Abstract
Acetaminophen (APAP) overdose is the leading cause of drug-induced acute liver failure in many developed countries. Mitochondrial oxidative stress is considered to be the predominant cellular event in APAP-induced liver injury. Accordingly, N-acetyl cysteine, a known scavenger of reactive oxygen species (ROS), is recommended as an effective clinical antidote against APAP-induced acute liver injury (AILI) when it is given at an early phase; however, the narrow therapeutic window limits its use. Hence, the development of novel therapeutic approaches that can offer broadly protective effects against AILI is clearly needed. To this end, it is necessary to better understand the mechanisms of APAP hepatotoxicity. Up to now, in addition to mitochondrial oxidative stress, many other cellular processes, including phase I/phase II metabolism, endoplasmic reticulum stress, autophagy, sterile inflammation, microcirculatory dysfunction, and liver regeneration, have been identified to be involved in the pathogenesis of AILI, providing new targets for developing more effective therapeutic interventions against APAP-induced liver injury. In this review, we summarize intracellular and extracellular events involved in APAP hepatotoxicity, along with emphatic discussions on the possible therapeutic approaches targeting these different cellular events.
Keywords: Acetaminophen, Mitochondrial oxidative stress, Endoplasmic reticulum stress, Autophagy, Sterile inflammation, Liver regeneration
1. Introduction
Acetaminophen (APAP) is one of the most frequently used drugs for its analgesic and antipyretic properties. It is safe and effective at recommended doses, whereas overdose may lead to hepatotoxicity and acute liver failure (ALF). In fact, APAP-induced hepatotoxicity remains the most common cause of ALF in many countries [70]. Given the public concern caused by APAP hepatotoxicity, great efforts have been made to understand the mechanisms of its toxic effects. Generally, APAP-induced oxidative stress and mitochondrial dysfunction plays the central role in the pathogenesis of AILI [50]. As such, the United States Food and Drug Administration recommends N-acetyl cysteine (NAC), a known antioxidant, as the only therapeutic option for APAP-overdosed patients; however, this medication has its limitations including adverse effects and narrow therapeutic window [31]. If the early and/or the most treatable stage is missed, liver transplantation is the only choice to improve survival in patients with AILI [23]. Hence, the development of new drugs that are superior to NAC, in terms of effectiveness and therapeutic time frame, is clearly needed. In recent years, there have been intensive studies demonstrating the protective effects of natural products against APAP-induced hepatotoxicity, providing considerable drug candidates for AILI. It has been recognized that the APAP toxicity consists of multi-stages and multi-signaling pathways, including APAP metabolism, oxidative stress, endoplasmic reticulum (ER) stress, autophagy, sterile inflammation, microcirculatory dysfunction, and compensatory liver repair and regeneration. Many genes or molecules have been identified to play pivotal roles in the regulation of APAP hepatotoxicity, so they are suggested to be potential targets for therapeutic intervention against APAP-induced liver injury.
This review summarizes the generally accepted mechanisms of APAP hepatotoxicity and highlights the key signaling pathways as targets for AILI therapeutic interventions.
2. Targeting the metabolism phase for AILI intervention
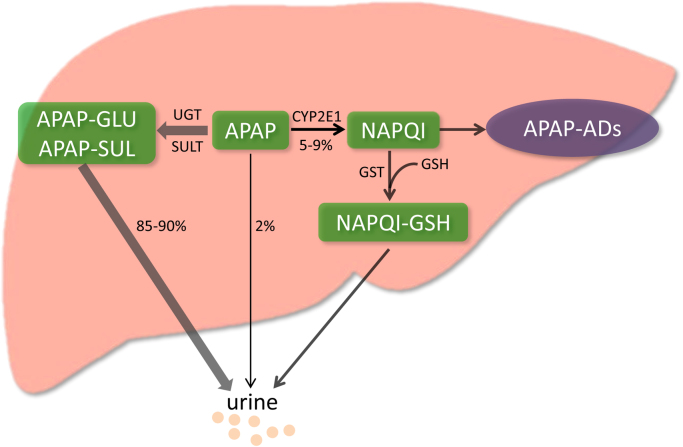
When taken at therapeutic doses, most of APAP is metabolized by phase II conjugating enzymes, mainly UDP-glucuronosyltransferase (UGT) and sulfotransferase (SULT), converting it to nontoxic compounds which are then excreted with the urine. Only a very small portion is excreted unchanged in the urine. The remaining APAP, approximately 5–9% is metabolized by the cytochrome P450 enzymes (CYPs), mainly CYP 2E1 into the highly reactive intermediate metabolite N-acetyl-p-benzoquinone imine (NAPQI) [66]. Generally, NAPQI is rapidly detoxified by conjugating with glutathione (GSH). However, when phase II metabolizing enzymes are saturated after APAP overdose, excessive NAPQI deplete GSH, leading to covalent binding of sulfhydryl groups in cellular proteins, especially mitochondrial proteins [95]. This results in mitochondrial oxidative stress and dysfunction, ultimately hepatocytes necrosis [50] (Fig. 1).
Fig. 1.
Metabolic activation of acetaminophen. 85–90% of APAP is primarily metabolized by phase II conjugating enzymes (mainly UGT and SULT). Only 2% is excreted unchanged in the urine. And approximately 5–9% is metabolized mainly by CYP 2E1 into the highly reactive intermediate metabolite NAPQI. In general, NAPQI is detoxified by conjugating with GSH. However, excessive NAPQI depletes GSH following APAP overdose, leading to formation of APAP-ADs through covalent binding of sulfhydryl groups in cellular proteins. APAP, acetaminophen; UGT, UDP-glucuronosyltransferase; SULT, sulfotransferase; CYP 2E1, Cytochrome P450 2E1; NAPQI, N-acetyl-p-benzoquinone imine; GSH, glutathione; APAP-ADs, APAP protein adducts.
Based on the pharmacokinetic studies of APAP, induction of anti-toxic phase II enzymes may accelerate the metabolic inactivation of this drug, leading to a reduction of APAP metabolized by cytochrome P450 enzymes to NAPQI. In another word, the regulation of APAP detoxification at the phase II level is beneficial in alleviating APAP hepatotoxicity. This hypothesis is consistent with the observation that activation of liver X receptors (LXR) prevented APAP-induced liver injury through induction of phase II conjugating enzymes, as well as suppression of pro-toxic phase I P450 enzymes [103]. Multiple studies have shown that expressions and activities of cytochrome P450 enzymes were also positively regulated by some nuclear receptors and transcription factors, including pregnane X receptor (PXR), constitutive androstane receptor (CAR), and retinoid X receptor α (RXRα) [124], [133], [38],]. Thus, any changes in the expression of these nuclear hormone receptors in response to lipids, cholesterols, bile acids, and xenobiotics could potentially alter APAP-induced toxicity through modulation of NAPQI generation. This assumption was verified in a follow-up study that activation of anti-viral responses by polyinosinic-polycytidylic acid attenuated APAP-induced hepatotoxicity through transcriptional down regulation of RXRα and PXR and their downstream CYPs [34]. Similarly, genetic deletion or pharmacological suppression of 5-lipoxygenase (5-LO) in mice led to induction of phase II detoxification enzyme SULT2A1 and inhibition of the pro-toxic phase I enzyme CYP 3A11, which subsequently reduced NAPQI formation and finally ameliorated AILI [94].
In addition to these genes and related proteins, pretreatment with some whole plant extracts or individual compounds would also alter APAP metabolic activation. It is, therefore, quite necessary and important to examine the expressions and activities of related cytochrome P450 enzymes, as well as the formation of NAPQI when natural products were tested in this model. In fact, many studies on natural products as potential therapy approaches against APAP hepatotoxicity were due to their enhancement effects on phase II metabolic enzymes and/or inhibitory effects on cytochrome P450 enzymes [125], [130], [17],]. In some cases, neglect of the impact on the metabolism phase might lead to ambiguous, even opposite conclusions. A case in point is the green tea extract, which has been shown to protect mice from APAP toxicity through its antioxidant activity [89], whereas this protection was actually more relevant to the reduction of APAP protein adducts caused by suppression of CYP 2E1 and CYP 1A2 [18]. Moreover, when the extract was administered followed by APAP as a therapeutic approach, it could even potentiate APAP-induced hepatotoxicity [106]. Taken together, pretreatment of natural products may cause drug-drug interactions with APAP more easily to happen, and that could possibly lead to unconvincing conclusions on the therapeutic effects and molecular mechanisms. More importantly, patients of AILI often present too late for medical intervention in the course of disease, after the metabolism phase and after liver injury has already developed. Hence, therapeutic approaches, rather than prophylactic treatment, would be more clinically relevant.
3. Targeting mitochondrial oxidative stress and dysfunction for AILI treatment
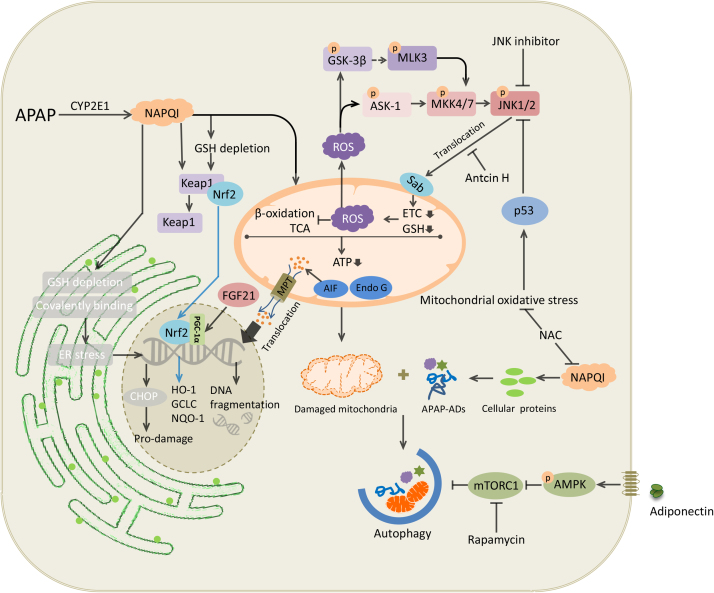
In the injury process of APAP hepatotoxicity, mitochondrial proteins are the main targets of NAPQI, compared with total protein-binding in hepatocytes, including housekeeping protein, glutathione peroxidase (GPx) and adenosine triphosphate (ATP) synthase α-subunit [95]. NAPQI also interferes with complex I/II of mitochondrial electron transport chain (ETC), causing leakage of electrons from the ETC to oxygen and thus forms superoxide radicals [32], [69]. Once formed, superoxide radicals are dismutated in mitochondria by manganese superoxide dismutase (MnSOD) to hydrogen peroxide (H2O2) and molecular oxygen (O2), or react with endogenous nitric oxide (NO) to form peroxynitrite (ONOO−) [50]. Then H2O2 is detoxified by GSH directly, or scavenged by a number of antioxidant enzymes in hepatocytes, such as catalase, glutathione peroxidase (GPx) and peroxiredoxin (Prx) [31]. ONOO− formed in mitochondria could also react with GSH to detoxify [61]. Consequently, GSH is depleted in response to these excessive free radicals, leading to accumulation of ONOO−, which causes formation of nitrotyrosine protein adducts and damage to mitochondrial DNA [22], [43]. Given the vital role of mitochondrial oxidative stress in the pathogenesis of AILI, NAC is used clinically as the only standard antidote to treat AILI, mainly via replenishing GSH to enhance detoxification of NAPQI. NAC also exerts protective effects when administered at the oxidative injury phase, when hepatocellular GSH is depleted, reactive oxygen species and peroxynitrite are generated in mitochondria [22], [60]. Moreover, surplus NAC in the liver could supply energy substrates for the Krebs cycle, thereby support maintenance of hepatic ATP levels and improve mitochondrial function [105]. There is no doubt that NAC is the mainstay of treatment against hepatotoxicity following APAP overdose; however, this medication has some drawbacks in terms of side effects and narrow therapeutic window. Generally, adverse effects of NAC are not life threatening, including nausea, vomiting, and anaphylactoid reactions with initial infusion [10]. The main caveat of NAC is the decreased efficacy when the treatment is initiated more than 8 h after poisoning [120]. Therefore, medications that still have therapeutic potential beyond early stage would be of great help, especially for those patients presenting at late stage. A number of molecules have been found to be involved in the regulation of APAP-induced oxidative stress, including JNK, p53 and Nrf2 (Fig. 2), which may serve as potential therapeutic targets for AILI.
Fig. 2.
Intracellular signaling events in acetaminophen hepatotoxicity. Excessive quantities of NAPQI generated by acetaminophen overdose deplete GSH in the cytoplasm, ER and mitochondria, leading to ER stress, mitochondrial oxidative stress and dysfunction. This induces TCA cycle and β-oxidation dysfunction, ATP depletion and the opening of the MPT pore, which, subsequently, leads to the translocation of mitochondrial proteins, such as AIF and Endo G, to the nucleus. This results in nuclear DNA fragmentation and ultimately necrotic cell death. ROS caused by NAPQI activates JNK signaling pathways. Sustained activation of JNK amplifies mitochondrial ROS and forms a self-sustaining activation loop. Nrf2, p53, adiponectin and FGF21 signaling pathways are activated to cope with cellular stress and injury. Similarly, autophagy alleviates AILI through removal of damaged mitochondria and detrimental APAP-ADs. NAPQI, N-acetyl-p-benzoquinone imine; ER, endoplasmic reticulum; TCA, tricarboxylic acid cycle; MPT, mitochondria permeability transition; AIF, apoptosis inducing factor; ROS, reactive oxygen species; JNK, c-Jun N-terminal kinase; AILI, acetaminophen-induced liver injury; APAP-ADs, acetaminophen protein adducts.
3.1. JNK
Depletion of GSH by NAPQI results in increased H2O2 release from mitochondria to cytoplasm, which could oxidize thioredoxin and cause thioredoxin to disassociate from apoptosis signaling-regulating kinase 1 (ASK-1) [41]. This triggers self-activation of ASK-1, which phosphorylates mitogen-activated protein kinase kinase 4/7 (MKK4/7), then activates c-Jun N-terminal kinase (JNK) [104], [84]. Once activated, p-JNK translocates to the mitochondria, where it binds to and phosphorylates SH3 homology-associated BTK-binding protein (Sab). This interaction leads to inactivation of intramitochondrial Src, which causes dysfunction of ETC and increase of ROS release. ROS continues to activate upstream mitogen-activated protein kinases, and then phosphorylates JNK. Sustained activation of JNK amplifies mitochondrial ROS and forms a self-sustaining activation loop [122]. p-JNK causes bax activation and translocation to mitochondria, which then triggers the opening of the mitochondrial permeability transition (MPT) pore in mitochondria with a collapse of the membrane potential and ATP depletion [37], [58]. MPT facilitates the release of mitochondrial intermembrane proteins, including endonuclease G and apoptosis-inducing factor, both of which translocate to the nucleus. This then causes DNA fragmentation, and finally necrosis [9].
SP600125, a classical ATP-competitive inhibitor of JNK, has been reported to protect against APAP-induced liver injury in vivo and in vitro [42], [67]. Importantly, its protection effect was even confirmed in primary human hepatocytes [126]. More importantly, 5 h delayed administration of SP600125 is more effective than NAC in AILI [42]. However, SP600125 was found to provide protection even in JNK-deleted hepatocytes, raising the possibility of off-target effects, e.g., on AMP-activated kinase (AMPK)-dependent survival signaling pathways [24]. Another type of JNK inhibitor that has been shown to protect against APAP hepatotoxicity is D-JNKI1 (JNK 1, D-stereoisomer), which is a peptide that inhibits the interaction of JNK with its substrates [42]. Considering JNK's function in liver regeneration and other possible protective effects (e.g., through activation of AP-1 transcription factor), strategies that inhibit JNK directly or indirectly may also inhibit the potential benefits of it, which may limit the therapeutic application of JNK inhibitors [107], [99]. Recently, a terpenoid compound isolated from Antrodia Camphorate, Antcin H, was found to protect against APAP hepatotoxicity through inhibiting the interaction between p-JNK and mitochondria protein Sab [44]. This agent is very promising for future clinical use since it perturbs the self-sustaining activation loop of JNK, instead of inhibiting JNK directly, and therefore would not be expected to inhibit the possible protective/beneficial effects of transient JNK activation in AILI [5]. However, given the possible high research costs to develop these compounds specifically for AILI, existing drugs with therapeutic potential in this hepatotoxicity model may be better choices. A case in point is metformin, a commonly used drug to treat type 2 diabetes mellitus. Convincing evidence has demonstrated that the protective and therapeutic effects of metformin against AILI were dependent on the inhibition of p-JNK through up-regulation of Gadd45β [59]. Interestingly, a follow-up study found that neither pre- nor post-treatment of metformin against APAP hepatotoxicity inhibited JNK activation or its mitochondrial translocation [31], [32]. Although AMPK dependent signaling pathway and autophagy were excluded from hepatoprotective mechanism of metformin [59], there are still many events probably being involved in the protective effects, including inhibition of hepatic gluconeogenesis, MPT and ROS production [55]. Taken together, further work will be needed to determine the exact mechanisms of metformin against APAP hepatotoxicity.
3.2. p53
The p53 tumor suppressor protein plays a crucial role in modulation of cell cycle, apoptosis, senescence and metabolism in response to diverse stimuli such as cellular stress and DNA damage [65]. Once activated, p53 promotes cell survival and repair of genetic damage (through target genes that mediate cell cycle arrest and facilitate DNA repair) in response to moderate cellular stress and damage; however, upon lethal levels of ROS and/or severe DNA damage, cells cannot be repaired and saved, then they would be eliminated permanently by high levels of p53-mediated cell death. This notion was confirmed in numerous conditions, under which the role of p53 was complicated and dependent on the pathology of disease [65]. Hence, not surprisingly, p53 is also activated by oxidative stress in APAP hepatotoxicity [116], and its role in pathogenesis is stage specific [14]. Activated p53 plays a protective role during injury phase of APAP hepatotoxicity through inhibiting the activation of JNK [46], whereas it delays progression of liver repair in regeneration phase [14]. As such, p53 may not be a promising target for AILI due to the dual role of p53 in the pathogenesis of APAP-induced hepatotoxicity.
3.3. Nrf2
Apart from p53, many other compensatory pro-survival signaling pathways are activated in response to oxidative stress following hepatotoxic doses of APAP. The most well-established among them appears to be nuclear factor erythroid 2-related factor 2 (Nrf2), which is likely activated by redox status changes of the kelch-like ECH associated protein 1 (Keap1) induced by NAPQI. Mechanistically, Nrf2 activation leads to transcriptional activation of antioxidant enzymes, including microsomal epoxide hydrolase, heme oxygense-1 (HO-1), NADPH: quinone oxidoreductase 1 (NQO-1), and glutamate cysteine ligase (GCL), which catalyzes the initial and rate-limiting step in GSH synthesis. These antioxidant enzymes activated by Nrf2 act as cell defense system to detoxify NAPQI [35]. In addition to being activated by NAPQI directly, some other mechanisms are also involved in the activation and/or regulation of Nrf2 signaling pathway, including protein tyrosine phosphatase 1B (PTP1B) [79], fibroblast growth factor 21 (FGF21) [128] and M1 muscarinic receptors (M1R) [114]. In mice that lack PTP1B or M1R, APAP hepatotoxicity was attenuated through enhanced hepatic Nrf2 system, suggesting that PTP1B and M1R may serve as novel therapeutic targets against AILI. As for FGF21, remarkable elevation of this hormone by APAP overdose enhanced Nrf2-mediated antioxidant capacity through peroxisome proliferator-activated receptor coactivator protein-1α (PGC-1α), representing a compensatory mechanism to protect against APAP hepatotoxicity [128]. Given the positive role of Nrf2 in alleviating oxidative stress, many bioactive components that could further activate this adaptive, protective signaling pathway are reported to exert protective effects against APAP-induced hepatotoxicity (Table 1). Of note, the effects of these natural products on APAP hepatotoxicity were tested only by pretreatment method; however, therapeutic strategies would be more clinically relevant as APAP intoxication is unpredictable and patients with AILI often present late for medical treatment. Therefore, further study is needed to establish their efficacy in the clinically relevant therapeutic models. Another concern regarding these studies is the omission of specific assessment of their impact on APAP metabolism. Given the fact that APAP toxicity is initiated by metabolism through cytochrome P450 enzymes, any subtle interference with the formation of reactive metabolite NAPQI and accumulation of APAP protein adducts (APAP-ADs) can produce a profound impact on the outcome of APAP-induced liver injury. This omission challenges the causal relationships between Nrf2 activation induced by these natural products and their hepatoprotective effects on AILI.
Table 1.
Protective effects of bioactive natural components in APAP-induced hepatotoxicity through Nrf2 activation.
| Bioactive components | Effective dose | Pretreatment | Animal models/APAP dose | Mechanisms | Refs. |
|---|---|---|---|---|---|
| Withaferin A | 7 mg/kg i.g. | 24 h | C57BL/6J mice, 250 mg/kg i.p. | Pten/PI3k/Akt dependent activation of Nrf2 | [90] |
| Tanshinone IIA | 10–30 mg/kg p.o. | 4 days | C57BL/6J, 300 mg/kg i.p. | Nrf2 activation | [118] |
| Quercitrin from Toona sinensis (Juss.) M.Roem. | 10–50 mg/kg i.g. | 7 days | Balb/c mice, 300 mg/kg i.p. | ↓Oxidative stress, inflammation | [112] |
| Caffeic acid | 10–30 mg/kg p.o. | 7 days | ICR mice, 400 mg/kg p.o. | Keap1/Nrf2/HO-1, NQO1 activation | [91] |
| Esculentoside A | 2.5 mg/kg i.p. | 24 and 12 h | Balb/c mice, 400 mg/kg | Activation of Nrf2 through AMPK/Akt/GSK3β | [117] |
| Carnosic acid | 100 mg/kg i.g. | 3 days | C57BL/6J mice,400 mg/kg i.p. | ↑Nrf2 nuclear translocation | [39] |
| Schisandrol B | 200 mg/kg, i.g. | 3 days | C57BL/6 mice, 400 mg/kg i.p. | Activation of Nrf2/ARE | [53] |
| Formononetin | 50–100 mg/kg p.o. | 7 days | Balb/c mice, 300 mg/kg i.p. | Nrf2 activation | [54] |
3.4. Therapeutic approaches targeting other signaling pathways
In light of previous studies, mitochondrial oxidative stress is recognized as the critical event in APAP hepatotoxicity. Therefore, researches toward therapeutic approaches mainly focused on how to alleviate this event. Based on above, a large number of natural compounds have been studied and showed the potential to treat APAP-induced liver injury through antioxidant capacity (Table 2). Although it was claimed in these studies that the antioxidant capacity of these natural products were due to the enhanced levels of GSH, superoxide dismutase, catalase and other antioxidant enzymes, these could be also attributed to the APAP metabolism inhibition or a faster recovery from hepatotoxicity due to less injury. A tome-course instead of a single time point study to monitor the dynamic changes of NAPQI formation would be helpful to determine the contributions of these mechanisms. Moreover, when compared with the current standard antidote NAC, they could not offer more outstanding therapeutic effects. Hence, compounds with new mechanisms to protect mitochondrial beyond NAC may be better choices. More recently, a mitochondrial targeted antioxidant Mito-Tempo was reported to protect against AILI even at 3 h post-treatment of APAP compared with NAC alone, suggesting it as a potential therapeutic option for patients at late phase in APAP poisoning [29]. Another existing drug being proved to have hepatoprotective properties in APAP model is methylene blue, a clinically used antidote that can permeate through mitochondrial membranes. This medication effectively restores the ETC function and maintains mitochondrial bioenergy homeostasis by acting as an electron carrier substitute for impaired complex II, thus protecting mice from AILI [69].
Table 2.
Antioxidant effects of bioactive natural components in APAP-induced hepatotoxicity.
| Bioactive components | Effective dose | Animals models/APAP dose | Mechanisms | Refs. |
|---|---|---|---|---|
| Magnolol | 0.01–1 μg/kg i.p. | Sprague-Dawley rat, 500 mg/kg i.p. | ↓TBARS; ↑GSH | [19] |
| Gallic acid | 100 mg/kg i.p. | Swiss albino mice, 900 mg/kg i.p. | ↑SOD, CAT, GPx, GR, GST and GSH | [97] |
| 6-Gingerol | 30 mg/kg i.p. | Swiss albino mice, 900 mg/kg i.p. | ↑SOD, CAT, GPx, GR, GST and GSH | [101] |
| Curcumin | 10–20 mg/kg i.p. | BALB/c mice, 300 mg/kg i.p. | ↑SOD; ↓MDA, apoptosis | [72] |
| Thearubigins | 60 mg/kg i.p. | Swiss mice, 300 mg/kg i.p. | ↓MDA, | [81] |
| Baicalin | 30 mg/kg i.p. | C57BL/6 mice, 300 mg/kg i.p. | ↓MDA, TNF-α, IL-17, IL-6 | [71] |
| Paeonol | 100 mg/kg i.g. | C57BL/6 mice, 400 mg/kg i.p. | ↑SOD, GPx and GSH; ↓MDA, inflammation | [28] |
| Methanol extract of Fagonia olivieri DC. | 200–400 mg/kg i.g. for 7 days | Sprague-Dawley rats, 700 mg/kg i.g. | ↑SOD, CAT,GPx, GR and GSH | [96] |
| Lophirones B and C | 20 mg/kg p.o. for 7 days | Swiss mice, 300 mg/kg | ↑SOD, CAT, GPx;↓inflammation | [3] |
| Hydroglycol extract of red rice | 128–512 mg/kg p.o. for 30 days | ICR mice, 60 mg/kg p.o. for 30 days | ↑GSH; ↓GSSH | [109] |
| Methanol extract of Adansonia digitata L. (Malvaceae) | 200 mg/kg p.o. for 7 days | Wistar rats,2 g/kg p.o. for 3 days | ↑SOD, CAT and GSH; ↓MDA | [40] |
| Quercitrin from Toona sinensis (Juss.) M.Roem. | 10–50 mg/kg i.g. | Balb/c mice, 300 mg/kg i.p. | ↑SOD, CAT and GPx; ↓inflammation | [112] |
| Methanol extract of pomegranate peels | 50 mg/kg p.o. for 14 days | Wistar rats, 750 mg/kg i.p. | ↓Oxidative stress | [2] |
| Withanolide | 50–200 mg/kg p.o. for 14 days | Wistar rats, 750 mg/kg p.o. | ↑SOD and GSH; ↓MDA, NO | [27] |
| Acetone extract of Passiflora subpeltata leaves | 200–400 mg/kg for 14 days | Wistar rats, 2 g/kg p.o. for 14 days | ↑SOD, CAT, GPx, GST and GSH | [108] |
| Opuntia robusta | 800 mg/kg p.o. for 5 days | Wistar rats, 500 mg/kg i.p. | ↑GSH | [36] |
| Aqueous extract of Zea mays L. (Poaceae) Stigma maydis | 200–400 mg/kg p.o. for 14 days | Wistar rats, 400 mg/kg p.o. for 14 days | ↑SOD, CAT, GPx, GR and GSH; ↓MDA | [102] |
| Silymarin | 25 mg/kg p.o. for 14 days | Wistar rats, 800 mg/kg i.p. for 3 days | ↑SOD, GPx; ↓NO | [88] |
| n-Hexane extract of Acrocarpus fraxinifolius leaves | 250–500 mg/kg p.o. for 14 days | Wistar rats, 400 mg/kg p.o. for 7 days | ↓MDA | [1] |
| Tormentic acid | 1.25–5 mg/kg i.p. for 6 days | ICR mice, 400 mg/kg i.p. | ↑SOD, CAT and GPx;↓NO, TNF-α, IL-1β | [52] |
| Methanol extract of Dicranopteris linearis L. leaves | 50–500 mg/kg p.o. for 7 days | Sprague-Dawley rats, 3 g/kg | ↑SOD, CAT | [132] |
| Methanol extract of black ginseng | 300–600 mg/kg i.g. for 7 days | ICR mice, 250 mg/kg i.p. | ↑GSH; ↓3-nitrotyrosine and MDA | [44] |
| Ginsenosides | 150–300 mg/kg i.g. for 7 days | ICR mice, 250 mg/kg i.p. | ↑GSH, SOD; ↓3-nitrotyrosine, MDA, COX-2 | [127] |
| Tannic acid | 25–50 mg/kg p.o. for 3 days | Kunming mice, 400 mg/kg i.p. | ↑SOD, CAT, GPx; ↓MDA, NO, TNF-α, IL-1β | [134] |
| Rice peptides | 100–500 mg/kg p.o. for 7 days | ICR mice, 700 mg/kg i.p. | ↑GSH | [57] |
| Cymbopogon citratus Essential Oil | 125–500 mg/kg for 7 days | Swiss mice, 250 mg/kg p.o. | ↓MPO, NO | [113] |
| Iridoid glycosides fraction of Veronica ciliata Fisch. | 150–450 mg/kg p.o. for 14 days | Kunming mice, 180 mg/kg i.p. | ↑SOD, GSH; ↓MDA, TNF-α | [111] |
| Rutin | 20 mg/kg p.o. for 11 days | Wistar rats, 500 mg/kg p.o. for 3 days | ↑SOD, CAT, GPx and GSH;↓MDA | [98] |
| Astaxanthin | 30–60 mg/kg i.g. for 14 days | C57BL/6, 300 mg/kg i.p. | ↑SOD and GSH;↓MDA | [135] |
TBARS, thiobarbituric acid reactive substances; SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase; GR, glutathione reductase; glutathione S-transferase; MDA, malonaldehyde; NO, nitric oxide; GSH, glutathione; GSSH, oxidized glutathione; IL-17, interleukin-17; IL-6, interleukin-6; TNF-α, tumor necrosis factor alpha; IL-1β, interleukin-1β; COX-2, cyclooxygenase 2.
4. Targeting autophagy for AILI treatment
Autophagy is a tightly regulated cellular process that renew cell through removing unwanted cytoplasmic contents, including misfolded or aggregated proteins, accumulated lipids, excessive or damaged organelles and harmful pathogens, via lysosomal degradation [80]. It is believed that APAP overdose can cause mitochondria damage and APAP-ADs accumulation in hepatocytes, resulting in hepatic necrosis and liver injury [75]. Therefore, it comes as no surprise that autophagy, serving as a protective mechanism to maintain cellular homeostasis, is activated in response to severe cellular stress caused by APAP. Moreover, pharmacological induction of autophagy by rapamycin alleviates APAP hepatotoxicity through removal of damaged mitochondria and detrimental APAP-ADs [86], [87], indicating the induction of autophagy as a novel therapeutic approach for AILI.
However, the exact molecular mechanisms involved in APAP-induced autophagy remain unclear. Generally, the energy sensor AMPK can activate autophagy through inhibition of mammalian target of rapamycin complex 1 (mTORC1). Indeed, mTORC1 is inhibited following APAP overdose. Moreover, a recent study has described a role of adiponectin in promoting autophagy through activation of the AMPK signaling pathway [73]. Conversely, in other studies, p-AMPK was inhibited in responds to APAP intoxication [47], [100]. Despite these contradictory observations, there is no doubt that activation of AMPK leads to energy generation and promotion of survival signaling pathways, which finally protect against APAP-induced hepatotoxicity (Fig. 2).
5. Targeting endoplasmic reticulum stress for AILI treatment
ER stress can be observed in various hepatic injury models, including alcoholic liver disease, nonalcoholic fatty liver disease, ischemia-reperfusion injury and cholestatic liver disease [26]. Hence, it is not surprising that ER stress plays a role in APAP hepatotoxicity. This stress respond is initiated by impaired protein folding process, which is triggered by covalent binding of NAPQI to the ER proteins [119]. It is also conceivable that GSH depletion in the ER results in intraluminal redox imbalance, leading to phosphorylation of eIF2α and activation of ATF6 and CHOP [82], [83]. Moreover, a recent study has found that CHOP plays a pro-damage role in response to APAP intoxication through inhibition of liver regeneration. This research, to some extent, clarified the role of unfolded protein response (UPR)/ER stress in mediating APAP-induced hepatotoxicity [115]. In light of these data, interventions to inhibit CHOP may be potential therapeutic strategies to treat APAP poisoning patients in whom liver damage has already been evident (Fig. 2).
6. Targeting glucose and lipid metabolism for AILI treatment
Mitochondria are key organelles that play an essential role in cell survival. One reason is that they are irreplaceable in control of many metabolic pathways, among which glucose metabolism and fatty acid β-oxidation are the most important. Hence, damaged mitochondria caused by APAP overdose inevitably lead to many metabolic changes in the liver. Metabolomics studies have shown that both the liver glucose and glycogen were depleted following APAP overdose [21]. There are two possible explanations for this observation: (1) both citric acid cycle and fatty acid β-oxidation are disrupted in response to mitochondria oxidative stress caused by APAP, resulting in ATP depletion. This mitochondrial malfunction then leads to compensatory increase of glycolysis [21]. (2) GSH depletion induced by APAP leads to decreased cellular NADPH/NADP+ ratios, which is known to activate glucose-6-phosphate dehydrogenase allosterically to enhance oxidative pentose phosphate pathway flux. The increased flux is an attempt to maintain cellular NADPH level to reduce oxidized glutathione [131]. Consistent with this, fructose and fructose-1, 6-diphosphate were found to protect against cell injury in APAP-treated rat liver slices via maintaining high ATP levels [74]. However, sufficient dATP/ATP switched the hepatocytes injury from necrosis to caspase-dependent apoptosis [62]. This means supplementing ATP merely cannot rescue cell death in APAP hepatotoxicity.
As mentioned above, fatty acid β-oxidation is inhibited by APAP treatment, concomitant with compensatory increase of peroxisomal activity. Peroxisome proliferator-activated receptor α (PPARα) encodes peroxisomal and mitochondrial enzymes that promote fatty acid catabolism, and its protective effects against APAP toxicity were confirmed in several studies [16], [85]. Although it was speculated that the protective effects of PPARα might depend on its role in facilitating fatty acid catabolism, a recent study showed that this protection is due in part to antioxidant function of the PPARα target gene mitochondrial uncoupling protein 2 (UCP2) [93].
7. Targeting sterile inflammation for AILI treatment
APAP-induced liver injury is characterized by hepatocyte necrosis, which causes release of cellular contents including nuclear DNA fragments [76], high mobility group box-1 protein [6], mitochondrial DNA [76], uric acid [63] and ATP [4]. These immune stimulatory factors function as damage-associated molecular patterns, which can transcriptionally activate pro-inflammatory cytokines (TNF-α, IL-1β, IL-6 and IL-10) and chemokines (MCP-1, MIP-2 and IL-8) [25], [51], [68]. As a result, neutrophils and later monocytes are activated and recruited to the damage area of the liver [68], [7]. Unquestionably, APAP overdose provoke an acute inflammatory response (Fig. 3). However, the pathophysiological role of this response to APAP is still controversial-whether it contributes to the progression of liver injury or acts as cellular defense against toxicity [13], [20], [48], [121],[121], [13], [20], [48]. Even though, there are still several studies targeting inflammation as therapeutic strategy. Benzyl alcohol (BA), often used as preservative when added to intravenous medication solutions, was shown to protect against AILI and reduce release of IL-1ß and IL-18 in a Toll-like receptor 4 (TLR4)-dependent manner [15]. However, mitochondrial toxic effect of this drug limits its clinical use for APAP overdose in patients [30]. Another case is resolvin, which was reported to attenuate APAP hepatotoxicity through inhibiting neutrophil migration into the liver. More importantly, resolvin given as late as 12 h after APAP was still protective, which means it extends the therapeutic window eight-fold compared to the standard antidote NAC [92].
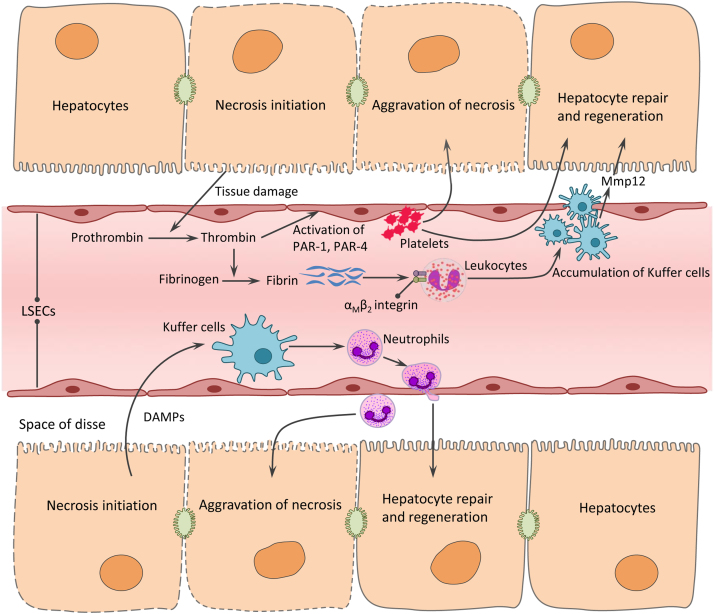
Fig. 3.
Sterile inflammation and microcirculatory dysfunction induced by acetaminophen hepatotoxicity. DAMPs (including DNA fragments, HMGB1, uric acid and ATP) released from necrotic hepatocytes, transcriptional activate pro-inflammatory cytokines and chemokines in Kupffer cells. This causes neutrophils activation and recruited to the damage area of the liver, resulting in aggravation of hepatocytes necrosis at first, but contributing to liver repair and regeneration at a late stage. Tissue damage caused by APAP activate the coagulation system, concomitant with the generation of thrombin and formation of soluble and insoluble fibrin. Thrombin activates the platelets through downstream PAR-1 and PAR-4 signaling pathways, thereby exacerbates liver injury. During late stage, fibrin induced by thrombin contributes to liver repair through engagement of the leukocyte αMβ2 integrin and subsequent induction of Mmp12. DAMP, damage associated molecular pattern; HMGB1, high mobility group box-1 protein; LSEC, liver sinusoidal endothelial cell; PAR-1, proteinase-activated receptor 1; PAR-4, proteinase-activated receptor 4; Mmp12, matrix metalloproteinases 12.
Apart from its possible contributions to the progression of AILI, inflammation also helps to clear up dead cells and fragments and stimulates liver to repair and regenerate at a late stage [123]. That means inflammation may play a distinct role in different phases of the pathological process in APAP hepatotoxicity-e.g., a promoter in the injury phase and a helper in the regeneration phase. Hence, blockade of inflammation may protect at first, but actually be detrimental to the final outcomes. Hence, conclusions that BA and resolvin protect against AILI only relied on a single time point (24 h), making the results unconvincing. Therapeutic effects at late regeneration phase (24–72 h in mice) also need to be experimentally verified. The protective role of inflammation in APAP hepatotoxicity was confirmed by lactoferrin, a multifunctional protein presented in human colostrum and cow milk. This globular glycoprotein has been demonstrated to regulate inflammatory responses, as it is part of the innate immunity defense system and can modulate immune cell functions. Consistent with this, lactoferrin was found to inhibit APAP-induced liver sinusoidal endothelial cell damage and improved hepatic microcirculatory dysfunction through activation of Kupffer cells [129]. Taken together, the role of inflammation in AILI remains under considerable debate, and this makes it controversial to be as a therapeutic target (Fig. 3).
8. Targeting microcirculatory dysfunction for AILI treatment
In addition to direct hepatocellular damage caused by APAP, hepatic microcirculatory dysfunction also plays a vital role in the pathogenesis of AILI. Liver sinusoidal endothelial cells (LSECs) are sensitive and damaged earlier than hepatocytes in man and animal models following APAP overdose [49]. LSECs and hepatocyte injury then lead to activation of the coagulation cascade and thrombocytopenia. Subsequently, disturbances of the hemostatic system contribute to AILI, at least in part via downstream protease-activated receptor-1 (PAR-1) signaling pathway. Consistent with this, pharmacological inhibition of coagulation by heparin and genetic approaches (mice deficient in either tissue factor or PAR-1) attenuate APAP hepatotoxicity at an early stage (6 h) [33]. However, PAR-1 mediates activation of platelets by thrombin neither in mouse nor in human; therefore, studies by Miyakawa et al. [78] defined the role of platelets in promoting AILI, depending on the action of PAR-4 on LSECs, as speculated by the authors (Fig. 3). In light of the previous studies, anticoagulation with thrombin inhibitor dabigatran etexilate (DABI) reduces the early hepatotoxicity in AILI, but significantly exacerbates liver injury at 24 h after APAP administration due to the attenuated hepatocyte proliferation [64]. Given the fact that anticoagulation can regulate AILI both positively and negatively, therapeutic strategies targeted on hemostatic system remain uncertain and need to be addressed in the future.
9. Targeting liver repair and regeneration for AILI treatment
Cellular stress and tissue injury induced by APAP overdose ultimately lead to compensatory liver repair and regeneration, which is a critical determinant of final outcome in patients of AILI. Though it is beneficial and important in recovery from AILI, specific details and mechanisms of this process have not been fully studied.
Epidermal growth factor receptor (EGFR) is a highly expressed tyrosine kinase receptor in the liver, and its ligands are direct mitogens for hepatocytes [77]. Therefore, it is not surprising that EGFR was found to promote liver regeneration after APAP overdose. However, it is worth noting that inhibition of EGFR activation at an early time remarkably attenuates AILI, suggesting a dual role of EGFR in both initiation of liver injury and subsequent regeneration in APAP hepatotoxicity [11]. Hence, it seems impossible to develop EGFR inhibitors as therapeutic medications for AILI, as undesirable outcomes may occur due to its opposite effects at late phase.
The Wnt family proteins are growth regulators acting as ligands of Frizzled receptors in the liver. They inhibit β-catenin ubiquitination in the cytoplasm, and then allow its translocation to the nucleus, where it initiates transcription of multiple genes related to cell proliferation (including EGFR, c-Myc and cyclin D1) [77]. Given the positive role of the Wnt/β-catenin signaling pathway in partial hepatectomy model, it was assumed that it might also contribute to liver regeneration in AILI, which was then confirmed in some previous studies [8], [11], [12], [110]. Furthermore, with glycogen synthase kinase 3 (GSK3) defined as canonical upstream regulator of β-catenin [12], sympathetic nervous system (SNS) has been found to stimulate the Wnt/β-catenin signaling pathway in AILI, at least in part via expansion of hepatic progenitor/oval cells, which are both targets and sources of Wnt ligands [110]. As such, SNS agonist isoproterenol (β-adrenoceptor agonist) showed its therapeutic potential against APAP hepatotoxicity. Moreover, in contrast to NAC, isoproterenol was still therapeutically effective even being administered in a late phase [110].
Although liver regeneration is generally undertaken by hepatocytes, there are still several other factors contributing to liver recovery from APAP hepatotoxicity, including hepatic microvasculature reconstitution and interactions between different cell types. Hepatocytes produce mitogenic growth factors for endothelial cells, such as vascular endothelial growth factor (VEGF), which was found to facilitate liver repair by reconstituting the sinusoids in AILI [56]. A recent study identified a crosstalk between intrahepatic coagulation and inflammation after APAP overdose, through which liver repair was stimulated. It is believed that repair in this case requires fibrin (ogen) (one of coagulation factors)-leukocyte αMβ2 integrin engagement and subsequent induction of matrix metalloproteinase 12 (Mmp12) [64]. However, the precise mechanism by which Mmp12 functions in liver repair has not been well understood. These results suggest the activation of fibrin(ogen)-αMβ2 integrin engagement as a therapeutic approach against APAP hepatotoxicity by stimulating liver repair and regeneration.
In short, as mentioned above, liver regeneration is a compensatory beneficial process in response to hepatocyte death and tissue injury, thus stimulation of this process would be a potential therapeutic strategy to manage APAP hepatotoxicity. However, it should be noted that prolonged liver regeneration triggered by inflammation, pathogens or toxins may have adverse effects leading to cirrhosis and even liver cancer [77], a concern that may also be reflected in preclinical and clinical use of stimulating liver regeneration to treat AILI.
10. Concluding remarks
The mechanisms of APAP-induced liver injury are highly complex and many intracellular and extracellular events are involved in this pathophysiological process, including metabolism of APAP, mitochondrial oxidative stress, ER stress, autophagy, sterile inflammation, microcirculatory dysfunction and liver regeneration. These events regulate various aspects of AILI, such as initiating the injury, mediating hepatocyte death directly, limiting cellular stress response, helping liver to repair and regenerate. Consequently, not only mitochondrial oxidative stress (well defined), but many other processes can be potential therapeutic targets to treat AILI. However, it should be noted that some cellular events might play paradoxical roles in different phases of AILI, making them both positively and negatively in regulating APAP hepatotoxicity. As such, therapeutic strategies targeted on these events may not be beneficial at last, a concern that has been reflected in certain preclinical studies. Overall, more studies are needed to further clarify the precise role of these time-dependent events in AILI, thus making the late phase therapy become possible in clinical.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Abd El-Ghffar E.A., El-Nashar H.A., Eldahshan O.A. GC-MS analysis and hepatoprotective activity of the n-hexane extract of Acrocarpus fraxinifolius leaves against paracetamol-induced hepatotoxicity in male albino rats. Pharm. Biol. 2017;55(1):441–449. doi: 10.1080/13880209.2016.1246575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad N., Tahir M., Lone K.P. Amelioration of acetaminophen induced hepatotoxicity by methanolic extract of pomegranate peels in rats. J. Pak. Med. Assoc. 2016;66(7):859–863. [PubMed] [Google Scholar]
- 3.Ajiboye T.O. Lophirones B and C attenuate acetaminophen-induced liver damage in mice: studies on hepatic, oxidative stress and inflammatory biomarkers. J. Biochem. Mol. Toxicol. 2016;30(10):497–505. doi: 10.1002/jbt.21814. [DOI] [PubMed] [Google Scholar]
- 4.Amaral S.S., Oliveira A.G., Marques P.E. Altered responsiveness to extracellular ATP enhances acetaminophen hepatotoxicity. Cell Commun. Signal. 2013;11(1):10. doi: 10.1186/1478-811X-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amir M., Liu K., Zhao E. Distinct functions of JNK and c-Jun in oxidant-induced hepatocyte death. J. Cell. Biochem. 2012;113(10):3254–3265. doi: 10.1002/jcb.24203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antoine D.J., Jenkins R.E., Dear J.W. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J. Hepatol. 2012;56:1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Antoniades C.G., Quaglia A., Taams L.S. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology. 2012;56:735–746. doi: 10.1002/hep.25657. [DOI] [PubMed] [Google Scholar]
- 8.Apte U., Singh S., Zeng G. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am. J. Pathol. 2009;175(3):1056–1065. doi: 10.2353/ajpath.2009.080976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bajt M.L., Cover C., Lemasters J.J. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol. Sci. 2006;94:217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- 10.Bateman D.N., Dear J.W., Thanacoody H.K. Reduction of adverse effects from intravenous acetylcysteine treatment for paracetamol poisoning: a randomised controlled trial. Lancet. 2014;383:697–704. doi: 10.1016/S0140-6736(13)62062-0. [DOI] [PubMed] [Google Scholar]
- 11.Bhushan B., Chavan H., Borude P. Dual role of epidermal growth factor receptor in liver Injury and regeneration after acetaminophen overdose in mice. Toxicol. Sci. 2017;155(2):363–378. doi: 10.1093/toxsci/kfw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhushan B., Poudel S., Manley M.W., Jr Inhibition of glycogen synthase kinase 3 accelerated liver regeneration after acetaminophen-induced hepatotoxicity in mice. Am. J. Pathol. 2017;187(3):543–552. doi: 10.1016/j.ajpath.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boess F., Bopst M., Althaus R. Acetaminophen hepatotoxicity in tumor necrosis factor/lymphotoxin-alpha gene knockout mice. Hepatology. 1998;27:1021–1029. doi: 10.1002/hep.510270418. [DOI] [PubMed] [Google Scholar]
- 14.Borude P., Bhushan B., Chavan H. p53 Regulates progression of injury and liver regeneration after acetaminophen overdose. FASEB J. 2017;(1):31. (Supplement 531.8) [Google Scholar]
- 15.Cai C., Huang H., Whelan S. Benzyl alcohol attenuates acetaminophen-induced acute liver injury in a toll-like receptor-4-dependent pattern in mice. Hepatology. 2014;60(3):990–1002. doi: 10.1002/hep.27201. [DOI] [PubMed] [Google Scholar]
- 16.Chen C., Hennig G.E., Whiteley H.E. Peroxisome proliferator-activated receptor alpha-null mice lack resistance to acetaminophen hepatotoxicity following clofibrate exposure. Toxicol. Sci. 2000;57(2):338–344. doi: 10.1093/toxsci/57.2.338. [DOI] [PubMed] [Google Scholar]
- 17.Chen L.C., Hu L.H., Yin M.C. Alleviative effects from boswellic acid on acetaminophen-induced hepatic injury. Biomedicine. 2016;6(2):12–19. doi: 10.7603/s40681-016-0009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X., Sun C.K., Han G.Z. Protective effect of tea polyphenols against paracetamol-induced hepatotoxicity in mice is significantly correlated with cytochrome P450 suppression. World J. Gastroenterol. 2009;15(15):1829–1835. doi: 10.3748/wjg.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y.H., Lin F.Y., Liu P.L. Antioxidative and hepatoprotective effects of magnolol on acetaminophen-induced liver damage in rats. Arch. Pharm. Res. 2009;32(2):221–228. doi: 10.1007/s12272-009-1139-8. [DOI] [PubMed] [Google Scholar]
- 20.Choi D.Y., Ban J.O., Kim S.C. CCR5 knockout mice with C57BL6 background are resistant to acetaminophen-mediated hepatotoxicity due to decreased macrophages migration into the liver. Arch. Toxicol. 2015;89(2):211–220. doi: 10.1007/s00204-014-1253-3. [DOI] [PubMed] [Google Scholar]
- 21.Coen M., Lenz E.M., Nicholson J.K. An integrated metabonomic investigation of acetaminophen toxicity in the mouse using NMR spectroscopy. Chem. Res. Toxicol. 2003;16(3):295–303. doi: 10.1021/tx0256127. [DOI] [PubMed] [Google Scholar]
- 22.Cover C., Mansouri A., Knight T.R. Peroxynitrite induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- 23.Craig D.G., Lee A., Hayes P.C. Review article: the current management of acute liver failure. Aliment. Pharmacol. Ther. 2010;31(3):345–358. doi: 10.1111/j.1365-2036.2009.04175.x. [DOI] [PubMed] [Google Scholar]
- 24.Cubero F.J., Zoubek M.E., Hu W. Combined activities of JNK1 and JNK2 in hepatocytes protect against toxic liver injury. Gastroenterology. 2016;150(4):968–981. doi: 10.1053/j.gastro.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dambach D.M., Watson L.M., Gray K.R. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology. 2002;35:1093–1103. doi: 10.1053/jhep.2002.33162. [DOI] [PubMed] [Google Scholar]
- 26.Dara L., Ji C., Kaplowitz N. The contribution of endoplasmic reticulum stress to liver diseases. Hepatology. 2011;53(5):1752–1763. doi: 10.1002/hep.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devkar S.T., Kandhare A.D., Zanwar A.A. Hepatoprotective effect of withanolide-rich fraction in acetaminophen-intoxicated rat: decisive role of TNF-α, IL-1β, COX-II and iNOS. Pharm. Biol. 2016;54(11):2394–2403. doi: 10.3109/13880209.2016.1157193. [DOI] [PubMed] [Google Scholar]
- 28.Ding Y., Li Q., Xu Y. Attenuating oxidative stress by paeonol protected against acetaminophen-induced hepatotoxicity in mice. PLoS One. 2016;11(5):e0154375. doi: 10.1371/journal.pone.0154375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du K., Farhood A., Jaeschke H. Mitochondria-targeted antioxidant mito-tempo protects against acetaminophen hepatotoxicity. Arch. Toxicol. 2017;91(2):761–773. doi: 10.1007/s00204-016-1692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du K., McGill M.R., Xie Y. Benzyl alcohol protects against acetaminophen hepatotoxicity by inhibiting cytochrome P450 enzymes but causes mitochondrial dysfunction and cell death at higher doses. Food Chem. Toxicol. 2015;86:253–261. doi: 10.1016/j.fct.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du K., Ramachandran A., Jaeschke H. Oxidative stress during acetaminophen hepatotoxicity: sources, pathophysiological role and therapeutic potential. Redox Biol. 2016;10:148–156. doi: 10.1016/j.redox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du K., Ramachandran A., Weemhoff J.L. Metformin protects against acetaminophen hepatotoxicity by attenuation of mitochondrial oxidant stress and dysfunction. Toxicol. Sci. 2016;154(2):214–226. doi: 10.1093/toxsci/kfw158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ganey P.E., Luyendyk J.P., Newport S.W. Role of the coagulation system in acetaminophen-induced hepatotoxicity in mice. Hepatology. 2007;46(4):1177–1186. doi: 10.1002/hep.21779. [DOI] [PubMed] [Google Scholar]
- 34.Ghaffari A.A., Chow E.K., Iyer S.S. Polyinosinic-polycytidylic acid suppresses acetaminophen-induced hepatotoxicity independent of type I interferons and toll-like receptor 3. Hepatology. 2011;53(6):2042–2052. doi: 10.1002/hep.24316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldring C.E., Kitteringham N.R., Elsby R. Activation of hepatic Nrf2 in vivo by acetaminophen in CD-1 mice. Hepatology. 2004;39(5):1267–1276. doi: 10.1002/hep.20183. [DOI] [PubMed] [Google Scholar]
- 36.González-Ponce H.A., Martínez-Saldaña M.C., Rincón-Sánchez A.R. Hepatoprotective effect of Opuntia robusta and Opuntia streptacantha fruits against acetaminophen-induced acute liver damage. Nutrients. 2016;8(10):607. doi: 10.3390/nu8100607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gunawan B.K., Liu Z.X., Han D. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131(1):165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 38.Guo G.L., Moffit J.S., Nicol C.J. Enhanced acetaminophen toxicity by activation of the pregnane X receptor. Toxicol. Sci. 2004;82(2):374–380. doi: 10.1093/toxsci/kfh286. [DOI] [PubMed] [Google Scholar]
- 39.Guo Q., Shen Z., Yu H. Carnosic acid protects against acetaminophen-induced hepatotoxicity by potentiating Nrf2-mediated antioxidant capacity in mice. Korean J. Physiol. Pharmacol. 2016;20(1):15–23. doi: 10.4196/kjpp.2016.20.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanafy A., Aldawsari H.M., Badr J.M. Evaluation of hepatoprotective activity of Adansonia digitata extract on acetaminophen-induced hepatotoxicity in rats. Evid. Based Complement. Altern. Med. 2016;2016:4579149. doi: 10.1155/2016/4579149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanawa N., Shinohara M., Saberi B. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 2008;283(20):13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henderson N.C., Pollock K.J., Frew J. Critical role of c-jun (NH2) terminal kinase in paracetamol-induced acute liver failure. Gut. 2007;56:982–990. doi: 10.1136/gut.2006.104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hinson J.A., Pike S.L., Pumford N.R. Nitrotyrosine-protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in mice. Chem. Res. Toxicol. 1998;11(6):604–607. doi: 10.1021/tx9800349. [DOI] [PubMed] [Google Scholar]
- 44.Hu J.N., Liu Z., Wang Z. Ameliorative effects and Possible molecular mechanism of action of black ginseng (Panax ginseng) on acetaminophen-mediated liver injury. Molecules. 2017;22(4):664. doi: 10.3390/molecules22040664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huo Y., Win S., Than T.A. Antcin H protects against acute liver injury through disruption of the interaction of c-Jun-N-terminal kinase with mitochondria. Antioxid. Redox Signal. 2017;26(5):207–220. doi: 10.1089/ars.2016.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huo Y., Yin S., Yan M. Protective role of p53 in acetaminophen hepatotoxicity. Free Radic. Biol. Med. 2017;106:111–117. doi: 10.1016/j.freeradbiomed.2017.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang J.H., Kim Y.H., Noh J.R. Enhanced production of adenosine triphosphate by pharmacological activation of adenosine monophosphate-activated protein kinase ameliorates acetaminophen-induced liver injury. Mol. Cells. 2015;38(10):843–850. doi: 10.14348/molcells.2015.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imaeda A.B., Watanabe A., Sohail M.A. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J. Clin. Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ito Y., Bethea N.W., Abril E.R. Early hepatic microvascular injury in response to acetaminophen toxicity. Microcirculation. 2003;10(5):391–400. doi: 10.1038/sj.mn.7800204. [DOI] [PubMed] [Google Scholar]
- 50.Jaeschke H., McGill M.R., Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev. 2012;44(1):88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.James L.P., Simpson P.M., Farrar H.C. Cytokines and toxicity in acetaminophen overdose. J. Clin. Pharmacol. 2005;45(10):1165–1171. doi: 10.1177/0091270005280296. [DOI] [PubMed] [Google Scholar]
- 52.Jiang W.P., Huang S.S., Matsuda Y. Protective effects of tormentic acid, a major component of suspension cultures of Eriobotrya japonica cells, on acetaminophen-induced hepatotoxicity in mice. Molecules. 2017;22(5):830. doi: 10.3390/molecules22050830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang Y.M., Wang Y., Tan H.S. Schisandrol B protects against acetaminophen-induced acute hepatotoxicity in mice via activation of the NRF2/ARE signaling pathway. Acta Pharmacol. Sin. 2016;37(3):382–389. doi: 10.1038/aps.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin F., Wan C., Li W. Formononetin protects against acetaminophen-induced hepatotoxicity through enhanced NRF2 activity. PLoS One. 2017;12(2):e0170900. doi: 10.1371/journal.pone.0170900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaplowitz N., Win S., Than T.A. Targeting signal transduction pathways which regulate necrosis in acetaminophen hepatotoxicity. J. Hepatol. 2015;63(1):5–7. doi: 10.1016/j.jhep.2015.02.050. [DOI] [PubMed] [Google Scholar]
- 56.Kato T., Ito Y., Hosono K. Vascular endothelial growth factor receptor-1 signaling promotes liver repair through restoration of liver microvasculature after acetaminophen hepatotoxicity. Toxicol. Sci. 2011;120(1):218–229. doi: 10.1093/toxsci/kfq366. [DOI] [PubMed] [Google Scholar]
- 57.Kawakami K., Moritani C., Uraji M. Hepatoprotective effects of rice-derived peptides against acetaminophen-induced damage in mice. J. Clin. Biochem. Nutr. 2017;60(2):115–120. doi: 10.3164/jcbn.16-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim B.J., Ryu S.W., Song B.J. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J. Biol. Chem. 2006;281(30):21256–21265. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- 59.Kim Y.H., Hwang J.H., Kim K.S. Metformin ameliorates acetaminophen hepatotoxicity via Gadd45β-dependent regulation of JNK signaling in mice. J. Hepatol. 2015;63(1):75–82. doi: 10.1016/j.jhep.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 60.Knight T.R., Kurtz A., Bajt M.L. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol. Sci. 2001;62:212–220. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- 61.Knight T.R., Ho Y.S., Farhood A. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J. Pharmacol. Exp. Ther. 2002;303:468–475. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- 62.Kon K., Kim J.S., Jaeschke H. Mitochondrial permeability transition in acetaminophen induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40(5):1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 63.Kono H., Chen C.J., Ontiveros F. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J. Clin. Invest. 2010;120:1939–1949. doi: 10.1172/JCI40124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kopec A.K., Joshi N., Cline-Fedewa H. Fibrin(ogen) drives repair after acetaminophen-induced liver injury via leukocyte αMβ2 integrin-dependent upregulation of Mmp12. J. Hepatol. 2017;66(4):787–797. doi: 10.1016/j.jhep.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kruiswijk F., Labuschagne C.F., Vousden K.H. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat. Rev. Mol. Cell Biol. 2015;16(7):393–405. doi: 10.1038/nrm4007. [DOI] [PubMed] [Google Scholar]
- 66.Lancaster E.M., Hiatt J.R., Zarrinpar A. Acetaminophen hepatotoxicity: an updated review. Arch. Toxicol. 2015;89(2):193–199. doi: 10.1007/s00204-014-1432-2. [DOI] [PubMed] [Google Scholar]
- 67.Latchoumycandane C., Goh C.W., Ong M.M. Mitochondrial protection by the JNK inhibitor leflunomide rescues mice from acetaminophen-induced liver injury. Hepatology. 2007;45(2):412–421. doi: 10.1002/hep.21475. [DOI] [PubMed] [Google Scholar]
- 68.Lawson J.A., Farhood A., Hopper R.D. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol. Sci. 2000;54(2):509–516. doi: 10.1093/toxsci/54.2.509. [DOI] [PubMed] [Google Scholar]
- 69.Lee K.K., Imaizumi N., Chamberland S.R. Targeting mitochondria with methylene blue protects mice against acetaminophen-induced liver injury. Hepatology. 2015;61(1):326–336. doi: 10.1002/hep.27385. [DOI] [PubMed] [Google Scholar]
- 70.Lee W.M. Drug-induced acute liver failure. Clin. Liver Dis. 2013;17:575–586. doi: 10.1016/j.cld.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liao C.C., Day Y.J., Lee H.C. Baicalin attenuates IL-17-mediated acetaminophen-induced liver injury in a mouse model. PLoS One. 2016;11(11):e0166856. doi: 10.1371/journal.pone.0166856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li G., Chen J.B., Wang C. Curcumin protects against acetaminophen-induced apoptosis in hepatic injury. World J. Gastroenterol. 2013;19(42):7440–7446. doi: 10.3748/wjg.v19.i42.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin Z., Wu F., Lin S. Adiponectin protects against acetaminophen-induced mitochondrial dysfunction and acute liver injury by promoting autophagy in mice. J. Hepatol. 2014;61(4):825–831. doi: 10.1016/j.jhep.2014.05.033. [DOI] [PubMed] [Google Scholar]
- 74.Martin F.L., McLean A.E. Comparison of protection by fructose against paracetamol injury with protection by glucose and fructose-1,6-diphosphate. Toxicology. 1996;108(3):175–184. doi: 10.1016/0300-483x(95)03280-s. [DOI] [PubMed] [Google Scholar]
- 75.McGill M.R., Lebofsky M., Norris H.R. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol. Appl. Pharmacol. 2013;269(3):240–249. doi: 10.1016/j.taap.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McGill M.R., Sharpe M.R., Williams C.D. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Michalopoulos G.K. Liver regeneration. J. Cell. Physiol. 2007;213(2):286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miyakawa K., Joshi N., Sullivan B.P. Platelets and protease-activated receptor-4 contribute to acetaminophen-induced liver injury in mice. Blood. 2015;126(15):1835–1843. doi: 10.1182/blood-2014-09-598656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mobasher M.A., González-Rodriguez Á., Santamaría B. Protein tyrosine phosphatase 1B modulates GSK3β/Nrf2 and IGFIR signaling pathways in acetaminophen-induced hepatotoxicity. Cell Death Dis. 2013;4(5):e626. doi: 10.1038/cddis.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moore M.N. Autophagy as a second level protective process in conferring resistance to environmentally-induced oxidative stress. Autophagy. 2008;4(2):254–256. doi: 10.4161/auto.5528. [DOI] [PubMed] [Google Scholar]
- 81.Murad H.A., Habib H., Kamel Y. Thearubigins protect against acetaminophen-induced hepatic and renal injury in mice: biochemical, histopathological, immunohistochemical, and flow cytometry study. Drug Chem. Toxicol. 2016;39(2):190–198. doi: 10.3109/01480545.2015.1070170. [DOI] [PubMed] [Google Scholar]
- 82.Nagy G., Kardon T., Wunderlich L. Acetaminophen induces ER dependent signaling in mouse liver. Arch. Biochem. Biophys. 2007;459(2):273–279. doi: 10.1016/j.abb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 83.Nagy G., Szarka A., Lotz G. BGP-15 inhibits caspase-independent programmed cell death in acetaminophen-induced liver injury. Toxicol. Appl. Pharmacol. 2010;243:96–103. doi: 10.1016/j.taap.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 84.Nakagawa H., Maeda S., Hikiba Y. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 85.Nguyen K.A., Carbone J.M., Silva V.M. The PPAR activator docosahexaenoic acid prevents acetaminophen hepatotoxicity in male CD-1 mice. J. Toxicol. Environ. Health A. 1999;58(3):171–186. doi: 10.1080/009841099157377. [DOI] [PubMed] [Google Scholar]
- 86.Ni H.M., Bockus A., Boggess N. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 2012;55(1):222–232. doi: 10.1002/hep.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ni H.M., McGill M.R., Chao X. Removal of acetaminophen protein adducts by autophagy protects against acetaminophen-induced liver injury in mice. J. Hepatol. 2016;65(2):354–362. doi: 10.1016/j.jhep.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Onaolapo O.J., Adekola M.A., Azeez T.O. L-Methionine and silymarin: a comparison of prophylactic protective capabilities in acetaminophen-induced injuries of the liver, kidney and cerebral cortex. Biomed. Pharmacother. 2017;85:323–333. doi: 10.1016/j.biopha.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 89.Oz H.S., McClain C.J., Nagasawa H.T. Diverse antioxidants protect against acetaminophen hepatotoxicity. J. Biochem. Mol. Toxicol. 2004;18(6):361–368. doi: 10.1002/jbt.20042. [DOI] [PubMed] [Google Scholar]
- 90.Palliyaguru D.L., Chartoumpekis D.V., Wakabayashi N. Withaferin A induces Nrf2-dependent protection against liver injury: role of Keap1-independent mechanisms. Free Radic. Biol. Med. 2016;101:116–128. doi: 10.1016/j.freeradbiomed.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pang C., Zheng Z., Shi L. Caffeic acid prevents acetaminophen-induced liver injury by activating the Keap1-Nrf2 antioxidative defense system. Free Radic. Biol. Med. 2016;91:236–246. doi: 10.1016/j.freeradbiomed.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 92.Patel S.J., Luther J., Bohr S. A novel resolvin-based strategy for limiting acetaminophen hepatotoxicity. Clin. Transl. Gastroenterol. 2016;7(3):e153. doi: 10.1038/ctg.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patterson A.D., Shah Y.M., Matsubara T. Peroxisome proliferator-activated receptor alpha induction of uncoupling protein 2 protects against acetaminophen-induced liver toxicity. Hepatology. 2012;56(1):281–290. doi: 10.1002/hep.25645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pu S., Ren L., Liu Q. Loss of 5-lipoxygenase activity protects mice against paracetamol-induced liver toxicity. Br. J. Pharmacol. 2016;173(1):66–76. doi: 10.1111/bph.13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qiu Y., Benet L.Z., Burlingame A.L. Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J. Biol. Chem. 1998;273:17940–17953. doi: 10.1074/jbc.273.28.17940. [DOI] [PubMed] [Google Scholar]
- 96.Rashid U., Khan M.R., Sajid M. Hepatoprotective potential of Fagonia olivieri DC. against acetaminophen induced toxicity in rat. BMC Complement. Altern. Med. 2016;16(1):449. doi: 10.1186/s12906-016-1445-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rasool M.K., Sabina E.P., Ramya S.R. Hepatoprotective and antioxidant effects of gallic acid in paracetamol-induced liver damage in mice. J. Pharm. Pharmacol. 2010;62(5):638–643. doi: 10.1211/jpp.62.05.0012. [DOI] [PubMed] [Google Scholar]
- 98.Reddy M.K., Reddy A.G., Kumar B.K. Protective effect of rutin in comparison to silymarin against induced hepatotoxicity in rats. Vet. World. 2017;10(1):74–80. doi: 10.14202/vetworld.2017.74-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sabapathy K., Hochedlinger K., Nam S.Y. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol. Cell. 2004;15(5):713–725. doi: 10.1016/j.molcel.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 100.Saberi B., Ybanez M.D., Johnson H.S. Protein kinase C (PKC) participates in acetaminophen hepatotoxicity through c-jun-N-terminal kinase (JNK)-dependent and-independent signaling pathways. Hepatology. 2014;59:1543–1554. doi: 10.1002/hep.26625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sabina E.P., Pragasam S.J., Kumar S. 6-Gingerol, an active ingredient of ginger, protects acetaminophen-induced hepatotoxicity in mice. Zhong Xi Yi Jie He Xue Bao. 2011;9(11):1264–1269. doi: 10.3736/jcim20111116. [DOI] [PubMed] [Google Scholar]
- 102.Saheed S., Frans Hendrik O., Tom A.A. Zea mays, stigma maydis prevents and extenuates acetaminophen-perturbed oxidative onslaughts in rat hepatocytes. Pharm. Biol. 2016;54(11):2664–2673. doi: 10.1080/13880209.2016.1178307. [DOI] [PubMed] [Google Scholar]
- 103.Saini S.P., Zhang B., Niu Y. Activation of liver X receptor increases acetaminophen clearance and prevents its toxicity in mice. Hepatology. 2011;54(6):2208–2217. doi: 10.1002/hep.24646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saitoh M., Nishitoh H., Fujii M. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saito C., Zwingmann C., Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010;51(1):246–254. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Salminen W.F., Yang X., Shi Q. Green tea extract can potentiate acetaminophen-induced hepatotoxicity in mice. Food Chem. Toxicol. 2012;50(5):1439–1446. doi: 10.1016/j.fct.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 107.Schwabe R.F., Bradham C.A., Uehara T. c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology. 2003;37:824–832. doi: 10.1053/jhep.2003.50135. [DOI] [PubMed] [Google Scholar]
- 108.Shanmugam S., Sivaraj D., Dos Santos Lima B. Polyphenols rich Passiflora leschenaultii leaves modulating farnesoid X receptor and pregnane X receptor against paracetamol-induced hepatotoxicity in rats. Biomed. Pharmacother. 2017;88:1114–1121. doi: 10.1016/j.biopha.2017.01.156. [DOI] [PubMed] [Google Scholar]
- 109.Sinthorn W., Chatuphonprasert W., Chulasiri M. Thai red rice extract provides liver protection in paracetamol-treated mice by restoring the glutathione system. Pharm. Biol. 2016;54(5):770–779. doi: 10.3109/13880209.2015.1079725. [DOI] [PubMed] [Google Scholar]
- 110.Soeda J., Mouralidarane A., Ray S. The β-adrenoceptor agonist isoproterenol rescues acetaminophen-injured livers through increasing progenitor numbers by Wnt in mice. Hepatology. 2014;60(3):1023–1034. doi: 10.1002/hep.27266. [DOI] [PubMed] [Google Scholar]
- 111.Tan S., Lu Q., Shu Y. Iridoid Glycosides Fraction Isolated from Veronica ciliata Fisch. Protects against Acetaminophen-Induced Liver Injury in Mice. Evid. Based Complement. Altern. Med. 2017;2017:6106572. doi: 10.1155/2017/6106572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Truong V.L., Ko S.Y., Jun M. Quercitrin from Toona sinensis (Juss.) M.Roem. Attenuates acetaminophen-induced acute liver toxicity in HepG2 cells and mice through induction of antioxidant machinery and inhibition of inflammation. Nutrients. 2016;8(7) doi: 10.3390/nu8070431. (pii: E431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Uchida N.S., Silva-Filho S.E., Aguiar R.P. Protective effect of Cymbopogon citratus essential oil in experimental model of acetaminophen-induced liver injury. Am. J. Chin. Med. 2017;45(3):515–532. doi: 10.1142/S0192415X17500318. [DOI] [PubMed] [Google Scholar]
- 114.Urrunaga N.H., Jadeja R.N., Rachakonda V. M1 muscarinic receptors modify oxidative stress response to acetaminophen-induced acute liver injury. Free Radic. Biol. Med. 2015;78:66–81. doi: 10.1016/j.freeradbiomed.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Uzi D., Barda L., Scaiewicz V. CHOP is a critical regulator of acetaminophen-induced hepatotoxicity. J. Hepatol. 2013;59(3):495–503. doi: 10.1016/j.jhep.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 116.Wan J., Bae M.A., Song B.J. Acetaminophen-induced accumulation of 8-oxode-oxyguanosine through reduction of Ogg1 DNA repair enzyme in C6 glioma cells. Exp. Mol. Med. 2004;36(1):71–77. doi: 10.1038/emm.2004.10. [DOI] [PubMed] [Google Scholar]
- 117.Wang L., Zhang S., Cheng H., Lv H., Cheng G., Ci X. Nrf2-mediated liver protection by esculentoside A against acetaminophen toxicity through the AMPK/Akt/GSK3β pathway. Free Radic. Biol. Med. 2016;101:401–412. doi: 10.1016/j.freeradbiomed.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 118.Wang W., Guan C., Sun X. Tanshinone IIA protects against acetaminophen-induced hepatotoxicity via activating the Nrf2 pathway. Phytomedicine. 2016;23(6):589–596. doi: 10.1016/j.phymed.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 119.Wang X., Thomas B., Sachdeva R. Mechanism of arylating quinone toxicity involving Michael adduct formation and induction of endoplasmic reticulum stress. Proc. Natl. Acad. Sci. USA. 2006;103(10):3604–3609. doi: 10.1073/pnas.0510962103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Whyte A.J., Kehrl T., Brooks D.E. Safety and effectiveness of acetadote for acetaminophen toxicity. J. Emerg. Med. 2010;39(5):607–611. doi: 10.1016/j.jemermed.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 121.Williams C.D., Antoine D.J., Shaw P.J. Role of the Nalp3 inflammasome in acetaminophen-induced sterile inflammation and liver injury. Toxicol. Appl. Pharmacol. 2010;252(3):289–297. doi: 10.1016/j.taap.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Win S., Than T.A., Min R.W. c-Jun N-terminal kinase mediates mouse liver injury through a novel Sab (SH3BP5)-dependent pathway leading to inactivation of intramitochondrial Src. Hepatology. 2016;63(6):1987–2003. doi: 10.1002/hep.28486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Woolbright B.L., Jaeschke H. Role of the inflammasome in acetaminophen-induced liver injury and acute liver failure. J. Hepatol. 2017;66(4):836–848. doi: 10.1016/j.jhep.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu Y., Zhang X., Bardag-Gorce F. Retinoid X receptor alpha regulates glutathione homeostasis and xenobiotic detoxification processes in mouse liver. Mol. Pharmacol. 2004;65(3):550–557. doi: 10.1124/mol.65.3.550. [DOI] [PubMed] [Google Scholar]
- 125.Xie W., Jiang Z., Wang J. Protective effect of hyperoside against acetaminophen (APAP) induced liver injury through enhancement of APAP clearance. Chem. Biol. Interact. 2016;246:11–19. doi: 10.1016/j.cbi.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 126.Xie Y., McGill M.R., Dorko K. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol. Appl. Pharmacol. 2014;279(3):266–274. doi: 10.1016/j.taap.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xu X.Y., Hu J.N., Liu Z. Saponins (Ginsenosides) from the leaves of panax quinquefolius ameliorated acetaminophen-induced hepatotoxicity in mice. J. Agric. Food Chem. 2017;65(18):3684–3692. doi: 10.1021/acs.jafc.7b00610. [DOI] [PubMed] [Google Scholar]
- 128.Ye D., Wang Y., Li H. Fibroblast growth factor 21 protects against acetaminophen-induced hepatotoxicity by potentiating peroxisome proliferator-activated receptor coactivator protein-1α-mediated antioxidant capacity in mice. Hepatology. 2014;60(3):977–989. doi: 10.1002/hep.27060. [DOI] [PubMed] [Google Scholar]
- 129.Yin H., Cheng L., Holt M. Lactoferrin protects against acetaminophen-induced liver injury in mice. Hepatology. 2010;51(3):1007–1016. doi: 10.1002/hep.23476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yuan J., Ge K., Mu J. Ferulic acid attenuated acetaminophen-induced hepatotoxicity though down-regulating the cytochrome P 2E1 and inhibiting toll-like receptor 4 signaling-mediated inflammation in mice. Am. J. Transl. Res. 2016;8(10):4205–4214. [PMC free article] [PubMed] [Google Scholar]
- 131.Yun J., Mullarky E., Lu C. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350(6266):1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zakaria Z.A., Kamisan F.H., Omar M.H. Methanol extract of Dicranopteris linearis L. leaves impedes acetaminophen-induced liver intoxication partly by enhancing the endogenous antioxidant system. BMC Complement. Altern. Med. 2017;17(1):271. doi: 10.1186/s12906-017-1781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang J., Huang W., Chua S.S., Wei P. Modulation of acetaminophen-induced hepatotoxicity by the xenobiotic receptor CAR. Science. 2002;298(5592):422–424. doi: 10.1126/science.1073502. [DOI] [PubMed] [Google Scholar]
- 134.Zhang J., Song Q., Han X. Multi-targeted protection of acetaminophen-induced hepatotoxicity in mice by tannic acid. Int. Immunopharmacol. 2017;47:95–105. doi: 10.1016/j.intimp.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 135.Zhang J., Zhang S., Bi J. Astaxanthin pretreatment attenuates acetaminophen-induced liver injury in mice. Int. Immunopharmacol. 2017;45:26–33. doi: 10.1016/j.intimp.2017.01.028. [DOI] [PubMed] [Google Scholar]