Abstract
Hedonic response is preserved in schizophrenia. However, it is unclear whether this is also true in individuals meeting criteria for “prodromal” psychosis, who are considered to be at symptomatic high risk for developing the disorder. In this study, we examined neurophysiological and self-reported response to emotional stimuli in UHR (n = 23) and healthy control (CN: n = 30) participants who passively viewed pleasant, unpleasant, and neutral images for 500 ms while the electroencephalogram was recorded and then provided self-reports of valence and arousal to the stimuli. The Late Positive Potential (LPP) event related potential (ERP) component was used as a neurophysiological marker of emotional reactivity. Results indicated that CN participants had higher LPP amplitude for pleasant and unpleasant compared to neutral stimuli; however, UHR youth displayed no differences in LPP amplitude among pleasant, unpleasant, and neutral stimuli. Self-report data mirrored neurophysiological data, as UHR youth had lower reports of positive emotion to pleasant stimuli and negative emotion to unpleasant stimuli compared to CN participants. Furthermore, the presence of a mood disorder diagnosis predicted reduced neurophysiological emotional reactivity in UHR youth.
Findings suggest that youth at UHR for psychosis display diminished subjective and neurophysiological reactivity to emotional stimuli, and that symptoms of depression may result in diminished emotional reactivity.
Keywords: Anhedonia, Emotion, Psychosis, Prodrome
1. Introduction
Anhedonia, traditionally defined as a diminished capacity for positive emotion (Rado, 1953), has been considered a core feature of schizophrenia (SZ) since the earliest conceptualizations of the disorder (Bleuler, 1950, Kraepelin, 1919). However, modern laboratory-based studies of affective response call the validity of this definition into question in SZ. Specifically, recent meta-analyses indicate that SZ patients and controls evidence comparable self-reports of valence (Cohen and Minor, 2010) and arousal (Llerena et al., 2012) to pleasant stimuli. Neuroimaging findings parallel the self-report data, with similar activation of key reward structures (e.g., ventral striatum) between SZ and control groups during the receipt of reward outcomes (Radua et al., 2015). Electrophysiological studies also indicate intact hedonic response, as indicated by comparable amplitude of the Late Positive Potential (LPP) and other ERP components between SZ patients and controls when participants are viewing pleasant stimuli (Horan et al., 2012, Horan et al., 2010). These findings suggest that at both subjective and objective levels of analysis, hedonic response may be intact in SZ. However, not all aspects of emotional response are normal in SZ. Compared to controls, SZ patients report greater intensity of negative emotion to unpleasant, neutral, and pleasant stimuli (Cohen and Minor, 2010), and display greater amygdala activation to unpleasant stimuli (Anticevic et al., 2010).
Few studies have examined whether youth at ultra-high risk (UHR) for developing a psychotic disorder also display intact hedonic response. The majority of prior studies have examined self-reported anhedonia assessed via trait questionnaires or clinical rating scales, finding that rates of self-reported anhedonia are elevated in UHR youth and that elevated reports reflect a latent vulnerability for developing schizophrenia-spectrum disorders (Meehl, 2001, Velthorst et al., 2009). Very few studies have examined anhedonia in UHR youth using laboratory-based paradigms. Of the three studies examining self-reported emotional experience in the prodromal phase of illness, UHR participants have consistently been found to report less positive emotion to pleasant stimuli and less negative emotion to unpleasant stimuli than CN participants (Gruber et al., 2017, Jhung et al., 2016, Yee et al., 2010). Furthermore, diminished emotional reactivity to both pleasant and unpleasant stimuli has been associated with greater severity of depression (Gruber et al. in press). This pattern of findings should be interpreted with the relatively low rate of conversion to a psychotic disorder among those deemed UHR in mind. Only approximately 37% of those identified as UHR will develop a psychotic disorder at four-year follow-up (Schultze-Lutter et al., 2015), with the majority going on to develop mood and anxiety disorders (Addington et al., 2011). Diminished self-reported emotional reactivity in the UHR group may therefore reflect a latent vulnerability for developing anhedonia and mood symptoms more generally, rather than schizophrenia specifically.
Although self-report data provides valuable information regarding a participant's perceived emotional experience, these reports are subject to certain reporting biases and demand characteristics (Robinson and Clore, 2002, Strauss and Gold, 2012). An important next step is therefore to determine whether objective indicators of hedonic response, such as neurophysiological measures, also indicate diminished responsiveness in UHR youth. The current study examined neurophysiological response to emotional stimuli in UHR youth and evaluated associations with clinical symptoms. Participants completed a Rapid Serial Visual Presentation task during which pleasant, unpleasant, and neutral photographs were presented while the electroencephalogram (EEG) was recorded. The LPP event related potential (ERP) component was used as an objective, neurophysiological marker of emotional reactivity. The LPP is a centroparietal midline ERP component that becomes evident at approximately 300 ms after stimulus onset and manifests as a greater relative positivity for both pleasant and unpleasant than neutral stimuli that persists throughout stimulus presentation (Hajcak et al., 2012). After the ERP task, participants made unipolar reports of positive emotion, negative emotion, and arousal to the stimuli. Based on results from prior self-report studies examining UHR youth (Gruber et al., 2017, Jhung et al., 2016, Yee et al., 2010), we hypothesized that the UHR group would display diminished self-reported positive emotion to pleasant stimuli and diminished negative emotion to unpleasant stimuli compared to controls. Our second hypothesis was that controls would evidence robust neurophysiological emotional reactivity, as indicated by significantly greater amplitude of the LPP for pleasant and unpleasant than neutral pictures. However, we predicted that UHR youth would evidence diminished neurophysiological emotional reactivity, as indicated by no significant differences in LPP amplitude among pleasant, unpleasant, and neutral stimulus conditions.
2. Method
2.1. Participants
Participants included 23 UHR youth and 30 healthy controls (CN). UHR participants were recruited from a psychosis risk evaluation program in New York state, which received referrals from local clinicians (e.g., Psychiatrists, Psychologists, Social Workers, School Psychiatrists) to perform diagnostic assessment and monitoring evaluations for youth displaying psychotic experiences. UHR youth were also recruited via online and print advertisements, in-person presentations to community mental health centers, and calls or in-person meetings with members of the local school system (e.g., superintendent, principals). UHR participants were included if they met criteria for a prodromal syndrome on the Structured Interview for Prodromal Syndromes (Miller et al., 1999). SIPS criteria included: 1) Attenuated Positive Symptoms (i.e., SIPS score of at least 3–5 on at least one positive symptom item, with worsening symptoms over the past year) (n = 19); 2) Genetic Risk and Deterioration Syndrome (i.e.,1st degree relative with a psychotic disorder and decline in global functioning over the past year) (n = 4). UHR youth did not meet lifetime criteria for a DSM-IV-TR psychotic disorder as determined via SCID interview (First et al., 2002) and had never been prescribed an antipsychotic.
CN participants were recruited from the local community using posted flyers, newspapers advertisements, and electronic advertisements. CN participants had no current Axis I or II DSM-IV diagnoses as established by the SCID-I and SCID-II (First et al., 2002, Pfohl et al., 1997), no family history of psychosis, and were not taking psychotropic medications. All participants were free from lifetime neurological disease. Moreover, participants provided written informed consent for a protocol approved by the Binghamton University Institutional Review Board and received monetary compensation for their participation. Groups did not significantly differ on age, ethnicity, sex, personal education, or parental education (see Table 1).
Table 1.
Participant demographics.
| UHR (n = 23) | CN (n = 30) | Test statistic, p-value | |
|---|---|---|---|
| Age | 19.6 (1.78) | 19.7 (1.37) | F (1,51) = 0.02, p = 0.89 |
| Participant education | 13.3 (1.69) | 13.6 (1.43) | F (1,51) = 0.59, p = 0.45 |
| Parental education | 14.8 (2.53) | 15.1 (2.36) | F (1,50) = 0.13, p = 0.71 |
| % Male | 30.4 | 23.3 | ×2 (1) = 0.34, p = 0.56 |
| Ethnicity % | ×2 (4) = 2.79, p = 0.59 | ||
| Caucasian | 65.2 | 73.3 | |
| African-American | 0.0 | 6.7 | |
| Latin-American | 13.0 | 6.7 | |
| Asian | 17.4 | 10.0 | |
| Native American | 0.0 | 0.0 | |
| Mixed-race | 4.3 | 3.3 | |
| Clinical symptoms | |||
| SIPS positive | 8.95 (3.83) | – | – |
| SIPS negative | 7.00 (5.48) | – | – |
| SIPS disorganized | 4.00 (2.49) | – | – |
| SIPS mood item | 1.95 (1.83) | – | – |
| PINS MAP | 9.19 (10.21) | – | – |
| PINS EXP | 6.00 (6.83) | – | – |
Note. UHR = Ultra High-Risk for Psychosis; CN = Healthy Control. SIPS = Structured Interview for Prodromal Syndromes; PINS = Prodromal Inventory for Negative Symptoms; MAP = PINS Motivation and Pleasure Subscale; EXP = PINS Diminished Expression Subscale; PINS MAP α = 0.92, α = EXP 0.95. In the UHR group, comorbid conditions included: major depressive disorder (MDD) (n = 6), bipolar disorder (n = 3), dysthymic disorder (n = 1), panic disorder (PD) (n = 7), social phobia (n = 2), obsessive compulsive disorder (OCD) (n = 4), generalized anxiety disorder (GAD) (n = 3), posttraumatic stress disorder (PTSD) (n = 1), substance use disorder (n = 2), borderline personality disorder (n = 1), bulimia nervosa (n = 1), attention deficit/hyperactivity disorder (ADHD) (n = 1). In the UHR group, psychiatric medications prescribed included: Clonazepam (n = 2), Fluvoxamine (n = 1), Hydroxyzine (n = 1), Fluoxetine (n = 1), Escitalopram (n = 2), Adderall (n = 2), Lithium (n = 1), Bupropion (n = 3).
2.2. Procedures
Prior to completing the behavioral and ERP tasks, examiners who were trained to reliability standards (ICC > 0.80), conducted a structured diagnostic interview with all participants to complete the SCID-I, SCID-II, and SIPS. SIPS training was provided by a clinical psychologist previously trained in SIPS assessment (GPS), using in-person and gold-standard training videos. SIPS interviews were either performed directly by the PI or by a clinical psychology doctoral student trained to reliability standards who consulted with the PI on all cases for consensus. A clinical interview was also completed to assess symptom severity in the UHR group, after which ratings were made on the Prodromal Inventory for Negative Symptoms (PINS: Pelletier-Baldelli et al., 2017).
2.3. ERP Task
Participants completed a Rapid Serial Visual Presentation (RSVP) task modeled after Hajcak and Olvet (2008) while the electroencephalogram (EEG) was recorded. Participants were told that they would be shown scenes depicting pleasant (e.g., cute puppies), unpleasant (e.g., snakes), and neutral (e.g., spoons) content, and that they were to simply view the images freely.
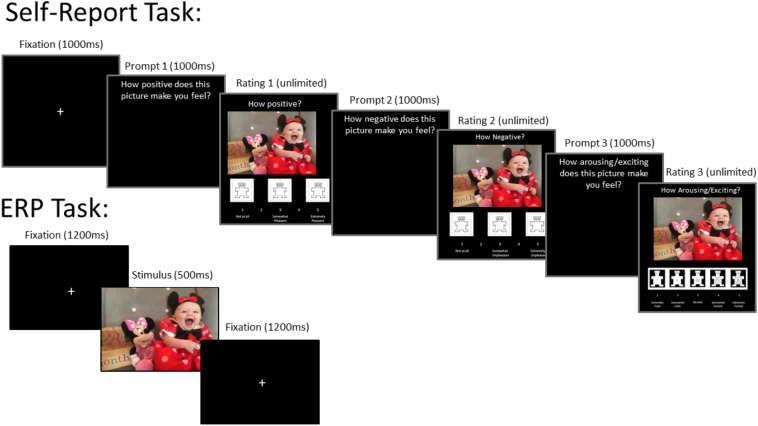
Each trial began with a white fixation cross presented against a black background for 1200 milliseconds (ms). Following the fixation cross, a color photograph was presented for 500 ms across the entirety of the screen (19′′ monitor, 1280 × 1024 resolution, 60 Hz refresh rate) at a viewing distance of approximately 70 cm (visual angle subtending 32.6° × 21.0°). After the stimulus presentation, the next trial began. All photographs were selected from the International Affective Picture System (IAPS) (Lang et al., 2008). There were 90 total stimuli: 30 pleasant, 30 unpleasant, and 30 neutral. Stimuli differed in normative IAPS valence ratings, such that unpleasant < neutral < pleasant. Stimuli differed in arousal, such that unpleasant and pleasant were higher than neutral; however, unpleasant and pleasant did not differ from each other. The three stimulus conditions did not significantly differ in lower-level visual features, including complexity, luminance, red/green/blue saturation (Nummenmaa et al., 2006). The order of stimulus presentation was randomized. See Fig. 1 for a trial diagram.
Fig. 1.
Trial Diagram for Event Related Potential and Self-Report Tasks.
Note. The Figure illustrates the trial sequence for the event related potential and self-report tasks. Stimuli from the International Affective Picture system (IAPS) library were used in the actual experiment. The image presented here is an example and not part of the IAPS set due to IAPS copyright.
2.4. EEG recording, data reduction, and analysis
2.4.1. EEG recording
The EEG was recorded from 64 Ag/AgCl electrodes mounted in an elastic cap from manufactured by BrainVision (ActiCap model). The signals were recorded online using a right mastoid reference electrode and re-referenced offline to the average of the left and right mastoid electrodes. The horizontal electrooculogram (EOG) was used to measure horizontal eye movements and was recorded as the voltage between electrodes placed lateral to the external canthi. The vertical EOG was used to detect eyeblinks and vertical eye movements and was recorded from electrodes above and beneath the left eye. All electrode impedances were maintained below 15KΩ. The EEG and EOG were amplified by a BrainVision actiCHamp amplifier with a gain of 5000, a bandpass filter of 0.05–100 Hz, and a 60-Hz notch filter. The amplified signals were digitized at 500 Hz and averaged offline.
2.4.2. EEG data reduction
All signal processing and analysis procedures were performed in Matlab using EEGLAB and the ERPLAB toolboxes (Lopez-Calderon and Luck, 2014). Data preprocessing included the removal of large muscle artifacts or extreme offsets (identified by visual inspection). Independent component analysis (ICA) was conducted on the continuous data to identify and correct eyeblink activity. The EEG was high-pass filtered with a cut-off of 0.1 Hz.
2.4.3. LPP measurement procedures
ERPs were constructed by separately averaging trials from the three conditions of interest. The ICA-corrected EEG data was divided into epochs that began 200 ms prior to the onset of the stimulus and continued for 700 ms (i.e., − 200–500 ms from stimulus onset), and was baseline corrected using a 200 ms pre-stimulus period. The LPP was calculated as the average amplitude at electrodes Cz, CP1, CP2, and Pz where the effect was maximal between 300 and 500 ms. Measurement procedures are consistent with prior work in this area and this task (e.g., Hajcak et al., 2006, Horan et al., 2013, Strauss et al., 2013).
2.5. Behavioral task
A separate behavioral self-report task was administered after the EEG task. During this task, participants were presented with a subset of the pleasant, unpleasant, and neutral IAPS stimuli presented during the EEG task. There were a total of 3 practice stimuli (1 pleasant, 1 unpleasant, and 1 neutral) and 30 experimental stimuli presented (10 pleasant, 10 unpleasant, 10 neutral). Participants made 3 self-reports for each stimulus: how positive they felt, how negative they felt, and arousal. Ratings for each report were made on a unipolar 1 (not at all) to 5 (extremely) scale that was anchored by the Self Assessment Manikin.
2.6. Data analysis
Analyses were conducted separately on behavioral and ERP data. Behavioral self-report data was analyzed using three separate 2 Group (CN, UHR) × 3 Valence (Pleasant, Unpleasant, Neutral) repeated measures ANOVA. Dependent variables used in these ANOVAs were: positive emotion, negative emotion, arousal. Significant interactions were followed-up by one-way ANOVAs and within-group paired samples t-tests.
ERP data was analyzed using a 2 Group (CN, UHR) × 3 Valence (Pleasant, Unpleasant, Neutral) repeated measures ANOVA. Mean LPP amplitude served as the dependent variable. Follow-up within-group paired samples t-tests were conducted using the following contrasts designed to evaluate emotional reactivity: pleasant vs neutral; unpleasant vs neutral; unpleasant vs pleasant. One-way ANOVAs were conducted to determine whether the two groups differed in the magnitude of the emotional reactivity difference score (pleasant – neutral; unpleasant – neutral).
To evaluate the role of symptoms, spearman correlations were calculated to determine associations between self-reported and ERP emotional reactivity variables and clinical measures. Regression was also used to specifically determine whether the diagnosis of MDD predicted LPP emotional reactivity difference scores (pleasant-neutral; unpleasant-neutral).
3. Results
3.1. Self-report
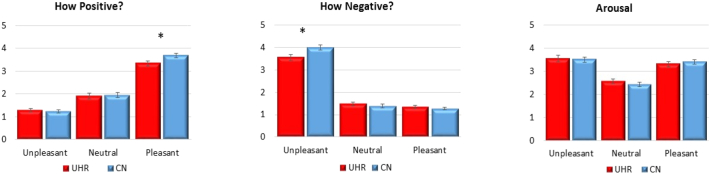
Three separate repeated measures ANOVAs were conducted to evaluate the effects of group on stimulus ratings to pleasant, unpleasant, and neutral stimuli. For reports of positive emotion and reports of negative emotion, there was a statistically significant Group X Valence interaction, as well as a significant Within-Subjects effect of Valence. The Between-Group effects were nonsignificant. Follow-up one-way ANOVAs and within-group paired-sample t-tests were performed. CN reported significantly more negative emotion to unpleasant stimuli and more positive emotion to pleasant stimuli than UHR. For reports of arousal, the Group X Valence interaction and Between-Subjects effect were nonsignificant; the Within-Subjects Effect was significant (see Table 2 and Fig. 2). Thus, UHR youth self-reported diminished emotional response compared to CN.
Table 2.
Omnibus ANOVA and post hoc results for self-report.
| Test-Statistic | P-Value | Cohen's d | |
|---|---|---|---|
| How Positive? | |||
| Group | F (1) = 1.04 | 0.31 | 0.16 |
| Valence | F(1.83) = 386.64 | < 0.001 | 5.69 |
| Group X valence | F (1.83) = 3.40 | 0.04 | 0.53 |
| Post hoc one-way ANOVAs | |||
| Pleasant | F(1,48) = 5.64 | 0.02 | 0.69 |
| Unpleasant | F(1,48) = 0.13 | 0.72 | 0.10 |
| Neutral | F(1,48) = 0.00 | 0.96 | 0.01 |
| Post hoc within-group paired-samples t-tests | |||
| Control | |||
| Pleasant vs. Neutral | t(26) = − 15.22 | < 0.001 | − 2.93 |
| Unpleasant vs. Neutral | t(26) = − 6.80 | < 0.001 | − 1.31 |
| Pleasant vs. Unpleasant | t(26) = − 22.29 | < 0.001 | − 4.29 |
| UHR | |||
| Pleasant vs. Neutral | t(22) = − 10.01 | < 0.001 | − 2.09 |
| Unpleasant vs. Neutral | t(22) = − 5.50 | < 0.001 | − 1.15 |
| Pleasant vs. Unpleasant | t(22) = − 15.52 | < 0.001 | − 3.24 |
| How Negative? | |||
| Group | F(1) = 0.37 | 0.55 | 0.18 |
| Valence | F(1.64) = 561.38 | < 0.001 | 6.86 |
| Group X Valence | F(1.64) = 7.33 | < 0.01 | 0.78 |
| Post hoc one-way ANOVAs | |||
| Pleasant | F(1,48) = 1.58 | 0.22 | 0.36 |
| Unpleasant | F(1,48) = 5.37 | 0.03 | 0.67 |
| Neutral | F(1,48) = 0.91 | 0.34 | 0.28 |
| Post hoc within-Group Paired-Samples t-tests | |||
| CN | |||
| Pleasant vs. Neutral | t(26) = 1.19 | 0.24 | 0.23 |
| Unpleasant vs. Neutral | t(26) = 22.80 | < 0.001 | 4.39 |
| Pleasant vs. Unpleasant | t(26) = 30.55 | < 0.001 | 5.88 |
| UHR | |||
| Pleasant vs. Neutral | t(22) = 1.01 | 0.32 | 0.21 |
| Unpleasant vs. Neutral | t(22) = 13.27 | < 0.001 | 2.77 |
| Pleasant vs. Unpleasant | t(22) = 13.70 | < 0.001 | 2.86 |
| Arousal | |||
| Group | F(1) = 0.07 | < 0.79 | 0.08 |
| Valence | F(1.84) = 69.92 | < 0.001 | 2.48 |
| Group X Valence | F(1.84) = 0.82 | 0.44 | 0.26 |
| Post hoc one-way ANOVAs | |||
| Pleasant | F(1,48) = 0.23 | 0.64 | 0.14 |
| Unpleasant | F(1,48) = 0.00 | 0.99 | 0.00 |
| Neutral | F(1,48) = 1.26 | 0.27 | 0.33 |
| Post Hoc within-Group Paired-Samples t-tests | |||
| CN | |||
| Pleasant vs. Neutral | t(26) = − 7.60 | < 0.001 | − 1.46 |
| Unpleasant vs. Neutral | t(26) = 8.14 | < 0.001 | 1.57 |
| Pleasant vs. Unpleasant | t(26) = 0.89 | 0.38 | 0.17 |
| UHR | |||
| Pleasant vs. Neutral | t(22) = − 6.99 | < 0.001 | − 1.46 |
| Unpleasant vs. Neutral | t(22) = 9.34 | < 0.001 | 1.94 |
| Pleasant vs. Unpleasant | t(22) = 1.53 | 0.14 | 0.31 |
Note. UHR = Ultra High-Risk for Psychosis; CN = Healthy Control.
Fig. 2.
Self-Reported Emotional Experience in CN and UHR Youth.
Note. UHR = Ultra High-Risk for Psychosis; CN = Healthy Control.
3.2. Late positive potential
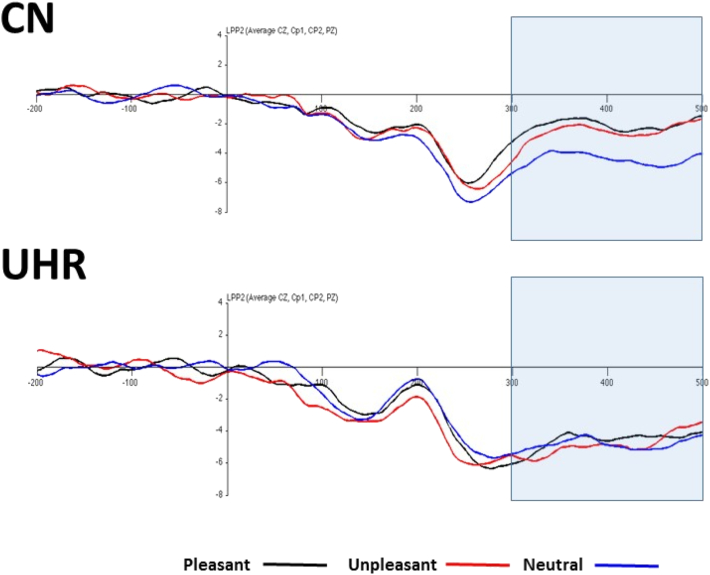
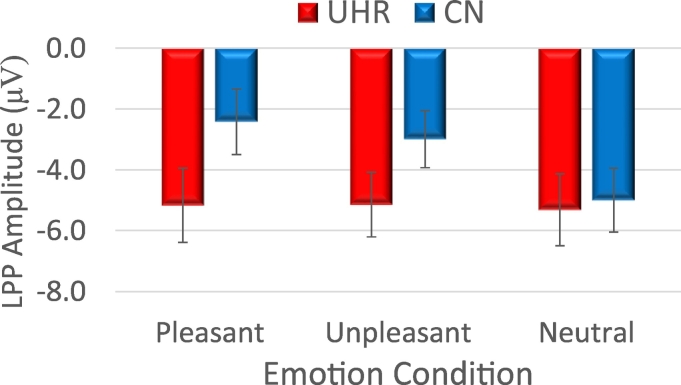
Repeated measures ANOVA indicated a significant Group X Valence interaction, as well as a significant main effect for Valence (see Fig. 3, Fig. 4).1 The between-Subjects effect was nonsignificant. Follow-up within-group paired samples t-tests confirmed that CN had higher amplitude for both pleasant and unpleasant than neutral, and pleasant and unpleasant did not significantly differ. In UHR youth, there were no differences among pleasant, unpleasant, and neutral conditions for LPP amplitude. Follow-up one-way ANOVAs using difference scores (emotional – neutral) as an index of emotional reactivity indicated that CN had significantly higher LPP amplitude than UHR youth for pleasant and unpleasant stimuli.2
Fig. 3.
LPP Grand Average Waveforms in CN and UHR Youth.
Note. UHR = Ultra High-Risk for Psychosis; CN = Healthy Control.
Fig. 4.
Mean LPP Scores in UHR Youth and CN Groups.
Note. UHR = Ultra High-Risk for Psychosis; CN = Healthy Control. Error bars reflect standard error.
Table 3.
Omnibus ANOVA and post hoc results for LPP amplitude.
| Test-statistic | P-value | Cohen's d | |
|---|---|---|---|
| Omnibus ANOVA | |||
| Group | F(1) = 1.43 | 0.24 | 0.35 |
| Valence | F(1.98) = 5.52 | 0.01 | 0.68 |
| Group X valence | F(1.98) = 4.17 | 0.02 | 0.59 |
| Post hoc one-way ANOVAs | |||
| Pleasant-neutral | F(1) = 6.26 | 0.02 | 0.72 |
| Unpleasant-neutral | F(1) = 6.46 | 0.01 | 0.74 |
| Post hoc within-group paired-samples t-tests | |||
| CN | |||
| Pleasant vs. Neutral | T(27) = 4.35 | < 0.001 | 0.82 |
| Unpleasant vs. Neutral | T(27) = 3.89 | < 0.001 | 0.74 |
| Pleasant vs. Unpleasant | T(27) = 0.72 | 0.48 | 0.14 |
| UHR | |||
| Pleasant vs. Neutral | T(21) = 0.41 | 0.69 | 0.09 |
| Unpleasant vs. Neutral | T(21) = − 0.07 | 0.95 | − 0.01 |
| Pleasant vs. Unpleasant | T(21) = 0.55 | 0.59 | 0.12 |
Note. UHR = Ultra High-Risk for Psychosis; CN = Healthy Control; Mean (SD) LPP Values: UHR—Pleasant − 4.58 (5.25), Unpleasant − 4.88(4.98), Neutral − 4.84 (5.33); CN—Pleasant − 2.13 (5.87), Unpleasant − 2.55 (4.81), Neutral − 4.44 (5.53). Mean (SD) LPP difference scores: UHR—Pleasant-Neutral 0.26 (2.96), Unpleasant-Neutral − 0.04(2.79); CN—Pleasant-Neutral 2.31 (2.81), Unpleasant – Neutral 1.90 (2.58).
3.3. The role of symptoms
Spearman correlations were conducted to account for positive skew of symptom data. In the UHR group, there was a significant correlation between self-reported positive emotion to pleasant stimuli and PINS Motivation dimension severity. LPP amplitude was not significantly correlated with other clinical variables or IAPS self-report (Table 4).
Table 4.
Correlations between emotion variables and clinical symptoms in UHR youth.
| SIPS Positive | SIPS Disorganized | PINS MAP | PINS EXP | SIPS Mood |
|
|---|---|---|---|---|---|
| LPP pleasant-neutral | 0.12 | − 0.04 | 0.02 | 0.15 | 0.07 |
| LPP unpleasant-neutral | 0.11 | − 0.05 | 0.07 | 0.17 | − 0.13 |
| Negative emotion to unpleasant stimuli | − 0.09 | 0.11 | − 0.02 | 0.02 | − 0.35 |
| Positive emotion to pleasant stimuli | 0.06 | − 0.13 | − 0.52* | − 0.49 | − 0.33 |
Note. * < 0.05; The significant correlation does not survive strict Bonferroni correction for multiple comparisons; SIPS = Structured Interview for prodromal Syndromes; PINS = Prodromal Inventory for Negative Symptoms; MAP = Motivation and Pleasure Subscale; EXP = Diminished Expression Subscale; SIPS Mood = Anxiety/Depression item.
Regression was used to determine whether the presence of a mood disorder diagnosis predicted LPP emotional reactivity difference scores (emotional – neutral) for pleasant and unpleasant stimuli. Mood disorder diagnosis significantly predicted 8% of the variance in LPP amplitude for pleasant stimuli [F (1, 48) = 4.13, p < 0.05] and 13% of the variance in LPP amplitude for unpleasant stimuli [F(1,48) = 6.91, p < 0.02]. Thus, mood diagnosis predicted diminished emotional reactivity.
4. Discussion
Consistent with hypotheses, UHR youth displayed diminished emotional reactivity compared to controls. Diminished reactivity was evident for both pleasant and unpleasant stimuli in the self-report and LPP data. These findings support prior studies indicating diminished self-reported positive and negative emotion in UHR youth (Gruber et al., 2017, Jhung et al., 2016, Yee et al., 2010), and extend them by indicating that diminished emotional response is also observed using objective neurophysiological measures.
Importantly, the pattern of results observed in our UHR group differs from SZ samples, which display levels of self-reported positive emotion and LPP amplitudes that are comparable to CN in response to pleasant stimuli (Kring and Moran, 2008). It is puzzling as to why SZ, which is more a more impairing and disabling phase of illness than the UHR phase in nearly every way, would show intact hedonic function while UHR youth would not. There are several plausible explanations. One possibility is that comorbid mood and anxiety symptoms, which are prevalent in the prodromal phase, but typically less severe in SZ, might drive diminished emotional response in the UHR group. Supporting this possibility is correlational data from past UHR studies (Gruber et al. in press), as well as the regression analyses in the current study. Additionally, meta-analytic results indicate that patients with major depressive disorder (MDD) display a profile of emotional experience that is similar to our UHR subjects (i.e., diminished positive emotion to pleasant stimuli and diminished negative emotion to unpleasant stimuli) (Bylsma et al., 2008). Given the high degree of similarity between profiles of self-report in MDD patients and UHR youth, this mood explanation seems plausible. Furthermore, few (~ 37%) of those identified as UHR ever develop a psychotic disorder (Schultze-Lutter et al., 2015). The majority go on to develop mood or anxiety disorders (Addington et al., 2011). Thus, the current findings may suggest that diminished emotional reactivity is a risk factor for the development of anhedonia more generally, rather than a psychotic disorder specifically.
A second possibility is that negative symptom phenomenology and pathophysiology changes throughout the course of psychotic illness. Studies have yet to follow the progression of negative symptoms and its pathophysiological correlates from prodromal to diagnosable phases of psychotic illness. Perhaps hedonic deficits are tightly coupled with volitional and expressive symptoms when attenuated psychosis develops during the prodromal phase, but the mechanisms underlying these symptoms become more disconnected over time as the illness becomes more established (Heerey and Gold, 2007). A third possibility is that antipsychotic and other psychotropic medications have direct or indirect effects on mechanisms underlying hedonic response, creating a normalizing effect in patients who are stably treated in later phases of illness. Neuroleptic-free UHR samples may therefore display hedonic deficits because they have not benefited from medication effects. Indeed, there is some evidence that second generation antipsychotics have a normalizing effect on ventral straitum activation during reward processing tasks in SZ (Nielsen et al., 2012).
There are several limitations to consider when interpreting these findings. The size of our UHR sample, although larger than some prior studies on UHR youth (e.g., n = 13, Yee et al., 2010), was relatively small. The study design was cross-sectional and longitudinal data predicting rates of conversion was not possible because the research team moved locations before follow-ups were completed. The effect of substance use and smoking on emotional reactivity could not be determined. ERPs have excellent temporal resolution regarding the time course of neural response; however, spatial resolution is poor. We were therefore unable to draw conclusions regarding neuroanatomical substrates of the observed results. Future longitudinal neuroimaging studies are therefore needed to extend these findings in larger samples. The LPP task involved passive viewing and no element was embedded within the task design to ensure that participants attended to the stimuli. Indeed, the LPP may be an indicator of attention to emotional stimuli. Future studies should directly evaluate the role of focused attention on emotional reactivity in UHR youth using combined methods, such as eye tracking and electroencephalography or fMRI.
Acknowledgments
Acknowledgments
We would like to acknowledge the participants who completed the research, those who facilitated recruitment and referrals at local institutions, and staff in Dr. Strauss' laboratory who contributed to this study.
Financial support
Research was supported by a Transdisciplinary Areas of Excellence Grant from the State University of New York to Dr. Strauss.
Conflicts of interest
The authors have no conflicts of interest related to the current research.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Footnotes
Research supported by a Transdisciplinary Areas of Excellence Grant from the State University of New York (SUNY) to Dr. Strauss.
Groups did not differ in the number of valid trials included in LPP analyses.
Analyses were also repeated on the 19 subjects meeting SIPS APS criteria alone compared to CN. The pattern of findings did not change.
References
- Addington J., Cornblatt B.A., Cadenhead K.S., Cannon T.D., McGlashan T.H., Perkins D.O.…Heinssen R. At clinical high risk for psychosis: outcome for nonconverters. Am. J. Psychiatr. 2011;168(8):800–805. doi: 10.1176/appi.ajp.2011.10081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A., Van Snellenberg J.X., Cohen R.E., Repovs G., Dowd E.C., Barch D.M. Amygdala recruitment in schizophrenia in response to aversive emotional material: a meta-analysis of neuroimaging studies. Schizophr. Bull. 2010;38(3):608–621. doi: 10.1093/schbul/sbq131. sbq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuler E. 1950. Dementia Praecox or the Group of Schizophrenias. [PubMed] [Google Scholar]
- Bylsma L.M., Morris B.H., Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clin. Psychol. Rev. 2008;28:676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Cohen A., Minor K. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr. Bull. 2010;36:143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Patient edition. Biometrics Research, New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis 1 Disorders, Research Version. (SCID-I/P) [Google Scholar]
- Gruber J.M., Strauss G.P., Dombrecht L., Mittal V.A. Neuroleptic-free youth at ultrahigh risk for psychosis evidence diminished Hedonic response that is predicted by depression and anxiety. Schizophr. Res. 2017 doi: 10.1016/j.schres.2017.08.013. in press. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Olvet D.M. The persistence of attention to emotion: brain potentials during and after picture presentation. Emotion. 2008;8:250. doi: 10.1037/1528-3542.8.2.250. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Moser J.S., Simons R.F. Attending to affect: appraisal strategies modulate the electrocortical response to arousing pictures. Emotion. 2006;6:517. doi: 10.1037/1528-3542.6.3.517. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Weinberg A., MacNamara A., Foti D. The Oxford Handbook of Event-related Potential Components. 2012. ERPs and the study of emotion; pp. 441–474. [Google Scholar]
- Heerey E.A., Gold J.M. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. J. Abnorm. Psychol. 2007;116:268–278. doi: 10.1037/0021-843X.116.2.268. [DOI] [PubMed] [Google Scholar]
- Horan W.P., Wynn J.K., Kring A.M., Simons R.F., Green M.F. Electrophysiological correlates of emotional responding in schizophrenia. J. Abnorm. Psychol. 2010;119:18. doi: 10.1037/a0017510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan W.P., Foti D., Hajcak G., Wynn J.K., Green M.F. Intact motivated attention in schizophrenia: evidence from event-related potentials. Schizophr. Res. 2012;135:95–99. doi: 10.1016/j.schres.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan W.P., Hajcak G., Wynn J.K., Green M.F. Impaired emotion regulation in schizophrenia: evidence from event-related potentials. Psychol. Med. 2013;43:2377–2391. doi: 10.1017/S0033291713000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhung K., Park J.Y., Song Y.Y., Kang J.I., Lee E., An S.K. Experiential pleasure deficits in the prodrome: a study of emotional experiences in individuals at ultra-high risk for psychosis and recent-onset schizophrenia. Compr. Psychiatry. 2016;68:209–216. doi: 10.1016/j.comppsych.2016.04.021. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. B. RM, Trans.; New York: 1919. Dementia Praecox and Paraphrenia. [Google Scholar]
- Kring A., Moran E. Emotional response deficits in schizophrenia: insights from affective science. Schizophr. Bull. 2008;34:819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. Technical Report A-8. University of Florida; Gainesville, FL: 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Llerena K., Strauss G.P., Cohen A.S. Looking at the other side of the coin: a meta-analysis of self-reported emotional arousal in people with schizophrenia. Schizophr. Res. 2012;142:65–70. doi: 10.1016/j.schres.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Calderon J., Luck S.J. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front. Hum. Neurosci. 2014;8:213. doi: 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl P.E. Primary and secondary hypohedonia. J. Abnorm. Psychol. 2001;110:188. doi: 10.1037//0021-843x.110.1.188. [DOI] [PubMed] [Google Scholar]
- Miller T.J., McGlashan T.H., Woods S.W., Stein K., Driesen N., Corcoran C.M., Hoffman R., Davidson L. Symptom assessment in schizophrenic prodromal states. Psychiatry Q. 1999;70:273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Nielsen M.O., Rostrup E., Wulff S., Bak N., Broberg B.V., Lublin H., Kapur S., Glenthoj B. Improvement of brain reward abnormalities by antipsychotic monotherapy in schizophrenia. Arch. Gen. Psychiatry. 2012;69:1195–1204. doi: 10.1001/archgenpsychiatry.2012.847. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L., Hyönä J., Calvo M.G. Eye movement assessment of selective attentional capture by emotional pictures. Emotion. 2006;6:257–268. doi: 10.1037/1528-3542.6.2.257. [DOI] [PubMed] [Google Scholar]
- Pelletier-Baldelli A., Strauss G.P., Visser K.H., Mittal V.A. Initial development and preliminary psychometric properties of the Prodromal Inventory of Negative Symptoms (PINS) Schizophr. Res. 2017;189:43–49. doi: 10.1016/j.schres.2017.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfohl B.M., Blum N., Zimmerman M. American Psychiatric Publishing, Inc; 1997. Structured Interview for DSM-IV Personality. [Google Scholar]
- Rado S. Dynamics and classification of disordered behaviors. Am. J. Psychiatry. 1953;110:406–416. doi: 10.1176/ajp.110.6.406. [DOI] [PubMed] [Google Scholar]
- Radua J., Schmidt A., Borgwardt S., Heinz A., Schlagenhauf F., McGuire P., Fusar-Poli P. Ventral striatal activation during reward processing in psychosis: a neurofunctional meta-analysis. JAMA Psychiat. 2015;72:1243–1251. doi: 10.1001/jamapsychiatry.2015.2196. [DOI] [PubMed] [Google Scholar]
- Robinson M.D., Clore G.L. Belief and feeling: evidence for an accessibility model of emotional self-report. Psychol. Bull. 2002;128:934–960. doi: 10.1037/0033-2909.128.6.934. [DOI] [PubMed] [Google Scholar]
- Schultze-Lutter F., Michel C., Schmidt S.J., Schimmelmann B.G., Maric N.P., Salokangas R.K.R.…Meneghelli A. EPA guidance on the early detection of clinical high risk states of psychoses. Eur. Psychiatry. 2015;30(3):405–416. doi: 10.1016/j.eurpsy.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Strauss G.P., Gold J.M. A new perspective on anhedonia in schizophrenia. Am. J. Psychiatry. 2012;169:364–373. doi: 10.1176/appi.ajp.2011.11030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss G.P., Kappenman E.S., Culbreth A.J., Catalano L.T., Lee B.G., Gold J.M. Emotion regulation abnormalities in schizophrenia: cognitive change strategies fail to decrease the neural response to unpleasant stimuli. Schizophr. Bull. 2013;39:872–883. doi: 10.1093/schbul/sbs186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velthorst E., Nieman D.H., Becker H.E., van de Fliert R., Dingemans P.M., Klaassen R., de Haan L., van Amelsvoort T., Linszen D.H. Baseline differences in clinical symptomatology between ultra high risk subjects with and without a transition to psychosis. Schizophr. Res. 2009;109:60–65. doi: 10.1016/j.schres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Yee C.M., Mathis K.I., Sun J.C., Sholty G.L., Lang P.J., Bachman P., Williams T.J., Bearden C.E., Cannon T.D., Green M.F. Integrity of emotional and motivational states during the prodromal, first-episode, and chronic phases of schizophrenia. J. Abnorm. Psychol. 2010;119:71. doi: 10.1037/a0018475. [DOI] [PMC free article] [PubMed] [Google Scholar]