google Scholar]
- 4.Escobar J., Cernada M., Vento M. Oxygen and oxidative stress in the neonatal period. NeoReviews. 2011;12:e613–e624. [Google Scholar]
- 5.Vento M., Moro M., Escrig R., Arruza L., Villar G., Izquierdo I., Roberts L.J., Arduini A., Escobar J.J., Sastre J., Asensi M.A. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics. 2009;124:e439–e449. doi: 10.1542/peds.2009-0434. [DOI] [PubMed] [Google Scholar]
- 6.Perrone S., Salvi G., Bellieni C.V., Buonocore G. Oxidative stress and nutrition in the preterm newborn. J. Pediatr. Gastroenterol. Nutr. 2007;45(Suppl 3):S178–S182. doi: 10.1097/01.mpg.0000302968.83244.d2. [DOI] [PubMed] [Google Scholar]
- 7.Hirano K., Morinobu T., Kim H., Hiroi M., Ban R., Ogawa S., Ogihara H., Tamai H., Ogihara T. Blood transfusion increases radical promoting non-transferrin bound iron in preterm infants. Arch. Dis. Child.-Fetal Neonatal Ed. 2001;84:F188–F193. doi: 10.1136/fn.84.3.F188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gathwala G., Sharma S. Phototherapy induces oxidative stress in premature neonates. Indian J. Gastroenterol. 2002;21:153–154. [PubMed] [Google Scholar]
- 9.Seema, Kumar R., Mandal R.N., Tandon A., Randhawa V.S., Mehta G., Batra S., Ray G.N., Kapoor A.K. Serum TNF-alpha and free radical scavengers in neonatal septicemia. Indian J. Pediatr. 1999;66:511–516. doi: 10.1007/BF02727159. [DOI] [PubMed] [Google Scholar]
- 10.Friel J.K., Diehl-Jones B., Cockell K.A., Chiu A., Rabanni R., Davies S.S., Roberts L.J. Evidence of oxidative stress in relation to feeding type during early life in premature infants. Pediatr. Res. 2011;69:160–164. doi: 10.1203/PDR.0b013e3182042a07. [DOI] [PubMed] [Google Scholar]
- 11.Negi R., Pande D., Kumar A., Khanna R.S., Khanna H.D. Evaluation of biomarkers of oxidative stress and antioxidant capacity in the cord blood of preterm low birth weight neonates. J. Matern. Fetal Neonatal Med. 2012;25:1338–1341. doi: 10.3109/14767058.2011.633672. [DOI] [PubMed] [Google Scholar]
- 12.Costeloe K.L., Hennessy E.M., Haider S., Stacey F., Marlow N., Draper E.S. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies) BMJ. 2012;345:e7976. doi: 10.1136/bmj.e7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comporti M., Signorini C., Leoncini S., Buonocore G., Rossi V., Ciccoli L. Plasma F2-isoprostanes are elevated in newborns and inversely correlated to gestational age. Free Radic. Biol. Med. 2004;37:724–732. doi: 10.1016/j.freeradbiomed.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Chakravartya S., Sontakkeb A.N. A correlation of antioxidants and lipid peroxidation between maternal and cord blood in full term and preterm deliveries. Curr. Pediatr. Res. 2012;16:167–174. [Google Scholar]
- 15.Buonocore G., Zani S., Perrone S., Caciotti B., Bracci R. Intraerythrocyte nonprotein-bound iron and plasma malondialdehyde in the hypoxic newborn. Free Radic. Biol. Med. 1998;25:766–770. doi: 10.1016/s0891-5849(98)00126-9. [DOI] [PubMed] [Google Scholar]
- 16.Negi R., Pande D., Kumar A., Khanna R.S., Khanna H.D. In vivo oxidative DNA damage and lipid peroxidation as a biomarker of oxidative stress in preterm low-birthweight infants. J. Trop. Pediatr. 2012;58:326–328. doi: 10.1093/tropej/fmr078. [DOI] [PubMed] [Google Scholar]
- 17.Abdel Ghany E.A.G., Alsharany W., Ali A.A., Younass E.R., Hussein J.S. Anti-oxidant profiles and markers of oxidative stress in preterm neonates. Paediatr. Int. Child Health. 2016;36:134–140. doi: 10.1179/2046905515Y.0000000017. [DOI] [PubMed] [Google Scholar]
- 18.Cipierre C., Haӱs S., phane S.S., Maucort-Boulch D., Steghens J.-P., Picaud J.-C. Malondialdehyde adduct to hemoglobin: a new marker of oxidative stress suitable for full-term and preterm neonates. Oxid. Med. Cell. Longev. 2013;2013:e694014. doi: 10.1155/2013/694014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochoa J.J., Ramirez-Tortosa M.C., Quiles J.L., Palomino N., Robles R., Mataix J., Huertas J.R. Oxidative stress in erythrocytes from premature and full-term infants during their first 72h of life. Free Radic. Res. 2003;37:317–322. doi: 10.1080/1071576021000050438. [DOI] [PubMed] [Google Scholar]
- 20.A. Agil, R. Fraile, D. Acuña Castroviejo, Changes in plasma susceptibility to lipid peroxidation and vitamin C in preterm and full-term neonates. 〈http://digibug.ugr.es/handle/10481/32804〉 (Accessed 25 February 2018).
- 21.Weber D., Stuetz W., Bernhard W., Franz A., Raith M., Grune T., Breusing N. Oxidative stress markers and micronutrients in maternal and cord blood in relation to neonatal outcome. Eur. J. Clin. Nutr. 2014;68:215–222. doi: 10.1038/ejcn.2013.263. [DOI] [PubMed] [Google Scholar]
- 22.Moison R.M.W., Palinckx J.J.S., Roest M., Houdkamp E., Berger H.M. Induction of lipid peroxidation of pulmonary surfactant by plasma of preterm babies. Lancet. 1993;341:79–82. doi: 10.1016/0140-6736(93)92557-a. [DOI] [PubMed] [Google Scholar]
- 23.Ledo A., Arduini A., Asensi M.A., Sastre J., Escrig R., Brugada M., Aguar M., Saenz P., Vento M. Human milk enhances antioxidant defenses against hydroxyl radical aggression in preterm infants. Am. J. Clin. Nutr. 2008;89:210–215. doi: 10.3945/ajcn.2008.26845. [DOI] [PubMed] [Google Scholar]
- 24.Yüksel S., Yiğit A.A. Malondialdehyde and nitric oxide levels and catalase, superoxide dismutase, and glutathione peroxidase levels in maternal blood during different trimesters of pregnancy and in the cord blood of newborns. Turk. J. Med. Sci. 2015;45:454–459. [PubMed] [Google Scholar]
- 25.Norishadkam M., Andishmand S., Zavar Reza J., Zare Sakhvidi M.J., Hachesoo V.R. Oxidative stress and DNA damage in the cord blood of preterm infants. Mutat. Res. 2017;824:20–24. doi: 10.1016/j.mrgentox.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Phylactos A., Leaf A.A., Costeloe K., Crawford M. Erythrocyte cupric/zinc superoxide dismutase exhibits reduced activity in preterm and low-birthweight infants at birth. Acta Paediatrica. 1995;84:1421–1425. doi: 10.1111/j.1651-2227.1995.tb13580.x. [DOI] [PubMed] [Google Scholar]
- 27.Brandt R.B., Mueller D.G., Schroeder J.R., Guyer K.E., Kirkpatrick B.V., Hutcher N.E., Ehrlich F.E. Serum vitamin A in premature and term neonates. J. Pediatr. 1978;92:101–104. doi: 10.1016/s0022-3476(78)80086-9. [DOI] [PubMed] [Google Scholar]
- 28.Shenai J.P., Chytil F., Jhaveri A., Stahlman M.T. Plasma vitamin A and retinol-binding protein in premature and term neonates. J. Pediatr. 1981;99:302–305. doi: 10.1016/s0022-3476(81)80484-2. [DOI] [PubMed] [Google Scholar]
- 29.Hustead V.A., Gutcher G.R., Anderson S.A., Zachman R.D. Relationship of vitamin A (retinol) status to lung disease in the preterm infant. J. Pediatr. 1984;105:610–615. doi: 10.1016/s0022-3476(84)80432-1. [DOI] [PubMed] [Google Scholar]
- 30.Lavoie J.-C., Chessex P. Gender and maturation affect glutathione status in human neonatal tissues. Free Radic. Biol. Med. 1997;23:648–657. doi: 10.1016/s0891-5849(97)00011-7. [DOI] [PubMed] [Google Scholar]
- 31.Berger T.M., Rifai N., Avery M.E., Frei B. Vitamin C in premature and full-term human neonates. Redox Report. 1996;2:257–262. doi: 10.1080/13510002.1996.11747058. [DOI] [PubMed] [Google Scholar]
- 32.Hung T.-H., Lo L.-M., Chiu T.-H., Li M.-J., Yeh Y.-L., Chen S.-F., Hsieh T.’sang-T’ang. A longitudinal study of oxidative stress and antioxidant status in women with uncomplicated pregnancies throughout gestation. Reprod. Sci. 2010;17:401–409. doi: 10.1177/1933719109359704. [DOI] [PubMed] [Google Scholar]
- 33.Wu F., Tian F.-J., Lin Y. Oxidative stress in placenta: health and diseases. BioMed. Res. Int. 2015;2015:e293271. doi: 10.1155/2015/293271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menon R. Oxidative stress damage as a detrimental factor in preterm birth pathology. Front Immunol. 2014;5:567. doi: 10.3389/fimmu.2014.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farkouh C.R., Merrill J.D., Ballard P.L., Ballard R.A., Ischiropoulos H., Lorch S.A. Urinary metabolites of oxidative stress and nitric oxide in preterm and term infants. Neonatology. 2006;90:233–242. doi: 10.1159/000093633. [DOI] [PubMed] [Google Scholar]
- 36.Shoji H. Suppressive effects of breast milk on oxidative DNA damage in very low birthweight infants. Arch. Dis. Child. - Fetal Neonatal Ed. 2004;89:136F–138F. doi: 10.1136/adc.2002.018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saugstad O.D. Update on oxygen radical disease in neonatology. Curr. Opin. Obstet. Gynecol. 2001;13:147–153. doi: 10.1097/00001703-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Fenton T.R., Nasser R., Eliasziw M., Kim J.H., Bilan D., Sauve R. Validating the weight gain of preterm infants between the reference growth curve of the fetus and the term infant. BMC Pediatr. 2013;13:92. doi: 10.1186/1471-2431-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gveric-Ahmetasevic S., Sunjic S.B., Skala H., Andrisic L., Stroser M., Zarkovic K., Skrablin S., Tatzber F., Cipak A., Jaganjac M., Waeg G., Gveric T., Zarkovic N. Oxidative stress in small-for-gestational age (SGA) term newborns and their mothers. Free Radic. Res. 2009;43:376–384. doi: 10.1080/10715760902783285. [DOI] [PubMed] [Google Scholar]
- 40.O’Donovan D.J., Fernandes C.J. Free radicals and diseases in premature infants. Antioxid. Redox Signal. 2004;6:169–176. doi: 10.1089/152308604771978471. [DOI] [PubMed] [Google Scholar]
- 41.Buonocore G., Perrone S., Longini M., Vezzosi P., Marzocchi B., Paffetti P., Bracci R. Oxidative stress in preterm neonates at birth and on the seventh day of life. Pediatr. Res. 2002;52:46–49. doi: 10.1203/00006450-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Nassi N., Ponziani V., Becatti M., Galvan P., Donzelli G. Anti-oxidant enzymes and related elements in term and preterm newborns. Pediatr. Int. 2009;51:183–187. doi: 10.1111/j.1442-200X.2008.02662.x. [DOI] [PubMed] [Google Scholar]
- 43.Filippone M., Bonetto G., Corradi M., Frigo A.C., Baraldi E. Evidence of unexpected oxidative stress in airways of adolescents born very pre-term. Eur. Respir. J. 2012;40:1253–1259. doi: 10.1183/09031936.00185511. [DOI] [PubMed] [Google Scholar]
- 44.Hadchouel A., Marchand-Martin L., Franco-Montoya M.-L., Peaudecerf L., Ancel P.-Y., Delacourt C., EPIPAGEADO study group Salivary telomere length and lung function in adolescents born very preterm: a prospective multicenter study. PLOS One. 2015;10:e0136123. doi: 10.1371/journal.pone.0136123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smeets C.C.J., Codd V., Samani N.J., Hokken-Koelega A.C.S. Leukocyte telomere length in young adults born preterm: supportsupport for accelerated biological ageing. PLOS One. 2015;10:e0143951. doi: 10.1371/journal.pone.0143951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parkinson J.R.C., Hyde M.J., Gale C., Santhakumaran S., Modi N. Preterm birth and the metabolic syndrome in adult life: a systematic review and meta-analysis. Pediatrics. 2013;131:e1240–1263. doi: 10.1542/peds.2012-2177. [DOI] [PubMed] [Google Scholar]
- 47.Sutherland M.R., Bertagnolli M., Lukaszewski M.-A., Huyard F., Yzydorczyk C., Luu T.M., Nuyt A.M. Preterm birth and hypertension risk: the oxidative stress paradigm. Hypertension. 2014;63:12–18. doi: 10.1161/HYPERTENSIONAHA.113.01276. [DOI] [PubMed] [Google Scholar]
- 48.Luo Z.C., Fraser W.D., Julien P., Deal C.L., Audibert F., Smith G.N., Xiong X., Walker M. Tracing the origins of “fetal origins” of adult diseases: programming by oxidative stress? Med. Hypotheses. 2006;66:38–44. doi: 10.1016/j.mehy.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 49.Li S., Zhang M., Tian H., Liu Z., Yin X., Xi B. Preterm birth and risk of type 1 and type 2 diabetes: systematic review and meta-analysis. Obes. Rev. 2014;15:804–811. doi: 10.1111/obr.12214. [DOI] [PubMed] [Google Scholar]
- 50.I. Narang, A. Al-Naimi, Preterm Birth and Long-Term Pulmonary Function, INTECH Open Access Publisher. 〈http://cdn.intechweb.org/pdfs/27132.pdf〉 (accessed 12 November 2016).
- 51.Touyz R.M., Briones A.M. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens. Res. 2011;34:5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- 52.Rains J.L., Jain S.K. Oxidative stress, insulin signaling and diabetes. Free Radic. Biol. Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park H.S., Kim S.R., Lee Y.C. Impact of oxidative stress on lung diseases. Respirology. 2009;14:27–38. doi: 10.1111/j.1440-1843.2008.01447.x. [DOI] [PubMed] [Google Scholar]
- 54.Powers S.K., Nelson W.B., Hudson M.B. Exercise-induced oxidative stress in humans: cause and consequences. Free Radic. Biol. Med. 2011;51:942–950. doi: 10.1016/j.freeradbiomed.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Powers S.K., Radak Z., Ji L.L. Exercise-induced oxidative stress: past, present and future: exercise-induced oxidative stress. J. Physiol. 2016;594:5081–5092. doi: 10.1113/JP270646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sen C.K. Oxidants and antioxidants in exercise. J. Appl. Physiol. 1995;79:675–686. doi: 10.1152/jappl.1995.79.3.675. [DOI] [PubMed] [Google Scholar]
- 57.Urso M.L., Clarkson P.M. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189:41–54. doi: 10.1016/s0300-483x(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 58.E.C. Gomes, A.N. Silva, M.R. de Oliveira, Oxidants, Antioxidants, and the Beneficial Roles of Exercise-Induced Production of Reactive Species, Oxidative Medicine and Cellular Longevity, 2012. 〈https://www.hindawi.com/journals/omcl/2012/756132/abs/〉 (Accessed 12 November 2017). [DOI] [PMC free article] [PubMed]
- 59.Svedenkrans J., Henckel E., Kowalski J., Norman M., Bohlin K. Long-Term impact of preterm birth on exercise capacity in healthy young men: a national population-based cohort study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clemm H.H., Vollsæter M., Røksund O.D., Eide G.E., Markestad T., Halvorsen T. Exercise capacity after extremely preterm birth. Development from adolescence to adulthood. Ann. ATS. 2014;11:537–545. doi: 10.1513/AnnalsATS.201309-311OC. [DOI] [PubMed] [Google Scholar]
- 61.Farrell E.T., Bates M.L., Pegelow D.F., Palta M., Eickhoff J.C., O’Brien M.J., Eldridge M.W. Pulmonary gas exchange and exercise capacity in adults born preterm. Ann. Am. Thorac. Soc. 2015;12:1130–1137. doi: 10.1513/AnnalsATS.201410-470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pialoux V., Brown A.D., Leigh R., Friedenreich C.M., Poulin M.J. Effect of cardiorespiratory fitness on vascular regulation and oxidative stress in postmenopausal women. Hypertension. 2009;54:1014–1020. doi: 10.1161/HYPERTENSIONAHA.109.138917. [DOI] [PubMed] [Google Scholar]
- 63.Chirico E.N., Martin C., Faës C., Féasson L., Oyono-Enguéllé S., Aufradet E., Dubouchaud H., Francina A., Canet-Soulas E., Thiriet P., Messonnier L., Pialoux V. Exercise training blunts oxidative stress in sickle cell trait carriers. J. Appl. Physiol. 2012;112:1445–1453. doi: 10.1152/japplphysiol.01452.2011. [DOI] [PubMed] [Google Scholar]
- 64.Subudhi A.W., Jacobs K.A., Hagobian T.A., Fattor J.A., Fulco C.S., Muza S.R., Rock P.B., Hoffman A.R., Cymerman A., Friedlander A.L. Antioxidant supplementation Does not attenuate oxidative stress at high altitude. Aviat., Space, Environ. Med. 2004;75:881–888. [PubMed] [Google Scholar]
- 65.Magalhães J., Ascensão A., Viscor G., Soares J., Oliveira J., Marques F., Duarte J. Oxidative stress in humans during and after 4h of hypoxia at a simulated altitude of 5500 m. Aviat. Space Environ. Med. 2004;75:16–22. [PubMed] [Google Scholar]
- 66.Pialoux V., Mounier R., Rock E., Mazur A., Schmitt L., Richalet J.P., Robach P., Coudert J., Fellmann N. Effects of acute hypoxic exposure on prooxidant/antioxidant balance in elite endurance athletes. Int. J. Sports Med. 2009;30:87–93. doi: 10.1055/s-0028-1103284. [DOI] [PubMed] [Google Scholar]
- 67.Faiss R., Pialoux V., Sartori C., Faes C., DéRiaz O., Millet G.P. Ventilation, oxidative stress, and nitric oxide in hypobaric versus normobaric hypoxia: Med. Sci. Sports Exerc. 2013;45:253–260. doi: 10.1249/MSS.0b013e31826d5aa2. [DOI] [PubMed] [Google Scholar]
- 68.Debevec T., Pialoux V., Mekjavic I.B., Eiken O., Mury P., Millet G.P. Moderate exercise blunts oxidative stress induced by normobaric hypoxic confinement. Med. Sci. Sports Exerc. 2014;46:33–41. doi: 10.1249/MSS.0b013e31829f87ef. [DOI] [PubMed] [Google Scholar]
- 69.Ribon A., Pialoux V., Saugy J.J., Rupp T., Faiss R., Debevec T., Millet G.P. Exposure to hypobaric hypoxia results in higher oxidative stress compared to normobaric hypoxia. Respir. Physiol. Neurobiol. 2016;223:23–27. doi: 10.1016/j.resp.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 70.Debevec T., Pialoux V., Ehrström S., Ribon A., Eiken O., Mekjavic I.B., Millet G.P. FemHab: the effects of bed rest and hypoxia on oxidative stress in healthy women. J. Appl. Physiol. 2016;120:930–938. doi: 10.1152/japplphysiol.00919.2015. [DOI] [PubMed] [Google Scholar]
- 71.Millet G.P., Faiss R., Pialoux V. Evidence for differences between hypobaric and normobaric hypoxia is conclusive. Exerc. Sport Sci. Rev. 2013;41 doi: 10.1097/JES.0b013e318271a5e1. [DOI] [PubMed] [Google Scholar]
- 72.Yuan G., Adhikary G., McCormick A.A., Holcroft J.J., Kumar G.K., Prabhakar N.R. Role of oxidative stress in intermittent hypoxia-induced immediate early gene activation in rat PC12 cells. J. Physiol. 2004;557:773–783. doi: 10.1113/jphysiol.2003.058503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mazzeo R.S., Child A., Butterfield G.E., Mawson J.T., Zamudio S., Moore L.G. Catecholamine response during 12 days of high-altitude exposure (4,300 m) in women. J. Appl. Physiol. 1998;84:1151–1157. doi: 10.1152/jappl.1998.84.4.1151. [DOI] [PubMed] [Google Scholar]
- 74.Kehrer J.P., Lund L.G. Cellular reducing equivalents and oxidative stress. Free Radic. Biol. Med. 1994;17:65–75. doi: 10.1016/0891-5849(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 75.Bates M.L., Farrell E.T., Eldridge M.W. Abnormal ventilatory responses in adults born prematurely. New Engl. J. Med. 2014;370:584–585. doi: 10.1056/NEJMc1311092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pialoux V., Hanly P.J., Foster G.E., Brugniaux J.V., Beaudin A.E., Hartmann S.E., Pun M., Duggan C.T., Poulin M.J. Effects of exposure to intermittent hypoxia on oxidative stress and acute hypoxic ventilatory response in humans. Am. J. Respir. Crit. Care Med. 2009;180:1002–1009. doi: 10.1164/rccm.200905-0671OC. [DOI] [PubMed] [Google Scholar]