Abstract
Manganese-based nanoparticles (NPs) have recently attracted much attention in the field of biomedical imaging due to their impressive enhanced T1 contrast ability. Although the reported manganese-based NPs have exhibited good imaging capabilities as contrast agents, it is still urgent to develop novel multifunctional manganese-based imaging probes for future biomedical imaging, especially PET/MRI probes. Herein, we present chelator-free zirconium-89 (89Zr, t1/2: 78.4 h) labeling of manganese oxide NPs (Mn3O4@PEG) with ~78% labeling yield and good stability. Serial positron emission tomography (PET) and magnetic resonance imaging (MRI) studies non-invasively assessed the biodistribution patterns of the NPs and the feasibility of in vivo dual-modality imaging and lymph-node mapping. Since Mn3O4 NPs exhibited desirable properties for enhanced T1 imaging and the simplicity of chelator-free radiolabeling, [89Zr]Mn3O4@PEG NPs offer a novel, simple, safe and accurate nanoplatforms for future precise cancer imaging and diagnosis.
Keywords: Manganese Oxide Nanoparticles, Positron Emission Tomography, Magnetic Resonance Imaging, Zirconium-89, Chelator-Free
INTRODUCTION
In the past decades, molecular imaging techniques have demonstrated great value in the early detection and diagnosis of disease, which is attributed to their fast and accurate diagnostic abilities at the molecular and cellular levels.1–3 Among them, the integration of PET and MRI has been under rapid development and is currently in clinical trials for applications in cancer detection and diagnosis due to the very high sensitivity of PET and the ultra-high spatial resolution of MRI.4,5 To take advantage of the hybrid PET/MRI imaging technology, design and synthesis of novel multifunctional PET/MRI nanoprobes has been a hot research topic since their emergence in the last decade.6–8 As one half of the PET/MRI nanoprobes, current MRI contrast agents are commonly in the forms of T1-positive contrast agents and T2-negative contrast agents.9 In particular. T2 contrast agents based on superparamagnetic iron oxide NPs (SPIONs) have been widely used as the MRI contrast component in PET/MRI probes over the last two decades and a few have advanced into clinical trials or received the approval of the Food and Drug Administration (FDA) of the United States.10–12 However, these magnetic-based PET/MRI nanoprobes have been somewhat limited in clinical applications due to their drawbacks of signal-decreasing effects and high susceptibility. Therefore, it is desirable to develop novel PET/MRI nanoprobes with higher T1 or T2 relaxivity to meet the future clinical requirements.
Recently, manganese-based NPs have emerged as a new class of T1-weighted contrast agents thanks to their relatively high magnetization spins and fast water proton exchange rates.13 Meanwhile, manganese-based agents also exhibit good biocompatibility and low side effects because manganese itself is an essential component of cells and a cofactor for many metabolic enzymes, which is attributed to the dissolution of the manganese oxide nanocrystal at low pH.13,14 Most importantly, manganese-based NPs with good crystallinity and uniformity have been demonstrated to be easily synthesized on a large scale under mild and ambient reaction conditions.15 This new promising class of MRI contrast agents, particularly manganese oxides, opens a new direction in biomedical imaging and tumor-targeted diagnosis for future medicine.14,16 Progress in developing multimodality imaging probes and their applications in biomedical imaging have been reported in recent years. For example, solid and hollow MnO-based T1 NP contrast agents have been reported for selectively imaging breast cancer cells and for drug delivery by several groups.17–22 Yang and co-workers further developed silica-coated Mn3O4 core-shell NPs for tumor folate-receptor-targeted MRI and fluorescent imaging in vitro and tumor aptamer-receptor-targeted MRI imaging in vivo.23–25 Despite many desirable properties for biomedical applications, there are currently few reports related to the fusion of PET tracers (radionuclides) and manganese oxide-based T1 contrast agents into one single probe for in vivo biomedical imaging. Therefore, it is of vital importance to develop novel dual-modality PET/MRI imaging probes based on manganese oxide NPs.
Chelator-based methods have been largely employed for radiolabeling NPs in the past. For example, by using NOTA (1,4,7-triazacyclononane-1,4,7-triacetic acid), DOTA(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid), or desferrioxamine B (DFO) as the chelator, Cu-64 and Zr-89 have been use to label graphene oxide,26 SPIONs,12 mesoporous silica NPs,27 and monocolonal antibodies (mAb),28 for preclinical studies. However, potential dissociation of these radionuclides from NPs could easily lead to false interpretation of PET imaging results in vivo. For example, significant bladder and bone uptake have been observed during in vivo PET imaging of 64Cu-DOTA-Au@PEG NPs29 and 89Zr-DFO-mAb30–32 respectively, indicating that the radionuclides dissociated from their respective chelator. To address this concern, recent successful syntheses of intrinsically radiolabeled NPs (i.e., without the use of a chelator) have been reported, such as 64Cu-labeled porphysomes33 and CuS NPs,34 72As and 69Ge-labeled SPIONs,35,36 and 89Zr-labeled mesoporous silica NPs,37 Gd2O2S:Eu NPs, and WS2/WOx nanodots.38–40 These NPs have exhibited great potential in providing a facile, faster, more stable, and more specific radiolabeling technique for future clinical applications. Inspired by these successes, we were encouraged to develop radio-labeled NPs with manganese oxide-based T1 contrast components for in vivo biomedical applications.
In this work, we hypothesized that mixing suitable water-soluble manganese oxide NPs with 89Zr would yield 89Zr-labeled manganese oxide ([89Zr]Mn3O4@PEG) NPs because of the specific affinity of 89Zr for the manganese oxide surface (Scheme 1).37–40 Subsequently, systematic in vivo PET/MRI imaging, biodistribution, and lymph node mapping studies were carried out in normal healthy BALB/c mice to evaluate their potential capabilities as novel dual-modality PET/MRI agents, and further validated through various in vitro and ex vivo experiments. Moreover, serum biochemistry assays and histological assessments were also carried out to determine the potential toxicity of these NPs.
Scheme 1.
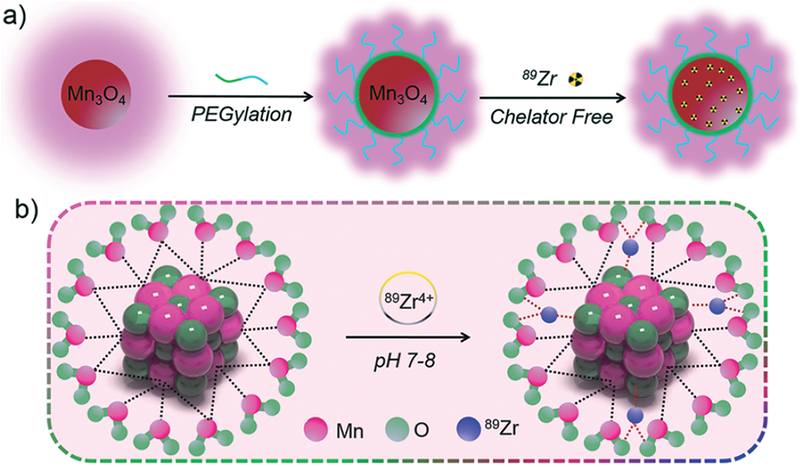
The synthetic process of [89Zr]Mn3O4@PEG NPs.
EXPERIMENTAL SECTION
Materials
Oleylamine (technical grade 90%), oleic acid (technical grade 90%), xylene (98%), manganese (II) acetate (98%), and the CCK-8 assay were all purchased from Sigma-Aldrich. DSPE-PEG5000-NH2 was purchased from Creative PEGworks (Winston Salem, NC). PD-10 desalting columns was acquired from GE Healthcare. All buffers and water were of Millipore grade. All chemicals were used as received without further purification.
Characterization
The size and morphology of Mn3O4 NPs were observed using an FEIT12 transmission electron microscope (TEM) operated at an accelerating voltage of 120 kV. X-ray diffraction (XRD) measurements were performed on a Bruker D8 diffractometer with Cu Kα radiation (λ = 0.15405 nm). The surface zeta potential and hydrodynamic size were measured using a Malvern Zetasizer Nano ZS. The T1-relaxivities were measured and T1-weighted images were acquired with a conventional spin echo acquisition (repetition time, TR, 1000 ms) with echo time, TE, of 50 ms, and a section thickness of 1 mm in a 7 T Bruker Micro-MR small animal scanner. Relaxivity values of r1 were calculated through curve fitting of 1/Tl relaxation time (s–1) versus the Mn concentration (mM).
Synthesis of the Mn3O4@PEG NPs
Mn3O4 NPs were prepared according to a previously reported method with slight modifications.14 In brief, manganese (II) acetate (1 mmol, 0.17 g) and a mixture of oleic acid (2 mmol, 0.57 g) and oleylamine (10 mmol, 2.67 g) were dissolved in 15 mL of xylene in air atmosphere. After slowly heating to 95 °C, 1 mL of DI water was injected into the solution under brisk stirring, and the resulting solution was aged at 95 °C for 3 h. A total of 100 mL of ethanol was then added to precipitate the nanocrystals, followed by centrifugation to retrieve the nanocrystals in powder form. 10 mg of Mn3O4 nanocrystals were dispersed in 1 mL of chloroform and then added to 20 mg of DSPE-PEG5000-NH2 in 2 mL chloroform. After stirring for 2–3 h at 25 °C and evaporating the solvent by argon blowing, the residue was kept at 60 °C in vacuum for 30 min. Upon the addition of 15 mL of DI water, a transparent brown solution was generated after sonication for 15 min. After filtration by centrifugation 3 or 4 times, excess DSPE-PEG5000-NH2 was removed and Mn3O4@PEG NPs were obtained.
89Zr Labeling and Serum Stability Studies
89Zr-oxalate was produced by the University of Wisconsin-Madison cyclotron group. Briefly, a natural yttrium-89 (89Y) foil (250 μm, 99.9%) was irradiated with a proton beam to create 89Zr via the 89Y (p, n) 89Zr reaction by using a 16 MeV GE PETtrace cyclotron. After isotope separation and purification, 89Zr-oxalate was obtained with a specific activity of >20 GBq/μmol. 200 μL of Mn3O4@PEG NPs (1 mg/mL) in HEPES buffer (0.1 M, pH 7.5) was mixed with 89Zr-oxalate at 75 °C. The final pH value of the mixture was adjusted to 7–8 by using 2 M Na2CO3. 89Zr labeling yield was monitored and quantified at different time points (from 3 min to 3 h) by using thin layer chromatography (TLC) and a final labeling time of 1 h was selected. The [89Zr]Mn3O4@PEG NPs could be easily collected by centrifugation at 15,000 rpm for 5 min. To ensure that [89Zr]Mn3O4@PEG NPs were sufficiently stable for in vivo applications, serum stability studies were carried out. [89Zr]Mn3O4@PEG NPs were incubated in complete mouse serum at 37 °C for up to 48 h, and analysis was performed as previously described.38 The percentage of retained 89Zr on the [89Zr]Mn3O4@PEG NPs was calculated according to the equation [(total radioactivity-radioactivity in filtrate)/total radioactivity] × 100%.
Cell Cytotoxicity Studies of Mn3O4@PEG NPs
The cytotoxicity of Mn3O4@PEG NPs was assessed with a CCK-8 assay using SGC-7901 cells and HEK-293 cells. Briefly, cells were seeded in 96-well plates at 20,000 cells per well in 200 μL culture medium. The cells were maintained in RPMI-1640 containing 10% fetal bovine serum (FBS) and incubated at 37 °C in a humidified cell culture incubator with 5% CO2 atmosphere for 24 h. Mn3O4@PEG NP solutions with different concentrations from 200 to 1000 μg/mL were added to each well, and the cells were subjected to a CCK-8 assay after being incubated for another 24 h. The cell viability was determined through measuring the absorption at 450 nm using a microplate reader. Cell viability was calculated using: cell viability (%) = (mean absorption value of treatment group/mean absorption value of control) × 100.
In Vivo Toxicity Studies of Mn3O4@PEG NPs
The toxicity of Mn3O4@PEG NPs to healthy male BALB/c mice was evaluated through injecting Mn3O4@PEG NPs (dose: 20 mg/kg) via the tail vein. Mice injected with only PBS served as a control group (n = 3). Three mice were sacrificed to collect blood for serum biochemistry assays on both day 7 and day 14 post-injection. At the same time, major organs from each mouse were harvested and fixed in 4% paraformaldehyde solution for 1 day. These tissues were then embedded in paraffin and stained with hematoxylin and eosin (H&E) and examined using a digital microscope (Leica DM5000). Examined tissues included the heart, liver, spleen, lung, and kidney. The serum chemistry data, including hepatic and kidney function markers, was measured by the University of Wisconsin-Madison Veterinary Hospital.
In Vivo PET/MRI Imaging and Biodistribution Studies
PET scans of BALB/c mice (n = 3 per group) at 0.5, 2, 12, and 48 h post-injection (p.i.) of [89Zr]Mn3O4@PEG NPs (~100 μ Ci or 2.7 MBq) were performed using an Inveon rodent model microPET/microCT scanner (Siemens Medical Solutions USA, Inc.) following tail vein injection. Detailed procedures for data acquisition and analysis of the PET data have been reported previously.38 Quantitative data are presented as percentage injected dose per gram of tissue (%ID/g). For in vivo lymph node mapping with PET, 40 μ L of [89Zr]Mn3O4@PEG NPs (~60 μ Ci or 0.81 MBq) was subcutaneously injected into the left footpad of healthy BALB/c mice. Time points of 0.5 h, 2 h and 6 h were selected for serial PET scans. In vivo T1-weighted MR imaging was performed at 0.5 h, 2 h and 6 h post-injection after intravenous injection of 200 μL Mn3O4@PEG with a Mn concentration of 1 mM and used a 7 T small animal scanner (Agilent Technologies, Santa Clara, CA) with the following parameters: TR = 500 ms; TE = 12 ms; flip angle = 120°; FOV = 40 mm × 40 mm; matrix = 256 × 256; NEX = 8; slice thickness = 1 mm for axial liver images and 0.5 mm for coronal and cross lymph node mapping. All the animal studies were conducted under a protocol approved by the University of Wisconsin Institutional Animal Care and Use Committee.
RESULTS AND DISCUSSION
Synthesis and Characterization
As shown in Scheme 1, [89Zr]Mn3O4@PEG NPs were prepared uising similar methods to those we have previously reported.37,38 The uniform Mn3O4 NPs were monodispersed in nonpolar organic solvent (Fig. 1(a), inset) and had a small spherical shape of approximately 8 nm, which was consistent with previous reports.14,24,25 Furthermore, the hydrophobic Mn3O4 NPs were successfully transferred to the aqueous phase through coating with amine-functionalized PEG lipids (DSPE-PEG5000-NH2) and showed good stability in aqueous solution (Fig. 1(b), inset). In addition, DLS measurements showed that Mn3O4@PEG had a hydrodynamic diameter of 10 ± 3.5 nm, similar to the observation from TEM. Meanwhile, the zeta-potential value of Mn3O4@PEG (23.7 ± 3.5 mV) further verified the existence of the NH2 group. Meanwhile, the stability of Mn3O4@PEG NPs was also investigated in PBS and 10% FBS at different temperatures. The results indicated that the particle size of the Mn3O4@PEG NPs did not change significantly after two weeks in different media. Figure 1(c) shows the XRD pattern of the Mn3O4 NPs. Clearly, all the diffraction peaks of the as-prepared Mn3O4 NPs can be indexed as a tetragonal Mn3O4 phase (Joint Committee for Powder Diffraction Standards (JCPDS) card no: 24–0734) without metallic manganese or other oxide phase, which indicates that the as-synthesized Mn3O4 NPs are crystalline and of high purity.
Figure 1.
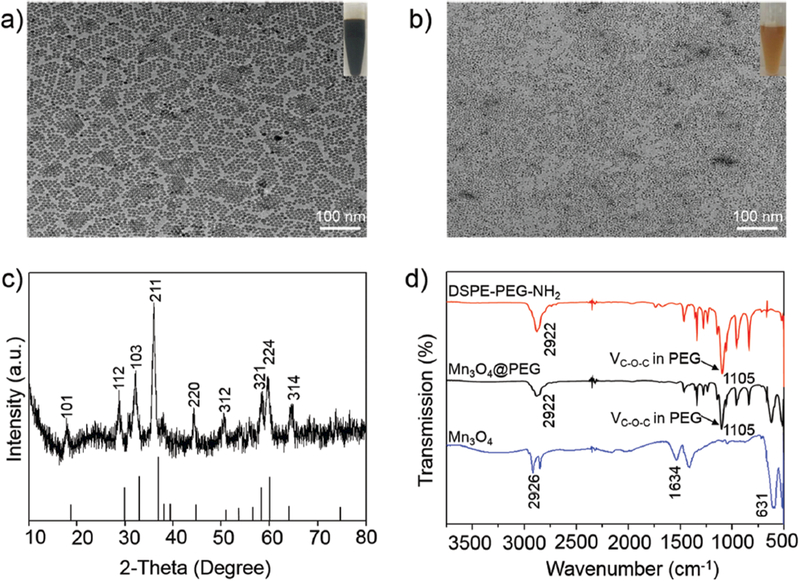
Characterization of Mn3O4@PEG NPs. (a) TEM images of Mn3O4; (b) TEM images of Mn3O4@PEG; (c) X-ray diffraction pattern of Mn3O4 NPs; (d) The FT-IR spectrum of DSPE-PEG-NH2 (red line), Mn3O4 (blue line) and Mn3O4@PEG (black line).
Further investigation of the FT-IR spectra of these NPs confirmed the presence of PEG on the surface of Mn3O4 NPs (Fig. 1(d)). The sample exhibited a strong band around v = 1105 cm–1, corresponding to the C-O-C asymmetric (vas) stretching vibration. To examine the effectiveness of the Mn3O4 NPs as positive MRI contrast agents, the relaxation properties of Mn3O4 NPs in aqueous media were measured by a 7 T MRI scanner. It was clear that Mn3O4@PEG NPs displayed signal enhancement in the T1-weighted MR images with increasing Mn concentration (Fig. 2(a)). The r1 value of the Mn3O4@PEG NPs was calculated from the linear fitting of the measured 1/T1 data versus Mn2+ concentration as 0.58 mM–1s–1 (Fig. 2(b)). These values are also similar to those of previously reported Mn3O4 NPs, verifying their potential use as a positive MRI contrast agent.20,23–25
Figure 2.
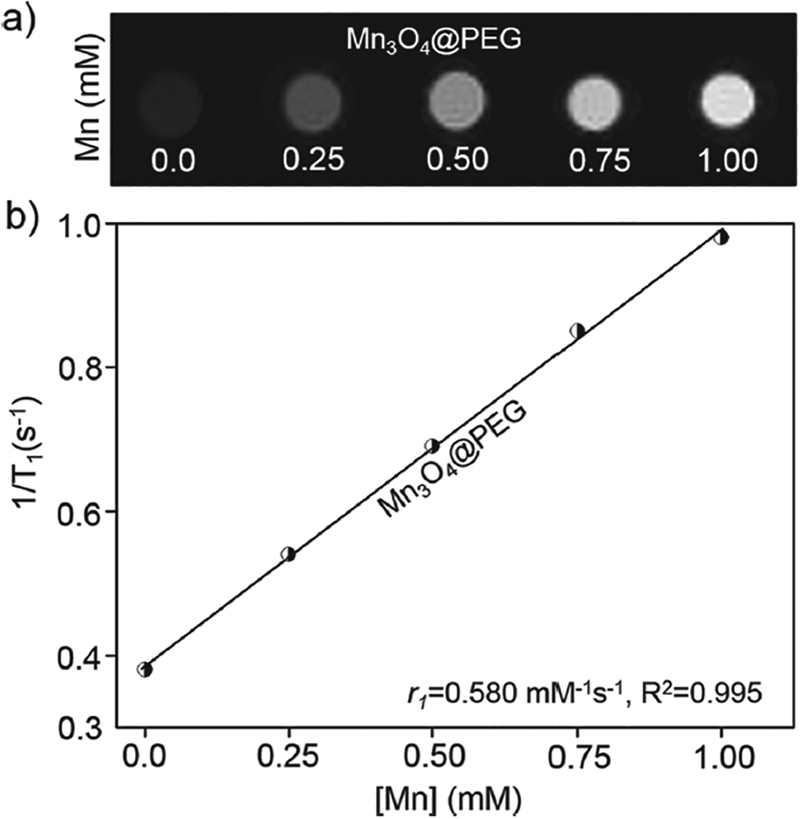
The relaxation properties of Mn3O4@PEG NPs. (a) T1.-weighted MR imaging of Mn3O4@PEG NPs; (b) T1 relaxivity plot of aqueous suspensions of Mn3O4@PEG NPs on a 7 T MRI system.
Chelator-Free 89Zr-Labeling and Serum Stability Studies
Inspired by our previous reports on the intrinsic radiolabeling of 89Zr to mesoporous silica NPs and Gd2O2S:Eu@PEG NPs37,38 and * As and 69Ge to iron oxide NPs35,36 we reasonably postulated that [89Zr]Mn3O4@PEG could be easily formed because of the strong and specific affinity of 89Zr for the Mn3O4 surface, which was possible attributhed to the formation of Zr complexes occupy vacant Mn3O4 tetrahedral sites on the octahedrally terminaged surface of the magnetite NPs (Scheme 1(b)).37 After mixing water-soluble Mn3O4@PEG with 89Zr4+ in HEPES buffer at pH 7–8, the radiolabeling yield of [89Zr]Mn3O4@PEG was then determined at different time intervals through radio-thin layer chromatography (radio-TLC), wherein [89Zr]Mn3O4@PEG (Rf = 0) could easily be distinguished from free 89Zr (Rf = 0.93) (Fig. 3(b)). The radiolabeling yield of [89Zr]Mn3O4@PEG was found to be over 78% within the first 30 min, and kept slightly increasing after 180 min of incubation (Fig. 3(a), black line). As expected, 89Zr-labeling was also concentration and temperature dependent, with higher concentration and higher incubation temperatures gaving higher labeling yield (Figs. 3(a and c)).
Figure 3.
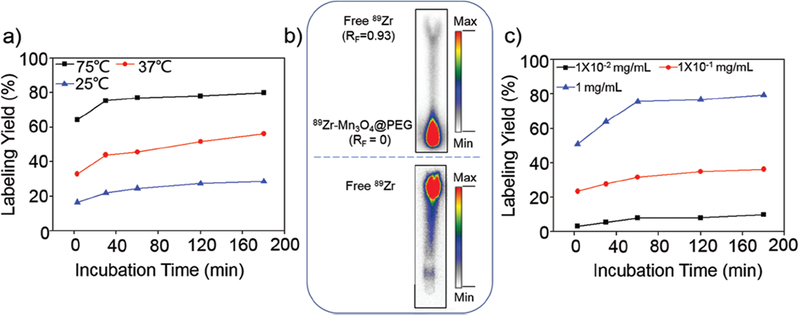
Radiolabeling yield of [89Zr]Mn3O4@PEG NPs. (a) Time-dependent 89Zr labeling yields of [89Zr]Mn3O4@PEG with varied temperature; (b) Autoradiography of TLC plates of [89Zr]Mn3O4@PEG (top) and free 89Zr (bottom); (c) 89Zr labeling yields of [89Zr]Mn3O4@PEG with varied concentrations.
To further investigate the stability of 89Zr labeling in vitro and the feasibility for in vivo applications, a serum stability study of the [89Zr]Mn3O4@PEG NPs was subsequently conducted. After incubation in complete mouse serum at 37 °C for 48 h, nearly 67% of 89Zr remained on the Mn3O4@PEG NPs, suggesting good stability of the radiolabeling on the Mn3O4@PEG NPs. Good radiostability in serum indicated that [89Zr]Mn3O4@PEG NPs would have desirable properties in vivo.
In Vitro Biocompatibility Studies of Mn3O4@PEG NPs
The cytotoxicity of Mn3O4@PEG NPs was evaluated by a CCK-8 assay with normal cells (HEK-293) and tumor cells (SGC-7901). The concentration-dependent effect of Mn3O4 conjugated NPs on the cell viability for 24 h and 48 h was determined. No obvious cytotoxicity of Mn3O4@PEG NPs to HEK-293 and SGC-7901 cells was observed at any studied concentration (from 0.2 to 1 mg mL–1, Fig. 4). Even at the concentration of 1 mg mL–1, the viability for both HEK-293 and SGC-7901 cells still remained above 80%, indicating that the Mn3O4@PEG NPs should have little cytotoxicity at the given concentration range.
Figure 4.
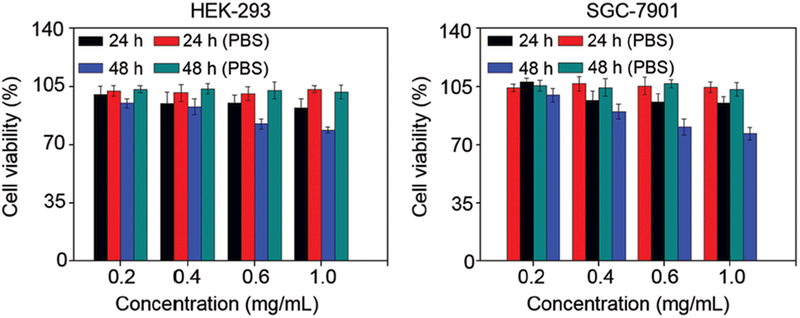
Viability of human embryonic kidney 293 cells (HEK-293) and human gastric cells (SGC-7901) incubated with Mn3O4@PEG NPs at different particle concentrations for 24 and 48 h. Black and blue bars indicate incubation with NPs, while red and green are controls.
In Vivo PET/MRI Imaging and Biodistribution Studies
As we all know, driven by the impending clinical requirement, the perfect combination of PET and MRI has been current under rapid development in clinical cancer detection and diagnosis due to its very high sensitivity of PET and ultra-high spatial resolution of MRI.4,5 To validate the feasibility of [89Zr]Mn3O4@PEG NPs for dual-modality PET/MRI imaging and to investigate their biodistribution pattern in vivo, [89Zr]Mn3O4@PEG NPs (200 μ L, 2.7 MBq) were intravenously injected into healthy BALB/c mice. According to our previously reported method37 time points of 0.5, 2, 24 and 48 h p.i. were chosen for serial PET scans, as seen in Figure 5(a). Since the hydrodynamic diameters of [89Zr]Mn3O4@PEG NPs are above the cutoff for renal filtration,41 the route of clearance was mainly through the hepatobiliary pathway for these nanoparticles. Because of this, strong uptake of [89Zr]Mn3O4@PEG NPs in the liver was observed, at 36.8 ± 2.3, 32.3 ± 1.8, 28.7 ± 0.8 and 25.6 ± 0.3 %ID/g at 0.5, 2, 24, and 48 h p.i. respectively, while the spleen radioactivity was also 23.4 ±0.3, 28.9 ± 0.5, 32.5 ± 0.2, and 37.1 ± 0.3%ID/g at 0.5, 2, 24, and 48 h p.i. respectively (n = 3). While PET imaging provides high sensitivity and quantitative tracking of radiotracers, essential anatomical information is also indispensable for accurate determination of the biodistribution patterns of [89Zr]Mn3O4@PEG. Biodistribution studies of [89Zr]Mn3O4@PEG NPs were also conducted at 48 h p.i. to validate the PET results. Dominant uptake of [89Zr]Mn3O4@PEG NPs by both the liver and spleen was observed, which confirmed that the serial in vivo PET imaging accurately reflected the distribution pattern of [89Zr]Mn3O4@PEG NPs in mice. To further supplement the PET results, MRI with high spatial resolution was used to investigate the Mn3O4@PEG NPs in BALB/c mice. As the Mn3O4@PEG NPs exhibited significant T1 signal enhancement in vitro. In vivo T1 MR imaging of BALB/c mice was conducted before and after intravenous injection of the Mn3O4@PEG NPs at a dose of 20 mg/kg NPs. Since the r1 value of the Mn3O4@PEG NPs was calculated as 0.58 mM−1s−1, the injection dose was adequate for in vivo MR imaging. In view of this, a positive T1 signal enhancement in the liver was observed at 0.5 h and 2 h post-injection of Mn3O4@PEG NPs. However, the signal enhancement in the liver was decreased at 24 h, which could be attributed to the biodegradation and clearance of the NPs (Fig. 5(b)). Moreover, no detectable signal change for the kidney, compared with the same mice prior to the injection of the NPs. In order to further quantify the consistencey between the PET and MRI, by extracting the region of interest (ROI) of MRI and compared with the PET, the ROI of PET and MRI was all gradually decreasing at 0.5 h, 2 h and 24 h. This positive correlation between PET and MRI further proved the feasibility of [89Zr]Mn3O4@PEG NPs for dual-modality PET/MR imaging.
Figure 5.
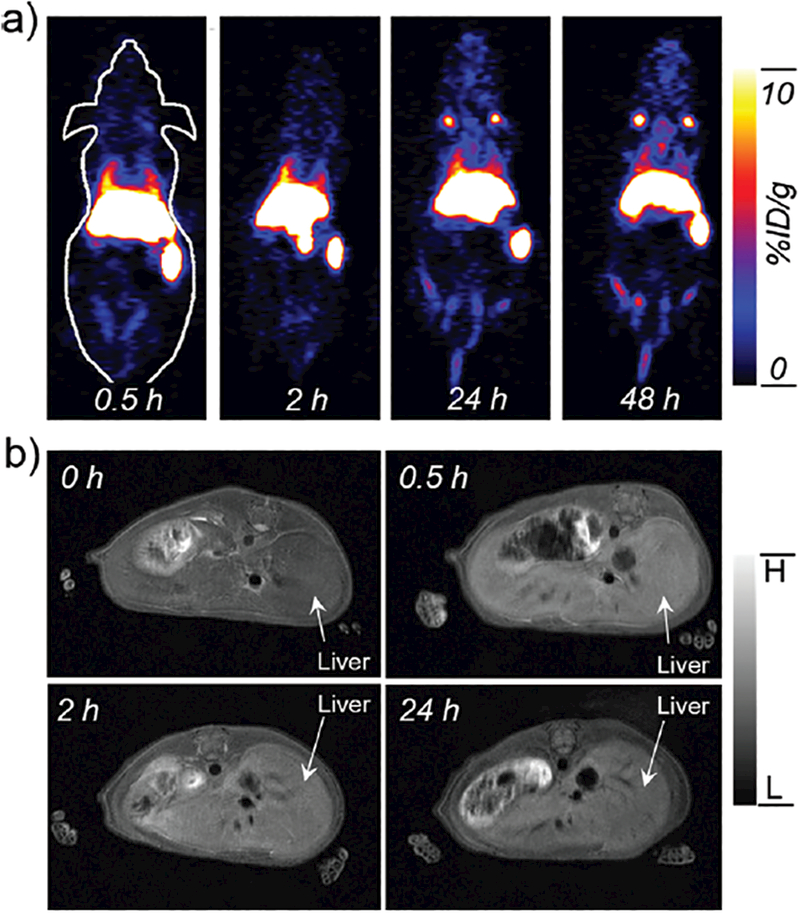
In vivo PET/MR imaging of [89Zr]Mn3O4@PEG NPs. (a) Serial in vivo PET imaging of [89Zr]Mn3O4@PEG in BALB/c mice at different post-injection time points; (b) Serial in vivo MR imaging of Mn3O4@PEG in BALB/c mice at different postinjection time points (n = 3 per group).
In Vivo PET/MRI Imaging of Lymph Node Mapping
The lymphatic system plays a vital role in resisting disease invasion, and is also a common site for tumor metastasis. Therefore, precise identification of sentinel lymph nodes is of vital importance in both the prediction of cancer metastasis as well as determination of treatment options. Considering the [89Zr]Mn3O4@PEG NPs as good dual-modality PET/MRI agent in biodistribution pattern in vivo, as a proof-of-concept, [89Zr]Mn3O4@PEG NPs were then used as non-invasive dual-modality PET/MRI probes for lymph node mapping. After [89Zr]Mn3O4@PEG NPs were subcutaneously injected into the left foot of normal healthy BALB/c mice (50 μL, 1.0 mM of Mn, ~60 μCi), serial PET scans were carried out. As seen from Figure 6(a), accumulation of [89Zr]Mn3O4@PEG NPs in the popliteal lymph node could clearly be seen at 0.5 h, 2 h and 6 h post-injection (red arrows), with the uptake of [89Zr]Mn3O4@PEG NPs evaluated to be 7.7 ± 1.3, 15.3 ± 3.6, and 10.5 ± 2.4 %ID g–1, respectively (n = 3) (Fig. 6(b)). On the contrary, the accumulation of [89Zr]Mn3O4@PEG NPs in paws showed gradual and prominent decreasing after injection of NPs. Meanwhile, the main organ uptake of [89Zr]Mn3O4@PEG NPs in vivo at different time points were also carried out. As seen in Figure 6(b), there was no obvious accumual-tion such as heart, liver, spleen, lung, kidney, and so on, which was compared with the lymph nodes. The above results further revealed that the accumulation of [89Zr]Mn3O4@PEG NPs in lymph nodes after injection was primarily due to the small size, which is well suited to uptake by the lymphatics.
Figure 6.
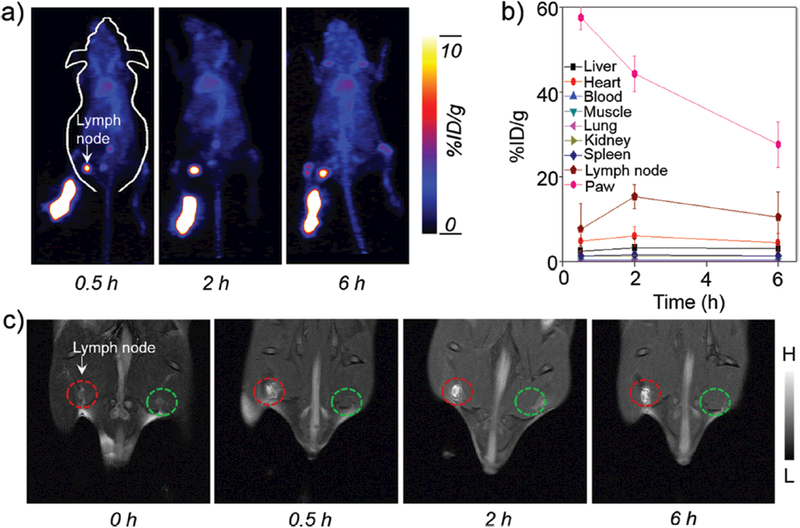
In vivo PET/MR imaging of lymph nodes with [89Zr]Mn3O4@PEG NPs. (a) In vivo PET imaging acquired after subcutaneous injection of [89Zr]Mn3O4@PEG NPs into the left footpad of the mouse (n = 3). Lymph nodes are indicated by arrows. (b) Quantification of the [89Zr]Mn3O4@PEG NPs uptake by the lymph node, paw, heart, liver, kidney, blood, lung, muscle and spleen (n = 3). (c) In vivo MR imaging of the lymph nodes before and after injection of Mn3O4@PEG NPs into the left footpad of the mouse (n = 3). Lymph nodes are indicated by circles.
Meanwhile, the accumulation of Mn3O4@PEG NPs in the sentinel lymph node could also be visualized by MRI, which showed gradual and prominent brightening of the lymph node after injection of Mn3O4@PEG NPs (50 μL, 1.0 mM of Mn; Fig. 6(c), red circle). As an internal contrast, the contralateral lymph node (Fig. 6(c), green circle) exhibited no obvious T1 contrast enhancement at all the same time points observed. In order to further testify the consistencey between the PET and MRI, by extracting the region of interest (ROI) of MRI and PET, the ROI of PET and MRI was all gradually increasing at 0.5 h and 2 h, whereas the signal was then decreased at 6 h respectively. This positive correlation between PET and MRI further proved the feasibility of [89Zr]Mn3O4@PEG NPs for dual-modality PET/MRI of lymph node mapping. Meanwhile, the ex vivo biodistribution of [89Zr]Mn3O4@PEG NPs was also further investigated at 6 h post-injection, and verified a dominant uptake of NPs in the lymph node. Moreover, once the lymph node was located by the PET/MRI images, the area of the lymph node was further dissected for histological analysis. No apparent histological changes, such as lymphocytes, macrophages, neutrophils, and eosinophils, were observed by hematoxylin and eosin (H&E) staining. While the PET/MRI imaging presented herein could only be acquired separately due to the lack of an integrated microPET/microMRI scanner, [89Zr]Mn3O4@PEG NPs offer huge potential for future cancer patient diagnosis. Meanwhile, through further conjugation with other specific targeting ligands, [89Zr]Mn3O4@PEG NPs could be additionally improved to enable accurate early diagnosis and targeted radiotherapy of cancers simultaneously.
In Vivo Biocompatibility Studies of Mn3O4@PEG NPs
To investigate the potential in vivo toxicity of Mn3O4@PEG NPs, histological assessment was carried out by injecting Mn3O4@PEG NPs (20 mg/kg) in healthy BALB/c mice via the tail vein. PBS injections served as the control. Two weeks after injection, mice were sacrificed and major organs (heart, liver, spleen, lung, and kidneys) of mice were sliced and stained by H&E for histology analysis. As shown in Figure 7, no noticeable tissue or cellular damage was observed in all major organs of mice, as compared to those obtained from the control group. There was no hydropic degeneration of cardiac muscle tissue in the heart samples. Normal liver macrophages also showed no evidence of inflammatory infiltrates. Meanwhile, there was no pathological changes and pulmonary fibrosis in the spleen and lung samples. Moreover, the glomerulus structure could be distinguished easily in the kidney samples. For further quantitative evaluation, serum biochemistry assays were then also conducted to investigate the influence of Mn3O4@PEG NPs, especially on potential hepatic injury and kidney functions (Fig. 8). Analysis of four primary hepatic function indicators including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and total bilirubin (TBIL), as well as two kidney function indicators including serum creatinine (CREA) and serum urea (UREA), demonstrated no obvious hepatic or kidney disorders in both the mice treated with Mn3O4@PEG NPs and the control injected with PBS on both day 7 and day 14 post-injection. Although there was somewhat decreased in the level of ALT (p < 0.05) and AST on day 7 and day 14, as compared to those obtained from the control group, it was still within the normal range. These above results also suggested that Mn3O4@PEG NPs demonstrated no obvious toxicity in mice and may be a safe agent for biomedical imaging.
Figure 7.
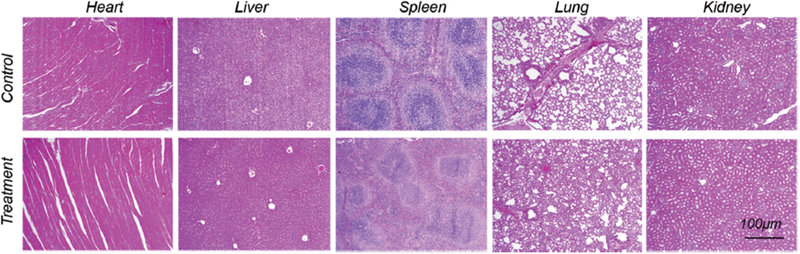
In vivo biocompatibility studies of Mn3O4@PEG in healthy mice. H&E staining of major organs from mice after injecting of Mn3O4@PEG NPs (dose: 20 mg/kg) at 14 d post-injection. Healthy mice treated with PBS were used as the control (n = 3).
Figure 8.
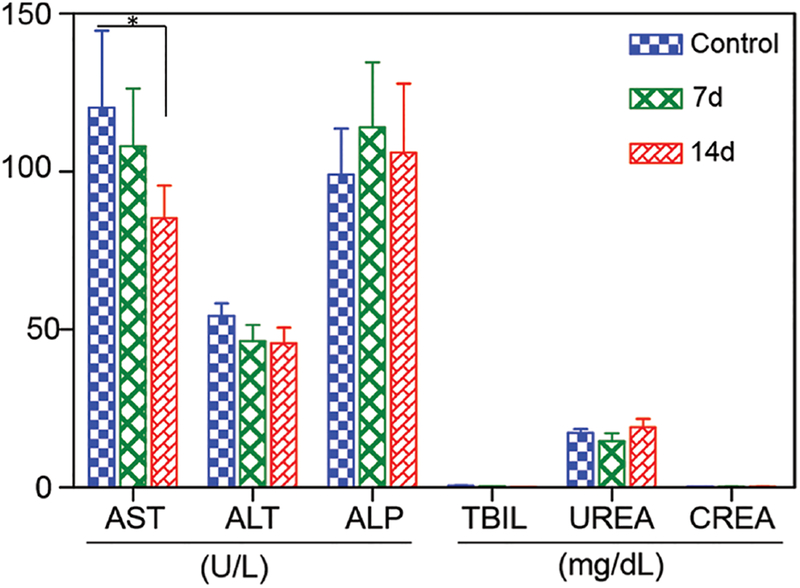
Serum biochemistry of liver and kidney function markers. Healthy BALB/c mice were intravenously injected with Mn3O4@PEG (dose: 20 mg kg−1), and sacrificed on day 7 and day 14 post-injection (n = 3), the difference between AST in control and the treatment group on 14 days were statistically significant (p < 0.05).
CONCLUSION
In conclusion, using Mn3O4 as a nanoplatform, we have developed a novel, efficient, chlealtor-free radiolabeling method to prepare the biocompatible T1-MRI and PET contrast agent, [89Zr]Mn3O4@PEG. Compared to other prevalent Mn3O4-based NPs reported to date, [89Zr]Mn3O4@PEG NPs make full use of the high sensitivity of PET and soft-tissue contrast of MRI. The utilization of [89Zr]Mn3O4@PEG for in vivo dual-modality PET/MR imaging and lymph node mapping provide successful examples for future clinical applications.
Acknowledgments
This work was supported, in part, by the University of Wisconsin-Madison, the National Institutes of Health (NIBIB/NCI1R01CA169365, 1R01CA205101, 1R01EB021336, T32GM008505, T32CA009206, and P30CA014520), the American Cancer Society (125246-RSG-13–099-01-CCE), the National Natural Science Foundation of China under Grant Nos. 81227901, 81571725, 81530058, 81230033, 31371006, 61405149, 81660505 and 81627807, the Natural Science Basic Research Plan in Shaanxi Province of China under Grant No. 2017JM8057, and the Fundamental Research Funds for the Central Universities (JB171204).
Footnotes
Conflict of Interest
The authors confirm that there are no known conflicts of interest associated with this publication. Funding sources had no involvement in study design; collection, analysis, and interpretation of data; writing of the report; and in the decision to submit the article for publication.
REFERENCES
- 1.Su T, Wang YB, Han D, Wang J, Qi S, Gao L, Shao YH, Qiao HY, Chen JW, Liang SH, Nie YZ, Li JY, and Cao F, Multimodality imaging of angiogenesis in a rabbit atherosclerotic model by GEBP11 peptide targeted nanoparticles. Theranostics 7, 4791 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su T, Wang Y, Wang J, Han D, Ma S, Cao J, Li X, Zhang R, Qiao H Liang J Liu G Yang B Liang S Nie Y Wu K Li J, and Cao F. In vivo magnetic resonance and fluorescence dual-modality imaging of tumor angiogenesis in rats using GEBP11 peptide targeted magnetic nanoparticles. J. Biomed. Nanotechnol 12 1011 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Zamboglou C, Drendel V, Jilg CA, Rischke HC, Beck TI, Schultze-Seemann W, Krauss T, Mix M, Schiller F, Wetterauer U, Werner M, Langer M, Bock M, Meyer PT, and Grosu AL, Comparison of 68Ga-HBED-CC PSMA-PET/CT and multiparametric MRI for gross tumour volume detection in patients with primary prostate cancer based on slice by slice comparison with histopathology. Theranostics 7, 228 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen B, Behera D, James ML, Reyes ST, Andrews L, Cipriano PW, Klukinov M, Lutz AB, Mavlyutov T, Rosenberg J, Ruoho E, McCurdy CR, Gambhir SS, Yeomans DC, Biswal S, and Chin FT, Visualizing nerve injury in a neuropathic pain model with [18F]FTC-146 PET/MRI. Theranostics 7, 2794 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Feng J, Zhang R, Wang J, Su T, Tian Z, Han D, Zhao C, Fan M, Li C, Liu B, Feng X, Nie Y, Wu K, Chen Y, Deng H, and Cao F, Quaternized chitosan/alginate-Fe3 O4 magnetic nanoparticles enhance the chemosensitization of multidrug-resistant gastric carcinoma by regulating cell autophagy activity in mice. J. Biomed. Nanotechnol 12, 948 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Glaus C, Rossin R, Welch MJ, and Bao G In vivo evaluation of Cu-64-labeled magnetic nanoparticles as a dual-modality PET/MR imaging agent. Bioconjugate. Chem 21, 715 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pichler BJ, Kolb A, Nagele T, and Schlemmer HP, PET/MRI: Paving the way for the next generation of clinical multimodality imaging applications. J. Nucl. Med 51, 333 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Tao W, Chen X, Farokhzad OC, and Liu G, Emerging advances in nanotheranostics with intelligent bioresponsive systems. Theranostics 7, 3915 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H, Lee E, Kim DK, Jang NK, Jeong YY, and Jon S, Antibiofouling polymer-coated superparamagnetic iron oxide nanoparticles as potential magnetic resonance contrast agents for in vivo cancer imaging. J. Am. Chem. Soc 128, 7383 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Ma LL, Feldman MD, Tam JM, Paranjape AS, Cheruku KK, Larson TA, Tam JO, Ingram DR, Paramita V, Villard JW, Jenkins JT, Wang T, Clarke GD, Asmis R, Sokolov K, Chandrasekar B, Milner TE, and Johnston KP, Small multifunctional nanoclusters (nanoroses) for targeted cellular imaging and therapy. Acs Nano 3, 2686 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong RM, Gilbert DA, Liu K, and Louie AY, Rapid size-controlled synthesis of dextran-coated, Cu-64-doped iron oxide nanoparticles. Acs Nano 6, 3461 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Yang XQ, Hong H, Grailer JJ, Rowland IJ, Javadi A, Hurley SA, Xiao YL, Yang YA, Zhang Y, Nickles R, Cai WB, Steeber DA, and Gong SQ, cRGD-functionalized, DOX-conjugatedd, and Cu-64-labeled superparamagnetic iron oxide nanoparticles for targeted anticancer drug delivery and PET/MR imaging. Biomaterials 32, 4151 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shapiro EM and Koretsky AP, Magnetic Resonance in Medicine 60, 265 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Na HB, Lee JH, An KJ, Park YI, Park M, Lee IS, Nam DH, Kim ST, Kim SH, Kim SW, Lim KH, Kim KS, Kim SO, and Hyeon T, Development of a T-1 contrast agent for magnetic resonance imaging using MnO nanoparticles. Angew. Chem. Int. Edit 46, 5397 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Yu T, Moon J, Park J, Park YI, Na HB, Kim BH, Song IC, Moon WK, and Hyeon T, Various-shaped uniform Mn3O4 nanocrystals synthesized at low temperature in air atmosphere. Chem. Mater 21, 2272 (2009). [Google Scholar]
- 16.Jun YW, Lee JH, and Cheon J, Chemical design of nanoparticle probes for high-performance magnetic resonance imaging. Angew. Chem. Int. Edit 47, 5122 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Bennewitz MF, Lobo TL, Nkansah MK, Ulas G, Brudvig GW, and Shapiro EM, Biocompatible and pH-sensitive PLGA encapsulated MnO nanocrystals for molecular and cellular MRI. ACS Nano 5, 3438 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ha TL, Kim HJ, Shin J, Im GH, Lee JW, Heo H, Yang J, Kang AM, Choe YS, Lee JH, and Lee IS, Development of target-specific multimodality imaging agent by using hollow manganese oxide nanoparticles as a platform. Chem. Commun 47, 9176 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Hao R, Yu J, Hou YL, and Sun SH, One-pot synthesis of hollow/porous Mn-based nanoparticles via a controlled ion transfer process. Chem. Commun 47, 9095 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Shin JM, Anisur RM, Ko MK, Im GH, Lee JH, and Lee S, Hollow manganese oxide nanoparticles as multifunctional agents for magnetic resonance imaging and drug delivery. Angew. Chem. Int. Edit 48, 321 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Huang CC, Khu NH, and Yeh CS, The characteristics of sub 10 nm manganese oxide T-1 contrast agents of different nanostructured morphologies. Biomaterials 31, 4073 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Huang J, Xie J, Chen K, Bu LH, Lee S, Cheng Z, Li XG, and Chen XY, HSA coated MnO nanoparticles with prominent MRI contrast for tumor imaging. Chem. Commun 46, 6684 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang XY, Zhou ZG, Wang L, Tang CZ, Yang H, and Yang SP, Folate conjugatedd Mn3O4@SiO2 nanoparticles for targeted magnetic resonance imaging in vivo. Mater. Res. Bull 57, 97 (2014). [Google Scholar]
- 24.Hu H, Dai AT, Sun J, Li XY, Gao FH, Wu LZ, Fang Y, Yang H, An L, Wu HX, and Yang SP, Aptamer-conjugatedd Mn3O4@SiO2 core-shell nanoprobes for targeted magnetic resonance imaging. Nanoscale 5, 10447 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Zhuang YM, Hu H, Du XX, Zhang CX, Shi XY, Wu HX, and Yang SP, Silica-coated manganese oxide nanoparticles as a platform for targeted magnetic resonance and fluorescence imaging of cancer cells. Adv. Funct. Mater 20, 1733 (2010). [Google Scholar]
- 26.Ranganathan A, Campo J, Myerson J, Shuvaev V, Zern B, Muzykantov V, and Eckmann DM, Fluorescence microscopy imaging calibration for quantifying nanocarrier binding to cells during shear flow exposure. J. Biomed. Nanotechnol 13, 737 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li N, Li T, Liu C, Ye S, Liang J, and Han H, Folic acid-targeted and cell penetrating peptide-mediated theranostic nanoplatform for high-efficiency tri-modal imaging-guided synergistic anticancer phototherapy. J. Biomed. Nanotechnol 12, 878 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Cheng L, Shen S, Jiang D, Jin Q, Ellison PA, Ehlerding EB, Goel S, Song G, Huang P, Barnhart TE, Liu Z, and Cai W, Chelator-free labeling of metal oxide nanostructures with zirconium-89 for positron emission tomography imaging. ACS Nano 11, 12193 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Liu Y, Luehmann H, Xia X, Brown P, Jarreau C, Welch M, and Xia Y, Evaluating the pharmacokinetics and in vivo cancer targeting capability of Au nanocages by positron emission tomography imaging. ACS Nano 6, 5880 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aerts HJ, Dubois L, Perk L, Vermaelen P, van Dongen GA, Wouters G, and Lambin P, Disparity between in vivo EGFR expression and 89Zr-labeled cetuximab uptake assessed with PET. J. Nucl. Med 50, 123 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Tinianow JN, Gill HS, Ogasawara A, Flores JE, Vanderbilt AN, Luis E, Vandlen R, Darwish M, Junutula JR, and Williams S-P, Site-specifically 89 Zr-labeled monoclonal antibodies for ImmunoPET. Nucl. Med. Biol 37, 289 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Holland JP, Divilov V, Bander NH, Smith-Jones PM, Larson SM, and Lewis JS, 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J. Nucl. Med 51, 1293 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng L, Yang K, Chen Q, and Liu Z, Organic stealth nanoparticles for highly effective in vivo near-infrared photothermal therapy of cancer. ACS Nano 6, 5605 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Zhou M, Zhang R, Huang M, Lu W, Song S, Melancon MP, Tian M, Liang D, and Li C, A chelator-free multifunctional [64Cu]CuS nanoparticle platform for simultaneous micro-PET/CT imaging and photothermal ablation therapy. J. Am. Chem. Soc 132, 15351 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen F, Ellison PA,Lewis CM, Hong H, Zhang Y, Shi S, Hernandez R, Meyerand ME, Barnhart TE,and Cai W, Chelator-free synthesis of a dual-modality PET/MRI agent. Angew. Chem. Int. Edit 52, 13319 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakravarty R, Valdovinos HF, Chen F, Lewis CM, Ellison PA, Luo H, Meyerand ME, Nickles RJ,and Cai W, Intrinsically germanium-69-labeled iron oxide nanoparticles: Synthesis and in-vivo dual-modality PET/MR imaging. Adv. Mater 5119 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen F, Goel S, Valdovinos HF, Luo H, Hernandez R, Barnhart TE, and Cai W In vivo integrity and biological fate of chelator-free zirconium-89-labeled mesoporous silica nanoparticles. ACS Nano 9, 7950 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhan Y, Ai F, Chen F, Valdovinos HF, Orbay H, Sun H, Liang J, Barnhart TE, Tian J, and Cai W, Intrinsically zirconium-89 labeled Gd2O2S: Eu nanoprobes for in vivo positron emission tomography and gamma-ray-induced radioluminescence imaging. Small 12, 2872 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ai F, Goel S, Zhan Y, Valdovinos HF, Chen F, Barnhart TE, and Cai W, Intrinsically 89Zr-labeled Gd2O2S: Eu nanophosphors with high in vivo stability for dual-modality imaging. Am. J. Transl. Res 8, 5591 (2016). [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng L, Kamkaew A, Shen S, Valdovinos HF, Sun H, Hernandez R, Goel S, Liu T, Thompson CR, and Barnhart TE, Facile preparation of multifunctional WS2/WOx nanodots for chelator-free 89Zr-labeling and in vivo PET imaging. Small 5750 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, and Frangioni JV, Renalclearance of quantum dots. Nat. Biotechnol 25, 1165 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]


