Abstract
Background
Disturbances in sleep and circadian rhythms are common among residents of long-term care facilities. In this systematic review, we aim to identify and evaluate the literature documenting the outcomes associated with non-pharmacological interventions to improve nighttime sleep among long-term care residents.
Methods
The Preferred Reporting Items for Systematic Reviews guided searches of five databases (MEDLINE, Embase, CINAHL, Scopus, and Cochrane Library) for articles reporting results of experimental or quasi-experimental studies conducted in long-term care settings (nursing homes, assisted-living facilities, or group homes) in which nighttime sleep was subjectively or objectively measured as a primary outcome. We categorized each intervention by its intended use and how it was administered.
Results
Of the 54 included studies evaluating the effects of 25 different non-pharmacological interventions, more than half employed a randomized controlled trial design (n = 30); the others used a pre-post design with (n = 11) or without (n = 13) a comparison group. The majority of randomized controlled trials were at low risk for most types of bias, and most other studies met the standard quality criteria. The interventions were categorized as environmental interventions (n = 14), complementary health practices (n = 12), social/physical stimulation (n = 11), clinical care practices (n = 3), or mind-body practices (n = 3). Although there was no clear pattern of positive findings, three interventions had the most promising results: increased daytime light exposure, nighttime use of melatonin, and acupressure.
Conclusions
Non-pharmacological interventions have the potential to improve sleep for residents of long-term care facilities. Further research is needed to better standardize such interventions and provide clear implementation guidelines using cost-effective practices.
Electronic supplementary material
The online version of this article (10.1186/s12877-018-0794-3) contains supplementary material, which is available to authorized users.
Keywords: Sleep, Circadian rhythms, Non-pharmacological intervention, Nursing homes
Background
Many long-term care residents have sleep and circadian rhythm disturbances [1, 2] due to advanced age, the effects of certain chronic illnesses and medications, declining brain health, diminished mobility, and other causes [3, 4]. Therefore, the American Geriatrics Society and the National Institute on Aging now recognize a geriatric syndrome in which physical and mental risk factors overlap to increase risk for sleep and circadian disturbances. The relationship between some risk factors and sleep disturbance is often bidirectional [3]. Numerous negative consequences are associated with sleep disturbances, including increases in cognitive decline, metabolic disease, high blood pressure, cardiovascular disease mortality, frailty, impaired quality of life, and hypersensitivity to pain [3].
Long-term care residents have a high prevalence of multimorbidity that includes both chronic physical (e.g., advanced cardiovascular or pulmonary disease, arthritis) and mental (e.g., dementia, depression) illnesses that are associated with sleep and circadian rhythm disturbances [4, 5]. More than a quarter of nursing-home residents and approximately 70% of assisted-living facility residents have been diagnosed with dementia [5, 6], and almost half of those will have sleep disturbance [7, 8]. Sleep disturbance in dementia patients is associated with anxiety and behavioral symptoms of agitation, aggressiveness, and disinhibition [7]. Sleep disturbances and accompanying symptoms often lead providers to prescribe psychoactive medications, including hypnotics. Sedative-hypnotic pharmaceuticals are commonly used for assisted-living facility residents with dementia [9]. Similarly, about 47% of nursing-home residents with dementia are prescribed sedative-hypnotics, especially when displaying anxiety and agitation [10].
However, both benzodiazepines and non-benzodiazepine receptor agonist hypnotics have been associated with an increased risk of fall and fractures in older adults [11–13]. These challenges reinforce the need to consider non-pharmacological approaches in the unique physical environment and institutional milieu of long-term care facilities [14]. The potential for sleep or circadian rhythm disturbance is linked to an increase in vulnerability to environmental challenges in these facilities [1]. For some residents, a non-stimulating environment may lead to excessive daytime and early evening napping that would exacerbate sleep disturbances. Others may experience an over-stimulating nighttime environment due to light and noise or exposure to disruptive behaviors, including pain, discomfort, repetitive vocalizations, and wandering. Staff routines such as nighttime incontinence care are also disruptive to sleep maintenance [15].
The problems associated with existing pharmacological treatments, coupled with institutional environments that can further disrupt sleep, mean that non-pharmacological interventions should be considered to help prevent or manage sleep disturbance [16]. Consistent with recommendations of the American Geriatrics Society and the National Institute on Aging [3], the objective of this systematic review is to identify and evaluate the literature documenting the outcomes associated with non-pharmacological interventions to improve nighttime sleep among long-term care residents.
Methods
Our original intent for this systematic review was to evaluate the literature addressing non-pharmacological interventions to promote sleep among adults across all institutional settings. As described below, our search was adjusted to focus on long-term care settings due to important differences between acute-care and residential settings, such as high medical acuity and short length of stay.
Search methodology
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement was used to guide this review [17]. A library specialist (KP) performed systematic searches of MEDLINE (Ovid), Embase (Ovid), CINAHL (EBSCOhost), Scopus (Elsevier), and the Cochrane Library (Wiley) between August and October of 2016, with weekly search updates for all five databases through December 2016. Major search terms for all databases were represented by both controlled vocabulary and keywords (Table 1) on the topic of sleep quality in institutional healthcare settings. Where appropriate, searches were restricted to human, adult, English-language studies but were not otherwise limited by study design or date of publication. Specific inclusion and exclusion criteria are listed in Table 2 for the original systematic review. Studies were then further limited to those located in nursing homes, assisted living facilities, and other long-term care facilities. The complete search strategies are available in the supplemental materials (Additional file 1: Table S1- Search Strategies-BMC).
Table 1.
Search terms
| Field | Key words | |
|---|---|---|
| Title | (sleep$ adj2 (disrupt$ or disturb$ or impair$ or interrupt$ or depriv$ or lack or poor or problem$)) | |
| OR | Title | (sleep$ adj2 (quality or quantity or duration or time$ or timing or pattern$ or rhythm$ or promotion or hygiene or efficiency or cycle$ or onset or health$ or hour$ or phase$ or support or help or initiat$)) |
| OR | Title | insomnia$ |
| OR | Title | circadian |
| OR | Title | (sleep$ adj5 “biological clock$”) |
| AND | All Text | (hospital$ or inpatient$ or institutional$ or “intensive care” or ward$ or hospice$ or “nursing home$” or “assisted living” or palliative or “end of life” or “end-of-life” or terminal or “health facilit$” or “residential facilit$” or icu or “critical care”) |
Table 2.
Inclusion and exclusion criteria
| Inclusion | Exclusion |
|---|---|
| English-language articles | Articles with only partial content in English |
| Intervention studies published in peer-reviewed journals | Dissertations, presentation/poster abstracts, brief reports in letters to the editors, editorials/commentaries, literature reviews, or meta-analyses |
| Non-pharmacological interventions, including food supplements and melatonin, conducted within or outside the United States | Pharmacological interventions, models of care (e.g., palliative care team), or respiratory interventions |
| Experimental or quasi-experimental design | Single case studies or cross-sectional design |
| Adult participants, including those with dementia | Participants being treated for a medical sleep disorder or those with a psychiatric disorder such as depression, schizophrenia, or addiction disorder |
| Any setting, including hospitals, long-term care settings (nursing homes, assisted-living facilities, or group homes), in-patient hospice or other institutional settings, or simulated hospital environments | Community settings including those involving interventions from home caregivers, outpatient clinics, dialysis centers, or adult foster homes, as well as psychiatric in-patient facilities |
| Nighttime sleep as a subjective (self-reported) or objective outcome (primary or secondary) | Sleep not measured with tool |
Study selection, data extraction, and analysis
After the removal of duplicates, the final set of 6747 articles was transferred to Covidence software for synthesis of the literature data. Two review authors (AB and NJ) independently assessed the eligibility of the retrieved articles by title and abstract. A third author (EC, RZ or AK) resolved all conflicts, with a total of 6302 articles excluded. The library specialist downloaded full-text versions of the remaining 445 articles. Teams of two review authors (AB, NJ, RZ or EC) then independently assessed the eligibility of the full-text articles. Any conflicts at this stage were resolved by another author (AK). Applying the exclusion criteria to this body of literature, another 311 articles were removed. The major reasons for exclusion, in order of frequency, were literature review, presentation abstract, wrong patient population, editorial, wrong outcomes (not sleep), and wrong setting.
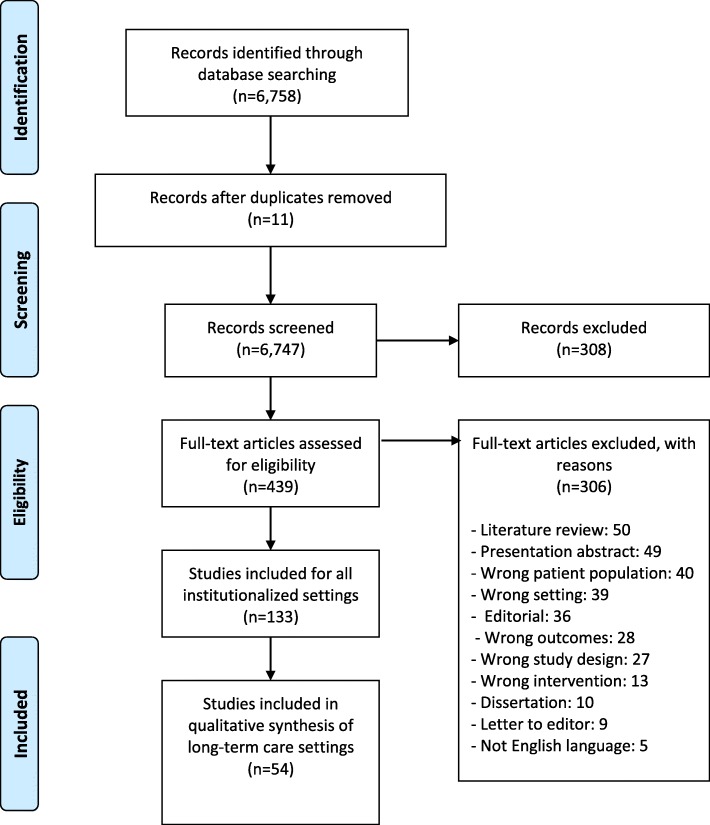
The resulting set of 134 articles was split between hospital-based studies (n = 79) and the final set of 54 articles focusing on long-term care settings that we used in this analysis. We used the details from the selection process in Covidence to complete a PRISMA flow diagram (Fig. 1) [17] and exported all titles and abstracts to EndNote software. Two review authors (AB and NJ) independently extracted study characteristics in Microsoft Excel and then summarized the characteristics of the included studies. A second review author (RZ or EC) checked the accuracy of the table against the original articles.
Fig. 1.
PRISMA flow diagram
We revised the intervention coding we used for an integrative review conducted by this research team that focused on non-pharmacological intervention for sleep in patients with advanced serious illness for this reviews’ population of long term care residents [18]. We used an iterative approach to code interventions. First, we examined the intent of the intervention, such as what risk factor for sleep disturbance was targeted for reduction or elimination (e.g., daytime physical exercise to address excessive daytime napping). The categories that emerged from the data were mostly interventions manipulated by staff or study personnel: environmental factors (external to the resident), complementary health practices (touch and oral supplements), social and physical stimulation (activities for exercise or engaging the resident cognitively), and clinical care practices (reducing sleep disruptions). The final category, mind-body practices, are those in which residents actively participate and include activities such as self-relaxation and meditation.
After evaluation of the interventions and intended outcomes, we then determined their overall effect on actual outcomes related to sleep disturbance. We categorized interventions as having a positive effect, a mixed effect (some positive and some inconclusive outcomes), no effect, or a negative effect. Our overall summary of the findings was based on nighttime sleep outcomes, although some studies also reported daytime sleep results. The risk of bias in the included studies was evaluated using the Cochrane Risk of Bias methodology for randomized clinical trials [19] and the Summary Quantitative Studies and Critical Appraisal Checklist for all other studies [20].
Results
Study characteristics are summarized in Tables 3 and 4 (multicomponent). Of the 54 studies, more than half employed a randomized controlled trial (RCT) design (n = 30); the others used a pre-post design with (n = 11) or without (n = 13) a comparison group.
Table 3.
Characteristics of Included Studies (except multi-component interventions; n = 43)
| First author, Year | Design, Number of groups, and Study type | Setting (number of facilities), Number of participants, Mean age, % male, and Inclusion/exclusion criteria | Description of intervention | Effect (positive, mixed, none, or negative), Measurement of sleep, Main finding(s) |
|---|---|---|---|---|
| Clinical care practices (n = 3) | ||||
| Kim, 2016 [73] | Quasi-experimental pre-post intervention with 3 groups (intervention, comparison with placebo of 36.5 ° C water and control) | Nursing home (n = 1), N = 30, mean age 85.9, 20% men | Adjust core body temperature: 30 min of warm (40 ° C) foot baths in the evening daily for 4 weeks | None; Actigraphy; No significant differences in total sleep amount, efficiency, or latency among the 3 groups |
| O’Rourke, 2001 [25] | Quasi-experimental pre-post intervention without comparison | Assisted-living facility (n = 2), N = 18, mean age 84.5, 22% men, with incontinence | Minimize clinical disruptions: 15 consecutive days alternating every 5 days between usual nighttime rounds and non-disruptive nighttime care | Positive; 24-h monitoring at 30-min intervals; Significant improvement in total nighttime sleep by 30 min (p = 0.01) |
| Matthews, 1996 [41] | Quasi-experimental pre-post intervention without comparison group | Nursing home (n = 1), N = 33, mean age 84.2, 36.3% men, with dementia | Individualize care: Four 4-week phases of changes in staff behavior from task-oriented to individualized (client-centered) care | None; Sleep subscale of Dementia Mood Assessment Scale; Nighttime sleep did not change significantly |
| Mind-body practices (n = 3) | ||||
| Chen, 2010 [22] | Quasi-experimental pre-post intervention with comparison | Assisted-living facility (n = 2), N = 55, mean age 75.4, 47.3% men | Relaxation techniques: 70-min sessions of Silver Hatha Yoga (adapted for older population) 3 times per week for 6 months | Positive; Pittsburgh Sleep Quality Index; Overall sleep quality significantly improved, and sleep disturbances and daytime dysfunction decreased significantly (p = 0.05) |
| Örsal, 2014 [58] | Quasi-experimental pre-post intervention with comparison | Nursing home (n = 1), N = 64, mean age 75.8, 57.8% men | Relaxation techniques: Progressive muscle relaxation exercises each night between 9 pm and 12 MN (for a total of 30 min/week) each night × 7 days | Positive; Pittsburgh Sleep Quality Index; Quality of sleep improved significantly (p = 0.000) |
| El Kady, 2012 [50] | Quasi-experimental pre-post intervention without comparison | Nursing home (n = 4), N = 210, mean age 72.2, 46.2% men, with sleep problems | Cognitive-behavioral therapy: Four 30-min sessions of cognitive behavioral sleep therapy using sleep hygiene education and stimulus control techniques | Positive; Pittsburgh Sleep Quality Index; Statistically higher improvement in sleep quality (percentage of poor sleepers decreased from 63.3 to 46.2%) |
| Social and physical stimulation (n = 11) | ||||
| Kuck, 2014 [51] | RCT clustered by nursing home | Nursing home (n = 20), N = 85, mean age 83.9, 75.5% men, with sleep problems | Combination (social/physical): 2 days per week of both two 45-min sessions of social activity and two sessions of physical exercise (balance & muscle strengthening) for 8 weeks | Mixed Actigraphy & Insomnia Severity Index; No improvement by actigraphy measures in the intervention group but subjective sleep quality increased post intervention (p = 0.04) |
| Lorenz, 2012 [55] | RCT, 4 groups (3 intervention and 1 control) | Nursing home (n = 13), N = 193, mean age 81.4, 36% men | Combination (social/physical): 3 intervention groups of exercise (3 days physical resistance training and 2 days walking per week), individualized social activity (1 h per day 5 days per week), or both for 7 weeks | None; Polysomnography; No relationship between change in everyday function (from interventions) and change in sleep parameters |
| Richards, 2011 [26] | RCT, 4 groups (3 intervention and 1 control) | Nursing home (n = 10) and assisted-living centers (3), N = 165, mean age 81.8, 39.9% men | Combination (social/physical): 3 intervention groups of exercise (3 days physical resistance training and 2 days walking per week), individualized social activity (1 h per day 5 days per week), or both for 7 weeks | Positive; Polysomnography; Group receiving both treatments showed a significantly greater increase in total nocturnal sleep time and sleep efficiency over the control condition, but the exercise and social activity alone groups did not |
| Richards, 2001 [42] | Quasi-experimental pre-post intervention without comparison | Nursing home (n = 1), N = 5, mean age 76.2, 100% men, with dementia | Social and cognitive activity: 15–30 min of individualized activity for 1 to 2 h per day for 3 days | Positive; Actigraphy; Percent of nocturnal time asleep significantly increased (p < 0.01) |
| Richards, 2005 [43] | RCT | Nursing home (n = 7), N = 139, mean age 79, 51.8% men, with dementia | Social and cognitive activity: 1–2 h of individualized social activity for 21 days | Mixed; Actigraphy; Significantly reduced minutes to sleep onset, significantly reduced minutes awake, and increased sleep among those with baseline poor sleep but not for total group; sleep efficiency not improved |
| Thodberg, 2015 [46] | Quasi-experimental pre-post intervention (dog visit) with comparison (robot seal or toy cat) | Nursing home (n = 4), N = 100, mean age 85.5, with dementia | Social and cognitive activity: 10-min biweekly visits by an “animal” (dog, robot seal, or toy cat) for 6 weeks | Mixed; Actigraphy; Sleep duration increased in the third week for the dog group compared to the robot seal or toy cat (p = 0.01); no effects were found in the sixth week or after the visit period had ended |
| Alessi, 1995 [33] | Quasi-experimental pre-post intervention with comparison | Nursing home (n = 7), N = 65, mean age 84.8, 85% men, with urinary incontinence or physically restrained | Physical exercises: Exercises (transfers, walking, and rowing) performed every 2 h (8 am-4 pm) 5 days a week for 9 weeks | None; Actigraphy; No significant improvement in nighttime or daytime sleep |
| Chen, 2015 [57] | RCT (clustered by nursing home) | Nursing home (n = 10), N = 127, mean age 79.2, 50.9% men, wheelchair bound | Physical exercises: 40-min elastic-band exercises (in wheelchair) 3 times per week for 6 months | Positive; Pittsburgh Sleep Quality Index; Intervention group had significantly longer sleep duration at 3 and 6 months and overall better sleep quality at 6 months |
| Eggermont, 2010 [67] | RCT | Nursing home (n = 19), N = 79, mean age 84.3, 20.3% men, with dementia | Physical exercises: Five 30-min walking sessions per week for 6 weeks (total of 30 sessions) | None; Actigraphy; No significant improvement in nighttime restlessness, sleep efficiency, number of waking bouts, or daytime activity |
| Taboonpong, 2010 [32] | Quasi-experimental pre-post intervention with comparison | Elderly residential center (n = 2), N = 50, 58% men | Physical exercises: Tai Chi exercise at least 3 times a week for 22 min for 12 weeks | Positive; Pittsburgh Sleep Quality Index; Significant improvement in sleep quality (p < 0.01) |
| Lee, 2008 [23] | Quasi-experimental pre-post intervention without comparison | Assisted-living facility (n = 1), N = 23, with dementia | Physical activity: Indoor gardening twice daily for 4 weeks | Positive; 24-h sleep diary; Significant improvement in wake after sleep onset, nocturnal sleep time, and sleep efficiency |
| Complementary health practices (n = 12) | ||||
| Soden, 2004 [29] | RCT (3 groups: aromatherapy and massage, massage, or control) | Assisted-living facility (n = 3), N = 42, 24% men | Combination (touch and aromatherapy): Massage with lavender oil and/or massage with inert oil, each for 30 min for four weeks | Mixed; Verran and Snyder-Halpern Sleep Scale; Statistically significant improvement in the massage (p = 0.02) and combined massage (p = 0.03) groups but not for the aromatherapy and massage group only |
| Gehrman, 2009 [68] | RCT | Nursing home (n = 1), N = 41, mean age 82.9, 31.7% men; with dementia | Oral supplement: Melatonin (8.5 mg immediate release and 1.5 mg sustained release) administered at 10 pm for 10 consecutive nights | None; Actigraphy; No significant differences between the groups for nighttime or daytime sleep |
| Rondanelli, 2011 [28] | RCT | Assisted-living facility (n = 1), N = 43, mean age 78.3, with insomnia | Oral supplement: Melatonin (5 mg) plus dietary supplement (magnesium 225 mg and zinc 11.25 mg) every day for 8 weeks | Positive; Pittsburgh Sleep Quality Index; Significantly better sleep (p < 0.001) |
| Valtonen, 2005 [47] | Quasi-experimental pre-post intervention with comparison, crossover design (3 groups: 2 intervention and 1 control) | Nursing home (n = 2), N = 81, mean age 82.8, 21% men, mild cognitive impairment | Oral supplement: 8 weeks of melatonin-rich (5–20 mg/day) milk then 8 weeks of normal milk (and conversely for the other intervention group) | Mixed; Sleep questionnaire; In one intervention group, sleep quality, morning activity, and evening activity all increased significantly (p < 0.001) when milk was consumed in the evening |
| Braun, 1986 [62] | Quasi-experimental pre-post intervention without comparison | Nursing home (n = 1), N = 6, mean age 85, 0% men | Touch: 5 min of talking and 5 min of therapeutic touch 6 in. above the solar plexus | Positive; Visser’s Sleep Quality Assessment; Improved sleep quality |
| Chen, 1999 [21] | RCT | Nursing home (n = 1), N = 84, mean age 79, 61.9% men | Touch: 15 min of acupressure, consisting of 5 min of finger massage and 10 min of acupoint massage between 1 pm and 10 pm 5 days per week for 3 weeks | Positive; Pittsburgh Sleep Quality Index; Significantly more positive sleep including quality, latency, duration, efficiency; reduced disturbances of sleep; and frequencies of nocturnal awakening and night wakeful time. |
| Harris, 2012 [69] | RCT | Nursing home (n = 4), N = 40, mean age 86, 22.5% men, with dementia | Touch: 3-min slow-stroke back massage at bedtime for 2 nights | None; Actigraphy; No significant increase in minutes of nighttime sleep |
| Nelson, 2010 [74] | RCT | Nursing home (n = 4), N = 28, mean age 69.5, 57.1% men | Touch: 15-min massage to head, neck shoulders, and back between 8 pm and 10 pm every night and 7 days | None; Observed 3 participants asleep following the intervention |
| Reza, 2010 [59] | RCT (3 groups: intervention, sham, and control) | Nursing home (n = 1), N = 77, mean age 75.2, 53.2% men | Touch: 3 sessions of acupressure (hands, head, ears, and feet) per week for 4 weeks | Positive; Pittsburgh Sleep Quality Index; Compared to controls, the acupressure group had significantly positive subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep sufficiency, and reduced sleep disturbance. No differences between the sham and control groups. |
| Simoncini, 2015 [60] | Quasi-experimental pre-post intervention without comparison | Nursing home (n = 2), N = 129, mean age 82.7 | Touch: Daily acupressure of 8 h continuously on the HT7 acupoint with a patch device for 8 weeks | Positive. Pittsburgh Sleep Quality Index; Significant improvement in ability to fall asleep and quality of sleep |
| Sun, 2010 [30] | RCT | Assisted-living facility (n = 2), N = 50, mean age 70.48, 64% men, with insomnia | Touch: 5 s of acupressure on HT7 acupoint of both wrists followed by 1-s rest repeated for 5-min before bedtime for 5 weeks | Positive; Athens Insomnia Scale-Taiwan Form; Significant improvement in sleep at 6 weeks post-intervention (p = 0.002) |
| Van Someren, 1998 [48] | RCT | Nursing home (n = 1), N = 14, mean age 84, 7.1% men, with early dementia | Touch: TENS (transcutaneous electrical nerve stimulation) between the shoulder blades for 30 min per day between 4 pm and 6 pm, 5 days a week for 6 weeks | Positive; Actigraphy; Post-treatment mean was significantly higher in the treatment group than both the pretreatment mean (p = 0.03) and the follow up mean (p = 0.03) |
| Environmental intervention (n = 14) | ||||
| Ancuelle, 2015 [56] | Quasi-experimental pre-post intervention without comparison | Nursing home (n = 1), N = 38, mean age 78.4, 44.7% men, with musculoskeletal pain | Ergonomic adjustment: 4 weeks of medium-firm mattress use | None. Pittsburg Sleep Quality Index and subsample with actigraphy. Sleep not significantly improved (p = 0.245). |
| Akyar, 2013 [49] | Quasi-experimental pre-post intervention without comparison | Nursing home (n = 1): N = 24, mean age 80, 33.3% men, with poor sleep quality | Increased light: 30 min of morning bright light (10,000 lx) for 30 days | Positive; Pittsburg Sleep Quality Index; Immediately and 4 weeks post-intervention, there was significant improvement on all sleep outcomes (p < 0.001) |
| Ancoli-Israel, 2002 [36] | RCT, 3 intervention groups (morning v. evening light) v. sleep restriction or comparison | Nursing homes (n = 2), N = 77, mean age 85.7, 24.7% men with dementia | Increased light: Bright light box (2500 lx) from 5:30 pm to 7:30 pm or 9:30 am to 11 am or daytime sleep restriction comparison with dim (50 lx) red light from 5:30 pm-7:30 pm for 10 days | None; Actigraphy; No improvements in nighttime sleep or daytime alertness in any of the treatment groups. (Note: Daytime restriction is comparison group.) |
| Ancoli-Israel, 2003 [35] | RCT, 3 groups (intervention of daytime or evening bright light with comparison of dim red light) | Nursing home (n = 2), N = 92, mean age 82.3, 31.5% men, with late-stage Alzheimer’s disease | Increased light: Either morning or evening bright light box (2500 lx) compared with morning dim (< 300 lx) red light for 10 days | Mixed; Actigraphy; Duration of maximum sleep bout significantly increased from 64.9 min to 88.4 min in the morning and evening bright light group. However, there was no effect on total sleep time or on night or day wake time. |
| Burns, 2009 [37] | RCT | Nursing home (n = 2), N = 48, mean age 83.5, 33% male; with dementia & agitation | Increased light: 2-h (10 am to 12 pm) bright light therapy (10,000 lx) for 2 weeks | None; Actigraphy; Mean duration of nocturnal sleep improved but not significantly |
| Calkins, 2007 [38] | RCT | Nursing home (n = 3), N = 17, 11.8% men, with dementia | Increased light: Outdoor daylight exposure for 30 min for 2 weeks | None; Actigraphy and Pittsburgh Sleep Quality Index; No significant improvement in sleep |
| Castor, 1991 [63] | Quasi-experimental pre-post intervention without comparison | Nursing home (n = 1), N = 12, mean age 70, 100% men | Increased light: Twice daily exposure (1 h in morning and 1 h in afternoon) to sunlight for 1 week | Positive; Nursing Assessment of Sleep; Significant improvement in uninterrupted sleep (p = 0.003) and mean sleep hours per 24 h (p = 0.052), as well as a decrease in night wake hours (p = 0.007) |
| Dowling, 2005 [40] | RCT | Assisted-living Facility (n = 2), N = 46, mean age 84, 22% men, with severe dementia | Increased light: 1 h of bright morning light (9:30 am to 10:30 am; 2500 lx) 5 days per week for 10 weeks | None; Actigraphy; No significant improvement in nighttime sleep efficiency, sleep time, wake time, or number of awakenings |
| Fetveit, 2003 [61] | Quasi-experimental pre-post intervention without comparison | Nursing home (n = 1), N = 11, mean age 86.1, 9.1% men, with dementia | Increased light: 2 h of morning (8 am to 11 am; 6000–8000 lx) bright light per day for 2 weeks | Positive; Actigraphy; Waking time within nighttime sleep reduced by 2 h and sleep efficiency improved from 73 to 86% |
| Fetveit, 2004 [64] | Quasi-experimental pre-post intervention without comparison | Nursing home (n = 1), N = 11, mean age 86.1, 9.1% men, with dementia | Increased light: 2 h of bright morning light (6000–8000 lx) per day for 2 weeks | Mixed; Actigraphy; Sleep efficiency remained higher than baseline for 4 weeks and sleep onset latency remained significantly reduced for 12 weeks |
| Figueiro, 2014 [53] | Quasi-experimental pre-post intervention without comparison | Nursing home (n = 1), N = 14, mean age 86.9, 37.7% men, with dementia | Increased light: 4 weeks of blush-white lighting (luminaires) in residents’ rooms with timer (from waking until 6 pm) for 4 weeks | Mixed; Daysimeter; Significant improvement in sleep efficiency (80 to 84%, p = 0.03) and sleep time (431 min to 460 min, p = 0.03) but not in sleep latency |
| Koyama, 1999 [71] | Quasi-experimental pre-post intervention without comparison | Nursing home (n = 2), N = 6, with dementia | Increased light: 1 or 2 h of late morning bright light (4000 lx) | None; sleep observation diary; Nighttime sleep maintained in 3 participants |
| Lyketsos, 1999 [24] | RCT | Assisted-living facility (n = 1), N = 8, mean age 80.8, 6.7% men, with dementia and agitated behaviors | Increased light: 1 h of morning bright light (10,000 lx) therapy for 4 weeks | Positive; sleep observation log, 8 pm-8 am; Statistically significant improvement in sleep duration from 6.4 h to 8.1 h (p < 0.05) |
| Wu, 2015 [31] | Quasi-experimental pre-post intervention with comparison (clustered by unit) | Assisted Living Facility (n = 1) N = 65, mean age 80, 57.1% men | Increased light: 30 min of morning (9:30 am–10 am) bright light (10,000 lx) therapy 3 times per week for 4 weeks | Mixed; sleep diary; Significant decrease in sleep disruptions in the experimental group from week 1 to week 4 (p = 0.02) but no significant difference between treatment and control groups |
RCT Randomized controlled trial
Table 4.
Characteristics of multi-component studies (n = 11)
| Environment | CHP | SPS | CCP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First author, Year) | Design, Number of groups, and type | Setting (number of facilities), Number of participants, Mean age, % Male, Inclusion/exclusion criteria | Description of intervention | Effect (positive, mixed, none, or negative), Measurement of sleep, Main finding(s) | Light | Noise | Melatonin | Physical activity | Individual care | Less disruptions | Mange Sleep wake |
| Alessi, 1999 [34] | RCT | Nursing home (n = 1); N = 29, mean age 88.3, 10% male, with incontinence | Daytime physical activity and nighttime program to decrease noise and decrease sleep-disruptive nursing care practices 5 days per week for 14 weeks | Positive; Actigraphy; Nighttime sleep increased from 51.7 to 62.5% (p = 0.045) | X | X | X | ||||
| Alessi, 2005 [65] | RCT | Nursing home (n = 4): N = 118, mean age 86.8, 45% men | Efforts to decrease daytime in-bed time, provide daily sunlight exposure for at least 30 min, increase physical activity, structure bedtime routines, and decrease nighttime noise and light for 5 consecutive days and nights | Mixed; Actigraphy; Modest decrease in nighttime awakenings (p = 0.04) but no effect on % of nighttime sleep or number of nighttime awakenings | X | X | X | X | X | ||
| Connel, 2007 [39] | RCT | Nursing home (n = 1), N = 20, mean age 79.7, 95% male, with dementia | 1 h of group outdoor structured activity for 10 days (compared with indoor structured activity) | Positive; Actigraphy; Maximum sleep duration increased and total sleep minutes significantly increased | X | X | |||||
| Dowling, 2008 [66] | RCT | Nursing home (n = 2), N = 50, mean age 86, 14% men, with dementia | Melatonin 5 mg & 1 h of morning bright light (≥ 2500 lx) for 5 mid-week days per week for 10 weeks | None; Actigraphy; No significant impact on sleep | X | X | |||||
| Gammack, 2009 [72] | RCT | Nursing homes (n = 1), N = 24, mean age 79.5, 37.5% men, without dementia | 60-min outdoor morning (between 7 am-12 pm) light and structured recreational activity for 21 days | None; Medical Outcome Study Sleep Scale; No differences in sleep scores between treatment and controls | X | X | |||||
| Ito, 2001 [70] | Quasi-experimental pre-post intervention with comparison (bright light only) | Nursing home, N = 28, mean age 78.3, 42.9% men, with Alzheimer’s disease | Daily Vitamin B12 (1.5 mg for 2 weeks then 3.0 mg for 2 weeks) and 2-h morning (9 am–11 am) bright light (3000 lx) therapy for 4 weeks | None; Actigraphy; No significant improvement in nighttime sleep outcomes | X | X | |||||
| Martin, 2007 [52] | RCT | Nursing home (n = 4) N = 118, mean age 87.05, 78% men, with sleep disruption | Exposure to outdoor bright light (at least 20,000 lx), efforts to keep residents out of bed during the day, bedtime routine, efforts to decrease nighttime noise and light, and structured physical activity for 10 min to 15 min 3 times per day for 5 days | None; Actigraphy; Significant change only in the active phase of the rest/activity rhythms. Not able to significantly reduce nighttime noise and light. | X | X | X | ||||
| Ouslander, 2006 [54] | RCT, clustered by facility | Nursing home (n = 8), N = 160, mean age 83.2, 25% men | Daytime activities, keep residents out of bed, evening bright light, consistent bedtime routine, nighttime care routines to minimize disruption, and strategies to reduce nighttime noise for 17 days | None; Actigraphy and polysomnography; No improvement in nighttime sleep | X | X | X | X | X | ||
| Riemersma-Van Der Lek, 2008 [27] | RCT, clustered by facility with 4 groups (bright light, melatonin, combination, or none) | Assisted-living facilities (n = 12), N = 189, mean age 85.8, 10% men, with dementia | Whole-day bright light (10,000 lx) and 2.5 mg melatonin for a mean of 15 months | Positive; Actigraphy; Combined treatment (light and melatonin) significantly ameliorated nocturnal restlessness, reduced awakenings, and increased sleep efficiency. Melatonin shortened sleep onset latency and increased sleep duration. | X | X | |||||
| Schnelle, 1998 [44] | Quasi-experimental pre-post intervention with comparison | Nursing home (n = 4), N = 92, mean age 87.3, 19% men, with incontinence | Individualized nighttime incontinence care (every 2 or 4 h and when awake) and minimized sleep disruption for 5 nights | Positive; Actigraphy; Significant reduction in awakenings due to light and sound (p < 0.001) | X | X | X | X | |||
| Schnelle, 1999 [45] | RCT | Nursing home (n = 8), N = 267, mean age 83.95, 82% men, with incontinence | Individualized nighttime incontinence care (every 2 or 4 h and when awake) and noise abatement and staff feedback to reduce noise for 5 nights | Mixed; Actigraphy; Significant reduction in sleep awakenings with noise and light abatement but not in % sleep or sleep duration. Significant reductions in light events but not noise. | X | X | X | X | |||
CHP Complementary Health Practices, SPS Social/Physical Stimulation, CCP Clinical Care Practices, RCT Randomized controlled trial
Sites and participants
The 54 studies included 3627 participants residing in nursing homes (n = 42), assisted-living facilities (n = 11) [21–31], and one elderly residential setting [32]. The facilities were located mostly in the United States (n = 25), Europe (n = 14) or Asia (n = 10). Most studies investigated one (n = 23) or two (n = 11) long-term care facilities, with a range from 1 to 20. The mean sample size was 66.5 patients (standard deviation [SD] = 58.6), with a range from 5 to 267 participants. The mean age was 81.9 years (SD = 4.4), and the study populations were 41.1% female on average. More than half (52%) of the studies included participants with dementia [23, 24, 27, 33–48], and six studies targeted patients with known sleep problems [28, 30, 49–52].
Measures
More than half (n = 28) of the included studies objectively measured sleep with wrist actigraphy or a daysimeter (measures both light and activity) [53], and one study supplemented these findings with polysomnography [54]. Two studies used polysomnography exclusively [26, 55]. The remainder used self-reporting or reports completed by research or clinical staff, most frequently with the valid and reliable Pittsburgh Sleep Quality Index (n = 11) [22, 28, 32, 38, 49, 50, 56–60].
Quality assessment
The risks of bias for randomized clinical trials and all other quantitative studies are summarized in Tables 5 (n = 30) and 6 (n = 24), respectively. Risks of bias for individual studies are available in the supplemental materials (Additional file 2: Table S2-BMC and Additional file 3: Table S3-BMC). Among the 30 RCTs, most studies had a low (n = 11) or unclear (n = 15) risk of selection bias, and a majority (n = 28) had a low risk of reporting bias because there was clarity in the reporting of all pre-specified outcomes. Most studies were at low risk for detection (n = 21) and attrition (n = 19) bias. However, only 7 studies were deemed at low risk for incomplete outcome data for periods longer than 6 weeks because most studies only reported data in the immediate post-intervention period. Regarding performance bias, 9 studies were at high risk because it was not possible to blind anyone to the intervention.
Table 5.
Summary of Cochrane Risk of Bias for Randomized Controlled Trials [19] (n = 30)
| Low Risk | High Risk | Unclear | Not Applicable | |
|---|---|---|---|---|
| Random sequence generation | 11 | 4 | 15 | 0 |
| Allocation concealment | 11 | 4 | 15 | 0 |
| Blinding of participants and personnel | 8 | 9 | 13 | 0 |
| Blinding of outcome assessment | 21 | 2 | 7 | 0 |
| Incomplete outcome data (2–6 weeks) | 19 | 5 | 6 | 0 |
| Incomplete outcome data (> 6 weeks) | 7 | 0 | 1 | 22 |
| Selective reporting | 28 | 0 | 2 | 0 |
For the 24 non-RCTs, most (n = 20) studies met at least 12 of the 17 criteria deemed most important for quality appraisal. Many studies, however, did not describe the statistical power of the study (n = 19), mention piloting of the intervention (n = 13), use valid or reliable measures (n = 8), or overstated study conclusions (n = 13). Few presented the ethical considerations of the study procedures or intervention (n = 19) (Table 6).
Table 6.
Summary Quantitative Studies and Critical Appraisal Checklista (n = 24)
| Criteria | Yes | No |
|---|---|---|
| 1. Are the aims and objectives of the study clearly stated? | 24 | 0 |
| 2. Are the hypotheses and research questions clearly specified? | 24 | 0 |
| 3. Are the dependent and independent variables clearly stated? | 23 | 1 |
| 4. Have the variables been adequately operationalized? | 23 | 1 |
| 5. Is the design of the study adequately described? | 24 | 0 |
| 6. Are the research methods appropriate? | 22 | 2 |
| 7. Were the instruments used appropriate and adequately tested for reliability and validity? | 16 | 8 |
| 8. Is there an adequate description of the source of the sample, inclusion and exclusion criteria, response rates, and (in the case of longitudinal research and post-test in experiments) sample attrition? | 20 | 4 |
| 9. Was the statistical power of the study to detect or reject differences (types I and II error) discussed critically? | 5 | 19 |
| 10. Are ethical considerations presented? | 5 | 19 |
| 11. Was the study piloted? | 11 | 13 |
| 12. Were the statistical analyses appropriate and adequate? | 22 | 2 |
| 13. Are the results clear and adequately reported? | 23 | 1 |
| 14. Does the discussion of the results report them in the light of the hypotheses of the study and other relevant literature? | 22 | 2 |
| 15. Are the limitations of the research and its design presented? | 20 | 4 |
| 16. Does the discussion generalize and draw conclusion beyond the limits of the data and number and type of people studied? | 11 | 13 |
| 17. Can the findings be generalized to other relevant population and time periods? | 21 | 3 |
| 18. Are the implications-practical or theoretical-of the research discussed? | 12 | 12 |
| 19. Who was the sponsor of the study, and was there a conflict of interest? | 11 | 2 (11 NI) |
NI Not indicated; aChecklist from Bowling A. Research methods in health: investigating health and health services. 4th ed. Maidenhead Berkshire, England: Open University Press, 2014
Outcomes
Most studies indicated positive findings (n = 24) of non-pharmacological interventions in improving nighttime sleep outcomes [21–28, 30, 32, 34, 39, 42, 44, 48–50, 57–63] whereas 11 studies reported mixed findings (both positive and none) [29, 31, 36, 43, 45–47, 51, 53, 64, 65]. Although reporting other positive outcomes, 19 studies found no change in nighttime sleep quality after the intervention [33, 35, 37, 38, 40, 41, 52, 54–56, 66–74]. The results differed among location sites, with studies conducted in assisted-living facilities reporting a higher proportion of positive findings than those in nursing homes (70 and 35.7%, respectively).
Study outcomes by intervention type
The interventions employed in the studies varied widely and included interventions in the following categories: clinical care practices (n = 3), mind-body practices (n = 3), social/physical stimulation (n = 11), complementary health practices (n = 12), and environmental interventions (n = 14). There were a total of 25 individual (same type, though differences in dose) interventions; 15 studies employed either a combination of interventions within a specific category (n = 4) or a multicomponent intervention consisting of two or more categories of non-pharmacological intervention (n = 11). The following sections summarize the results for each intervention category (Table 3).
Clinical care practices (n = 3) [25, 41, 73]
Practices implemented by nurses included administering a warm evening foot bath to adjust core body temperature [73], providing individualized care (e.g., residents have choice regarding bedtime) [41], and minimizing nighttime disruptions [25]. These interventions had no, mixed, and positive findings, respectively. All three studies used a pre-post design with sample sizes of 30, 33, and 18, respectively, and none of the authors described sample size calculation. All three studies used quasi-experimental designs, with only one including a comparison/control group as well as objectively measuring sleep with actigraphy [73]. Seven multicomponent studies utilized the clinical care practices of minimizing clinical disruptions [44, 45, 54, 65] and/or sleep-wake time management [34, 52, 54, 65]. Among these multicomponent studies, six incorporated clinical care practices to minimize disruptions [44, 45, 54, 65] and/or manage sleep-wake [34, 52, 54, 65].
Mind-body practices (n = 3) [22, 50, 58]
These interventions require some active involvement by the participant, and each of the three studies included only cognitively intact residents. One study was conducted with assisted-living facility residents in Taiwan [22]; the other two were in nursing homes in Egypt [50] and Turkey [58]. Two relaxation strategies had positive results: progressive muscle relaxation [58] and the meditative practice of yoga [22]. This was an adaptation of hatha yoga specifically developed for the reduced flexibility and exercise tolerance of older adults. All three were well-conducted, quasi-experimental studies using the Pittsburgh Sleep Quality Index subjective measure. However, the study examining cognitive-behavioral therapy did not include a comparison group [50]. No mind-body practices were used in the multicomponent studies.
Social and physical stimulation (n = 11) [23, 26, 32, 33, 42, 43, 46, 51, 55, 57, 67]
Eleven studies utilized interventions that prompted participants to engage in a physical or social activity meant to stimulate cognition, mobility, or both. The latter included three RCTs using actigraphy [51] or polysomnography [26, 55] with low risk of bias; however, the findings were not consistent, with positive [26], none [55], and mixed findings [51]. Of three studies testing social and cognitive activities on nursing-home residents with dementia, one reported improved sleep [42], and the other two studies reported mixed findings [43, 46]. The remaining five studies employed physical exercise/activity and varied in quality. Three studies reported improved sleep [23, 32, 57] and two reported no changes in sleep [32, 33]. Six multicomponent studies included physical activity.
Complementary health practices (n = 12) [21, 28–30, 47, 48, 59, 60, 62, 68, 69, 74]
These are interventions that originated outside mainstream medicine, are administered by a practitioner or clinical staff member, and are received by touch, smell, or ingestion. Two studies examining the effect of massage alone [69, 74] did not find improvements in sleep, and one study combining massage with lavender aromatherapy [29] reported mixed findings. Other touch modalities positively improved sleep, including transcutaneous electrical nerve stimulation [48] and therapeutic touch [62]. However, these studies tested only 14 and 6 participants, respectively. Of the four studies of acupressure, three employed a RCT design with low risk of bias [21, 30, 59], while the fourth evaluated 8-h continuous acupressure using a pre-post design with 129 residents [60]. All four reported positive sleep outcomes. The three studies evaluating melatonin use reported no change in sleep (melatonin dose: 8.5 immediate + 1.5 mg sustained release) [68], mixed findings (melatonin dose: 5 mg to 20 mg of melatonin-rich milk) [41], and better sleep (5 mg + magnesium and zinc) [28]. Two multicomponent RCTs included melatonin (2.5 mg and 5 mg) and found positive [27] and no [66] changes in sleep, respectively.
Environment (n = 14) [24, 31, 35–38, 40, 49, 53, 56, 61, 63, 64, 71]
With the exception of one study evaluating the use of a medium-firmness mattress (no improvement in sleep) [56], all studies of the environment focused on increasing light exposure via natural (outdoor) [38, 63] or bright artificial light illumination during the day. The “dose” of light varied considerably from 2500 lx to 10,000 lx administered for 30 min to 8 h per day for 10 days to 10 weeks. It was not possible to correlate dose with findings of no [35–38, 40, 71], positive [24, 49, 61, 63], or mixed [31, 36, 53, 64] improvement in sleep outcomes. Similarly, there was no relationship between study design/risk of bias and outcomes. Light was also included in most (n = 8) multicomponent studies, using either natural light [39, 52, 65, 72] or a bright light source of 2500 lx to 10,000 lx [27, 54, 66, 70] for a range of time periods.
Multicomponent (n = 11) [27, 34, 39, 44, 45, 52, 54, 65, 66, 70, 72]
Table 4 provides a summary of the characteristics and components of each multicomponent intervention. These studies included an average of 3 (SD = 1.2) interventions with a range of 2 to 5. All included an environmental intervention: increased light (n = 8) [27, 39, 52, 54, 65, 66, 70, 72] and/or reduced noise (n = 5) [34, 44, 45, 54, 65]. Three included the use of melatonin [27, 66] or a vitamin B12 supplement [70]. Two studies included 5 interventions (light, noise, activity, fewer disruptions, and sleep-wake management) [54, 65], and two other studies investigated 4 interventions (light, noise, individual care, and sleep-wake management) [44, 45], but the findings were not consistent. Physical activity was investigated in six studies [34, 39, 52, 54, 65, 72], and four studies employed the clinical care practices of individual care [44, 45], fewer disruptions [44, 45, 54, 65], and sleep-wake management [34, 52, 54, 65]. Most studies (n = 9) were RCTs, and many of these had a low risk of bias [27, 45, 52, 54, 65]. No clear pattern of intervention combinations emerged among studies with no [52, 54, 66, 70, 72], positive [27, 34, 39, 44], or mixed [45, 65] effect on sleep. The highest proportion of RCTs with several areas of high risk of bias was found among this category of studies [34, 45, 52, 54, 65, 72].
Discussion
Despite the minimization of the use of physical restraint, the promotion of function-focused care, and the growing trend of culture change in nursing homes, residents spend considerable time inactive, including large amounts of time in bed [75, 76]. Moreover, the institutional environment provides little opportunity for residents to synchronize their circadian clock to the solar day, which is necessary to support alertness during the day and the consolidation of sleep at night. During the night, residents may experience frequent awakenings and fragmented sleep from clinical care practices that increase light and noise. These factors together contribute to the sleep and circadian rhythm disturbances frequently encountered among long-term care residents. In this systematic review of non-pharmacological interventions to improve sleep among long-term care residents, it was found that nearly three-quarters (n = 37) of the studies aimed to normalize circadian rhythms by increasing daytime activity (100% of social and physical stimulation category), increasing daytime light (93% of environment category), improving nighttime staff routines to minimize disruptions (67% of clinical care practices), or a combination of these interventions (100% of multicomponent). Although there is sound evidence to support these strategies, the variation in how the interventions were delivered (type of daytime activity or dose of light) reduces the ability to draw definitive conclusions.
Given the functional and cognitive limitations of long-term care residents, it is not surprising that the most frequently studied interventions were largely passive in nature: environmental interventions, complementary health practices, and social/physical stimulation. Daytime light therapy was highly correlated with improved sleep [77], including in those with dementia [78], but more evidence-based guidance is needed regarding dose, delivery, frequency, and duration [79]. Because exposure to natural daylight is often not feasible due to location or building design, supplementing the environment with bright artificial light is a feasible option. Current room lighting systems that eliminate safety concerns (excessive heat or UV rays) can be incorporated in high-use areas such as day and dining rooms. However, this intervention was not found in this review. Other environmental interventions, such as control of ambient temperature with a cooler nighttime temperature, were not found in this review and deserve to be explored in future research [80].
Many single and most multicomponent studies also aimed to “reset” residents’ circadian rhythm with stimulating activities during the day and/or strategies that promote relaxation or deter sleep disruption at night. Fewer than half of those studies that tested either exercise or passive social stimulation reported positive findings [23, 32, 34, 39, 42, 57], although several were well-executed RCTs [26, 39, 57]. Most were conducted by research staff to establish intervention efficacy; thus, translating these time-consuming strategies to current staff levels and roles needs careful consideration. The low number of studies directed toward changing clinical care practices to promote sleep suggests difficulty in altering entrenched routines [52]. These concerns underscore the need to consider intervention feasibility in a low-resource practice environment. A community-participatory approach that actively includes equitable input from long-term care staff, residents, and their families may be needed to overcome challenges to practice change [81]. For example, there is considerable evidence to support acupressure [82], but further research is needed with nursing staff to understand how easily (or not) this practice could be incorporated within their nighttime care routines. Another aspect of feasibility that was absent from the reviewed studies is a cost/benefit analysis, which is needed for buy-in from administrators.
Complementary health practices, although not commonly employed in nursing homes, represented more than a quarter of the included studies and were associated with a high proportion of positive outcomes for both acupressure and melatonin in well-executed studies. Although there were no consistent findings with melatonin in this review, possibly because the dose regimen was quite variable, melatonin is considered a safe and effective approach to improve sleep in older adults [83], including those with dementia [84].
Because some of the studies evaluating mind-body techniques were performed in nursing homes outside the United States, their findings may not be fully generalizable, as their population included more cognitive and physically able residents [50, 58, 75], who could be actively involved in the practice of sleep hygiene principles [50] or self-relaxation techniques [58, 75]. Studies using these interventions may demonstrate better outcomes among the growing population in assisted-living facilities, which has similar characteristics.
The overall assessment of the methodological quality of the included studies revealed that the majority of RCTs were at low risk for most types of risk of bias and most non-RCTs met the standard quality criteria. There were several quality concerns, however, for both study types because we chose to include studies that were underpowered, had a high dropout rate, did not include a control/comparison group, and/or did not collect long-term outcomes.
Also, in some cases, it was difficult to identify which component(s) of a multicomponent intervention contributed to the outcome [85]. These complex interventions, as well as single-component interventions, require considerable resident or staff effort, resulting in participant attrition. Treatment adherence is an important aspect of any proposed intervention, indicating its acceptability to both the participant and the staff. Some interventions require equipment that needs to be maintained by staff and may not be readily available. Correct use of an intervention, whether equipment-based or staff-delivered, necessitates staff/provider training. Also, regular supervision is needed to ensure continued accurate execution, and a quality-improvement program is needed to monitor institution-based outcomes. Intervention integrity was not assessed in this review because only a few studies documented any aspect of treatment fidelity.
Conclusions
This systematic review located 54 articles evaluating the effects of 25 different non-pharmacological interventions aimed at improving nighttime sleep in long-term care settings. The analysis of these interventions, applied either in isolation or combination, did not reveal a clear pattern of positive findings. Three interventions had the most promising results: increased daytime light exposure (n = 21), nighttime use of melatonin (n = 6), and acupressure prior to sleep (n = 4).
This review highlights the need for further research to help standardize non-pharmacological interventions to improve sleep in institutionalized settings, including dose and timing of light and melatonin use and site of acupressure, and to determine the optimal combination of interventions. Furthermore, more consistent outcome measurements and identification of sub-groups that would best benefit from certain interventions, along with detailed analyses of cost/benefit ratios and feasibility are needed. In summary, non-pharmacological interventions have the potential to improve sleep and circadian rhythm disturbances in residents of long-term care facilities; however, further research is needed to better standardize such interventions and provide clear implementation guidelines using cost-effective practices.
Additional files
Table S1. Detailed search strategies. Detailed search strategies for each database: Cochrane Library (Wiley), Ovid MEDLINE, Ovid Embase, CINAHL, and Scopus. (PDF 89 kb)
Table S2. Individual study results for Cochrane Risk of Bias for Randomized Controlled Trials [89] (n = 30). Provides details (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting) for individual studies. (DOC 75 kb)
Table S3. Individual studies’ results for the quantitative studies and critical appraisal checklist (n = 24). Provides details for each individual study using the nineteen criteria from the Summary Quantitative Studies and Critical Appraisal Checklist. (DOC 109 kb)
Acknowledgements
We are grateful to Paul Eshelman, MFA, and Jennifer Tiffany, RN, MRP, PhD, of Cornell University for their substantive input to the overall design of the grants supporting this work. We acknowledge the assistance of Cynthia Lien, MD; Eugenia L. Siegler, MD; and Dale Johnson for input on research scope. Special thanks to Hessam Sadatsafavi for grant submission management, Kimia Erfani for assistance with the PRISMA flow chart, and Melissa Kuhnell for manuscript preparation.
Funding
This study was supported by the New York State Department of Food and Agriculture’s Smith Lever Fund, the Building Faculty Connections Program Fund of Cornell University’s College of Human Ecology, and the Professional Staff Congress at CUNY. These funders had no role in the study design, the collection, analysis and interpretation of data, the writing of the report or the decision to submit the article for publication.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- RCT
Randomized controlled trial
- SD
Standard deviation
Authors’ contributions
Each author took an active role in the literature data collection, evaluation, and synthesis. RSZ, ACK and EC worked closely with KP to develop the search strategies. After KP located articles, all other co-authors reviewed the studies based on inclusion/exclusion criteria. Research assistants AB and NZJ developed the tables under our supervision and we all contributed to the reporting of the results. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12877-018-0794-3) contains supplementary material, which is available to authorized users.
Contributor Information
Elizabeth Capezuti, Phone: 212-396-7155, Email: ec773@hunter.cuny.edu.
Rana Sagha Zadeh, Phone: 607-255-1946, Email: rzadeh@cornell.edu.
Kevin Pain, Phone: 646-962-2553, Email: kjpain@med.cornell.edu.
Aleksa Basara, Phone: 607-255-1946, Email: ab2295@cornell.edu.
Nancy Ziyan Jiang, Phone: 607-255-1946, Email: nzj2@cornell.edu.
Ana C. Krieger, Email: ack2003@med.cornell.edu
References
- 1.Fung CH, Martin JL, Chung C, Fiorentino L, Mitchell M, Josephson KR, et al. Sleep disturbance among older adults in assisted living facilities. Am J Geriatr Psychiatry. 2012;20:485–493. doi: 10.1097/JGP.0b013e318252e3e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neikrug AB, Ancoli-Israel S. Sleep disturbances in nursing homes. J Nutr Health Aging. 2010;14:207. doi: 10.1007/s12603-010-0051-8. [DOI] [PubMed] [Google Scholar]
- 3.Fung CH, Vitiello MV, Alessi CA, Kuchel GA. AGS/NIA Sleep Conference Planning Committee and Faculty. Report and research agenda of the American Geriatrics Society and National Institute on Aging bedside-to-bench conference on sleep, circadian rhythms, and aging: new avenues for improving brain health, physical health, and functioning. J Am Geriatr Soc. 2016;64:238–247. doi: 10.1111/jgs.14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smagula SF, Stone KL, Fabio A, Cauley JA. Risk factors for sleep disturbances in older adults: evidence from prospective studies. Sleep Med Rev. 2016;25:21–30. doi: 10.1016/j.smrv.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore KL, Boscardin WJ, Steinman MA, Schwartz JB. Patterns of chronic co-morbid medical conditions in older residents of US nursing homes: differences between the sexes and across the agespan. J Nutr Health Aging. 2014;18(4):429–436. doi: 10.1007/s12603-014-0001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmerman S, Sloane PD, Reed D. Dementia prevalence and care in assisted living. Health Aff (Millwood). 2014;33:658–666. doi: 10.1377/hlthaff.2013.1255. [DOI] [PubMed] [Google Scholar]
- 7.Cipriani G, Lucetti C, Danti S, Nuti A. Sleep disturbances and dementia. Psychogeriatrics. 2015;15:65–74. doi: 10.1111/psyg.12069. [DOI] [PubMed] [Google Scholar]
- 8.Saeed Y, Abbott SM. Circadian disruption associated with Alzheimer’s disease. Curr Neurol Neurosci Rep. 2017;17(4):29. doi: 10.1007/s11910-017-0745-y. [DOI] [PubMed] [Google Scholar]
- 9.Sloane PD, Zimmerman S, Brown LC, Ives TJ, Walsh JF. Inappropriate medication prescribing in residential care/assisted living facilities. J Am Geriatr Soc. 2002;50:1001–1011. doi: 10.1046/j.1532-5415.2002.50253.x. [DOI] [PubMed] [Google Scholar]
- 10.Maust DT, Langa KM, Blow FC. Psychotropic use and associated neuropsychiatric symptoms among patients with dementia in the USA. Int J Geriatr Psychiatry. 2017;32:164–174. doi: 10.1002/gps.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Geriatrics Society 2015 Beers Criteria Update Expert Panel American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227–2246. doi: 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- 12.Berry SD, Lee Y, Cai S, Dore DD. Non-benzodiazepine sleep medications and hip fractures in nursing home residents. JAMA Intern Med. 2013;173:754–761. doi: 10.1001/jamainternmed.2013.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saarelainen L, Tolppanen AM, Koponen M, Tanskanen A, Sund R, Tiihonen J, et al. Risk of hip fracture in benzodiazepine users with and without Alzheimer disease. J Am Med Dir Assoc. 2017;18:87–e15. doi: 10.1016/j.jamda.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Bicket MC, Samus QM, McNabney M, Onyike CU, Mayer LS, Brandt J, et al. The physical environment influences neuropsychiatric symptoms and other outcomes in assisted living residents. Int J Geriatr Psychiatry. 2010;25:1044–1054. doi: 10.1002/gps.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cruise PA, Schnelle JF, Alessi CA, Simmons SF, Ouslander JG. The nighttime environment and incontinence care practices in nursing homes. J Am Geriatr Soc. 1998;46:181–186. doi: 10.1111/j.1532-5415.1998.tb02536.x. [DOI] [PubMed] [Google Scholar]
- 16.Cabrera E, Sutcliffe C, Verbeek H, Saks K, Soto-Martin M, Meyer G, et al. Non-pharmacological interventions as a best practice strategy in people with dementia living in nursing homes. A systematic review. Eur Geriatr Med. 2015;6:134–150. doi: 10.1016/j.eurger.2014.06.003. [DOI] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;6:e100010. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Capezuti E, Sagha Zadeh R, Woody N, Basara A, Krieger AC. An integrative review of nonpharmacological interventions to improve sleep among adults with advanced serious illness. Palliat Med. 2018. [DOI] [PMC free article] [PubMed]
- 19.Higgins JP, Altman DG, Gøtzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowling A. Research methods in health: investigating health and health services. 4. Maidenhead Berkshire: Open University Press; 2014. [Google Scholar]
- 21.Chen ML, Lin LC, Wu SC, Lin JG. The effectiveness of acupressure in improving the quality of sleep of institutionalized residents. J Gerontol A Biol Sci Med Sci. 1999;54:M389–M394. doi: 10.1093/gerona/54.8.M389. [DOI] [PubMed] [Google Scholar]
- 22.Chen KM, Chen MH, Lin MH, Fan JT, Lin HS, Li CH. Effects of yoga on sleep quality and depression in elders in assisted living facilities. J Nurs Res. 2010;18:53–61. doi: 10.1097/JNR.0b013e3181ce5189. [DOI] [PubMed] [Google Scholar]
- 23.Lee Y, Kim S. Effects of indoor gardening on sleep, agitation, and cognition in dementia patients—a pilot study. Int J Geriatr Psychiatry. 2008;23:485–489. doi: 10.1002/gps.1920. [DOI] [PubMed] [Google Scholar]
- 24.Lyketsos CG, Lindell Veiel L, Baker A, Steele C. A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. Int J Geriatr Psychiatry. 1999;14(7):520–525. doi: 10.1002/(SICI)1099-1166(199907)14:7<520::AID-GPS983>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 25.O’Rourke DJ, Klaasen KS, Sloan JA. Redesigning nighttime care for personal care residents. J Gerontol Nurs. 2001;27:30–37. doi: 10.3928/0098-9134-20010701-10. [DOI] [PubMed] [Google Scholar]
- 26.Richards KC, Lambert C, Beck CK, Bliwise DL, Evans WJ, Kalra GK, et al. Strength training, walking, and social activity improve sleep in nursing home and assisted living residents: randomized controlled trial. J Am Geriatr Soc. 2011;59:214–223. doi: 10.1111/j.1532-5415.2010.03246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riemersma-Van Der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299:2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 28.Rondanelli M, Opizzi A, Monteferrario F, Antoniella N, Manni R, Klersy C. The effect of melatonin, magnesium, and zinc on primary insomnia in long-term care facility residents in Italy: a double-blind, placebo-controlled clinical trial. J Am Geriatr Soc. 2011;59:82–90. doi: 10.1111/j.1532-5415.2010.03232.x. [DOI] [PubMed] [Google Scholar]
- 29.Soden K, Vincent K, Craske S, Lucas C, Ashley S. A randomized controlled trial of aromatherapy massage in a hospice setting. Palliat Med. 2004;18:87–92. doi: 10.1191/0269216304pm874oa. [DOI] [PubMed] [Google Scholar]
- 30.Sun JL, Sung MS, Huang MY, Cheng GC, Lin CC. Effectiveness of acupressure for residents of long-term care facilities with insomnia: a randomized controlled trial. Int J Nurs Stud. 2010;47:798–805. doi: 10.1016/j.ijnurstu.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Wu MC, Sung HC, Lee WL, Smith GD. The effects of light therapy on depression and sleep disruption in older adults in a long-term care facility. Int J Nurs Pract. 2015;21:653–659. doi: 10.1111/ijn.12307. [DOI] [PubMed] [Google Scholar]
- 32.Taboonpong S, Puthsri N, Kong-In W, Saejew A. The effects of Tai Chi on sleep quality, well-being and physical performances among older adults. Thai J Nurs Res. 2010;12:1–13. [Google Scholar]
- 33.Alessi CA, Schnelle JF, MacRae PG, Ouslander JG, Al-Samarrai N, Simmons SF, et al. Does physical activity improve sleep in impaired nursing home residents? J Am Geriatr Soc. 1995;43:1098–1102. doi: 10.1111/j.1532-5415.1995.tb07007.x. [DOI] [PubMed] [Google Scholar]
- 34.Alessi CA, Yoon EJ, Schnelle JF, Al-Samarrai NR, Cruise PA. A randomized trial of a combined physical activity and environmental intervention in nursing home residents: do sleep and agitation improve? J Am Geriatr Soc. 1999;47:784–791. doi: 10.1111/j.1532-5415.1999.tb03833.x. [DOI] [PubMed] [Google Scholar]
- 35.Ancoli-Israel S, Gehrman P, Martin JL, Shochat T, Marler M, Corey-Bloom J, et al. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer's disease patients. Behav Sleep Med. 2003;1:22–36. doi: 10.1207/S15402010BSM0101_4. [DOI] [PubMed] [Google Scholar]
- 36.Ancoli-Israel S, Martin JL, Kripke DF, Marler M, Klauber MR. Effect of light treatment on sleep and circadian rhythms in demented nursing home patients. J Am Geriatr Soc. 2002;50:282–289. doi: 10.1046/j.1532-5415.2002.50060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burns A, Allen H, Tomenson B, Duignan D, Byrne J. Bright light therapy for agitation in dementia: a randomized controlled trial. Int Psychogeriatr. 2009;21:711–721. doi: 10.1017/S1041610209008886. [DOI] [PubMed] [Google Scholar]
- 38.Calkins M, Szmerekovsky JG, Biddle S. Effect of increased time spent outdoors on with dementia residing in nursing homes. J Hous Elderly. 2007;21:211–228. doi: 10.1300/J081v21n03_11. [DOI] [Google Scholar]
- 39.Connell BR, Sanford JA, Lewis D. Therapeutic effects of an outdoor activity program on nursing home residents with dementia. J Hous Elderly. 2007;21:194–209. doi: 10.1300/J081v21n03_10. [DOI] [Google Scholar]
- 40.Dowling GA, Hubbard EM, Mastick J, Luxenberg JS, Burr RL, Van Someren EJ. Effect of morning bright light treatment for rest–activity disruption in institutionalized patients with severe Alzheimer's disease. Int Psychogeriatr. 2005;17:221–236. doi: 10.1017/S1041610205001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthews EA, Farrell GA, Blackmore AM. Effects of an environmental manipulation emphasizing client-centered care on agitation and sleep in dementia sufferers in a nursing home. J Adv Nurs. 1996;24:439–447. doi: 10.1046/j.1365-2648.1996.02102.x. [DOI] [PubMed] [Google Scholar]
- 42.Richards KC, Sullivan SC, Phillips RL, Beck CK, Overton-McCoy AL. The effect of individualized activities on the sleep of nursing home residents who are cognitively impaired: a pilot study. J Gerontol Nurs. 2001;27:30–37. doi: 10.3928/0098-9134-20010901-07. [DOI] [PubMed] [Google Scholar]
- 43.Richards KC, Beck C, O'Sullivan PS, Shue VM. Effect of individualized social activity on sleep in nursing home residents with dementia. J Am Geriatr Soc. 2005;53:1510–1517. doi: 10.1111/j.1532-5415.2005.53460.x. [DOI] [PubMed] [Google Scholar]
- 44.Schnelle JF, Cruise PA, Alessi CA, Al-Samarrai N, Ouslander JG. Individualizing nighttime incontinence care in nursing home residents. Nurs Res. 1998;47:197–204. doi: 10.1097/00006199-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Schnelle JF, Alessi CA, Al-Samarrai NR, Fricker RD, Jr, Ouslander JG. The nursing home at night: effects of an intervention on noise, light and sleep. J Am Geriatr Soc. 1999;47:430–438. doi: 10.1111/j.1532-5415.1999.tb07235.x. [DOI] [PubMed] [Google Scholar]
- 46.Thodberg K, Sørensen LU, Christensen JW, Poulsen PH, Houbak B, Damgaard V, et al. Therapeutic effects of dog visits in nursing homes for the elderly. Psychogeriatrics. 2016;16:289–297. doi: 10.1111/psyg.12159. [DOI] [PubMed] [Google Scholar]
- 47.Valtonen M, Niskanen L, Kangas AP, Koskinen T. Effect of melatonin-rich night-time milk on sleep and activity in elderly institutionalized subjects. Nord J Psychiatry. 2005;59:217–221. doi: 10.1080/08039480510023034. [DOI] [PubMed] [Google Scholar]
- 48.Van Someren EJ, Scherder EJ, Swaab DF. Transcutaneous electrical nerve stimulation (TENS) improves circadian rhythm disturbances in Alzheimer disease. Alzheimer Dis Assoc Disord. 1998;12(2):114–118. doi: 10.1097/00002093-199806000-00010. [DOI] [PubMed] [Google Scholar]
- 49.Akyar I, Akdemir N. The effect of light therapy on the sleep quality of the elderly: an intervention study. Aust J Adv Nurs. 2013;31:31. [Google Scholar]
- 50.El Kady HM, Ibrahim HK, Mohamed SG. Cognitive behavioral therapy for institutionalized elders complaining of sleep disturbance in Alexandria, Egypt. Sleep Breath. 2012;16:1173–1180. doi: 10.1007/s11325-011-0629-3. [DOI] [PubMed] [Google Scholar]
- 51.Kuck J, Pantke M, Flick U. Effects of social activation and physical mobilization on sleep in nursing home residents. Geriatr Nurs. 2014;35:455–461. doi: 10.1016/j.gerinurse.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 52.Martin JL, Marler MR, Harker JO, Josephson KR, Alessi CA. A multicomponent nonpharmacological intervention improves activity rhythms among nursing home residents with disrupted sleep/wake patterns. J Gerontol A Biol Sci Med Sci. 2007;62:67–72. doi: 10.1093/gerona/62.1.67. [DOI] [PubMed] [Google Scholar]
- 53.Figueiro MG, Plitnick BA, Lok A, Jones GE, Higgens P, Hornick TR, et al. Tailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer’s disease and related dementia living in long-term care facilities. Clin Interv Aging. 2014;9:1527–1537. doi: 10.2147/CIA.S68557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ouslander JG, Connell BR, Bliwise DL, Endeshaw Y, Griffiths P, Schnelle JF. A nonpharmacological intervention to improve sleep in nursing home patients: results of a controlled clinical trial. J Am Geriatr Soc. 2006;54:38–47. doi: 10.1111/j.1532-5415.2005.00562.x. [DOI] [PubMed] [Google Scholar]
- 55.Lorenz RA, Gooneratne N, Cole CS, Kleban MH, Kalra GK, Richards KC. Exercise and social activity improve everyday function in long-term care residents. Am J Geriatr Psychiatry. 2012;20:468–476. doi: 10.1097/JGP.0b013e318246b807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ancuelle V, Zamudio R, Mendiola A, Guillen D, Ortiz PJ, Tello T, et al. Effects of an adapted mattress in musculoskeletal pain and sleep quality in institutionalized elders. Sleep Sci. 2015;8:115–120. doi: 10.1016/j.slsci.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen KM, Huang HT, Cheng YY, Li CH, Chang YH. Sleep quality and depression of nursing home older adults in wheelchairs after exercises. Nurs Outlook. 2015;63:357–365. doi: 10.1016/j.outlook.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 58.Örsal Ö, Alparslan GB, Özkaraman A, Sönmez N. The effect of relaxation exercises on quality of sleep among the elderly: holistic nursing practice review copy. Holist Nurs Pract. 2014;28:265–274. doi: 10.1097/HNP.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 59.Reza H, Kian N, Pouresmail Z, Masood K, Sadat Seyed Bagher M, Cheraghi MA. The effect of acupressure on quality of sleep in Iranian elderly nursing home residents. Complement Ther Clin Pract. 2010;16:81–85. doi: 10.1016/j.ctcp.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Simoncini M, Gatt A, Quirico PE, Balla S, Capellero B, Obialero R, et al. Acupressure in insomnia and other sleep disorders in elderly institutionalized patients suffering from Alzheimer’s disease. Aging Clin Exp Res. 2015;27:37–42. doi: 10.1007/s40520-014-0244-9. [DOI] [PubMed] [Google Scholar]
- 61.Fetveit A, Skjerve A, Bjorvatn B. Bright light treatment improves sleep in institutionalised elderly—an open trial. Int J Geriatr Psychiatry. 2003;18:520–526. doi: 10.1002/gps.852. [DOI] [PubMed] [Google Scholar]
- 62.Braun C, Layton J, Braun J. Therapeutic touch improves residents' sleep. J Am Health Care Assoc. 1986;12:48. [PubMed] [Google Scholar]
- 63.Castor D, Woods D, Pigott K. Effect of sunlight on sleep patterns of the elderly. J Am Acad Physician Assist. 1991;4:321–326. [Google Scholar]
- 64.Fetveit A, Bjorvatn B. The effects of bright-light therapy on actigraphical measured sleep last for several weeks post-treatment. A study in a nursing home population. J Sleep Res. 2004;13:153–158. doi: 10.1111/j.1365-2869.2004.00396.x. [DOI] [PubMed] [Google Scholar]
- 65.Alessi CA, Martin JL, Webber AP, Cynthia Kim E, Harker JO, Josephson KR. Randomized, controlled trial of a nonpharmacological intervention to improve abnormal sleep/wake patterns in nursing home residents. J Am Geriatr Soc. 2005;53:803–810. doi: 10.1111/j.1532-5415.2005.53251.x. [DOI] [PubMed] [Google Scholar]
- 66.Dowling GA, Burr RL, Van Someren EJ, Em H, Luxenberg JS, Mastick J, et al. Melatonin and bright-light treatment for rest–activity disruption in institutionalized patients with Alzheimer's disease. J Am Geriatr Soc. 2008;56:239–246. doi: 10.1111/j.1532-5415.2007.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eggermont LH, Blankevoort CG, Scherder EJ. Walking and night-time restlessness in mild-to-moderate dementia: a randomized controlled trial. Age Ageing. 2010;9:746–749. doi: 10.1093/ageing/afq115. [DOI] [PubMed] [Google Scholar]
- 68.Gehrman PR, Connor DJ, Martin JL, Shochat T, Corey-Bloom J, Ancoli-Israel S. Melatonin fails to improve sleep or agitation in double-blind randomized placebo-controlled trial of institutionalized patients with Alzheimer disease. Am J Geriatr Psychiatry. 2009;17:166–169. doi: 10.1097/JGP.0b013e318187de18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harris M, Richards KC, Grando VT. The effects of slow-stroke back massage on minutes of nighttime sleep in persons with dementia and sleep disturbances in the nursing home: a pilot study. J Holist Nurs. 2012;30:255–263. doi: 10.1177/0898010112455948. [DOI] [PubMed] [Google Scholar]
- 70.Ito T, Yamadera H, Ito R, Suzuki H, Asayama K, Endo S. Effects of vitamin B12 on bright light on cognitive and sleep–wake rhythm in Alzheimer-type dementia. Psychiatry Clin Neurosci. 2001;55:281–282. doi: 10.1046/j.1440-1819.2001.00860.x. [DOI] [PubMed] [Google Scholar]
- 71.Koyama E, Matsubara H, Nakano T. Bright light treatment for sleep–wake disturbances in aged individuals with dementia. Psychiatry Clin Neurosci. 1999;53:227–229. doi: 10.1046/j.1440-1819.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- 72.Gammack JK, Burke JM. Natural light exposure improves subjective sleep quality in nursing home residents. JAMDA. 2009;10(6):440–441. doi: 10.1016/j.jamda.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 73.Kim HJ, Lee Y, Sohng KY. The effects of footbath on sleep among the older adults in nursing home: a quasi-experimental study. Complement Ther Med. 2016;26:40–46. doi: 10.1016/j.ctim.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 74.Nelson R, Coyle C. Effects of a bedtime massage on relaxation in nursing home residents with sleep disorders. Act Adapt Aging. 2010;34(3):216–231. [Google Scholar]
- 75.Anderiesen H, Scherder EJ, Goossens RH, Sonneveld MH. A systematic review–physical activity in dementia: the influence of the nursing home environment. Appl Ergon. 2014;45:1678–1686. doi: 10.1016/j.apergo.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 76.Resnick B, Galik E, Boltz M. Function focused care approaches: literature review of progress and future possibilities. J Am Med Dir Assoc. 2013;14:313–318. doi: 10.1016/j.jamda.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 77.Van Maanen A, Meijer AM, van der Heijden KB, Oort FJ. The effects of light therapy on sleep problems: a systematic review and meta-analysis. Sleep Med Rev. 2016;29:52–62. doi: 10.1016/j.smrv.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 78.Chiu HL, Chan PT, Chu H, Hsiao SS, Liu D, Lin CH, et al. Effectiveness of light therapy in cognitively impaired persons: a metaanalysis of randomized controlled trials. J Am Geriatr Soc. 2017;65:2227–2234. doi: 10.1111/jgs.14990. [DOI] [PubMed] [Google Scholar]
- 79.Ploeg ES, O'Connor DW. Methodological challenges in studies of bright light therapy to treat sleep disorders in nursing home residents with dementia. Psychiatry Clin Neurosci. 2014;68:777–784. doi: 10.1111/pcn.12192. [DOI] [PubMed] [Google Scholar]
- 80.Lack LC, Gradisar M, Van Someren EJ, Wright HR, Lushington K. The relationship between insomnia and body temperatures. Sleep Med Rev. 2008;12:307–317. doi: 10.1016/j.smrv.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 81.Burns D, Hyde P, Killett A, Poland F, Gray R. Participatory organizational research: examining voice in the co-production of knowledge. Br J Manag. 2014;25:133–144. doi: 10.1111/j.1467-8551.2012.00841.x. [DOI] [Google Scholar]
- 82.Waits A, Tang YR, Cheng HM, Chen-Jei T, Chien L-Y. Acupressure effect on sleep quality: a systematic review and meta-analysis. Sleep Med Rev. 2016. Available at: 10.1016/j.smrv.2016.12.004; [Epub ahead of print] [DOI] [PubMed]
- 83.Xu J, Wang LL, Dammer EB, Li CB, Xu G, Chen SD, et al. Melatonin for sleep disorders and cognition in dementia: a meta-analysis of randomized controlled trials. Am J Alzheimers Dis Other Demen. 2015;30:439–447. doi: 10.1177/1533317514568005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang YY, Zheng W, Ng CH, Ungvari GS, Wei W, Xiang YT. Meta-analysis of randomized, double-blind, placebo-controlled trials of melatonin in Alzheimer's disease. Int J Geriatr Psychiatry. 2016;32:50–57. doi: 10.1002/gps.4571. [DOI] [PubMed] [Google Scholar]
- 85.Petticrew M, Anderson L, Elder R, Grimshaw J, Hopkins D, Hahn R, et al. Complex interventions and their implications for systematic reviews: a pragmatic approach. Int J Nurs Stud. 2015;52:1211–1216. doi: 10.1016/j.ijnurstu.2015.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Detailed search strategies. Detailed search strategies for each database: Cochrane Library (Wiley), Ovid MEDLINE, Ovid Embase, CINAHL, and Scopus. (PDF 89 kb)
Table S2. Individual study results for Cochrane Risk of Bias for Randomized Controlled Trials [89] (n = 30). Provides details (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting) for individual studies. (DOC 75 kb)
Table S3. Individual studies’ results for the quantitative studies and critical appraisal checklist (n = 24). Provides details for each individual study using the nineteen criteria from the Summary Quantitative Studies and Critical Appraisal Checklist. (DOC 109 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.