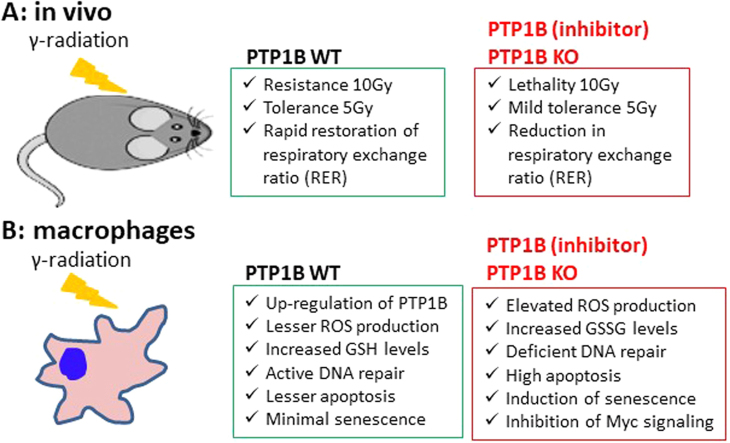
Abstract
Protein tyrosine phosphatase 1B (PTP1B) is widely expressed in mammalian tissues, in particular in immune cells, and plays a pleiotropic role in dephosphorylating many substrates. Moreover, PTP1B expression is enhanced in response to pro-inflammatory stimuli and to different cell stressors. Taking advantage of the use of mice deficient in PTP1B we have investigated the effect of γ-radiation in these animals and found enhanced lethality and decreased respiratory exchange ratio vs. the corresponding wild type animals. Using bone-marrow derived macrophages and mouse embryonic fibroblasts (MEFs) from wild-type and PTP1B-deficient mice, we observed a differential response to various cell stressors. PTP1B-deficient macrophages exhibited an enhanced response to γ-radiation, UV-light, LPS and S-nitroso-glutathione. Macrophages exposed to γ-radiation show DNA damage and fragmentation, increased ROS production, a lack in GSH elevation and enhanced acidic β-galactosidase activity. Interestingly, these differences were not observed in MEFs. Differential gene expression analysis of WT and KO macrophages revealed that the main pathways affected after irradiation were an up-regulation of protein secretion, TGF-β signaling and angiogenesis among other, and downregulation of Myc targets and Hedgehog signaling. These results demonstrate a key role for PTP1B in the protection against the cytotoxicity of irradiation in intact animal and in macrophages, which might be therapeutically relevant.
Abbreviations: DE, Differentially Expressed Gen; FDR, False Discovery Rates; GSH, glutathione; GSNO, S-nitroso-glutathione; GSEA, Gene Set Enrichment Analysis; JNK, c-Jun N-terminal kinase; KEGG, Kyoto encyclopedia of genes and genomes; LPS, lipopolysaccharide; KO, knockout; MAPK, mitogen-activated protein kinase; MnTBAP, Mn-7-hydroxyflavone complex; NAC, N-acetyl-L-cysteine; qRT-PCR, quantitative real-time (RT) polymerase chain reaction; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; WT, wild-type
Keywords: Protein tyrosine phosphatase, Cell viability, Irradiation sensitivity, Lethality, p53
Graphical abstract
Highlights
-
•
Absence of PTP1B activity enhances sensitivity to ionizing radiation.
-
•
Animals lacking PTP1B show decreased metabolic parameters after sub-lethal γ-irradiation.
-
•
Myeloid cells show an upregulation in the expression of PTP1B when challenged with sub-lethal γ-irradiation.
-
•
Macrophages deficient in PTP1B show elevated ROS production and reduced capacity to repair DNA damage.
-
•
TGF-β signaling, angiogenesis, Wnt/β-catenin and protein secretion pathways are up-regulated after γ-irradiation.
1. Introduction
PTP1B is a ubiquitously expressed protein tyrosine phosphatase that has emerged as a key regulator of several signaling pathways activated by members of the tyrosine kinase superfamily [1], [2], [3], [4]. Studies on PTP1B inhibitors have received attention in different areas of research and the number of inhibitors of this phosphatase is continuously increasing [5]. In the field of diabetes, due to the ability of PTP1B to dephosphorylate and inactivate the insulin receptor, these inhibitors have been considered as potential therapeutic drugs [6], [7], [8]. Indeed, PTP1B-deficient mice are a unique model of insulin hypersensitivity due to enhanced insulin action [9], [10], [11], since these mice are protected against diet [9], [11] and age-induced obesity and insulin resistance [12], [13]. PTP1B is also involved in the regulation of cytokine signaling pathways, controlling in this way cell outcomes [14], [15], [16], [17]. Moreover, PTP1B mRNA levels are regulated by different pro- and anti-inflammatory stimuli, such as LPS or IL4 [18], [19], [20], [21]. Through these mechanisms PTP1B has been involved in atherogenesis and cardiovascular diseases [4], [22], [23], liver diseases [1], neuroinflammation [3] and in endoplasmic reticulum stress [24]; however, conflictive data have been reported regarding its involvement in cancer [18], [25], [26], [27], [28].
We have previously described that PTP1B is highly expressed in monocytes and that mice lacking PTP1B, as well as human monocytes depleted of this protein, exhibited enhanced pro-inflammatory responses, and an attenuated capacity to express anti-inflammatory and pro-resolution mediators, resulting in a low-grade pro-inflammatory phenotype [20]. Indeed, other groups have reported an increase in myeloid precursors in the spleen and bone marrow in PTP1B-deficient mice, an effect probably due to the sustained activation of the M-CSF receptor [21]. Experiments intended to ablate the bone marrow from PTP1B-deficient mice showed that these animals were more sensitive to irradiation-induced injury than the corresponding wild-type (WT), resulting in significant higher death rates [20]. In the present work, we have investigated the effect of γ-radiation on the overall metabolic behavior of WT and PTP1B-deficient mice. In addition to this and taking into account that PTP1B is highly expressed in the myeloid lineage, we determined the impact of different stressors (γ-radiation, UV-light, LPS and GSNO) in the response of macrophages. Our data show a depressed metabolic activity of PTP1B-deficient animals after irradiation and a lesser capacity of macrophages to regulate oxidative stress. Moreover, ROS scavengers protected against the drop in GSH levels in PTP1B-deficient macrophages, preserving cell viability. These data suggest a relevant role for PTP1B in the mechanisms of defense against γ-radiation.
2. Materials and methods
2.1. Materials
Common reagents were from Sigma-Aldrich-Merck (St Louis, MO, USA) or Roche (Darmstadt, Germany). Murine or human cytokines were obtained from PeproTech (London, UK). Antibodies were from Ambion (Austin, TX, USA), Abcam (Cambridge, UK) or Cell signaling (Danvers, MA, USA). Reagents for electrophoresis were from Bio-Rad (Hercules, CA, USA). Tissue culture dishes were from Falcon (Lincoln Park, NJ, USA), and serum and culture media were from Invitrogen (Life Technologies/Thermo-Fisher, Madrid, Spain).
2.2. Animal care and preparation of macrophages
Male and female PTP1B heterozygous mice [29], maintained on mixed genetic background (C57BL/6×129sv), were intercrossed to yield three genotypes of mice (WT, HET, KO). In this study, we used 12-week-old WT and PTP1B KO male mice housed under 12 h light/dark cycle and food and water was provided ad libitum. Animals were cared for according to the protocol approved by the Ethical Committee of our institution (following directive 2010/63/EU of the European Parliament). Bone marrow derived macrophages (BMDMs) were obtained from male mice by flushing pelvises, femurs, and tibiae with DMEM. Bone marrow mononuclear phagocytic precursor cells were propagated in suspension by culturing in DMEM containing 10% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin, and 0.2 nM recombinant murine M-CSF (PeproTech) in tissue-culture plates. The precursor cells became adherent within 7 days of culture. BMDMs were maintained in RPMI 1640 medium supplemented with 10% FBS for 14 h prior to use.
2.3. Animal and cell irradiation
PTP1B WT and KO male mice were irradiated with the indicated doses in a two-sources Cs-137 irradiator (JL Shepherd, San Fernando, CA, USA; model Mark-1 30 A; 29,6 TBq of total activity). When PTP1B was inhibited, the drug was intraperitoneally administered 1 h prior to irradiation (15 mg/kg) followed by daily administrations for 3 days. Cells were irradiated in a similar way.
2.4. Evaluation of animal metabolic profile
Animals were maintained in Phenomaster Metabolic Cages (TSE Systems International Group) for two days prior to irradiation and then evaluated for 5 additional days. The movement, drinking, eating, respiration (O2 and CO2), weight and heat production were monitored.
2.5. Determination of myeloid cell populations
Animals were irradiated (5 Gy) and the distribution of myeloid cells was determined by flow cytometry at 24 h in the spleen and bone marrow. This dose of irradiation preserved viability of the animals. The markers used were: CD45, CD115, CD11b (monocytes), Ly6G (neutrophils), Ly6Clow (patrolling/tissue repair) Ly6Cmedium/high (pro-inflammatory) and F4/80 (macrophages) and combinations of florescence dyes was used to evaluate different cell subsets.
2.6. Measurement of cell viability
Macrophage viability was determined by flow cytometry as previously described [20] in a BD-Canto flow cytometer (BD Biosciences; San Jose, CA). In addition to this, cells were stained with annexin V-PE (Immunostep; Salamanca, Spain).
2.7. Measurement of oxidized guanine species
Cells were treated with the indicated stressors and the amount of 8-hydroxyguanine, 8-hydroxyguanosine and 8-hydroxy-2-deoxyguanosine were determined at 72 h using a DNA/RNA oxidative damage kit (Cayman-Vitro, Madrid, Spain).
2.8. Comet assay
Cells were irradiated and the DNA was analyzed at 72 h using the OxiSelect Comet assay kit (Cell Biolabs, San Diego, CA), following the instructions of the supplier.
2.9. Measurement of GSH and GSSG levels
Cells were treated with the indicated stressors and the amount of GSH and GSSG were determined at 24 h using a commercial kit (Enzo, Farmingdale, NY).
2.10. Determination of senescence
Cells were treated with the indicated stressors for the indicated times and the staining for acidic β-galactosidase activity, or the measurement of the β-galactosidase activity were determined using specific kits (Merck-Millipore), following the instructions of the supplier.
2.11. Determination of glutathione synthetase (GS) activity
GS was measured in macrophage extracts that were filtered through Sephadex G-25 columns to remove low-weight metabolites. The reaction was carried out at 25 °C in the presence of 1 mM γ-glutamylcysteine, 1 mM glycine and 1 mM MgATP at pH 7.4, and measuring the synthesis of GSH at 10 min. The ATP was regenerated from the ADP formed in the presence of 0.5 mM phospho(enol)pyruvate. One unit of GS is the amount of enzyme that synthetizes one µmol of GSH per 10 min.
2.12. Preparation of total protein cell extracts
Macrophages were homogenized in a buffer containing 10 mM Tris-HCl, pH 7.5; 1 mM MgCl2, 1 mM EGTA, 10% glycerol, 0.5% CHAPS, 1 mM β-mercaptoethanol and a protease and phosphatase inhibitor cocktail (Sigma). The extracts were vortexed for 30 min at 4 °C and after centrifuging for 20 min at 13,000 g, the supernatants were stored at − 20 °C. Protein levels were determined using Bradford reagent (Bio-Rad).
2.13. RNA isolation and RT-PCR analysis
RNA was extracted with TRIzol Reagent (Thermo-Fisher, Carlsbad, CA) and reverse transcribed using Transcriptor First Strand cDNA Synthesis Kit for RT-PCR following the indications of the manufacturer (Thermo-Fisher). Real-time PCR was conducted with SYBR Green Master (Thermo-Fisher) on a MyiQ Real-Time PCR System (Bio-Rad). Primer oligonucleotide sequences are available on request. Validation of amplification efficiency was performed for each pair of primers [30]. PCR thermocycling parameters were 95 °C for 10 min, 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. Each sample was run in duplicate and was normalized vs. 36B4. The fold induction (FI) was determined in a ΔΔCt based fold-change calculation.
2.14. Western blotting
Protein extracts were boiled in loading buffer (250 mM Tris–HCl; pH 6.8, 2% SDS, 10% glycerol and 2% β-mercaptoethanol) and 30 µg of protein were subjected to 8–10% SDS-PAGE electrophoresis gels. Proteins were transferred into polyvinylidene difluoride (PVDF) membranes (GE Healthcare). Membranes were incubated for 1 h with low-fat milk powder (5%) in PBS containing 0.1% Tween-20. Blots were incubated for 2 h or overnight at 4 °C with primary antibodies at the dilutions recommended by the suppliers. The blots were developed with ECL Advance protocol (GE Healthcare) and different exposure times were performed for each blot in an ImageQuant analyzer (LAS 500, GE) to ensure the linearity of the band intensities. Blots were normalized for lane charge using antibodies against GAPDH.
2.15. Microarray analysis
Normalized expression data in macrophages after 24 h of stimulation were obtained in our core facility, using a mouse microarray platform (Agilent-014868 Whole Mouse Genome Microarray 4×44K G4122F). Quality of the samples was ensured in a Bioanalyzer 2100. Processing, normalization and differential expression was performed using Limma Bioconductor package [31]. Enrichment of gene sets of interest from KEGG and REACTOME was accomplished using the GSEA software as described by Mootha et al. [32].
2.16. Statistical analysis
The values in graphs correspond to the means±SD. The statistical significance was determined with Student t-test for unpaired observation. Data were analyzed by the SPSS for Windows statistical package, v21.
3. Results
3.1. PTP1B protects against γ-radiation
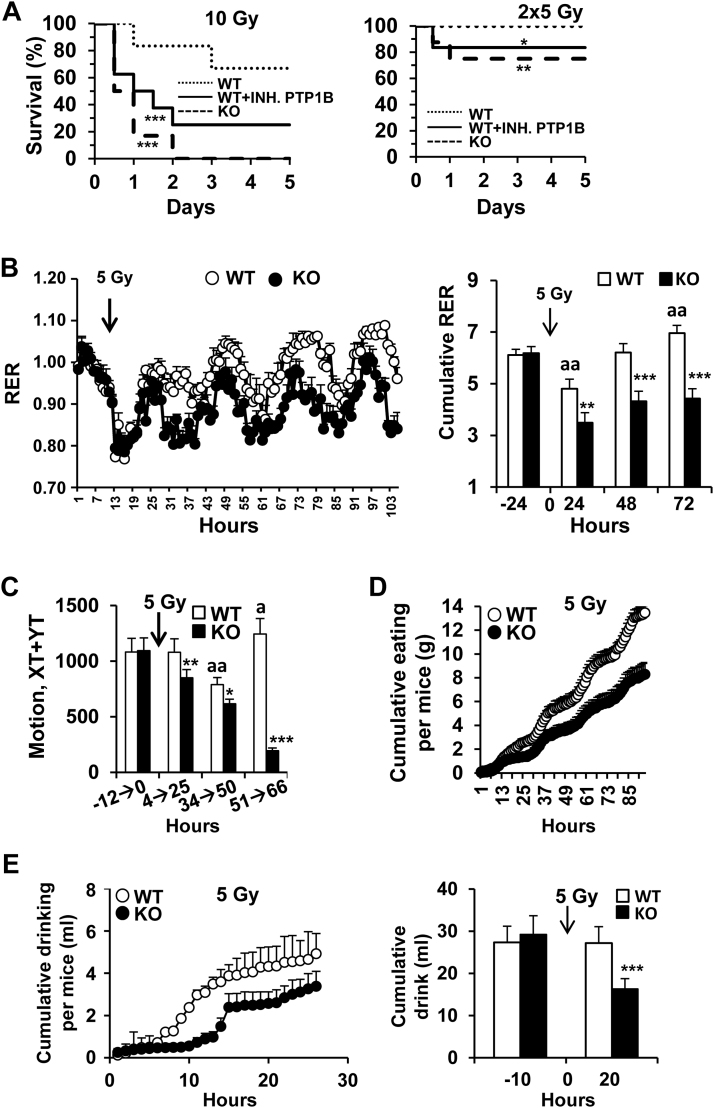
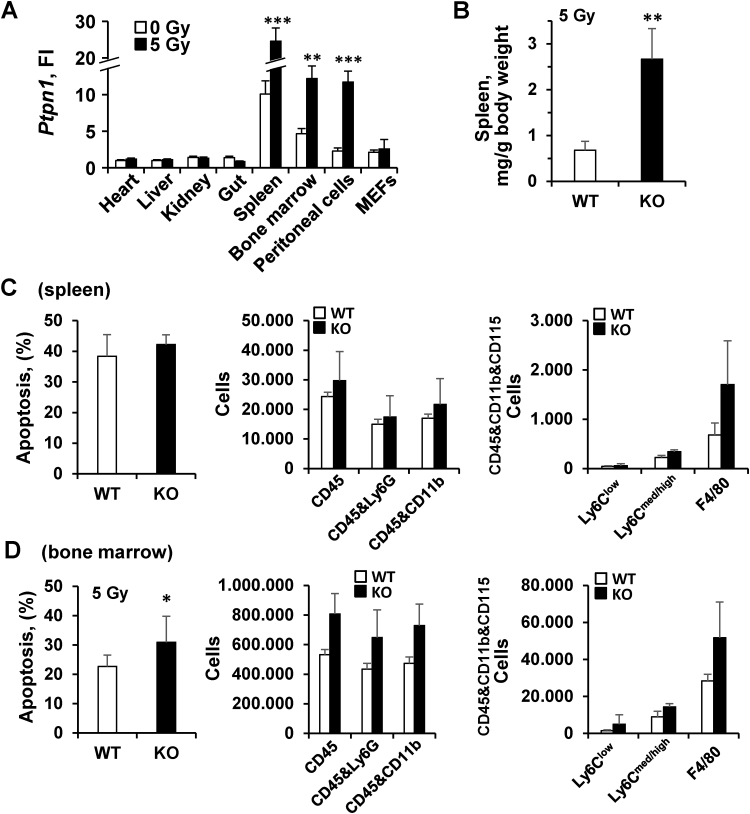
Since previous data suggested that animals lacking PTP1B were more sensitive to irradiation than the WT counterparts [20] we investigated the contribution of PTP1B to this protection. To this end, PTP1B WT and KO mice were irradiated with 10 Gy in a single dose. As Fig. 1A shows, animals lacking PTP1B or treated with a PTP1B inhibitor prior to irradiation exhibited a higher morbidity than the WT counterparts. When the 10 Gy were administered in two sessions of 5 Gy each in an 18 h-interval, animal survival increased, but still a statistically significant difference between the WT and the PTP1B inhibited or the PTP1B KO condition was observed (Fig. 1A, right panel). However, irradiation with a single dose of 5 Gy did not affect viability in any of the groups (results not shown). Next, we evaluated the response of PTP1B WT and KO animals to 5 Gy irradiation by performing indirect calorimetry. As Fig. 1B,C show, after 5 Gy irradiation PTP1B KO animals exhibited lower respiratory exchange ratio (RER), and decreased locomotor activity, food intake (Fig. 1D) and drinking (Fig. 1E) compared to the WT counterparts. The relative mRNA levels of Ptpn1 in different tissues after exposure to 5 Gy were determined (Fig. 2A). Interestingly, 5 Gy irradiation increased Ptpn1 mRNA levels mainly in immune organs and cells.
Fig. 1.
PTP1B deficiency increases the susceptibility to γ-radiation, depresses RER and drinking in mice after 5 Gy irradiation. (A) PTP1B WT animals (n = 12) untreated or treated with PTP1B inhibitor XXII (n = 8; 15 mg/kg) or PTP1B KO mice (n = 12) were submitted to 10 Gy irradiation administered in a single dose or in two doses of 5 Gy in a 18 h period and survival of the animals was evaluated. (B) PTP1B WT or KO mice (n = 10) were submitted to 5 Gy irradiation and the plural effects on RER, motion (C), eating (D) and drinking (E) were determined in ‘Phenomaster Metabolic Cages’. Results show the mean±SD of two independent experiments. *P < 0.05; **P < 0.01; * **P < 0.005 vs. the same condition in PTP1B WT animals; aP < 0.05; aaP < 0.01 vs. the ‘untreated’ condition of WT animals.
Fig. 2.
PTP1B is highly expressed in immune cells and its absence decreases viability of myeloid cells afterγ-irradiation. (A) PTP1B mRNA basal levels of different organs from WT animals (n = 5) or MEFs isolated from these animals, or at 6 h after exposure to 5 Gy. The indicated tissues were isolated, the RNA extracted and analyzed for the levels of Ptpn1 vs. the corresponding unexposed (n = 5) counterparts (Fold Induction, FI, was calculated vs. the liver Ptpn1 levels from untreated animals). (B) Spleens of irradiated animals were excised off and expressed vs. the body weight of the animals (24 h). (C,D) The percentage of apoptotic cells in the spleen (per mg of tissue) and bone marrow, as well as the distribution of different subsets of myeloid cells were determined 24 h after 5 Gy irradiation of WT or PTP1B KO mice (n = 4–5). Results show the mean±SD of two independent experiments. *P < 0.05; **P < 0.01; ***P < 0.005 vs. the same condition in WT animals.
Irradiated PTP1B KO animals (5 Gy) exhibited a significant spleen enlargement (Fig. 2B), with a high content of apoptotic cells (Fig. 2C), although no statistically significant differences were observed in the cell populations analyzed. Moreover, analysis of myeloid precursors in the bone marrow showed a statistically significant increase of apoptotic cells, suggesting a higher sensitivity of PTP1B KO cells to irradiation (Fig. 2D).
3.2. Macrophages from PTP1B-deficient mice sense γ-radiation, UV and oxidative/nitrosative stress
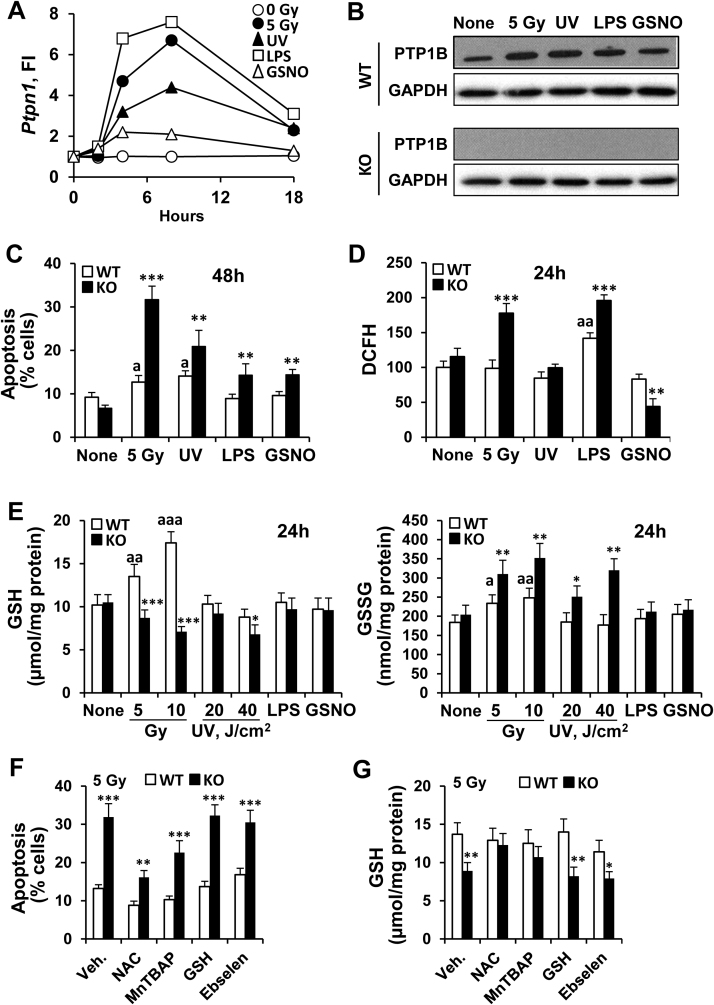
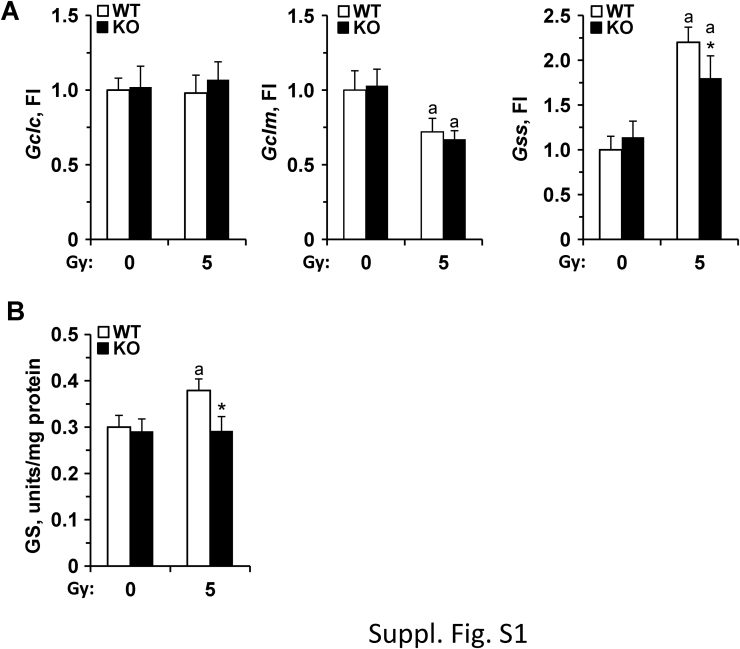
To evaluate the molecular mechanisms responsible for the enhanced sensitivity of PTP1B-deficient mice to different stressors compromising animal survival or cell function, macrophages –as an immune cell type expressing high levels of Ptpn1- and MEFs –as a non-immune cell with significant levels of Ptpn1- were used. As Fig. 3A-B shows, bone marrow-derived macrophages (BMDMs) from PTP1B WT mice show an increase of Ptpn1 mRNA and protein levels after exposure to γ-radiation, UV or LPS, and to a lesser extent after incubation with the NO-donor GSNO. At the same time, PTP1B-deficient macrophages exhibited higher apoptotic rates (48 h) under these conditions, including after GSNO incubation (Fig. 3C). When ROS production was measured we found that whereas irradiated cells from WT animals were not affected (Fig. 3D), those from PTP1B KO mice exhibited a significant increase and this effect occurred after LPS challenge. PTP1B KO macrophages treated with GSNO exhibited a decrease in ROS, probably due to the formation of peroxynitrite between O2- and NO. Since γ-radiation and LPS exposure produced the major impact on ROS synthesis, we determined the levels of GSH and GSSG under the different conditions assayed. As Fig. 3E shows, GSH levels increased in irradiated WT macrophages but not in those deficient in PTP1B. Moreover, GSSG concentrations were higher in the corresponding PTP1B KO macrophages than in the WT cells after irradiation or UV exposure. None of these effects were found in MEFs (results not shown). To evaluate the role of ROS on the apoptosis of PTP1B KO macrophages, cells were incubated with ROS scavengers (NAC, MnTBAP and ebselen) and GSH in the extracellular medium. As Fig. 3F shows, viability was significantly improved in irradiated PTP1B KO macrophages treated with NAC and the permeable superoxide dismutase mimetic MnTBAP, but not by ebselen (probably due to scavenging NO) nor GSH. Moreover, the intracellular levels of GSH were higher in NAC- and MnTBAP-treated cells (Fig. 3G). Analysis of the mRNA levels of the enzymes involved in GSH biosynthesis did not show significant changes in glutamate-cysteine ligase catalytic subunit (Gclc), a decrease in the regulatory subunit (Gclm), and a lesser increase in glutathione synthetase (Gss), compatible with modest differences in the enzymatic activity of glutathione synthetase (GS) from irradiated PTP1B KO macrophages (Suppl. Fig. S1A,B).
Fig. 3.
Macrophages from PTP1B-deficient mice exhibit increased apoptosis and phagocytosis, and fail to restore GSH levels after 5 Gy irradiation. (A) BMDM from WT animals were exposed to 5 Gy, 20 J/cm2 of UV light, 200 ng/ml of LPS or 0.5 mM GSNO and the time-course of Ptpn1 mRNA levels was determined. (B) The protein levels of PTP1B were determined after 24 h of treatment by immunoblot. (C,D) The extent of apoptotic cells (48 h) and production of reactive oxygen species (24 h) were determined under these conditions. (E) The dose-dependent effect of γ-radiation (5 Gy and 10 Gy), UV exposure (20 and 40 J/cm2), LPS and GSNO on the GSH and GSSG levels were determined 24 h after exposure to these stressors. (F) Macrophages from WT (n = 3) or from PTP1B-deficient mice (n = 4) were isolated, incubated for 60 min with 5 mM N-acetyl-cysteine (NAC), 0.2 µM MnTBAP, 10 mM GSH or 0.1 µM ebselen and exposed to 5 Gy. After 48 h the percentage of apoptotic cells and the levels of GSH (G) were determined. Results show the mean of two experiments (A), a representative blot (B) and the mean±SD of three independent experiments (C-G), *P < 0.05; **P < 0.01; ***P < 0.005 vs. the same condition in WT cells or in the absence of challenge; aP < 0.05; aaP < 0.01; aaaP < 0.005 vs. the ‘none’ condition of WT cells.
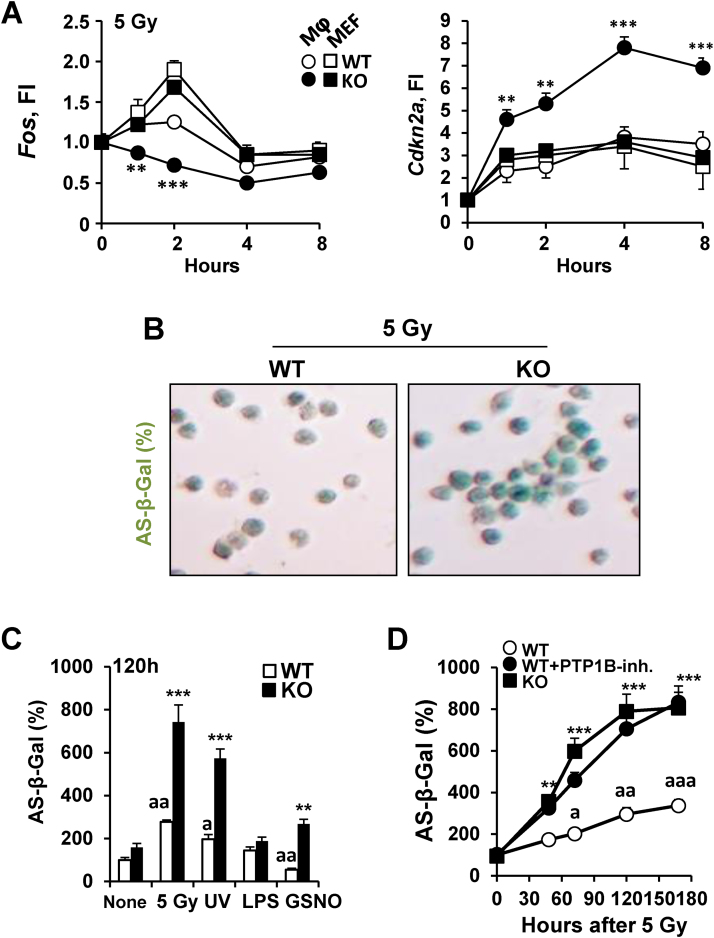
3.3. Absence of PTP1B promotes senescence in macrophages after challenge with different stressors
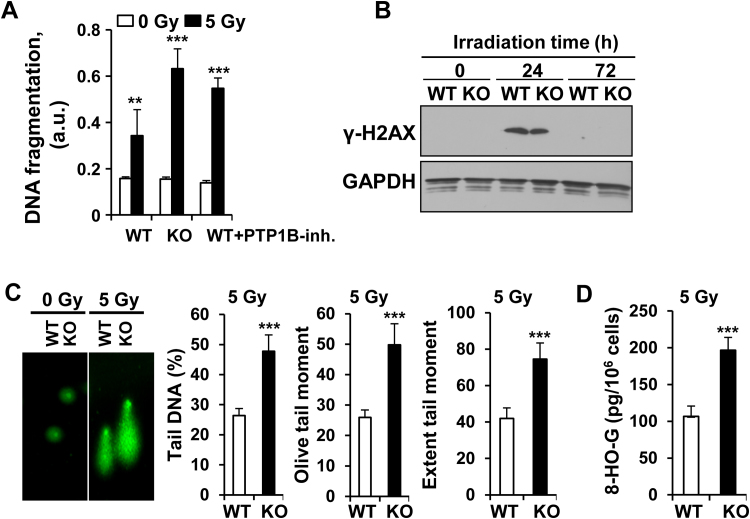
Since morphological differences were observed between WT and PTP1B KO macrophages challenged with different stressors, such as cell and nuclear enlargement (data not shown), as well as molecular markers of senescence (Fos and Cdkn2a; Fig. 4A), the occurrence of senescence was investigated under these conditions. Exposure to γ-radiation and UV and, to a lesser extend to GSNO resulted in an increase in senescence-associated acidic β-galactosidase activity in macrophages lacking PTP1B or in WT cells after inhibition of PTP1B (Fig. 4B–D). In addition to these changes, increased DNA fragmentation 72 h after irradiation was observed in PTP1B KO macrophages (Fig. 5A). However, the phosphorylation of histone H2AX (γ-H2AX) as an indicator of DNA damage was observed at 24 h after irradiation and was similar in WT and PTP1B KO macrophages (Fig. 5B). This is despite the increased DNA damage in PTP1B KO macrophages as determined at 72 h by the Comet assay (Fig. 5C), and by the increase in 8-hydroxyguanine, 8-hydroxy-2′-deoxyguanosine and 8-hydroxyguanosine species (8-HO-G) after 5 Gy irradiation (Fig. 5D). These results indicate that the absence of PTP1B favors an increase in oxidative stress, but also a lack in the capacity to prevent the harmful effects associated to these radicals as evidenced by the DNA damage and the accumulation of oxidized nucleotide species.
Fig. 4.
PTP1B deficiency enhances senescence in macrophages submitted to 5 Gy irradiation or UV exposure. (A) BMDMs and MEFs from WT or PTP1B-deficient mice were maintained at subconfluence and then submitted to 5 Gy irradiation and at the indicated times the mRNA levels of Fos and Cdkn2a were determined by qPCR. (B) Macrophages from WT and PTP1B KO mice were irradiated (5 Gy) and the staining for acidic β-galactosidase was determined. (C-D) Macrophages from PTP1B WT mice in the absence or presence of 5 µM PTP1B inhibitor XXII and PTP1B KO mice were submitted to 5 Gy, 20 J/cm2 of UV light, 200 ng/ml of LPS or 0.5 mM GSNO and after 5 days the acidic β-galactosidase activity, and (D) activity at the indicated times was measured. Results show the mean±SD of three independent experiments, or a representative experiment out of three (B). *P < 0.05; **P < 0.01; ***P < 0.005 vs. the same condition in the WT cells; aP < 0.05; aaP < 0.01; aaaP < 0.005 vs. the ‘none’ condition of WT cells. Fold induction (F.I., A).
Fig. 5.
PTP1B deficiency enhances DNA damage in macrophages submitted to 5 Gy irradiation. (A) BMDMs from WT or PTP1B-deficient mice were exposed to 5 Gy and the DNA fragmentation was determined at 72 h in these cells. The PTP1B inhibitor XXII was used at 5 µM. (B) The phosphorylation of histone H2AX was determined by immunoblot at the indicated times. (C) Comet assay of macrophages 72 h after exposure to 5 Gy irradiation. (D) Quantification of oxidized guanine species (72 h post-irradiation). Results show the mean±SD of three independent experiments, or a representative experiment out of three (B,C). **P < 0.01; ***P < 0.005 vs. the same condition in the WT cells.
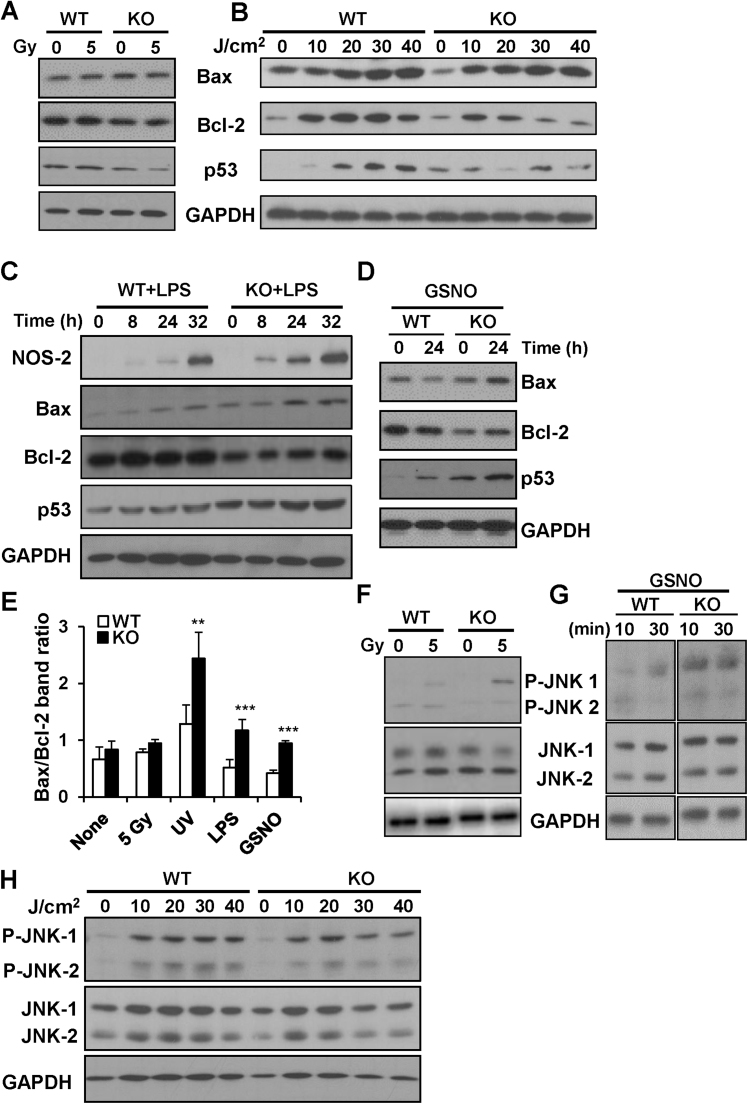
3.4. PTP1B deficiency results in a Bcl-2 decrease and increase in Bax levels in response to different stressors in macrophages
The loss in viability of PTP1B-deficient macrophages after irradiation reported in Fig. 2C was associated with a significant decrease in Bcl-2 and a rise in Bax levels 24 h after 5 Gy exposure (Fig. 6A). Moreover, after UV challenge Bax levels increased similarly in WT and PTP1B-deficient macrophages whereas a dose-dependent increase in Bcl-2 was observed only in WT cells (Fig. 6B). In the same way, the rise in Bax levels after treatment with LPS or GSNO was higher in PTP1B KO cells vs. WT (Fig. 6C,D), resulting in a higher increase in the Bax/Bcl-2 ratio (Fig. 6E). Regarding p53 levels, whereas UV treatment enhanced its expression mainly in WT cells, this effect was attenuated in PTP1B KO macrophages. In addition, incubation with LPS resulted in higher levels of p53 in PTP1B KO cells, probably due to the increased production of reactive oxygen and nitrogen species (Fig. 6C,D, and Fig. 3D). Regarding MAPKs signaling, we have analyzed P-ERK and P-p38 in a previous work [20] and they exhibited minimal changes in response to LPS. Here we show that P-JNK1/2 levels were higher after irradiation or GSNO treatment of PTP1B KO macrophages, but remained similar in WT and PTP1B KO cells exposed to UV (Fig. 6F–H).
Fig. 6.
PTP1B-deficient macrophages exhibit higher Bax/Bcl-2 ratios in response to different stressors. Macrophages from PTP1B WT or PTP1B KO mice were submitted to 5 Gy, UV light, 200 ng/ml of LPS or 0.5 mM GSNO and the levels of Bax, Bcl-2, p53, GAPDH and NOS-2 (in the case of LPS challenge) were determined after 24 h (A,B) or at the indicated times (C,D). (F-H) The levels p-JNK1/2 after 30 min or at the indicated times were determined. Results show a representative experiment out of three and (E) the mean±SD of the Bax/Bcl-2 ratios at 24 h (30 J/cm2 in the case of UV light). **P < 0.01; ***P < 0.005; vs. the same condition in the WT cells.
3.5. Gene expression analysis of the response of macrophages from PTP1B-deficient mice to γ-radiation
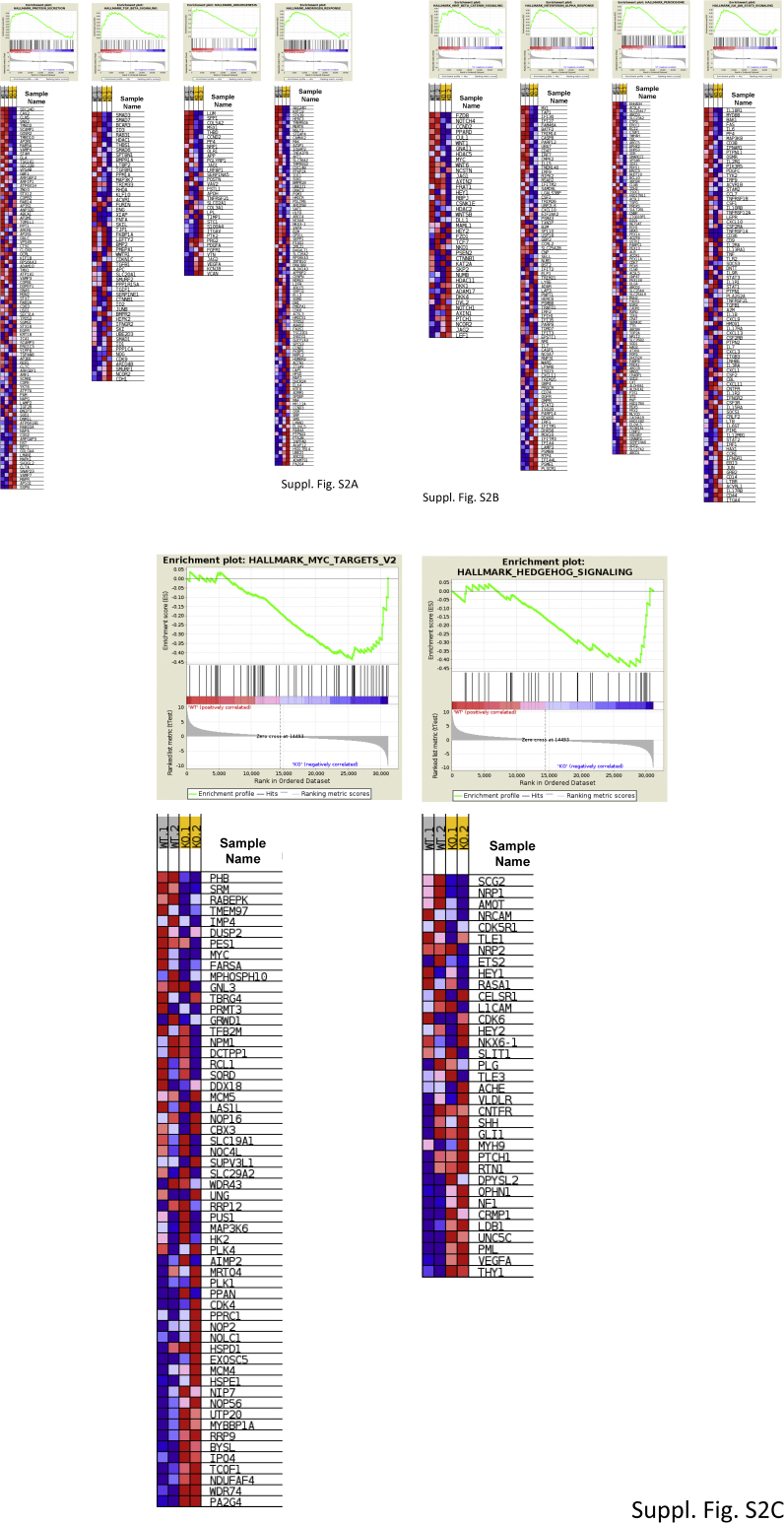
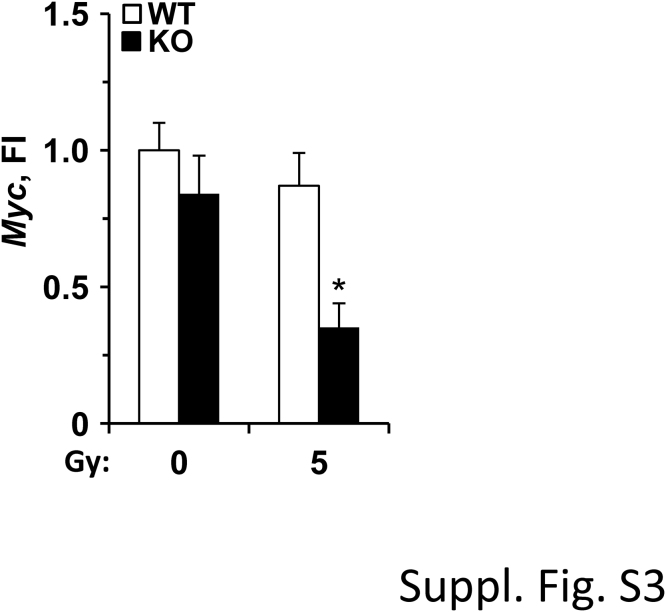
Because PTP1B-deficient macrophages have an enhanced response to 5 Gy irradiation, a microarray gene expression analysis comparing PTP1B WT and KO macrophages was carried out. As Table 1 and Suppl. Fig. S2A–C show, 8 pathways were significantly upregulated and two down-regulated in PTP1B KO macrophages vs. the WT counterparts, after GSEA analysis. Among these pathways, the protein secretion route, the TGF-β signaling, angiogenesis and Wnt/β-catenin pathways are of special relevance in the pathophysiological role of macrophages in different contexts (see in Discussion), and the same holds true for the downregulated Myc and Hedgehog signaling. Indeed, Myc levels were determined by qPCR confirming the microarray data, and exhibited a significant decrease in irradiated PTP1B KO macrophages (Suppl. Fig. S3).
Table 1.
GSEA analysis of microarray data from macrophages from PTP1B WT and PTP1B KO mice exposed to 5 Gy irradiation. Cells from 4 independent animals for each condition were maintained in culture for 24 h and after analysis of the RNA quality in a Bioanalyzer, equal amounts of RNA were pooled and submitted to microarray hybridization. Gene Set Enrichment Analysis (GSEA) was performed and the quantitative parameters of the significant pathways (FDR < 0.25) are provided. Results show the data from two independent experiments as reflected in Supplementary Fig. S1.
| Pathway | Enrichment score | Normal p-value | FDR q-value |
|---|---|---|---|
| Upregulated | |||
| Protein secretion | 0.374 | 0.0106 | 0.2331 |
| TGF-β signaling | 0.418 | 0.0191 | 0.1216 |
| Angiogenesis | 0.442 | 0.0445 | 0.1124 |
| Androgen receptor | 0.336 | 0.0720 | 0.2194 |
| Wnt/β-Catenin | 0.386 | 0.1013 | 0.2093 |
| IFN-α response | 0.316 | 0.0972 | 0.2385 |
| Peroxisome | 0.309 | 0.1037 | 0.2257 |
| IL-6/Jak/Stat-3 | 0.308 | 0.1507 | 0.2338 |
| Downregulated | |||
| Myc targets | − 0.436 | 0.0111 | 0.0434 |
| Hedgehog signaling | − 0.443 | 0.0482 | 0.0746 |
4. Discussion
Work form several groups has demonstrated that protein tyrosine phosphatases play a relevant role in the process of macrophage polarization acting, not only as negative regulators of pro-inflammatory signaling, but also as attenuators of anti-inflammatory pathways [21], [33], [34], [35], [36], [37]. Moreover, in addition to immune regulation, an essential role for PTP1B in obesity-induced chronic low-grade inflammation and in other pathologies including diabetes, cancer, neuroinflammation, liver diseases or Rett syndrome, has been described as evidenced by the use of selective PTP1B inhibitors or animal models targeted for PTP1B [12], [18], [20], [22], [38], [39], [40], [41].
In this work, we confirmed the enhanced sensitivity of PTP1B-deficient mice to γ-radiation. Interestingly enough, 10 Gy exerted a deleterious effect on these animals in a time shorter than 24 h. These animals exhibited hemorrhages in organs such as liver, a situation not found in their age-matched WT counterparts. Pharmacological inhibition of PTP1B partially mimicked the PTP1B-deficient genotype, with a prominent death in the initial 24 h period. In addition to this, we have evaluated metabolic parameters in animals submitted to a single 5 Gy administration, a situation in which all animals survived. Under these conditions, the severity of the irradiation was significantly higher in PTP1B-deficient mice as reflected by a reduced locomotor activity and impaired physiological functions (i.e., eating and drinking), and by an attenuated RER. The latter effect could be explained by lower carbohydrate consumption due to decreases in food intake. Moreover, this mild irradiation significantly decreased myeloid cell viability in the bone marrow, in addition to splenic enlargement. Thus, PTP1B seems to be a relevant target in the protection against ionizing radiation. Indeed, absence of PTP1B reduces life-span and enhances the appearance of lymphoproliferative disorders in mice [18]. Also, these results are in agreement with previous data on the role of myeloid cell irradiation (macrophages among them) in oncogenic treatments [42], [43], [44].
Although PTP1B is present in almost all tissues analyzed, myeloid cells, particularly monocyte/macrophages exhibited high levels of Ptpn1 mRNA, and both mRNA and protein increased in response to different stressors, such as ionizing radiation, UV exposure or LPS challenge. It is worthy to mention that cytokines involved in the differentiation to anti-inflammatory profile of macrophages, such as IL-4, also promote elevations in PTP1B protein and enzymatic activity, suggesting a main role for this protein tyrosine phosphatase as modulator of the resolution phase of the inflammatory response [19], [45]. Conversely, absence of PTP1B impairs the well-known anti-inflammatory action of IL-4, reinforcing its role in the regulation of macrophage polarization [20]. In addition to PTP1B, other protein tyrosine phosphatases, such as the T-cell protein tyrosine phosphatase (TC-PTP) that are relevant in immune cell development, play non-redundant roles in macrophage function, reflecting the singularity of these PTPs [36].
Macrophages were used to gain insight on the mechanisms controlled by PTP1B and involved in the maintenance of cell viability after irradiation or exposure to other stressors. One of the most characteristic features of irradiated macrophages from PTP1B KO mice was an enhanced production of ROS together with a loss in cell viability. When GSH levels were measured, a reduced capacity of PTP1B-deficient macrophages to restore GSH concentration after irradiation was observed, a situation not found in the WT counterparts. Interestingly, incubation of cells with ROS scavengers significantly protected cell viability and preserved GHS levels after irradiation of PTP1B KO macrophages. This effect was not mimicked by GSH in the extracellular milieu. Moreover, PTP1B KO cells exhibited increased DNA and RNA oxidative damage as reflected by the Comet assay, by the formation of 8-hydroxy derivatives of guanine and guanosine species and by an induction of the senescence program in the surviving cells. The latter phenotype was manifested by the appearance of acidic β-galactosidase activity, and by a drop in Fos and a rise in Cdkn2a mRNA in the PTP1B KO macrophages. In endothelial cells, absence of PTP1B also promoted senescence and favored neointimal hyperplasia [46]. Since histone H2AX phosphorylation was similar in PTP1B WT and KO cells after irradiation, it can be concluded that the repair mechanisms are defective in the PTP1B KO counterparts, probably due to the decreased Myc signaling, as observed in the microarray analysis [47]. Indeed, the cross-talk between the c-Myc and p53 pathways upon irradiation are very cell dependent [48]. Here we show an up-regulation of p53 in PTP1B KO macrophages that may explain the decrease the repression of Myc after irradiation [49]. However, the situation was more complex since examination of pathways involved in DNA repair, such as p53 were defective in PTP1B KO macrophages exposed to irradiation or UV, but not after incubation with LPS or GSNO, suggesting the existence of time-dependent specific mechanisms involved in the up-regulation of p53 levels in PTP1B KO cells after irradiation. In addition to this, Bax levels increased and Bcl-2 levels were reduced in PTP1B KO cells exposed to these stressors suggesting a role for these proteins in cell viability in macrophages lacking PTP1B.
In a previous work comparing macrophages from WT and PTP1B-deficient mice the microarray analysis at 24 h after LPS challenge showed main differences in gene expression in the p53 and DNA excision/repair pathways, but not in genes related to pro-inflammatory pathways, probably because they occurred at earlier times [20]. Thus, the link between the p53 pathway and the DNA excision/repair pathway fits with the observed loss of viability of PTP1B-deficient macrophages. This role of PTP1B in protecting mice against irradiation injury is a relevant issue of the present work and we suggest that strategies aimed to induce a transient PTP1B activity would prevent adverse effects in case of harmful exposure and, alternatively, inhibition of this activity may enhance the therapeutic action of irradiation in cancer radiotherapy. Moreover, the levels of PTP1B are consistently increased after stressing macrophages, probably as a compensatory response to improve cell viability under conditions of intense synthesis of reactive oxygen and/or nitrogen species that may damage the DNA. Microarray analysis of macrophages from WT and PTP1B KO mice 24 h after 5 Gy irradiation reflected an up-regulation of several pathways, among them protein secretion, the Wnt/β-catenin pathway, the TGF-β signaling pathway and the IL-6/Jak/Stat3 pathway. In agreement to these changes, an increase in the Wnt/β-catenin pathway [43] and vascular development [42], [50] have been described. Although these pathways regulate many macrophage activities, it should be expected a specific phenotype in the irradiated cells lacking PTP1B. In the same way, the Myc and the Hedgehog signaling pathways are repressed in PTP1B KO macrophages, and confirm the deficient DNA repair and the relevant pathophysiological role of macrophages after irradiation [47], [51], [52]. Noteworthy, the p53 and the DNA excision/repair pathways that are upregulated in the LPS condition in PTP1B KO cells are not altered after irradiation; the possibility exists that the gene changes occurred earlier than the 24 h of analysis performed in our arrays.
In conclusion, our data show an enhanced sensitivity to γ-radiation and to other stressors in mice and macrophages lacking PTP1B and exposed to irradiation. These results might be relevant for patients submitted to irradiation for therapeutic purposes, or for personnel on irradiation risk. Under these circumstances, favoring PTP1B activation might reduce the deleterious effects in immune cells. Unraveling the exact contribution of PTP1B activity to these situations might help to develop both protocols for protection or increased immune damage in these interventions.
5. Conclusions
The results presented describe a relevant role for PTP1B in the protection against ionizing irradiation, and can be used either to enhance sensitivity, by using selective inhibitors, or to increase resistance after induction and/or activation of this phosphatase.
Acknowledgements
This work was supported by grants SAF2017-82436R, SAF2016-75004R and SAF2015-65267 from MINIECO, S2017/BMD-3686 and BMD3684 from Comunidad de Madrid, Fundación Ramón Areces (2016/CIVP18A3864) and Cibercv, Ciberdem and Ciberehd (funded by the Instituto de Salud Carlos III and by Fondos FEDER). We also acknowledge H2020 Marie Sklodowska-Curie ITN-TREATMENT (Grant Agreement number 721236) (European Commission). P.R. was recipient a Juan de la Cierva postdoctoral contract from MINIECO (IJCI-2014-19381).
Acknowledgments
Authors disclosure statement
None.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.04.018.
Contributor Information
Angela M. Valverde, Email: avalverde@iib.uam.es.
Lisardo Boscá, Email: lbosca@iib.uam.es.
Appendix A. Supplementary material
Fig S1.
Effect of irradiation on GSH biosynthesis in macrophages from PTP1B WT and PTP1B KO mice. (A) Cells were exposed to 5 Gy and the mRNA levels of the catalytic and regulatory subunits of γ-glutamyl-cysteine ligase (Gclc and Gclm) and glutathione synthetase (Gss) were determined at 24 h. (B) The activity of glutathione synthetase (GS) was determined in cell extracts. Results show the mean±SD of three experiments. *P < 0.05 vs. the same condition in the WT cells; aP < 0.05; vs. the 0 Gy condition. Fold induction (F.I., A).
Fig S2.
Microarrayanalysis of macrophages from PTP1B WT and PTP1B KO mice. Cells were treated as described in Table 1 and the GSEA analysis resulted in 8 pathways up-regulated in the PTP1B KO (A-B) and two pathways down-regulated. (C) The graphs on the top show the gene set enrichment plots, with black horizontal lines indicating the gene ranking positions (see http://software.broadinstitute.org/gsea/doc/GSEAUserGuideFrame.html for details). At the bottom, we show the expression levels of the genes in each pathway as heatmaps, where red indicates up-regulation and blue down-regulation.
Fig S3.
Irradiation decreased Myc levels in PTP1B KO mice. Macrophages from WT or PTP1B KO mice were submitted to 5 Gy irradiation and the Myc mRNA levels were determined at 24 h after treatment. Results show the mean±SD of three experiments. *P < 0.05 vs. the same condition in the WT cells.
References
- 1.Chen P.J., Cai S.P., Huang C., Meng X.M., Li J. Protein tyrosine phosphatase 1B (PTP1B): a key regulator and therapeutic target in liver diseases. Toxicology. 2015;337:10–20. doi: 10.1016/j.tox.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Haj F.G., Markova B., Klaman L.D., Bohmer F.D., Neel B.G. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatase-1B. J. Biol. Chem. 2003;278:739–744. doi: 10.1074/jbc.M210194200. [DOI] [PubMed] [Google Scholar]
- 3.Song G.J., Jung M., Kim J.H., Park H., Rahman M.H., Zhang S., Zhang Z.Y., Park D.H., Kook H., Lee I.K., Suk K. A novel role for protein tyrosine phosphatase 1B as a positive regulator of neuroinflammation. J. Neuroinflamm. 2016;13:86. doi: 10.1186/s12974-016-0545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thiebaut P.A., Besnier M., Gomez E., Richard V. Role of protein tyrosine phosphatase 1B in cardiovascular diseases. J. Mol. Cell Cardiol. 2016;101:50–57. doi: 10.1016/j.yjmcc.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Du Y., Zhang Y., Ling H., Li Q., Shen J. Discovery of novel high potent and cellular active ADC type PTP1B inhibitors with selectivity over TC-PTP via modification interacting with C site. Eur. J. Med. Chem. 2017;144:692–700. doi: 10.1016/j.ejmech.2017.12.064. [DOI] [PubMed] [Google Scholar]
- 6.Salmeen A., Andersen J.N., Myers M.P., Tonks N.K., Barford D. Molecular basis for the dephosphorylation of the activation segment of the insulin receptor by protein tyrosine phosphatase 1B. Mol. Cell. 2000;6:1401–1412. doi: 10.1016/s1097-2765(00)00137-4. [DOI] [PubMed] [Google Scholar]
- 7.Seely B.L., Staubs P.A., Reichart D.R., Berhanu P., Milarski K.L., Saltiel A.R., Kusari J., Olefsky J.M. Protein tyrosine phosphatase 1B interacts with the activated insulin receptor. Diabetes. 1996;45:1379–1385. doi: 10.2337/diab.45.10.1379. [DOI] [PubMed] [Google Scholar]
- 8.Krishnan N., Konidaris K.F., Gasser G., Tonks N.K. A potent, selective and orally bioavailable inhibitor of the protein tyrosine phosphatase PTP1B improves insulin and leptin signaling in animal models. J. Biol. Chem. 2017 doi: 10.1074/jbc.C117.819110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elchebly M., Payette P., Michaliszyn E., Cromlish W., Collins S., Loy A.L., Normandin D., Cheng A., Himms-Hagen J., Chan C.C., Ramachandran C., Gresser M.J., Tremblay M.L., Kennedy B.P. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 10.Haj F.G., Zabolotny J.M., Kim Y.B., Kahn B.B., Neel B.G. Liver-specific protein-tyrosine phosphatase 1B (PTP1B) re-expression alters glucose homeostasis of PTP1B-/-mice. J. Biol. Chem. 2005;280:15038–15046. doi: 10.1074/jbc.M413240200. [DOI] [PubMed] [Google Scholar]
- 11.Klaman L.D., Boss O., Peroni O.D., Kim J.K., Martino J.L., Zabolotny J.M., Moghal N., Lubkin M., Kim Y.B., Sharpe A.H., Stricker-Krongrad A., Shulman G.I., Neel B.G., Kahn B.B. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol. Cell Biol. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Rodriguez A., Mas-Gutierrez J.A., Mirasierra M., Fernandez-Perez A., Lee Y.J., Ko H.J., Kim J.K., Romanos E., Carrascosa J.M., Ros M., Vallejo M., Rondinone C.M., Valverde A.M. Essential role of protein tyrosine phosphatase 1B in obesity-induced inflammation and peripheral insulin resistance during aging. Aging Cell. 2012;11:284–296. doi: 10.1111/j.1474-9726.2011.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Rodriguez A., Escribano O., Alba J., Rondinone C.M., Benito M., Valverde A.M. Levels of protein tyrosine phosphatase 1B determine susceptibility to apoptosis in serum-deprived hepatocytes. J. Cell Physiol. 2007;212:76–88. doi: 10.1002/jcp.21004. [DOI] [PubMed] [Google Scholar]
- 14.Adachi M., Sekiya M., Arimura Y., Takekawa M., Itoh F., Hinoda Y., Imai K., Yachi A. Protein-tyrosine phosphatase expression in pre-B cell NALM-6. Cancer Res. 1992;52:737–740. [PubMed] [Google Scholar]
- 15.Yi T., Cleveland J.L., Ihle J.N. Identification of novel protein tyrosine phosphatases of hematopoietic cells by polymerase chain reaction amplification. Blood. 1991;78:2222–2228. [PubMed] [Google Scholar]
- 16.Myers M.P., Andersen J.N., Cheng A., Tremblay M.L., Horvath C.M., Parisien J.P., Salmeen A., Barford D., Tonks N.K. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J. Biol. Chem. 2001;276:47771–47774. doi: 10.1074/jbc.C100583200. [DOI] [PubMed] [Google Scholar]
- 17.Aoki N., Matsuda T. A cytosolic protein-tyrosine phosphatase PTP1B specifically dephosphorylates and deactivates prolactin-activated STAT5a and STAT5b. J. Biol. Chem. 2000;275:39718–39726. doi: 10.1074/jbc.M005615200. [DOI] [PubMed] [Google Scholar]
- 18.Le Sommer S., Morrice N., Pesaresi M., Thompson D., Vickers M.A., Murray G.I., Mody N., Neel B.G., Bence K.K., Wilson H.M., Delibegovic M. Deficiency in protein tyrosine phosphatase PTP1B shortens lifespan and leads to development of acute leukemia. Cancer Res. 2018;78:75–87. doi: 10.1158/0008-5472.CAN-17-0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu X., Malumbres R., Shields B., Jiang X., Sarosiek K.A., Natkunam Y., Tiganis T., Lossos I.S. PTP1B is a negative regulator of interleukin 4-induced STAT6 signaling. Blood. 2008;112:4098–4108. doi: 10.1182/blood-2008-03-148726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Traves P.G., Pardo V., Pimentel-Santillana M., Gonzalez-Rodriguez A., Mojena M., Rico D., Montenegro Y., Cales C., Martin-Sanz P., Valverde A.M., Bosca L. Pivotal role of protein tyrosine phosphatase 1B (PTP1B) in the macrophage response to pro-inflammatory and anti-inflammatory challenge. Cell Death Dis. 2014;5:e1125. doi: 10.1038/cddis.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinonen K.M., Dube N., Bourdeau A., Lapp W.S., Tremblay M.L. Protein tyrosine phosphatase 1B negatively regulates macrophage development through CSF-1 signaling. Proc. Natl. Acad. Sci. USA. 2006;103:2776–2781. doi: 10.1073/pnas.0508563103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson D., Morrice N., Grant L., Le Sommer S., Lees E.K., Mody N., Wilson H.M., Delibegovic M. Pharmacological inhibition of protein tyrosine phosphatase 1B protects against atherosclerotic plaque formation in the LDLR(-/-) mouse model of atherosclerosis. Clin. Sci. 2017;131:2489–2501. doi: 10.1042/CS20171066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson D., Morrice N., Grant L., Le Sommer S., Ziegler K., Whitfield P., Mody N., Wilson H.M., Delibegovic M. Myeloid protein tyrosine phosphatase 1B (PTP1B) deficiency protects against atherosclerotic plaque formation in the ApoE(-/-) mouse model of atherosclerosis with alterations in IL10/AMPKalpha pathway. Mol. Metab. 2017;6:845–853. doi: 10.1016/j.molmet.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeon Y.M., Lee S., Kim S., Kwon Y., Kim K., Chung C.G., Lee S., Lee S.B., Kim H.J. Neuroprotective effects of protein tyrosine phosphatase 1B inhibition against ER stress-induced toxicity. Mol. Cells. 2017;40:280–290. doi: 10.14348/molcells.2017.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bettaieb A., Koike S., Chahed S., Bachaalany S., Griffey S., Sastre J., Haj F.G. Pancreatic protein tyrosine phosphatase 1B deficiency exacerbates acute pancreatitis in mice. Am. J. Pathol. 2016;186:2043–2054. doi: 10.1016/j.ajpath.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X., Chen Q., Hu X.G., Zhang X.C., Fu T.W., Liu Q., Liang Y., Zhao X.L., Zhang X., Ping Y.F., Bian X.W. PTP1B promotes aggressiveness of breast cancer cells by regulating PTEN but not EMT. Tumour Biol. 2016;37:13479–13487. doi: 10.1007/s13277-016-5245-1. [DOI] [PubMed] [Google Scholar]
- 27.Mei W., Wang K., Huang J., Zheng X. Cell transformation by PTP1B truncated mutants found in human colon and thyroid tumors. PLoS One. 2016;11:e0166538. doi: 10.1371/journal.pone.0166538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zahn M., Marienfeld R., Melzner I., Heinrich J., Renner B., Wegener S., Miessner A., Barth T.F., Dorsch K., Bruderlein S., Moller P. A novel PTPN1 splice variant upregulates JAK/STAT activity in classical Hodgkin lymphoma cells. Blood. 2017;129:1480–1490. doi: 10.1182/blood-2016-06-720516. [DOI] [PubMed] [Google Scholar]
- 29.Yata K., Matchett G.A., Tsubokawa T., Tang J., Kanamaru K., Zhang J.H. Granulocyte-colony stimulating factor inhibits apoptotic neuron loss after neonatal hypoxia-ischemia in rats. Brain Res. 2007;1145:227–238. doi: 10.1016/j.brainres.2007.01.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez-Prados J.C., Traves P.G., Cuenca J., Rico D., Aragones J., Martin-Sanz P., Cascante M., Bosca L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J. Immunol. 2010;185:605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- 31.Smyth G.K. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Springer; New York: 2005. Limma: linear models for microarray data. [Google Scholar]
- 32.Mootha V.K., Lindgren C.M., Eriksson K.F., Subramanian A., Sihag S., Lehar J., Puigserver P., Carlsson E., Ridderstrale M., Laurila E., Houstis N., Daly M.J., Patterson N., Mesirov J.P., Golub T.R., Tamayo P., Spiegelman B., Lander E.S., Hirschhorn J.N., Altshuler D., Groop L.C. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 33.Berdnikovs S., Pavlov V.I., Abdala-Valencia H., McCary C.A., Klumpp D.J., Tremblay M.L., Cook-Mills J.M. PTP1B deficiency exacerbates inflammation and accelerates leukocyte trafficking in vivo. J. Immunol. 2012;188:874–884. doi: 10.4049/jimmunol.1004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carbone C.J., Zheng H., Bhattacharya S., Lewis J.R., Reiter A.M., Henthorn P., Zhang Z.Y., Baker D.P., Ukkiramapandian R., Bence K.K., Fuchs S.Y. Protein tyrosine phosphatase 1B is a key regulator of IFNAR1 endocytosis and a target for antiviral therapies. Proc. Natl. Acad. Sci. USA. 2012;109:19226–19231. doi: 10.1073/pnas.1211491109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu H., An H., Hou J., Han C., Wang P., Yu Y., Cao X. Phosphatase PTP1B negatively regulates MyD88- and TRIF-dependent proinflammatory cytokine and type I interferon production in TLR-triggered macrophages. Mol. Immunol. 2008;45:3545–3552. doi: 10.1016/j.molimm.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Heinonen K.M., Bourdeau A., Doody K.M., Tremblay M.L. Protein tyrosine phosphatases PTP-1B and TC-PTP play nonredundant roles in macrophage development and IFN-gamma signaling. Proc. Natl. Acad. Sci. USA. 2009;106:9368–9372. doi: 10.1073/pnas.0812109106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhee I., Veillette A. Protein tyrosine phosphatases in lymphocyte activation and autoimmunity. Nat. Immunol. 2012;13:439–447. doi: 10.1038/ni.2246. [DOI] [PubMed] [Google Scholar]
- 38.Gogiraju R., Schroeter M.R., Bochenek M.L., Hubert A., Munzel T., Hasenfuss G., Schafer K. Endothelial deletion of protein tyrosine phosphatase-1B protects against pressure overload-induced heart failure in mice. Cardiovasc Res. 2016;111:204–216. doi: 10.1093/cvr/cvw101. [DOI] [PubMed] [Google Scholar]
- 39.Hilmarsdottir B., Briem E., Halldorsson S., Kricker J., Ingthorsson S., Gustafsdottir S., Maelandsmo G.M., Magnusson M.K., Gudjonsson T. Inhibition of PTP1B disrupts cell-cell adhesion and induces anoikis in breast epithelial cells. Cell Death Dis. 2017;8:e2769. doi: 10.1038/cddis.2017.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krishnan N., Koveal D., Miller D.H., Xue B., Akshinthala S.D., Kragelj J., Jensen M.R., Gauss C.M., Page R., Blackledge M., Muthuswamy S.K., Peti W., Tonks N.K. Targeting the disordered C terminus of PTP1B with an allosteric inhibitor. Nat. Chem. Biol. 2014;10:558–566. doi: 10.1038/nchembio.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maheswari N., Karthikeyan C., Trivedi P., Moorthy N.S. Recent advances in protein tyrosine phosphatase 1B targeted drug discovery for type II diabetes and obesity. Curr. Drug Targets. 2017 doi: 10.2174/1389450118666170222143739. [DOI] [PubMed] [Google Scholar]
- 42.Brown J.M., Recht L., Strober S. The promise of targeting macrophages in cancer therapy. Clin. Cancer Res. 2017;23:3241–3250. doi: 10.1158/1078-0432.CCR-16-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakamaki A., Katsuragi Y., Otsuka K., Tomita M., Obata M., Iwasaki T., Abe M., Sato T., Ochiai M., Sakuraba Y., Aoyagi Y., Gondo Y., Sakimura K., Nakagama H., Mishima Y., Kominami R. Bcl11b SWI/SNF-complex subunit modulates intestinal adenoma and regeneration after γ-irradiation through Wnt/β-catenin pathway. Carcinogenesis. 2015;36:622–631. doi: 10.1093/carcin/bgv044. [DOI] [PubMed] [Google Scholar]
- 44.Takebe N., Miele L., Harris P.J., Jeong W., Bando H., Kahn M., Yang S.X., Ivy S.P. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat. Rev. Clin. Oncol. 2015;12:445–464. doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon S., Martinez F.O. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Jager M., Hubert A., Gogiraju R., Bochenek M.L., Munzel T., Schafer K. Inducible knockdown of endothelial protein tyrosine phosphatase-1B promotes neointima formation in obese mice by enhancing endothelial senescence. Antioxid. Redox Signal. 2018 doi: 10.1089/ars.2017.7169. [DOI] [PubMed] [Google Scholar]
- 47.Phesse T.J., Myant K.B., Cole A.M., Ridgway R.A., Pearson H., Muncan V., van den Brink G.R., Vousden K.H., Sears R., Vassilev L.T., Clarke A.R., Sansom O.J. Endogenous c-Myc is essential for p53-induced apoptosis in response to DNA damage in vivo. Cell Death Differ. 2014;21:956–966. doi: 10.1038/cdd.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rupnow B.A., Alarcon R.M., Giaccia A.J., Knox S.J. p53 mediates apoptosis induced by c-Myc activation in hypoxic or gamma irradiated fibroblasts. Cell Death Differ. 1998;5:141–147. doi: 10.1038/sj.cdd.4400328. [DOI] [PubMed] [Google Scholar]
- 49.Ho J.S., Ma W., Mao D.Y., Benchimol S. p53-Dependent transcriptional repression of c-myc is required for G1 cell cycle arrest. Mol. Cell Biol. 2005;25:7423–7431. doi: 10.1128/MCB.25.17.7423-7431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown J.M. Vasculogenesis: a crucial player in the resistance of solid tumours to radiotherapy. Br. J. Radiol. 2014;87:20130686. doi: 10.1259/bjr.20130686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meng E., Hanna A., Samant R.S., Shevde L.A. The impact of hedgehog signaling pathway on DNA repair mechanisms in human cancer. Cancers. 2015;7:1333–1348. doi: 10.3390/cancers7030839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meng X., Cai J., Liu J., Han B., Gao F., Gao W., Zhang Y., Zhang J., Zhao Z., Jiang C. Curcumin increases efficiency of gamma-irradiation in gliomas by inhibiting Hedgehog signaling pathway. Cell Cycle. 2017;16:1181–1192. doi: 10.1080/15384101.2017.1320000. [DOI] [PMC free article] [PubMed] [Google Scholar]