Abstract
Background and purpose
Oxidative stress has been implicated in the pathogenesis of amyotrophic lateral sclerosis (ALS). Edaravone, a free radical scavenger, was approved as a therapeutic drug for ALS in 2015 in Japan. A phase 3 clinical trial demonstrated a smaller decline in ALS functional scale scores compared with placebo. However, the long-term effects of edaravone on ALS patients remain unclear. This study aimed to retrospectively investigate the long-term effects of edaravone on the survival of ALS patients.
Methods
We retrospectively analyzed 27 consecutive patients with ALS who were treated with edaravone and 30 consecutive ALS patients who were not treated with edaravone between 2010 and 2016.
Results
The differences of ALSFRS-R scores from baseline to 6 months was significantly reduced in the edaravone group, compared to the control group. The changes in serum creatinine, as a possible marker of ALS severity, from baseline to 6 and 12 months were significantly improved in the edaravone group, compared to the control group. The survival rate was significantly improved in the edaravone group compared with control patients.
Conclusion
Our retrospective single-center analysis suggests slower progression and better prognosis of ALS patients with edaravone treatment. Further investigation, including prospective multicenter analysis, is warranted to confirm the usefulness of edaravone for a better prognosis of ALS.
Keywords: Amyotrophic lateral sclerosis, Oxidative stress, Edaravone, Long-term effect, Survival
Abbreviations: ALS, amyotrophic lateral sclerosis; 3-NT, 3-nitrotyrosine; CSF, cerebrospinal fluid; ALSFRS-R, revised ALS functional rating scale; TPPV, tracheostomy positive pressure ventilation
Highlights
-
•
We retrospectively investigate the effects of edaravone on ALS patients.
-
•
The differences of ALSFRS-R scores were reduced in the edaravone group.
-
•
The changes in serum creatinine were improved in the edaravone group.
-
•
The survival rate was improved in the edaravone group.
-
•
A prospective multicenter analysis is warranted to confirm usefulness of edaravone.
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive, fatal disease characterized by chronic degeneration of upper and lower motor neurons. ALS leads to progressive muscle waste and weakness of the upper and lower extremities, bulbar palsy, and finally death within 3–5 years due to respiratory failure. Although the mechanisms by which ALS leads to motor neuron degeneration remain unclear, various mechanisms including the deposition of intranuclear and cytosolic protein and RNA aggregates, disturbances of protein degradative mechanisms, mitochondrial dysfunction, endoplasmic reticulum stress, defective nucleocytoplasmic trafficking, altered neuronal excitability, altered axonal transport, and excessive oxidative stress have been implicated as involved in the pathogenesis of ALS [1].
Regarding oxidative stress [2], post-mortem studies have revealed increased concentrations of 3-nitrotyrosine (3-NT), a specific marker for oxidative stress, in the lumbar and thoracic spinal cord of ALS patients and increased 3-NT immunoreactivity in motor neurons of both sporadic and familial ALS patients [3]. The concentration of 3-NT and the 3-NT/tyrosine ratio have been shown to be increased seven-fold in the cerebrospinal fluid (CSF) of patients with sporadic ALS compared to levels in controls, suggesting the significance of peroxynitrite-mediated oxidative damage in the pathogenesis of sporadic ALS [4]. A recent study demonstrated that the magnitude of oxidative stress as measured by positron emission tomography of the brain with [62Cu]diacetyl-bis (N4-methylthiosemicarbazone) correlated with clinical severity in ALS [5]. Oxidative stress can thus be a critical factor for the development of neurodegeneration in ALS.
A biochemical study showed that 3-methyl-1-phenyl-2-pyrazolin-5-one (edaravone), a free radical scavenger, has been shown to eliminate peroxynitrite as well as lipid peroxyl radicals [6]. An open-label phase 2 study demonstrated that the decline in the revised ALS functional rating scale (ALSFRS-R) score was significantly less than that in the six months prior to edaravone administration, and that the concentration of 3-NT in CSF was markedly reduced to almost undetectable levels at the end of the six-month treatment period [7]. Subsequently, the first placebo-controlled phase 3 study of edaravone over 24 weeks of treatment revealed that the reduction of ALSFRS-R was smaller in the edaravone group than in the placebo group, but failed to show significant efficacy of edaravone in the treatment of ALS [8]. However, post-hoc analysis revealed that patients with early-stage ALS showed a greater degree of therapeutic effect compared to the full study population. A second randomized, double-blinded, placebo-controlled phase 3 trial examined patients with grade 1 or 2 ALS in the Japan ALS severity classification, scores of ≥2 points on all 12 items of the ALSFRS-R, forced vital capacity ≥80%, definite or probable ALS according to the revised El Escorial criteria, and disease duration of ≤2 years, showing a significantly smaller decline in ALSFRS-R score compared with placebo [9]. Based on these clinical trials, edaravone was finally approved as a therapeutic drug for ALS in 2015 in Japan. However, the evaluation for long-term effects of edaravone on patients with ALS is taken over by the post-marketing surveillance study.
The present study aimed to retrospectively investigate long-term effects of edaravone on ALS patients in advance of the future results of post-marketing surveillance.
2. Methods
2.1. Protocol approvals
This study was approved by the institutional ethics committee at Kumamoto Saishunso National Hospital. We obtained oral informed consent from each patient before enrolment and participation in the study.
2.2. Patients and study design
Fifty-seven consecutive patients with ALS were examined between 2010 and 2016, and classified into two groups based on the presence or absence of edaravone medication: the edaravone group and the control group. Since Edaravone was approved as a therapeutic drug for ALS at June 2015 in Japan, all the patients before June 2015 were allocated to the control group. Edaravone was administered once a day via 60-min intravenous infusion. Edaravone was initially administered for 14 consecutive days followed by a 2-week drug-free period, and subsequently administered for 10 days within a 2-week period according to the standard protocol. All patients were diagnosed with ALS according to the revised El Escorial criteria [10]. For patients who had already taken riluzole, the same drug was continuously prescribed without changing the dosages.
Patients were categorized with limb-onset type, bulbar-onset type, or flail-arm type based on the clinical features at onset. We compared Japan ALS severity classifications, ALSFRS-R scores, respiratory function, and biochemical data between groups. We evaluated differences of ALSFRS-R scores from baseline (within 1 week at the initiation of edaravone) to 6, 12, and 18 months in the edaravone group, compared to those from baseline (at the first visit to our institution) to 6, 12, and 18 months in the control group. Changes in serum creatinine from baseline to 6 and 12 months were examined in the both groups. We set the primary endpoint as tracheostomy-free survival or death, and the secondary endpoints as changes in ALSFRS-R and serum creatinine, a possible marker for ALS severity [11]. Safety endpoints included the incidence of adverse events, adverse drug reactions, and abnormal biochemical data.
2.3. Statistics
Differences in clinical characteristics between groups were analyzed using the chi-square test and/or Fisher's exact test. Comparisons of differences in ALSFRS-R and creatinine were analyzed by two-way ANOVA. Survival from onset to the primary endpoint was analyzed using Kaplan-Meier curves and log-rank tests.
3. Results
Among 57 ALS patients, 27 patients (15 men, 12 women; mean age, 62 ± 9.4 years) were treated with edaravone for a mean duration of 8.8 ± 6.0 months, and 30 patients (19 men, 11 women, mean age, 67 ± 9.7 years) were not treated with edaravone at all (Table 1). Differences in the clinical characteristics of patients did not differ significantly between the edaravone and control groups. However, initial symptoms showed the following tendency (p = .0519): limb-onset-type patients were more frequently included in the edaravone group, whereas bulbar-onset-type patients were more frequently included in the control group. Four of the 27 patients in the edaravone group and 6 of the 30 patients in the control group fulfilled the inclusion criteria for the second phase 3 trial: patients with ALS of grade 1 or 2 in the Japan ALS severity classification, scores of ≥2 points on all 12 items of the ALSFRS-R, forced vital capacity ≥80%, definite or probable ALS according to the revised El Escorial criteria, and disease duration of ≤2 years.
Table 1.
Clinical characteristics of patients with ALS in the present study.
| Edaravone group (n = 27) | Control group (n = 30) | P | |
|---|---|---|---|
| Sex | |||
| Male | 15 (56%) | 19 (63%) | 0.5974 |
| Female | 12 (44%) | 11 (37%) | |
| Age, years | 62.0 ± 9.4 | 67.2 ± 9.7 | |
| <65 years | 15 (56%) | 11 (37%) | 0.1887 |
| ≥65 years | 12 (44%) | 19 (63%) | |
| Disease duration, months | 22.6 ± 15.3 | 15.0 ± 11.5 | |
| ALS severity | 2.7 ± 1.0 | 2.7 ± 0.8 | 0.2360 |
| Grade 1 | 2 (7%) | 2 (7%) | |
| Grade 2 | 12 (44%) | 9 (30%) | |
| Grade 3 | 6 (22%) | 15 (50%) | |
| Grade 4 | 6 (22%) | 4 (13%) | |
| Grade 5 | 1 (4%) | 0 (0%) | |
| ALSFRS-R | 30.0 ± 12.1 | 38.7 ± 6.3 | 0.0034 |
| Initial symptoms | |||
| Limb-onset type | 18 (67%) | 11 (37%) | 0.0519 |
| Bulbar-onset type | 3 (11%) | 10 (33%) | |
| Flail-arm type | 6 (22%) | 9 (30%) | |
| Creatinine (mg/dl) | 0.44 ± 0.15 | 0.57 ± 0.15 | 0.0017 |
| Use of riluzole | 25 (93%) | 23 (77%) | 0.0997 |
| Use of PEG | 15 (56%) | 20 (67%) | 0.3896 |
| Use of NPPV | 9 (33%) | 12 (40%) | 0.6024 |
PEG, percutaneous endoscopic gastrostomy; NPPV, noninvasive positive pressure ventilation.
We first investigated whether edaravone indeed reduced the rate of decline in ALSFRS-R among the population including patients at both early and advanced stages of ALS. ALSFRS-R scores at baseline were more severe in the edaravone group (30.0 ± 12.1) than those in the control group (38.7 ± 6.3) (p = .0034, Table 1). Among the total population, the differences of ALSFRS-R scores from baseline to 6 months was significantly reduced in the edaravone group, compared to the control group whereas changes in ALSFRS-R scores were not significant at 12 and 18 months between the groups (p < .05, Fig. 1).
Fig. 1.
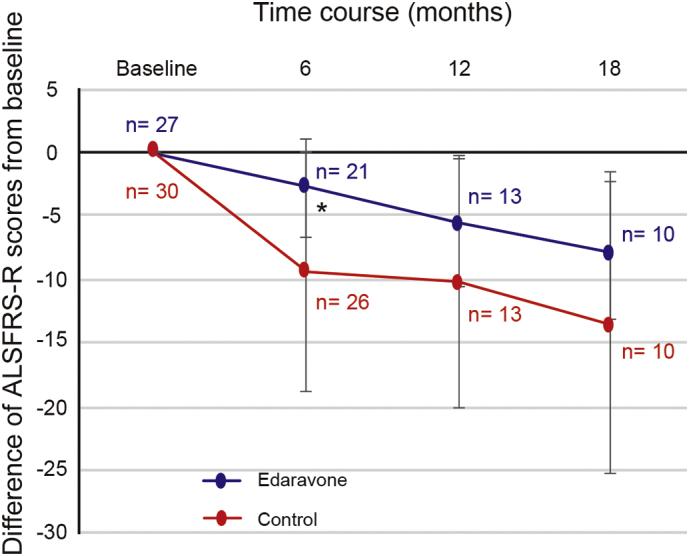
Changes of ALSFRS-R scores from baseline to 6, 12, and 18 months in the edaravone and control groups. *, p < .05.
We next evaluated whether edaravone affected the change in serum creatinine, as a possible marker of ALS severity. Serum creatinine levels at baseline were lower in the edaravone group (0.44 ± 0.15 mg/dl) than those in the control group (0.57 ± 0.15 mg/dl) (p = .0017, Table 1). Serum creatinine levels at 6 months were significantly increased in the edaravone group whereas those were rather decreased in the control group (Fig. 2). The changes in serum creatinine from baseline to 12 months were significantly reduced in the edaravone group, compared to the control group (p < .05, Fig. 2).
Fig. 2.
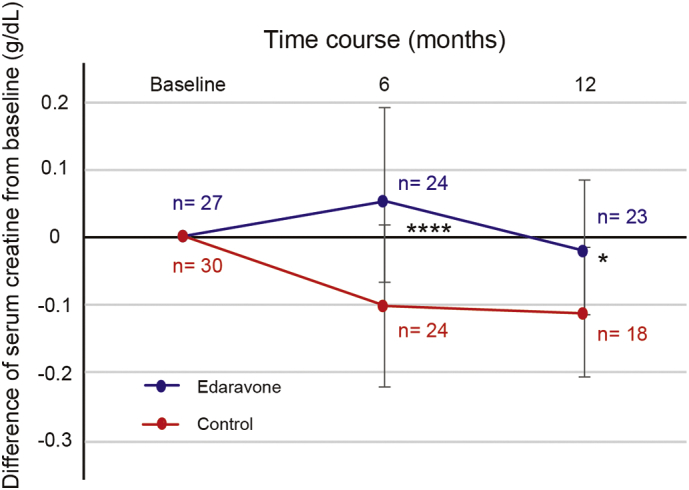
Changes in serum creatinine from baseline to 6 and 12 months in the edaravone and control groups. ****, p < .0001; *, p < .05.
Ten of the 27 patients treated with edaravone discontinued this medication. We then investigated the reasons and timing of discontinuation. Only one patient stopped edaravone treatment due to a side effect, namely, renal dysfunction with the combination of antibiotics (Table 2). Other reasons for discontinuation included vein inflammation, worsening of the disease (pneumonia), difficulty with vascular access, and patient request. After discontinuation of edaravone, four patients died at 30, 31, 43, and 61 months from onset whereas 2 patients survived at 41 and 57 months, not reaching to the primary endpoint.
Table 2.
Reasons for discontinuation of edaravone treatment.
| Reasons | n = 27 |
|---|---|
| Renal dysfunction | 1 (4%) |
| Vein inflammation | 1 (4%) |
| Worsening of disease (pneumonia) | 3 (11%) |
| Difficulty in vascular access | 2 (7%) |
| Patient request | 3 (11%) |
We finally investigated the long-term effects on survival rate in ALS patients. Kaplan-Meier curves showed that the survival rate until the defined primary endpoint was significantly improved in the edaravone group compared to the control group (p = .0046, Fig. 3). Median survival durations were 61.0 and 32.5 months in the edaravone and control groups, respectively (HR 0.37, 95% CI 0.20–0.74). Of the 4 patients who fulfilled the inclusion criteria for the second phase 3 trial in the edaravone group, 2 patients have been continuing edaravone treatment, 1 patient died at 29 months, and 1 patient underwent TPPV at 23 months.
Fig. 3.
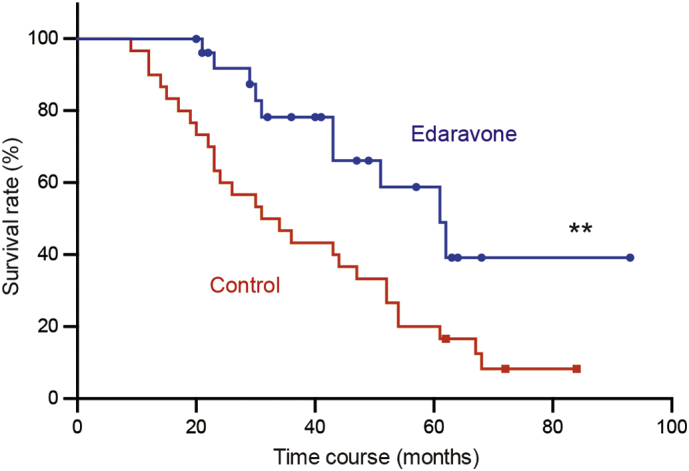
Survival rates in edaravone-treated and control groups. **, P = .0046.
4. Discussion
Our retrospective, single-center analysis revealed that the rate of decline in ALS functional scores was significantly reduced at 6 months from edaravone initiation among the population including both early and advanced stages of ALS. The changes in serum creatinine concentration, as a possible marker of ALS severity, were significantly improved at 6 and 12 months in the edaravone-treated group. The survival rate until the primary endpoint was significantly improved in the edaravone group.
To date, the long-term effects of edaravone on patients with ALS have not been fully evaluated. In an open-label, 24-week extension study of the second phase 3 study, time to death or a specified state of disease progression that included disability of independent ambulation, loss of upper-limb function, tracheotomy, use of a respirator, use of tube feeding, and loss of useful speech were analyzed [12]. The data revealed 15 events in 10 patients in the edaravone-edaravone group and 20 events in 19 patients in the placebo-edaravone group without once mentioning significant differences.
The previous phase 3 clinical trial revealed the efficacy of edaravone for maintaining physical function and quality of life in patients with early-stage ALS. In the present study, the primary outcome measure was defined as tracheostomy-free survival or death, because TPPV prevents death from respiratory failure and has a huge positive effect on survival rate.
The present study has several limitations. First, the control group included a higher number of patients with bulbar-onset-type ALS, which often manifests with a more aggressive clinical course [13]. Second, patients in the edaravone-treated group might have been more motivated, leading to emotional rewards. Lastly, patients in the edaravone-treated group might have obtained more appropriate medical care due to a multidisciplinary approach to the treatment with edaravone [14]. Because this study was a retrospective single-center analysis with a relatively small number of ALS patients, a prospective study on a larger scale is warranted. An observational study (SUNRISE Japan) is ongoing, which will evaluate the safety and efficacy under the actual clinical use, and the effect on long-term prognosis in patients who receive a treatment with edaravone for ALS.
5. Conclusion
Our retrospective, single-center analysis suggests slower progression and better prognosis of ALS patients with edaravone treatment. Further investigation including prospective multicenter analysis is needed to confirm the usefulness of edaravone for achieving better prognosis of ALS.
Conflict of interest
The authors have no conflicts of interest to disclose.
Contributor Information
Satoshi Yamashita, Email: y-stsh@kumamoto-u.ac.jp.
Hidetsugu Ueyama, Email: hueyama@saisyunsou1.hosp.go.jp.
References
- 1.Brown R.H., Al-Chalabi A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2017;377:162–172. doi: 10.1056/NEJMra1603471. [DOI] [PubMed] [Google Scholar]
- 2.Kaur C., Ling E.A. Antioxidants and neuroprotection in the adult and developing central nervous system. Curr. Med. Chem. 2008;15:3068–3080. doi: 10.2174/092986708786848640. [DOI] [PubMed] [Google Scholar]
- 3.Beal M.F., Ferrante R.J., Browne S.E. Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann. Neurol. 1997;42:644–654. doi: 10.1002/ana.410420416. [DOI] [PubMed] [Google Scholar]
- 4.Tohgi H., Abe T., Yamazaki K. Remarkable increase in cerebrospinal fluid 3-nitrotyrosine in patients with sporadic amyotrophic lateral sclerosis. Ann. Neurol. 1999;46:129–131. doi: 10.1002/1531-8249(199907)46:1<129::aid-ana21>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 5.Ikawa M., Okazawa H., Tsujikawa T. Increased oxidative stress is related to disease severity in the ALS motor cortex: a PET study. Neurology. 2015;84:2033–2039. doi: 10.1212/WNL.0000000000001588. [DOI] [PubMed] [Google Scholar]
- 6.Fujisawa A., Yamamoto Y. Edaravone, a potent free radical scavenger, reacts with peroxynitrite to produce predominantly 4-NO-edaravone. Redox Rep. 2016;21:98–103. doi: 10.1179/1351000215Y.0000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshino H., Kimura A. Investigation of the therapeutic effects of edaravone, a free radical scavenger, on amyotrophic lateral sclerosis (phase II study) Amyotroph. Lateral Scler. 2006;7:241–245. doi: 10.1080/17482960600881870. [DOI] [PubMed] [Google Scholar]
- 8.Abe K., Itoyama Y., Sobue G. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler. Frontotemporal. Degener. 2014;15:610–617. doi: 10.3109/21678421.2014.959024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Writing G., Edaravone A.L.S.S.G. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16:505–512. doi: 10.1016/S1474-4422(17)30115-1. [DOI] [PubMed] [Google Scholar]
- 10.Brooks B.R., Miller R.G., Swash M. World Federation of Neurology Research Group on motor neuron D. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 11.Tetsuka S., Morita M., Ikeguchi K. Utility of cystatin C for renal function in amyotrophic lateral sclerosis. Acta Neurol. Scand. 2013;128:386–390. doi: 10.1111/ane.12134. [DOI] [PubMed] [Google Scholar]
- 12.Writing group on behalf of the edaravone Als 19 study G. open-label 24-week extension study of edaravone (MCI-186) in amyotrophic lateral sclerosisAmyotroph. Lateral Scler. Frontotemporal Degener. 2017;18:55–63. doi: 10.1080/21678421.2017.1364269. [DOI] [PubMed] [Google Scholar]
- 13.Fogh I., Lin K., Tiloca C. Association of a Locus in the CAMTA1 gene with survival in patients with sporadic amyotrophic lateral sclerosis. JAMA Neurol. 2016;73:812–820. doi: 10.1001/jamaneurol.2016.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traynor B.J., Alexander M., Corr B. Effect of a multidisciplinary amyotrophic lateral sclerosis (ALS) clinic on ALS survival: a population based study, 1996-2000. J. Neurol. Neurosurg. Psychiatry. 2003;74:1258–1261. doi: 10.1136/jnnp.74.9.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]


