Abstract
Cyclin-dependent kinase 5 (cdk5) is a proline-directed serine/threonine kinase that is activated mostly by association with its activators, p35 and p39. Initially projected as a neuron-specific kinase, cdk5 is expressed ubiquitously and its kinase activity solely depends on the presence of its activators, which are also found in some non-neuronal tissues. As a multifunctional protein, cdk5 has been linked to axonogenesis, cell migration, exocytosis, neuronal differentiation and apoptosis. Cdk5 plays a critical role in functions other than normal physiology, especially in neurodegeneration. Its contribution to both normal physiological as well as pathological processes is mediated by its specific substrates. Cdk5-null mice are embryonically lethal, therefore making it difficult to study precisely what cdk5 does to the nervous system at early stages of development, be it neuron development or programmed cell death. Zebrafish model system bypasses the impediment, as it is amenable to reverse genetics studies. One of the functions that we have followed for the cdk5 ortholog in zebrafish in vivo is its effect on the Rohon-Beard (RB) neurons. RB neurons are the primary sensory spinal neurons that die during the first two days of zebrafish development eventually to be replaced by the dorsal root ganglia (DRG). Based on ours studies and others’, here we discuss possible mechanisms that may be involved in cdk5’s role in RB neuron development and survival.
Keywords: Rohon-Beard neuron, Neurogenesis, Protein kinases, Gene knockdown, Cell fate
Introduction
Cyclin-dependent kinase 5 (cdk5) belongs to the family of serine/threonine cyclin-dependent kinases and was initially identified as a member of the cyclin-dependent kinase family [1]. Although cdk5 is found in mitotic cells [2], it is not involved in the cell cycle. Despite its high degree of homology to other cyclin-dependent kinases, cdk5 activity is stimulated not by an associated cyclin [3] but by neuron-specific activators, p35, and p39, and possibly p67 (Munc-18) [2, 4, 5]. The cdk5 activators p25 and p29 are cleavage products of p35 and p39, respectively, generated by calpain-mediated proteolysis. The major activator, p35, is expressed predominantly in post-mitotic neurons of the central nervous system, and correlates well with cdk5 kinase activity [6–8]. Although cdk5 is expressed in many tissues, its highest kinase activity is observed mainly in post-mitotic neurons of the CNS due to the expression of the activators p35 and p39 in these neurons. Cdk5 activity is controlled by regulatory phosphorylation, subcellular localization, and association with other proteins [9–14]. Although the various activators have been shown to differentially influence the kinetics and substrate specificity of the cdk5 [9, 15, 16], the significance of cdk5 activation by a range of activators is unknown. It is possible that differences in anatomical and subcellular localization of the activators may serve specific physiological functions.
Cdk5 Function in the Nervous System
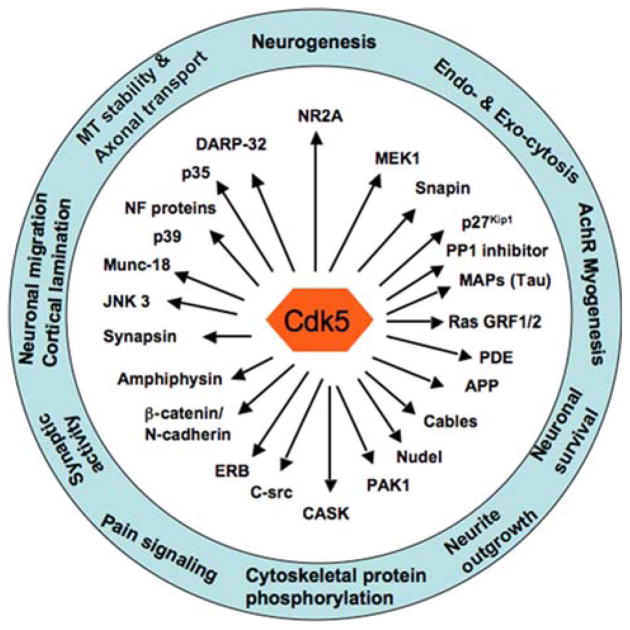
Cdk5 is a multifunctional protein kinase (Fig. 1). It is known to affect neurite outgrowth and axonogenesis in both mammalian and Drosophila development [17–22]. Cdk5 knockout mice exhibit defects in organization of the cortex and cerebellum and are embryonically lethal [19]. In addition, cdk5 plays an important role in a range of physiological and pathological processes that include involvement in nervous system development, dopaminergic function and neurodegeneration [2, 23–27]. Recent studies support potential roles of cdk5 in regulated exocytosis of growth factors [28, 29].
Fig. 1.
Cdk5 is a multi-functional kinase. Cdk5 is involved in a wide range of neuronal functions. These functions are mediated by proteins that are phosphorylated by cdk5. Some of the cdk5 substrates are indicated by arrows
Furthermore, cdk5 has been implicated in the connectivity of developing neurons. Disturbed fasciculation of axonal tracts, such as the corpus callosum in p35 null mutant mice, together with effects of cdk5 on growth cone collapse and neurite actin dynamics, provide evidence for a role of cdk5 in axon guidance [2, 26, 27]. Recent investigations have focused on the role of cdk5 in synaptogenesis, another aspect of connectivity. Work in models of synapse formation has demonstrated that cdk5 promotes the formation of synaptic structures, possibly by inducing the expression and clustering of acetylcholine receptors in the post-synaptic membrane [30–32].
Cdk5 has been associated with neuronal differentiation. Loss of cdk5 during development leads to an inability of neurons to exit the cell cycle, coupled with their incomplete differentiation [33]. This is consistent with data from other cell types that suggest a general role for cdk5 in cellular differentiation [34].
The development of the CNS requires the programmed migration, differentiation and connection of neurons to form functional circuits capable of expressing synaptic plasticity. Phenotypically, cdk5 null mutant mice, as well as p35/p39 double null mutants, exhibit a reversal of the normal cortical laminar architecture, as well as cytoarchitectural disturbances in the cerebellum, brainstem and hippocampus [19, 35], implicating cdk5 in neuronal migration. Cdk5 phosphorylates a large number of proteins, including the neurofilaments and tau [36–43]. Cdk5-mediated phosphorylation of a broad range of substrates including proteins involved in cytoskeletal dynamics and axonal transport suggests its role in neuronal migration (Fig. 1). Moreover, mice lacking p35 reveal cortical lamination defects [44]. Additional evidences suggest that p35 null mutant mice may carry confounding developmental defects, such as aberrant hippocampal circuitry forming excitatory feedback loops [45]. In Xenopus, cdk5 has two p35 homologs, and plays a role in eye, muscle and neuronal development [46–48].
Regulated and deregulated cdk5 kinase activity has been implicated in neuronal survival and death, respectively. Amyloid-β peptide and ischemic insults [49, 50] induce the conversion of p35 to its truncated form, p25, which causes apoptosis in neurons. In contrast, the association of p35 and cdk5 is required for neuronal survival. Cdk5-mediated kinase crosstalk regulates neuronal survival [51–53]. Much less is known about the proteolytically cleaved activators p25 and p29, than their parent molecules, p35 and p39. The formation of p25 has so far been demonstrated only in neurotoxic and degenerative situations such as Alzheimer’s disease, although there is still controversy on the latter issue [54]. Whether p25 and p29 serve any normal physiological function remain to be elucidated.
Cdk5 and Neuronal Death
During vertebrate nervous system development, as many as half of the developing neurons die [55–57]. An anti-apoptotic role for cdk5 has been reported in mammalian systems [52, 58, 59], although its precise role in apoptosis is still uncertain. On the other hand, cdk5 has been implicated in the induction of apoptosis [58, 60–64], while others have reported a reduction in cdk5/p35 in apoptotic cells [65]. Furthermore, in the mammalian system, based on studies using siRNA and anti-sense RNA, it has been suggested that cdk5 activation is not the inducer of cell death, but is a result of cell death, thus placing cdk5 activation downstream of cell death [66].
Inhibition of de-regulated cdk5 activity has been shown to promote cell survival of neurons challenged by β-amyloid peptide [67–69]. However, cdk5-deficient cultured cortical neurons exhibit increased sensitivity to apoptotic stimuli [52]. How precisely cdk5 regulates cell survival or death seems cell-specific and probably requires a tight regulation that would conceivably depend on the coactivator level and the existing signaling molecules or circuitry to propagate its effect. Apparently, even if cdk5 activity were the same in various cells, its downstream or parallel signaling factors would be critical in determining the ultimate outcome.
RB Neuron Development and Apoptosis
Death is an essential part of the developmental process. Several hypotheses have been proposed to explain the purpose of this death: reduction in the number of neurons to match target tissue size, elimination of neurons that make connectivity errors, and failure to obtain adequate trophic support [70]. Irrespective of the mechanism, elimination of neurons eventually results in a nervous system comprising the right number of neurons having error-proof pre- and post-synaptic connections.
Therefore, death serves to eliminate an obsolete or unnecessary cell type [66]. One such population is RB spinal sensory neurons. RB cells have been described in amphibians [55, 71–73]. Zebrafish RB neurons share the same attributes as amphibian RB neurons: large cell bodies with huge nuclei and granular cytoplasm [74]. RB neurons are derived from the same neural plate domain that generates neural crest cells [75], and manipulation of Delta/Notch signaling, as in the mind bomb mutants, reveals that RB neurons are the preferred fate, and neural crest cells the nonpreferred fate within this domain [75]. Why RB neurons die during development is unknown. In amphibians, the RB neurons die gradually and their death coincides with dorsal root ganglion (DRG) development [71, 73, 76].
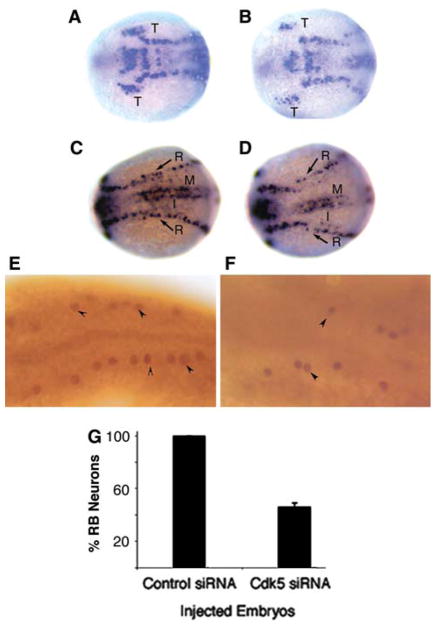
Although our studies have shown that cdk5 is essential to maintain the RB neurons, as analyzed in 24 h embryos [77, 78], it’s likely that cdk5 also plays a role during differentiation of RB neurons from neuronal precursors. During neuronal differentiation, proneural genes, such as neurogenin1 (ngn1), induce downstream bHLH genes, such as neuroD, to elicit the transition from proliferative neural precursor cells to postmitotic neurons that express neuron-specific markers, N tubulin, and HuC [79–82]. In Xenopus, however, cdk5 activation has been shown to be downstream from the expression of inducers of terminal neural differentiation, ngn1and neuroD [48], suggesting that cdk5 activity is not required for neuronal differentiation. However, in zebrafish, when we analyzed the expression of HuC by in situ hybridization at 11.5 h of development, there was a significant difference in the number of HuC-expressing cells between control siRNA-injected and cdk5 siRNA-injected embryos (Fig. 2a–d). The trigeminal ganglion primordia (T) were smaller in the cdk5knockdown embryos compared to those in the control embryos. Primary neurons include neurons in the trigeminal ganglia (T), RB neurons in the lateral neural plate, and primary interneurons (I) and motoneurons (M) in the intermediate and medial neural plates, respectively [83]. In our studies, a similar reduction in the number of early-born RB neurons (R), were also observed in the cdk5 knockdown embryos. Later at 24 h of development, immunostaining of embryos with an antibody against islet-1, a marker of primary neurons, revealed a significant reduction in RB neurons in the cdk5 siRNA-injected embryos compared to the control siRNA-injected embryos (Fig. 2e, f). Quantitative analyzes of islet-1 positive neurons showed a significant (~50%) reduction in the number of RB neurons in the cdk5 siRNA-injected embryos (Fig. 2g). In this context, the question arises, whether cdk5 is involved in RB neuron differentiation.
Fig. 2.
Cdk5 is required for primary sensory neurogenesis. (a–d) Whole-mount in situ hybridization was carried out as described [102] by using digoxigenin-11-UTP-labeled probes (Roche). The antisense probe for HuC (marker for primary neurons) was obtained from the HuC gene with 3′UTR cloned in pGEMT vector. The anitsense DIG-labeled RNA was synthesized by using SP6 Polymerase after linearization with NcoI. The results show HuC mRNA expression at 11.5 h embryos (dorsal views) injected with control siRNA (a, c) or cdk5 siRNA (b, d). T indicates trigeminal ganglion placode. Arrows (R) indicate Rohon-Beard (RB) neurons. R, RB neurons; M, motor neurons and I, interneurons. (e–g) Immunostaining of 24 hpf embryos with an antibody against islet-1 marks (lateral views of the anterior regions of the embryos) RB neurons in control siRNA-injected (e), cdk5 siRNA-injected (f) embryos. Arrowheads indicate some of the RB neurons. Quantitative analyzes of islet-1 positive neurons are presented (g)
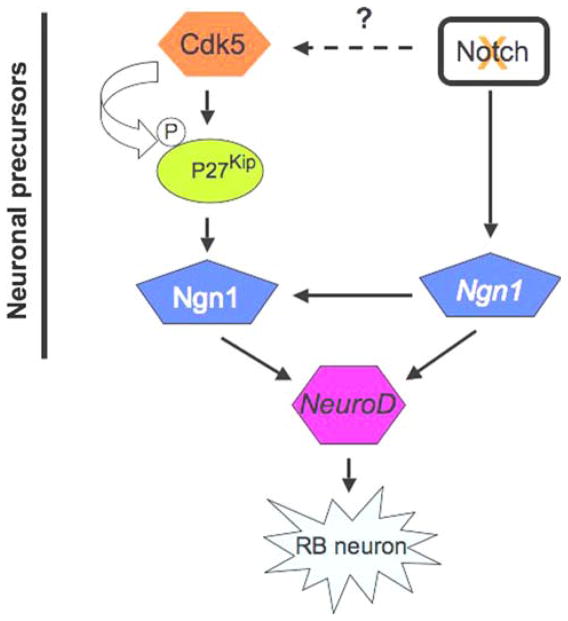
Most recently, phosphorylation and stabilization of p27Kip1 by cdk5 has been reported in the mammalian system [[84], and our unpublished data]. It’s homolog, the cdk inhibitor p27Xic1 in Xenopus, stabilizes neurogenin1 and promotes early neuron development [85], whereas Notch suppresses p27Xic1 expression in both the myotome and the lateral stripe of primary neurons [86]. Mammals express three members of the Cip/Kip family members, p21Cip1, p27Kip1 and p57Kip2 [87]. However, redundancy and inaccessibility has made it impossible to precisely determine the function of these Cip/Kip family members in nervous system development of null mouse models [88–90]. Whether cdk5 regulates early neurogenesis in zebrafish by modulating p27Kip function warrants further investigation. Here, we present a possible scenario of how cdk5 might be involved in zebrafish RB neuron differentiation from neuronal precursors along with the well-studied pathway of Notch inhibition resulting in RB neuron differentiation (Fig. 3). It is known that Notch inhibition induces Ngn1 gene expression, which, in turn, can translate Ngn1 protein that has been shown in Xenopus to be stabilized by p27Kip. Ngn1 is an inducer of NeuroD and both are key players in RB neuron differentiation.
Fig. 3.
Proposed model shows a possible role of cdk5 in Rohon-Beard neuron differentiation. Cdk5 stabilizes p27Kip by phosphorylating it in mammalian cells. On the other hand, in Xenopus, p27Kip has been shown to stabilize Ngn1 protein leading to neuronal differentiation. Ngn1 is known to induce NeuroD. This model is presented with an established pathway, in which inhibition of Notch signaling is known to promote RB neuron generation via upregulation of Ngn1 and subsequently, neuroD
Recently, we have shown that a Notch inhibitor, DAPT, downregulates cdk5 activity in rat primary neurons [91]. However, it’s not known whether DAPT inhibits cdk5 activity in neuronal precursors and what would the fate of the neuronal precursors be, if such were the case. It is likely that in the neuronal precursors, Notch inhibition, during the process of inducing neuronal differentiation, might not suppress cdk5 activity. This assumption is based on the fact that cdk5 is essential for complete differentiation of neurons from the neuronal precursors in mammals [33]. During neuronal differentiation, it is possible that the impact of the proneural gene Ngn1 expression, induced by Notch inhibition, becomes more pronounced by stabilization of Ngn1 protein by p27Kip1, the latter being stabilized by cdk5 phosphorylation. Thus, a synergistic effect of Notch inhibition that regulates the expression of the pro-neural gene, Ngn1, and cdk5 activity that could stabilize the Ngn1 protein, during RB neuron differentiation seems plausible (Fig. 3).
Cdk5 and RB Neuron Death
As in amphibians, zebrafish RB neurons die over a protracted period of time [92, 93]. A number of factors may be responsible for the death of the RB neurons. It has been shown recently that zebrafish RB neuron death is correlated with reduced expression of neurotrophin-3 (NT-3) receptor, trkC1, suggesting that RB neuron survival involves the neurotrophin NT-3 [92, 93]. RB neuron death also appears delayed by inhibiting caspase activity, suggesting that RB death is caspase-dependent [92, 93]. A decrease in sodium current-mediated electrical activity has been shown to cause RB neuron death [94]. Recently, our data suggest that a reduction in cdk5 levels results in RB neuron death as seen in 24 h zebrafish embryos [77] and also causes a reduction in the trigeminal ganglia primary sensory neurons [78]. Although zebrafish RB neurons begin showing signs of programmed cell death, such as DNA fragmentation, as early as the first day of development, the cells remain present for considerably longer and degenerate over a protracted period of time and unlike in amphibians, independent of any developmental link to DRG neurons [93].
The DNA of RB neurons is fragmented before neuronal degeneration and this can be detected using the TUNEL assay [95]. It is also of interest to note that a drastic reduction in TUNEL-positive RB neurons in the cdk5 siRNA and cdk5 mRNA-co-injected embryos occurs compared to the cdk5 siRNA-injected embryos, coincidentally with an up-regulation in cdk5 catalytic activity [77]. These results support the notion that cdk5 may have a role in RB neuron survival. A tight regulation of cdk5 expression during RB neuron development, and their (RB neurons’) disappearance in two days, must occur for the normal development of zebrafish embryos. In this context, other signaling cascades, along with cdk5, could have a major role in the proper development of the spinal sensory nervous system.
Previously, we cloned the zebrafish ortholog of human cdk5 [78]. The zebrafish cdk5 is strikingly homologous (97% identity) to all vertebrate cdk5s, conservation consistent with the view that this kinase plays a major role in development and function of the vertebrate nervous systems. Compared with other vertebrates, particularly the closely related Xenopus [96], zebrafish cdk5 protein is expressed very early during cleavage, before the mid-blastula transition. Moreover, cdk5 kinase activity, although low, was measurable during the first 12 h of development. Cdk5 is present maternally in the early embryos much before muscle or neuronal differentiation occurs [78]. In contrast, cdk5 in Xenopus, first appears after the mid blastula transition [46]. Using siRNA-mediated cdk5 knockdown and cdk5 mRNA misexpression studies, we have shown that cdk5 influences RB neuron survival although the exact mechanism remains to be understood [77]. Immunostaining with the islet-1 antibody revealed the reduction of RB neurons in the cdk5 siRNA-injected embryos that can be rescued when cdk5 mRNA is coinjected. These studies were further supported by our observation that the primary sensory neurons of the trigeminal ganglia of the peripheral nervous system were also depleted in the cdk5 siRNA-injected embryos [78].
How cdk5 regulates RB neuron death is not known. Whether during RB neuron death, cdk5 level decreases prior to the onset of apoptosis remains to be elucidated. This issue is complicated since in situ hybridization analyzes do not reveal a detectable expression of cdk5 in the RB neurons of zebrafish at 24 h of development. In this context, whether cdk5 functions cell autonomously or non-autonomously needs to be examined. Another possibility is that the effect of cdk5 could be indirect based on cdk5’s role in exocytosis of growth factors [28, 97–99]. P39-mediated cdk5 activation seems to positively regulate glucose-dependent insulin secretion, but p35-mediated cdk5 activation seems to negatively regulate glucose-dependent insulin secretion [99–101]. Cdk5/p39 stimulates Ca2+-dependent exocytosis in primary β-cells via the phosphorylation of Munc18-1 [99]. It has been reported that antibodies, which deplete NT-3, induce RB cell death while exogenous application of NT-3 reduces death and RB neurons express the neurotrophin receptor trkC1 [93]. Thus, Neurotrophin-3-triggered RB neuron survival may be mediated via cdk5. For example, if the neurotrophin secretion is compromised in the neighboring cells in cdk5 knockdown embryos, the RB neuron survival may be in jeopardy, suggesting a cell non-autonomous mechanism triggered by cdk5 in RB neuron survival. Yet another possibility is the upregulation of the anti-apoptotic molecule, Bcl-2, by cdk5. Inhibition of cdk5 has been shown to attenuate Bcl-2 expression in mammalian cells [53]. The possibility of cdk5-induced Bcl-2 expresssion in RB neurons thus leading to their survival in a cell-autonomous manner remains to be tested. Based on the available studies on the programmed cell death of RB neurons, a summary of possible scenarios of potential pathways operating to bring about this important developmental process is presented in Fig. 4, where we show a potential involvement of cdk5 in RB neuron survival dependent on the regulation of neurotrophin secretion from adjacent non-neuronal cells.
Fig. 4.
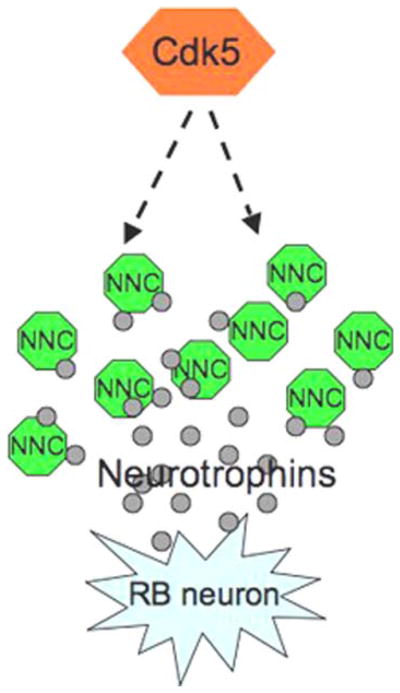
Model shows a potential role of cdk5 in Rohon-Beard (RB) neuron survival in a cell non-autonomous pathway. Neurotrophin (especially NT-3) secretion by non-neuronal neighboring cells (NNC), such as muscle cells, may be regulated by cdk5, thus providing trophic support to the embedded RB neurons
Conclusions
Cdk5’s resume as a multifunctional kinase that includes a range of its participation in cell death (pro-apoptotic), cell survival (anti-apoptotic), neuron migration, and nervous system development, is topped by its general regulation of endocytosis/exocytosis in both neuronal and non-neuronal cells. The latter, which could regulate neuronal growth factor secretion, may also have a major role in neuronal survival. In zebrafish, although we have reported that in 24 h of development, RB neurons are scanty in the siRNA-mediated cdk5 knockdown embryos, suggesting a role of cdk5 in RB neuron survival, the exact mechanism(s) of this process remains to be determined. In 11.5 h of development, when early neurons begin to develop, we observed that the primary neurons were significantly less in the trigeminal ganglion placode of the cdk5 knockdown embryos. RB primary neurons were also reduced in number in these embryos, suggesting a possible role of cdk5 during early primary neuron differentiation. We speculate that cdk5 may impart dual functions on RB neuron development; an early role during their differentiation from the neuronal precursors, and a later role in their survival as fully differentiated neurons.
Acknowledgments
This work was supported by intramural funds from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, USA. We thank Drs. Ajay Chitnis and Moloy Goswami (NICHD, NIH) for providing the HuC plasmid and helpful discussions.
Contributor Information
Jyotshnabala Kanungo, Laboratory of Neurochemistry, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bldg. 49, Rm 2A28, 9000 Rockville Pike, Bethesda, MD 20892-4130, USA.
Ya-Li Zheng, Laboratory of Neurochemistry, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bldg. 49, Rm 2A28, 9000 Rockville Pike, Bethesda, MD 20892-4130, USA.
Bibhutibhushan Mishra, Laboratory of Developmental Neurobiology, Georgetown University School of Medicine, 3900 Reservoir Road NW, Medical/Dental Building, Room NW 209-213, Washington, DC 20007, USA.
Harish C. Pant, Laboratory of Neurochemistry, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bldg. 49, Rm 2A28, 9000 Rockville Pike, Bethesda, MD 20892-4130, USA
References
- 1.Meyerson M, Enders GH, Wu CL, et al. A family of human cdc2-related protein kinases. EMBO J. 1992;11(8):2909–2917. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2(10):749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Kipreos ET. Evolution of cyclin-dependent kinases (CDKs) and CDK-activating kinases (CAKs): differential conservation of CAKs in yeast and metazoa. Mol Biol Evol. 2000;17(7):1061–1074. doi: 10.1093/oxfordjournals.molbev.a026387. [DOI] [PubMed] [Google Scholar]
- 4.Veeranna, Grant P, Pant HC. Expression of p67 (Munc-18), Cdk5, P-NFH and syntaxin during development of the rat cerebellum. Dev Neurosci. 1997;19(2):172–183. doi: 10.1159/000111203. [DOI] [PubMed] [Google Scholar]
- 5.Veeranna, Shetty KT, Amin N, Grant P, Albers RW, Pant HC. Inhibition of neuronal cyclin-dependent kinase-5 by staurosporine and purine analogs is independent of activation by Munc-18. Neurochem Res. 1996;21(5):629–636. doi: 10.1007/BF02527763. [DOI] [PubMed] [Google Scholar]
- 6.Lew J, Huang QQ, Qi Z, et al. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994;371(6496):423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- 7.Tsai LH, Delalle I, Caviness VS, Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371(6496):419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 8.Zheng M, Leung CL, Liem RK. Region-specific expression of cyclin-dependent kinase 5 (cdk5) and its activators, p35 and p39, in the developing and adult rat central nervous system. J Neurobiol. 1998;35(2):141–159. doi: 10.1002/(SICI)1097-4695(199805)35:2<141::AID-NEU2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Ching YP, Pang AS, Lam WH, Qi RZ, Wang JH. Identification of a neuronal Cdk5 activator-binding protein as Cdk5 inhibitor. J Biol Chem. 2002;277(18):15237–15240. doi: 10.1074/jbc.C200032200. [DOI] [PubMed] [Google Scholar]
- 10.Lim HY, Seow KT, Li Q, Kesuma D, Wang JH, Qi RZ. Structural insights into Cdk5 activation by a neuronal Cdk5 activator. Biochem Biophys Res Commun. 2001;285(1):77–83. doi: 10.1006/bbrc.2001.5086. [DOI] [PubMed] [Google Scholar]
- 11.Moorthamer M, Chaudhuri B. Identification of ribosomal protein L34 as a novel Cdk5 inhibitor. Biochem Biophys Res Commun. 1999;255(3):631–638. doi: 10.1006/bbrc.1999.0145. [DOI] [PubMed] [Google Scholar]
- 12.Moorthamer M, Zumstein-Mecker S, Chaudhuri B. DNA binding protein dbpA binds Cdk5 and inhibits its activity. FEBS Lett. 1999;446(2–3):343–350. doi: 10.1016/S0014-5793(99)00248-3. [DOI] [PubMed] [Google Scholar]
- 13.Saito T, Hisanaga S. Regulation of Cdk5 activity in post-mitotic neurons. Seikagaku. 2001;73(4):276–278. [PubMed] [Google Scholar]
- 14.Zhu YS, Saito T, Asada A, Maekawa S, Hisanaga S. Activation of latent cyclin-dependent kinase 5 (Cdk5)-p35 complexes by membrane dissociation. J Neurochem. 2005;94(6):1535–1545. doi: 10.1111/j.1471-4159.2005.03301.x. [DOI] [PubMed] [Google Scholar]
- 15.Kawauchi T, Chihama K, Nishimura YV, Nabeshima Y, Hoshino M. MAP1B phosphorylation is differentially regulated by Cdk5/p35, Cdk5/p25, and JNK. Biochem Biophys Res Commun. 2005;331(1):50–55. doi: 10.1016/j.bbrc.2005.03.132. [DOI] [PubMed] [Google Scholar]
- 16.Liu SJ, Fang ZY, Yang Y, Deng HM, Wang JZ. Alzheimer-like phosphorylation of tau and neurofilament induced by cocaine in vivo. Acta Pharmacol Sin. 2003;24(6):512–518. [PubMed] [Google Scholar]
- 17.Connell-Crowley L, Le Gall M, Vo DJ, Giniger E. The cyclin-dependent kinase Cdk5 controls multiple aspects of axon patterning in vivo. Curr Biol. 2000;10(10):599–602. doi: 10.1016/s0960-9822(00)00487-5. [DOI] [PubMed] [Google Scholar]
- 18.Nikolic M, Dudek H, Kwon YT, Ramos YF, Tsai LH. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996;10(7):816–825. doi: 10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- 19.Ohshima T, Ward JM, Huh CG, et al. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA. 1996;93(20):11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith PD, Crocker SJ, Jackson-Lewis V, et al. Cyclin-dependent kinase 5 is a mediator of dopaminergic neuron loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci USA. 2003;100(23):13650–13655. doi: 10.1073/pnas.2232515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai LH, Takahashi T, Caviness VS, Jr, Harlow E. Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development. 1993;119(4):1029–1040. doi: 10.1242/dev.119.4.1029. [DOI] [PubMed] [Google Scholar]
- 22.Sharma M, Sharma P, Pant HC. CDK-5-mediated neurofilament phosphorylation in SHSY5Y human neuroblastoma cells. J Neurochem. 1999;73(1):79–86. doi: 10.1046/j.1471-4159.1999.0730079.x. [DOI] [PubMed] [Google Scholar]
- 23.Benavides DR, Bibb JA. Role of Cdk5 in drug abuse and plasticity. Ann N Y Acad Sci. 2004;1025:335–344. doi: 10.1196/annals.1316.041. [DOI] [PubMed] [Google Scholar]
- 24.Bibb JA, Snyder GL, Nishi A, et al. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402(6762):669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- 25.Shelton SB, Johnson GV. Cyclin-dependent kinase-5 in neurodegeneration. J Neurochem. 2004;88(6):1313–1326. doi: 10.1111/j.1471-4159.2003.02328.x. [DOI] [PubMed] [Google Scholar]
- 26.Hahn CM, Kleinholz H, Koester MP, Grieser S, Thelen K, Pollerberg GE. Role of cyclin-dependent kinase 5 and its activator P35 in local axon and growth cone stabilization. Neuroscience. 2005;134(2):449–465. doi: 10.1016/j.neuroscience.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 27.Kwon YT, Tsai LH, Crandall JE. Callosal axon guidance defects in p35(−/−) mice. J Comp Neurol. 1999;415(2):218–229. doi: 10.1002/(SICI)1096-9861(19991213)415:2<218::AID-CNE6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 28.Amin ND, Zheng YL, Kesavapany S, et al. Cyclin-dependent kinase 5 phosphorylation of human septin SEPT5 (hCDCrel-1) modulates exocytosis. J Neurosci. 2008;28(14):3631–3643. doi: 10.1523/JNEUROSCI.0453-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosales JL, Lee KY. Extraneuronal roles of cyclin-dependent kinase 5. Bioessays. 2006;28(10):1023–1034. doi: 10.1002/bies.20473. [DOI] [PubMed] [Google Scholar]
- 30.Fu AK, Ip FC, Fu WY, et al. Aberrant motor axon projection, acetylcholine receptor clustering, and neurotransmission in cyclin-dependent kinase 5 null mice. Proc Natl Acad Sci USA. 2005;102(42):15224–15229. doi: 10.1073/pnas.0507678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansson JU, Lilja L, Chen XL, et al. Cyclin-dependent kinase 5 activators p35 and p39 facilitate formation of functional synapses. Brain Res Mol Brain Res. 2005;138(2):215–227. doi: 10.1016/j.molbrainres.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Lin W, Dominguez B, Yang J, et al. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron. 2005;46(4):569–579. doi: 10.1016/j.neuron.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Cicero S, Herrup K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J Neurosci. 2005;25(42):9658–9668. doi: 10.1523/JNEUROSCI.1773-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazaro JB, Kitzmann M, Poul MA, Vandromme M, Lamb NJ, Fernandez A. Cyclin dependent kinase 5, cdk5, is a positive regulator of myogenesis in mouse C2 cells. J Cell Sci. 1997;110(Pt 10):1251–1260. doi: 10.1242/jcs.110.10.1251. [DOI] [PubMed] [Google Scholar]
- 35.Ko J, Humbert S, Bronson RT, et al. p35 and p39 are essential for cyclin-dependent kinase 5 function during neuro-development. J Neurosci. 2001;21(17):6758–6771. doi: 10.1523/JNEUROSCI.21-17-06758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ackerley S, Thornhill P, Grierson AJ, et al. Neurofilament heavy chain side arm phosphorylation regulates axonal transport of neurofilaments. J Cell Biol. 2003;161(3):489–495. doi: 10.1083/jcb.200303138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bu B, Li J, Davies P, Vincent I. Deregulation of cdk5, hyperphosphorylation, and cytoskeletal pathology in the Niemann-Pick type C murine model. J Neurosci. 2002;22(15):6515–6525. doi: 10.1523/JNEUROSCI.22-15-06515.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grant P, Sharma P, Pant HC. Cyclin-dependent protein kinase 5 (Cdk5) and the regulation of neurofilament metabolism. Eur J Biochem. 2001;268(6):1534–1546. doi: 10.1046/j.1432-1327.2001.02025.x. [DOI] [PubMed] [Google Scholar]
- 39.Li BS, Zhang L, Gu J, Amin ND, Pant HC. Integrin alpha(1) beta(1)-mediated activation of cyclin-dependent kinase 5 activity is involved in neurite outgrowth and human neurofilament protein H Lys-Ser-Pro tail domain phosphorylation. J Neurosci. 2000;20(16):6055–6062. doi: 10.1523/JNEUROSCI.20-16-06055.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pant AC, Veeranna, Pant HC, Amin N. Phosphorylation of human high molecular weight neurofilament protein (hNF-H) by neuronal cyclin-dependent kinase 5 (cdk5) Brain Res. 1997;765(2):259–266. doi: 10.1016/S0006-8993(97)00561-1. [DOI] [PubMed] [Google Scholar]
- 41.Sharma P, Sharma M, Amin ND, Albers RW, Pant HC. Regulation of cyclin-dependent kinase 5 catalytic activity by phosphorylation. Proc Natl Acad Sci USA. 1999;96(20):11156–11160. doi: 10.1073/pnas.96.20.11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shea TB, Yabe JT, Ortiz D, et al. Cdk5 regulates axonal transport and phosphorylation of neurofilaments in cultured neurons. J Cell Sci. 2004;117(Pt 6):933–941. doi: 10.1242/jcs.00785. [DOI] [PubMed] [Google Scholar]
- 43.Shea TB, Zheng YL, Ortiz D, Pant HC. Cyclin-dependent kinase 5 increases perikaryal neurofilament phosphorylation and inhibits neurofilament axonal transport in response to oxidative stress. J Neurosci Res. 2004;76(6):795–800. doi: 10.1002/jnr.20099. [DOI] [PubMed] [Google Scholar]
- 44.Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18(1):29–42. doi: 10.1016/S0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- 45.Patel LS, Wenzel HJ, Schwartzkroin PA. Physiological and morphological characterization of dentate granule cells in the p35 knock-out mouse hippocampus: evidence for an epileptic circuit. J Neurosci. 2004;24(41):9005–9014. doi: 10.1523/JNEUROSCI.2943-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gervasi C, Szaro BG. The Xenopus laevis homologue to the neuronal cyclin-dependent kinase (cdk5) is expressed in embryos by gastrulation. Brain Res Mol Brain Res. 1995;33(2):192–200. doi: 10.1016/0169-328X(95)00109-6. [DOI] [PubMed] [Google Scholar]
- 47.Philpott A, Porro EB, Kirschner MW, Tsai LH. The role of cyclin-dependent kinase 5 and a novel regulatory subunit in regulating muscle differentiation and patterning. Genes Dev. 1997;11(11):1409–1421. doi: 10.1101/gad.11.11.1409. [DOI] [PubMed] [Google Scholar]
- 48.Philpott A, Tsai L, Kirschner MW. Neuronal differentiation and patterning in Xenopus: the role of cdk5 and a novel activator xp35.2. Dev Biol. 1999;207(1):119–132. doi: 10.1006/dbio.1998.9146. [DOI] [PubMed] [Google Scholar]
- 49.Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402(6762):615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Liu S, Fu Y, Wang JH, Lu Y. Cdk5 activation induces hippocampal CA1 cell death by directly phosphorylating NMDA receptors. Nat Neurosci. 2003;6(10):1039–1047. doi: 10.1038/nn1119. [DOI] [PubMed] [Google Scholar]
- 51.Harada T, Morooka T, Ogawa S, Nishida E. ERK induces p35, a neuron-specific activator of Cdk5, through induction of Egr1. Nat Cell Biol. 2001;3(5):453–459. doi: 10.1038/35074516. [DOI] [PubMed] [Google Scholar]
- 52.Li BS, Zhang L, Takahashi S, et al. Cyclin-dependent kinase 5 prevents neuronal apoptosis by negative regulation of c-Jun N-terminal kinase 3. EMBO J. 2002;21(3):324–333. doi: 10.1093/emboj/21.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y, Xie WY, He Y, et al. Role of CDK5 in neuro-protection from serum deprivation by mu-opioid receptor agonist. Exp Neurol. 2006;202(2):313–323. doi: 10.1016/j.expneurol.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Giese KP, Ris L, Plattner F. Is there a role of the cyclin-dependent kinase 5 activator p25 in Alzheimer’s disease? NeuroReport. 2005;16(16):1725–1730. doi: 10.1097/01.wnr.0000185019.67434.d2. [DOI] [PubMed] [Google Scholar]
- 55.Clarke JD, Hayes BP, Hunt SP, Roberts A. Sensory physiology, anatomy and immunohistochemistry of Rohon-Beard neurones in embryos of Xenopus laevis. J Physiol. 1984;348:511–525. doi: 10.1113/jphysiol.1984.sp015122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cowan WM, Fawcett JW, O’Leary DD, Stanfield BB. Regressive events in neurogenesis. Science. 1984;225(4668):1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- 57.Oppenheim RW, Prevette D, Yin QW, Collins F, MacDonald J. Control of embryonic motoneuron survival in vivo by ciliary neurotrophic factor. Science. 1991;251(5001):1616–1618. doi: 10.1126/science.2011743. [DOI] [PubMed] [Google Scholar]
- 58.Neystat M, Rzhetskaya M, Oo TF, et al. Expression of cyclin-dependent kinase 5 and its activator p35 in models of induced apoptotic death in neurons of the substantia nigra in vivo. J Neurochem. 2001;77(6):1611–1625. doi: 10.1046/j.1471-4159.2001.00376.x. [DOI] [PubMed] [Google Scholar]
- 59.Zhu Y, Lin L, Kim S, Quaglino D, Lockshin RA, Zakeri Z. Cyclin dependent kinase 5 and its interacting proteins in cell death induced in vivo by cyclophosphamide in developing mouse embryos. Cell Death Differ. 2002;9(4):421–430. doi: 10.1038/sj.cdd.4400967. [DOI] [PubMed] [Google Scholar]
- 60.Henchcliffe C, Burke RE. Increased expression of cyclin-dependent kinase 5 in induced apoptotic neuron death in rat substantia nigra. Neurosci Lett. 1997;230(1):41–44. doi: 10.1016/S0304-3940(97)00472-2. [DOI] [PubMed] [Google Scholar]
- 61.Morris EJ, Keramaris E, Rideout HJ, et al. Cyclin-dependent kinases and P53 pathways are activated independently and mediate Bax activation in neurons after DNA damage. J Neurosci. 2001;21(14):5017–5026. doi: 10.1523/JNEUROSCI.21-14-05017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shirvan A, Ziv I, Zilkha-Falb R, Machlyn T, Barzilai A, Melamed E. Expression of cell cycle-related genes during neuronal apoptosis: is there a distinct pattern? Neurochem Res. 1998;23(5):767–777. doi: 10.1023/A:1022415611545. [DOI] [PubMed] [Google Scholar]
- 63.Zhang BF, Peng FF, Zhang W, Shen H, Wu SB, Wu DC. Involvement of cyclin dependent kinase 5 and its activator p35 in staurosporine-induced apoptosis of cortical neurons. Acta Pharmacol Sin. 2004;25(9):1105–1111. [PubMed] [Google Scholar]
- 64.Zheng YL, Kesavapany S, Gravell M, et al. A Cdk5 inhibitory peptide reduces tau hyperphosphorylation and apoptosis in neurons. EMBO J. 2005;24(1):209–220. doi: 10.1038/sj.emboj.7600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kerokoski P, Suuronen T, Salminen A, Soininen H, Pirttila T. The levels of cdk5 and p35 proteins and tau phosphorylation are reduced during neuronal apoptosis. Biochem Biophys Res Commun. 2001;280(4):998–1002. doi: 10.1006/bbrc.2001.4240. [DOI] [PubMed] [Google Scholar]
- 66.Penaloza C, Lin L, Lockshin RA, Zakeri Z. Cell death in development: shaping the embryo. Histochem Cell Biol. 2006;126(2):149–158. doi: 10.1007/s00418-006-0214-1. [DOI] [PubMed] [Google Scholar]
- 67.Alvarez A, Toro R, Caceres A, Maccioni RB. Inhibition of tau phosphorylating protein kinase cdk5 prevents beta-amyloid-induced neuronal death. FEBS Lett. 1999;459(3):421–426. doi: 10.1016/S0014-5793(99)01279-X. [DOI] [PubMed] [Google Scholar]
- 68.Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405(6784):360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- 69.Zheng YL, Li BS, Amin ND, Albers W, Pant HC. A peptide derived from cyclin-dependent kinase activator (p35) specifically inhibits Cdk5 activity and phosphorylation of tau protein in transfected cells. Eur J Biochem. 2002;269(18):4427–4434. doi: 10.1046/j.1432-1033.2002.03133.x. [DOI] [PubMed] [Google Scholar]
- 70.Davies AM. Regulation of neuronal survival and death by extracellular signals during development. EMBO J. 2003;22(11):2537–2545. doi: 10.1093/emboj/cdg254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hughes A. The development of the primary sensory system in Xenopus laevis (Daudin) J Anat. 1957;91(3):323–338. [PMC free article] [PubMed] [Google Scholar]
- 72.Kollros JJ, Bovbjerg AM. Growth and death of Rohon-Beard cells in Rana pipiens and Ceratophrys ornata. J Morphol. 1997;232(1):67–78. doi: 10.1002/(SICI)1097-4687(199704)232:1<67::AID-JMOR4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 73.Lamborghini JE. Disappearance of Rohon-Beard neurons from the spinal cord of larval Xenopus laevis. J Comp Neurol. 1987;264(1):47–55. doi: 10.1002/cne.902640105. [DOI] [PubMed] [Google Scholar]
- 74.Metcalfe WK, Myers PZ, Trevarrow B, Bass MB, Kimmel CB. Primary neurons that express the L2/HNK-1 carbohydrate during early development in the zebrafish. Development. 1990;110(2):491–504. doi: 10.1242/dev.110.2.491. [DOI] [PubMed] [Google Scholar]
- 75.Cornell RA, Eisen JS. Delta signaling mediates segregation of neural crest and spinal sensory neurons from zebrafish lateral neural plate. Development. 2000;127(13):2873–2882. doi: 10.1242/dev.127.13.2873. [DOI] [PubMed] [Google Scholar]
- 76.Eichler VB, Porter RA. Rohon-Beard cells in frog development: a study of temporal and spatial changes in a transient cell population. J Comp Neurol. 1981;203(1):121–130. doi: 10.1002/cne.902030110. [DOI] [PubMed] [Google Scholar]
- 77.Kanungo J, Li BS, Zheng Y, Pant HC. Cyclin-dependent kinase 5 influences Rohon-Beard neuron survival in zebrafish. J Neurochem. 2006;99(1):251–259. doi: 10.1111/j.1471-4159.2006.04114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kanungo J, Li BS, Goswami M, Zheng YL, Ramchandran R, Pant HC. Cloning and characterization of zebrafish (Danio rerio) cyclin-dependent kinase 5. Neurosci Lett. 2007;412(3):233–238. doi: 10.1016/j.neulet.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blader P, Fischer N, Gradwohl G, Guillemot F, Strahle U. The activity of neurogenin1 is controlled by local cues in the zebrafish embryo. Development. 1997;124(22):4557–4569. doi: 10.1242/dev.124.22.4557. [DOI] [PubMed] [Google Scholar]
- 80.Kim CH, Bae YK, Yamanaka Y, et al. Overexpression of neurogenin induces ectopic expression of HuC in zebrafish. Neurosci Lett. 1997;239(2–3):113–116. doi: 10.1016/S0304-3940(97)00908-7. [DOI] [PubMed] [Google Scholar]
- 81.Lee JE. Basic helix-loop-helix genes in neural development. Curr Opin Neurobiol. 1997;7(1):13–20. doi: 10.1016/S0959-4388(97)80115-8. [DOI] [PubMed] [Google Scholar]
- 82.Ma Q, Kintner C, Anderson DJ. Identification of neurogenin, a vertebrate neuronal determination gene. Cell. 1996;87(1):43–52. doi: 10.1016/S0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- 83.Kimmel CB, Hatta K, Eisen JS. Genetic control of primary neuronal development in zebrafish. Development Suppl. 1991;2:47–57. [PubMed] [Google Scholar]
- 84.Kawauchi T, Chihama K, Nabeshima Y, Hoshino M. Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat Cell Biol. 2006;8(1):17–26. doi: 10.1038/ncb1338. [DOI] [PubMed] [Google Scholar]
- 85.Vernon AE, Devine C, Philpott A. The cdk inhibitor p27Xic1 is required for differentiation of primary neurones in Xenopus. Development. 2003;130(1):85–92. doi: 10.1242/dev.00193. [DOI] [PubMed] [Google Scholar]
- 86.Vernon AE, Movassagh M, Horan I, Wise H, Ohnuma S, Philpott A. Notch targets the Cdk inhibitor Xic1 to regulate differentiation but not the cell cycle in neurons. EMBO Rep. 2006;7(6):643–648. doi: 10.1038/sj.embor.7400691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13(12):1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 88.Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82(4):675–684. doi: 10.1016/0092-8674(95)90039-X. [DOI] [PubMed] [Google Scholar]
- 89.Nakayama K, Ishida N, Shirane M, et al. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85(5):707–720. doi: 10.1016/S0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 90.Yan Y, Frisen J, Lee MH, Massague J, Barbacid M. Ablation of the CDK inhibitor p57Kip2 results in increased apoptosis and delayed differentiation during mouse development. Genes Dev. 1997;11(8):973–983. doi: 10.1101/gad.11.8.973. [DOI] [PubMed] [Google Scholar]
- 91.Kanungo J, Zheng YL, Amin ND, Pant HC. The Notch signaling inhibitor DAPT down-regulates cdk5 activity and modulates the distribution of neuronal cytoskeletal proteins. J Neurochem. 2008;106(5):2236–2248. doi: 10.1111/j.1471-4159.2008.05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cole LK, Ross LS. Apoptosis in the developing zebrafish embryo. Dev Biol. 2001;240(1):123–142. doi: 10.1006/dbio.2001.0432. [DOI] [PubMed] [Google Scholar]
- 93.Williams JA, Barrios A, Gatchalian C, Rubin L, Wilson SW, Holder N. Programmed cell death in zebrafish rohon beard neurons is influenced by TrkC1/NT-3 signaling. Dev Biol. 2000;226(2):220–230. doi: 10.1006/dbio.2000.9860. [DOI] [PubMed] [Google Scholar]
- 94.Svoboda KR, Linares AE, Ribera AB. Activity regulates programmed cell death of zebrafish Rohon-Beard neurons. Development. 2001;128(18):3511–3520. doi: 10.1242/dev.128.18.3511. [DOI] [PubMed] [Google Scholar]
- 95.Reyes R, Haendel M, Grant D, Melancon E, Eisen JS. Slow degeneration of zebrafish Rohon-Beard neurons during programmed cell death. Dev Dyn. 2004;229(1):30–41. doi: 10.1002/dvdy.10488. [DOI] [PubMed] [Google Scholar]
- 96.Szaro BG, Lee VM, Gainer H. Spatial and temporal expression of phosphorylated and non-phosphorylated forms of neurofilament proteins in the developing nervous system of Xenopus laevis. Brain Res Dev Brain Res. 1989;48(1):87–103. doi: 10.1016/0165-3806(89)90095-3. [DOI] [PubMed] [Google Scholar]
- 97.Chergui K, Svenningsson P, Greengard P. Cyclin-dependent kinase 5 regulates dopaminergic and glutamatergic transmission in the striatum. Proc Natl Acad Sci USA. 2004;101(7):2191–2196. doi: 10.1073/pnas.0308652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee HY, Jung H, Jang IH, Suh PG, Ryu SH. Cdk5 phosphorylates PLD2 to mediate EGF-dependent insulin secretion. Cell Signal. 2008;20(10):1787–1794. doi: 10.1016/j.cellsig.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 99.Lilja L, Johansson JU, Gromada J, et al. Cyclin-dependent kinase 5 associated with p39 promotes Munc18-1 phosphorylation and Ca(2+)-dependent exocytosis. J Biol Chem. 2004;279(28):29534–29541. doi: 10.1074/jbc.M312711200. [DOI] [PubMed] [Google Scholar]
- 100.Lilja L, Yang SN, Webb DL, Juntti-Berggren L, Berggren PO, Bark C. Cyclin-dependent kinase 5 promotes insulin exocytosis. J Biol Chem. 2001;276(36):34199–34205. doi: 10.1074/jbc.M103776200. [DOI] [PubMed] [Google Scholar]
- 101.Wei FY, Nagashima K, Ohshima T, et al. Cdk5-dependent regulation of glucose-stimulated insulin secretion. Nat Med. 2005;11(10):1104–1108. doi: 10.1038/nm1299. [DOI] [PubMed] [Google Scholar]
- 102.Wilkinson DG, Nieto MA. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-W. [DOI] [PubMed] [Google Scholar]