Summary
Angiotensin II-preconditioning (APC) has been shown to reproduce the cardioprotective effects of ischaemic preconditioning (IPC), however, the molecular mechanisms mediating the effects of APC remain unknown. In this study, Langendorff-perfused rat hearts were subjected to IPC, APC or both (IPC/APC) followed by ischaemia-reperfusion (IR), to determine translocation of PKCε, PKCδ, Akt, Erk1/2, JNK, p38 MAPK and GSK-3β to mitochondria as an indicator of activation of the protein kinases. In agreement with previous observations, IPC, APC and IPC/APC increased the recovery of left ventricular developed pressure (LVDP), reduced infarct size (IS) and lactate dehydrogenase (LDH) release, compared to controls. These effects were associated with increased mitochondrial PKCε/PKCδ ratio, Akt, Erk1/2, JNK, and inhibition of permeability transition pore (mPTP) opening. Chelerythrine, a pan-PKC inhibitor, abolished the enhancements of PKCε but increased PKCδ expression, and inhibited Akt, Erk1/2, and JNK protein levels. The drug had no effect on the APC- and IPC/APC-induced cardioprotection as previously reported, but enhanced the post-ischaemic LVDP in controls. Losartan, an angiotensin II type 1 receptor (AT1-R) blocker, abolished the APC-stimulated increase of LVDP and reduced PKCε, Akt, Erk1/2, JNK, and p38. Both drugs reduced ischaemic contracture and LDH release, and abolished the inhibition of mPTP by the preconditioning. Chelerythrine also prevented the reduction of IS by APC and IPC/APC. These results suggest that the cardioprotection induced by APC and IPC/APC involves an AT1-R-dependent translocation of PKCε and survival kinases to the mitochondria leading to mPTP inhibition. In chelerythrine-treated hearts, however, alternate mechanisms appear to maintain cardiac function.
Keywords: Angiotensin II, cardioprotection, ischaemia-reperfusion, ischaemic preconditioning, MAPKs, prosurvival kinases, protein kinase C
1 | INTRODUCTION
Angiotensin II (Ang II), a key hormone involved in cardiovascular homeostasis, has been found to protect the heart against ischaemia-reperfusion (IR) injury. Several studies,1-6 including a recent report from our laboratory,7 demonstrate that acute exposure of the heart to Ang II exerts ischaemic preconditioning (IPC)-like effects and improves post-ischaemic ventricular recovery, reduces lactate dehydrogenase (LDH) release and infarct size, among others.1,3,4 Indeed, Ang II-preconditioning (APC) and IPC apparently share common signalling pathways including activation of G protein-coupled receptors (GPCR), multiple kinase cascade systems, reactive oxygen species (ROS), and mitochondrial KATP (mitoKATP) channels.8-10 These mediators regulate cellular metabolism,8,11 ATP synthesis,8 and cell death through regulation of apoptotic proteins (Bax, Bcl-2, and caspase 3)12,13 to promote cell survival and tissue integrity.
Protein kinase C (PKC) appears to be a central player mediating cardioprotective effects of IPC and APC because inhibition of this kinase abolishes the beneficial effects of both preconditioning.14-16 By contrast, stimulation of PKC activity with phorbol esters17,18 replicate cardioprotective signals against IR injury. Indeed, GPCRs agonists including Ang II,1 appear to mimic IPC through PKC. From 11 known PKC isoforms, two seem to play key roles in cardioprotection. These isoforms, PKCε and PKCδ, act on effectors such as the mitoKATP channels, mitogen-activated protein kinases (MAPKs), and the mitochondrial permeability transition pore (mPTP).19 PKC isoforms are activated by 3-phosphoinositide-dependent protein kinase-1 (PDK1)-dependent phosphorylation20 and subsequently translocate to sub-cellular organelles during the preconditioning or at the beginning of reperfusion.21 The process is isoform specific, and through a cytoskeleton mediated process, results in phosphorylation and activation of the effectors of preconditioning.16 For this reason, the translocation of PKC is considered a hallmark for PKC activation and has been used as an indicator of activation following preconditioning stimuli.22 PKCε has been reported to exert cardioprotection by opening the mitoKATP channel and inhibiting the mPTP,23 while PKCδ is associated with increased superoxide anion generation, loss of mitochondrial function, as well as the enhanced release of cytochrome c and downstream pro-apoptotic factors.24,25 The design of isoform-specific peptides that inhibit PKCε translocation has confirmed the requirement of this step in preconditioning.26 By contrast, peptides that promote translocation of PKCε have been shown to induce cardioprotection against IR-mediated damage.27
Accumulating evidence shows that ROS production from Ang II type 1 receptor (AT1-R) stimulation results from activation of NADPH oxidase.10 Indeed, studies have demonstrated that Ang II increases the activity of subunits gp91phox, gp22phox and p47phox.6,28,29 Similar to IPC, Ang II also stimulates mitochondrial ROS production, which may further stimulate NADPH oxidase expression and activity.30,31 Besides, MAPKs,10,29,32 such as Erk1/2, JNK and p38 MAPK are main targets of ROS signalling and AT1-R activation,9 but their role in IPC and APC is still not fully understood. Indeed, conflicting data33 exists on the role of JNK and p38 MAPK in cardioprotection. These kinases are part of the family of stress-activated MAPKs implicated in cell death.19,34 However, they may also promote cardioprotection depending on the dynamics of activation and their interaction with protective pathways.33, Therefore, there is a need to evaluate the role of stress-activated MAPKs and the prosurvival kinase pathway35 (PI3K, Akt and Erk1/2) in APC.
Previous work from our laboratory,7 demonstrated that APC enhances the post-ischaemic recovery of the heart, reduces infarct size and LDH release, and improves mitochondrial function. However, treatment with chelerythrine during preconditioning did not affect the post-ischaemic recovery of cardiac function. These findings suggested that the post-ischaemic cardiac recovery in chelerythrine-treated hearts relies on PKC-independent pathways or, PKC-dependent signalling pathways activated at reperfusion. However, the lack of data on PKC-dependent signalling pathways in APC in the presence or absence of chelerythrine precluded the evaluation of these possibilities.
In this work, we evaluated further the mechanisms involved in APC, IPC, and their combination, to clarify the role of PKC and its downstream signalling targets in these processes. Protein kinases can be affected by different types of post-translational modifications (phosphorylation, nitrosylation, SUMOylation, etc.) that do not necessarily result in kinase activation.19 We chose to evaluate the translocation of protein kinases to the mitochondria because this process is an indicator of PKC activation during IPC.22 Thus, the protein levels of PKCε, PKCδ, Erk1/2, Akt, JNK, p38 MAPK and GSK-3β, were determined in mitochondria to assess the APC- and IPC-induced translocation to these organelles. The role of PKC and AT1-R in APC and IPC/APC was evaluated by using chelerythrine and losartan, respectively. Also, the rate of mitochondrial swelling was used to determine the status of mPTP. Our results demonstrate that both APC and IPC/APC increase the AT1-R-dependent translocation of PKCε to mitochondria with no effect on PKCδ. A high PKCε/PKCδ ratio was associated with increased translocation of Erk1/2, Akt, and JNK to mitochondria and inhibition of mPTP. Inhibition of PKC with chelerythrine, however, blocked the signalling cascade but preserved the post-ischaemic cardiac recovery possibly by reducing the contracture-induced damage at reperfusion and increasing PKCδ expression.
2 | RESULTS
2.1 | Cardiac function in APC and IPC/APC hearts: effects of chelerythrine and losartan
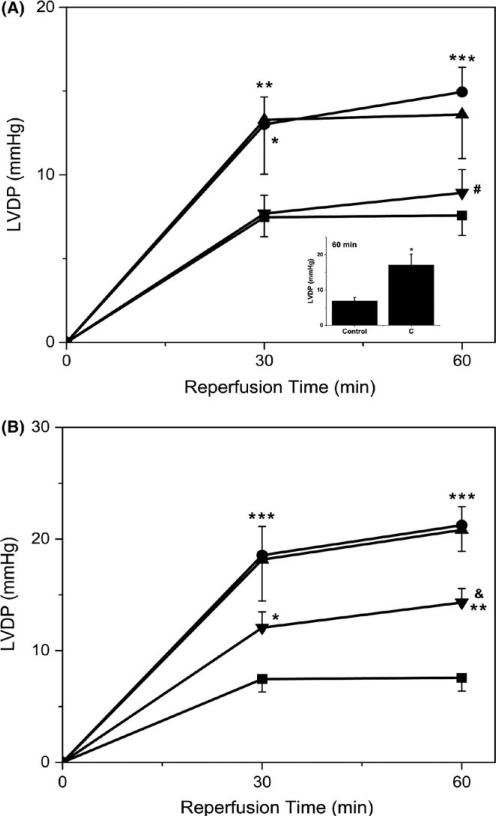
Figure 2 depicts the recovery of cardiac function (LVDP) after IR as a function of time in hearts subjected to APC or IPC/APC in the presence or absence of chelerythrine (5 μmol/L) and losartan (10 μmol/L). As depicted in Figure 2A, LVDP was stimulated by APC compared with controls (90% at 60 minutes, P <.001). A similar increase in this parameter was observed with IPC (data not shown). Figure 2B illustrates that IPC/APC markedly increased LVDP (2.8-fold at 60 minutes, P <.001) over control values. Chelerythrine did not affect the increase of LVDP induced by APC or IPC/APC, but enhanced it in the control group (2.47-fold, P <.05, Figure 2A inset). By contrast, losartan significantly decreased LVDP by 82% (P <.05) in APC and 51% (P <.01) in IPC/APC when compared with their respective controls at 60 minutes. These results agree with a previous study from our laboratory7 and support the view that cardioprotection by APC is an AT1-R-dependent event that is chelerythrine-insensitive under our experimental conditions.
FIGURE 2.
Effects of preconditioning protocols on LVDP in Langendorff-perfused rat hearts after IR: Effects of chelerythrine and losartan. LVDP following global ischaemia was determined at 0, 30, and 60 minutes of reperfusion. LVDP values at 90 minutes were not significantly different from those at 60 minutes. The values shown are means ± SEM of 5-14 experiments per group. The inset shows the effect of chelerythrine in control hearts at 60 minutes (n = 4). *P <.05, **P <.01, ***P < .001 vs Control; #P <.05 vs A; &P <.01 vs IA. LVDP, Left ventricular developed pressure; C, chelerythrine; A, APC; IA, IPC/APC; A+C, APC+chelerythrine; IA+C, IPC/APC+chelerythrine; A+L, APC+losartan; IA+L, IPC/APC+losartan. The number of experiments per group (in parenthesis): Control (14), A (12), IA (10), A+C (6), IA+C (5), A+L (5), IA+L (7). (A) ( ) Control; (
) Control; ( ) A; (
) A; ( ) A+C; (
) A+C; ( ) A+L. (B) (
) A+L. (B) ( ) Control; (
) Control; ( ) IA; (
) IA; ( ) IA+C; (
) IA+C; ( ) IA+L
) IA+L
2.2 | Effect of chelerythrine and losartan on ischaemic contracture and LDH release from hearts in both APC and IPC/APC groups
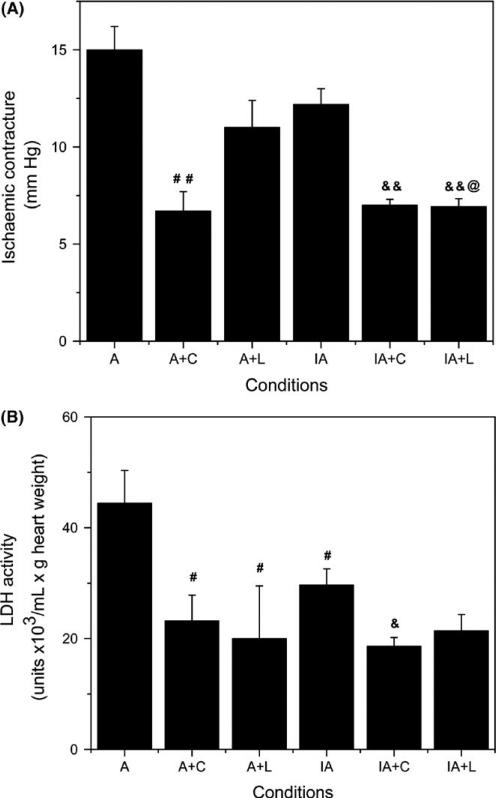
In control animals, 5 μmol/L chelerythrine administered prior to global ischaemia reduced 22% (P <.05) the ischaemic contracture (from 19 ± 2 to 15 ± 1 mmHg, n = 4) and 68% (P <.01) LDH activity in ventricular effluents at the start of reperfusion (from 100 ± 15 to 32 ± 8 units × 103/mL × g heart weight, n = 4). In APC and IPC/APC hearts (Figure 3A), the drug also inhibited the ischaemic contracture by 55% (P <.001) in APC and 43% (P <.001) in the IPC/APC group. Similarly, LDH release from perfused hearts treated with chelerythrine decreased 48% (P <.05) in APC and 37% (P <.01) in IPC/APC (Figure 3B). By contrast, losartan reduced ischaemic contracture by 27% (NS, P = .08) and 43% (P <.001) in APC and IPC/APC, respectively (Figure 3A). LDH release (Figure 3B) was also reduced by losartan 55% (P <.05) in APC and 28% (NS, P = .07) in IPC/APC. The data are consistent with reduced myocardial calcium during global ischaemia and diminished myocardial damage at the initiation of the reperfusion by chelerythrine and losartan treatments in both basal and following preconditionings.
FIGURE 3.
Effect of chelerythrine and losartan on ischaemic contracture and LDH activity in APC and IPC/APC treated rat hearts. (A) Ischaemic contracture during global ischaemia. (B) LDH activity in cardiac effluents collected at 5 minutes after the initiation of reperfusion. The values shown are means ± SEM of 5-12 experiments per group. #P <.05, ##P <.01 vs A; &P <.01, &&P <.001 vs IA; @P <.01 vs A+L. A, APC; A+C, APC+chelerythrine; A+L, APC+losartan; IA, IPC/APC; IA+C, IPC/APC+chelerythrine; IA+L, IPC/APC+losartan. The number of experiments per group (in parenthesis): A (12), IA (10), A+C (6), IA+C (5), A+L (5), IA+L (7)
2.3 | Effect of chelerythrine and losartan on mPTP opening
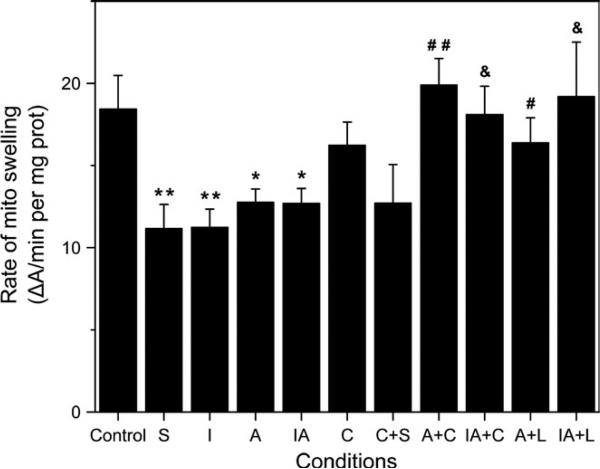
Figure 4 shows the effect of chelerythrine and losartan on the rate of mitochondrial swelling as an indicator of mPTP opening in APC and IPC/APC hearts. The rate of mitochondrial swelling was significantly inhibited 39% (P <.01) by sanglifehrin A, an immunosupressor (analogue of cyclosporine A) and inhibitor of mPTP, indicating the presence of pore opening. Chelerythrine had no effect on mitochondrial swelling in control hearts in the presence or absence of sanglifehrin A. The rate of swelling, however, was inhibited 40% by IPC (P <.01), and 32% by APC or IPC/APC (P <.01 for both) compared to the control group. Both Chelerythrine and losartan abolished the effects of APC and IPC/APC on the swelling rate, suggesting a role of mPTP opening in AT1-R-mediated preconditioning.
FIGURE 4.
Effect of chelerythrine and losartan on mPTP opening. Measurement of mitochondrial swelling, as an indicator of mPTP opening under de-energized conditions, was performed by monitoring the Ca2+-induced decrease in light scattering (A520) as a function of time. The figure also shows the effect of sanglifehrin A on mitochondrial swelling in the presence and absence of chelerythrine. Data are shown as ΔA520/minutes per mg of mitochondrial protein. The values shown are the means ± SEM of 4-14 experiments per group. Sanglifehrin A was added in vitro to control and chelerythrine mitochondrial samples. *P <.05, **P <.01 vs Control; #P <.05, ##P <.01 vs A; &P <.05 vs IA. S, sanglifehrin A; I, IPC; A, APC; IA, IPC/APC; C, chelerythrine; C+S, chelerythrine+sangliferhin A; A+C, APC+chelerythrine; IA+C, IPC/APC+chelerythrine; A+L, APC+losartan; IA+L, IPC/APC+losartan. The number of experiments per group (in parenthesis): Control (14), S (14) A (12), I (9), IA (10), C (4), C+S (4), A+C (6), IA+C (5), A+L (5), IA+L (7)
2.4 | Effect of chelerythrine on infarct size determinations in APC and IPC/APC hearts
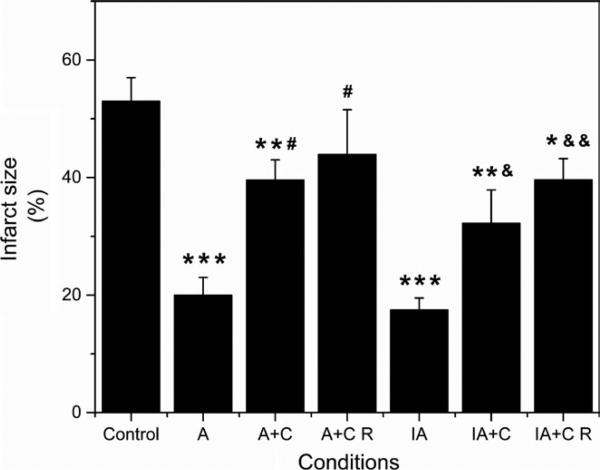
Figure 5 shows the effect of chelerythrine when added during the preconditioning protocols or at reperfusion, on infarct size measured by the triphenyltetrazolium chloride (TTC) staining. Infarct size was reduced 61% and 67% by APC (P <.001) and IPC/APC (P <.001), respectively. In the presence of chelerythrine, however, the effect of APC was inhibited 59% and 72% (P <.05 for both) at preconditioning and reperfusion, respectively. Similar inhibitory effects of chelerythrine were observed on the reduction of infarct size induced by IPC/APC. These observations indicate that cardioprotection by APC and IPC/APC is mediated through PKC-dependent signalling pathways.
FIGURE 5.
Effect of chelerythrine on infarct size in APC and IPC/APC treated rat hearts. Infarct size is expressed in percent of total area (whole heart). The values are shown as means ± SEM of six experiments per group. *P <.05, **P <.01, ***P <.001 vs Control; #P <.05 vs A; &P <.05, &&P <.01 vs IA. A, APC; A+C, APC+chelerythrine; A+C R, APC+chelerythrine during reperfusion; IA, IPC/APC; IA+C, IPC/APC+chelerythrine; IA+C R, IPC/APC+chelerythrine during reperfusion
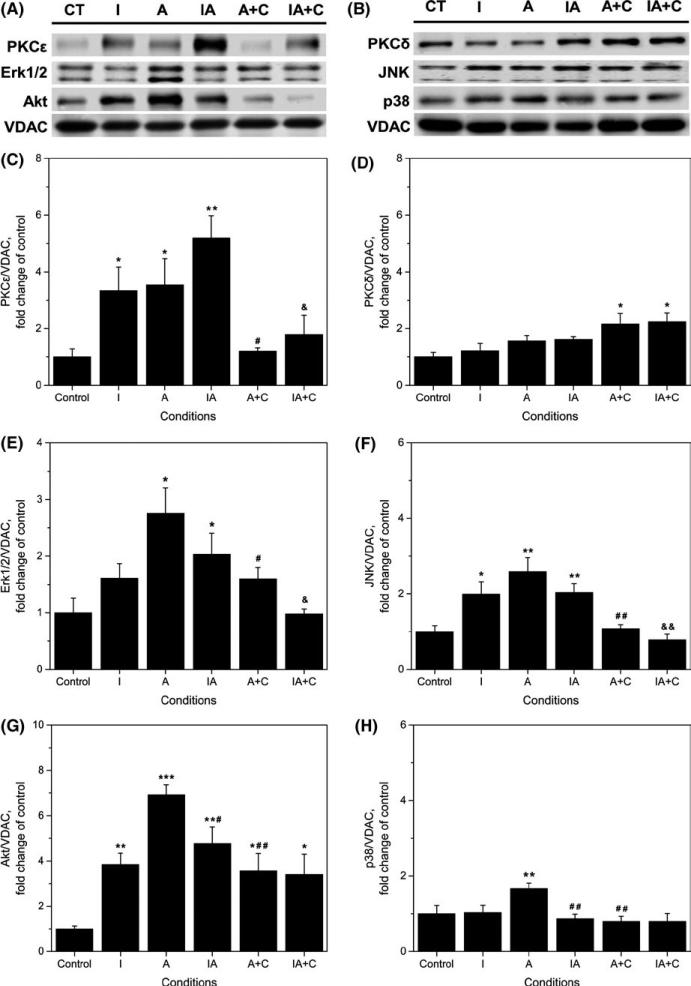
2.5 | Effect of chelerythrine on PKCε, PKCδ, Erk1/2, Akt, JNK, p38 MAPK and GSK-3β protein in mitochondrial fractions
Figure 6A-H depicts the results of experiments in which the protein levels of PKCε, PKCδ, Erk1/2, Akt, JNK, and p38 MAPK in mitochondria were evaluated in controls, IPC, APC and IPC/APC-hearts. As shown in the figure, PKCε, Erk1/2, and Akt protein increased markedly in these organelles compared to controls. The mitochondrial PKCε isoform response pattern (Figure 6C) follows that observed for cardiac function studies following global ischaemia (Figure 2A,B). Both IPC and APC increased PKCε protein levels about 3.5-fold (P <.05 for both) and the combination IPC/APC further enhanced it 5-fold (P <.01) compared with controls. Treatment with 5 μmol/L chelerythrine abolished the enhanced PKCε protein in mitochondria by APC and IPC/APC.
FIGURE 6.
Effect of chelerythrine on protein levels of PKCε, PKCδ, Erk1/2, JNK, Akt, and p38 MAPK in isolated mitochondria. (A, B) Representative western blots and quantitative data of total; (C) PKCε; (D) PKCδ; (E) Erk1/2; (F) JNK; (G) Akt; (H) p38. Proteins were calculated as the ratio of total protein to VDAC, and normalized to control for each kinase. The values are shown as means ± SEM of five experiments per group. *P <.05, **P <.01, ***P <.001 vs Control; #P <.05, ##P <.01 vs A; &P <.05, &&P <.01 vs IA. CT, control; I, IPC; A, APC; IA, IPC/APC; A+C, APC+chelerythrine; IA+C, IPC/APC+chelerythrine
Significant increases in mitochondrial Erk1/2 (Figure 6E) and Akt (Figure 6G) with IPC, APC and IPC/APC were observed (from 1.6 to 6.9-fold, P <.05 for each) compared with controls. However, in these studies chelerythrine was less effective in preventing the enhanced protein levels. Indeed, Akt protein was inhibited 49% (P <.01) by the drug in APC and 29% in IPC/APC although this value did not reach statistical significance. Significant inhibition by chelerythrine was also observed with Erk1/2 protein in APC (42%, P <.05) and the combined IPC/APC treatment (52%, P <.05).
At variance with PKCε, Erk1/2 and Akt proteins, the effects of treatments on PKCδ (Figure 6D), JNK (Figure 6F), and p38 (Figure 6H) were noticeably smaller. With the exception of JNK, which increased about 2-fold in mitochondrial fractions with IPC, APC, and IPC/APC (P <.05 for all), mitochondrial PKCδ and p38 were unaffected by treatments. Only APC increased p38 protein levels (67%, P <.01). Chelerythrine treatment inhibited JNK protein in mitochondria by APC (72%, P <.01), and IPC/APC (62%, P <.01) and abolished p38 protein levels in APC (P <.01). GSK-3β was not significantly affected (data not shown) by IPC, APC or their combination. These studies demonstrate that translocation to the mitochondria of PKCε, Erk1/2 and Akt (which occurs upon their activation) is involved in the cardioprotection induced by both preconditionings.
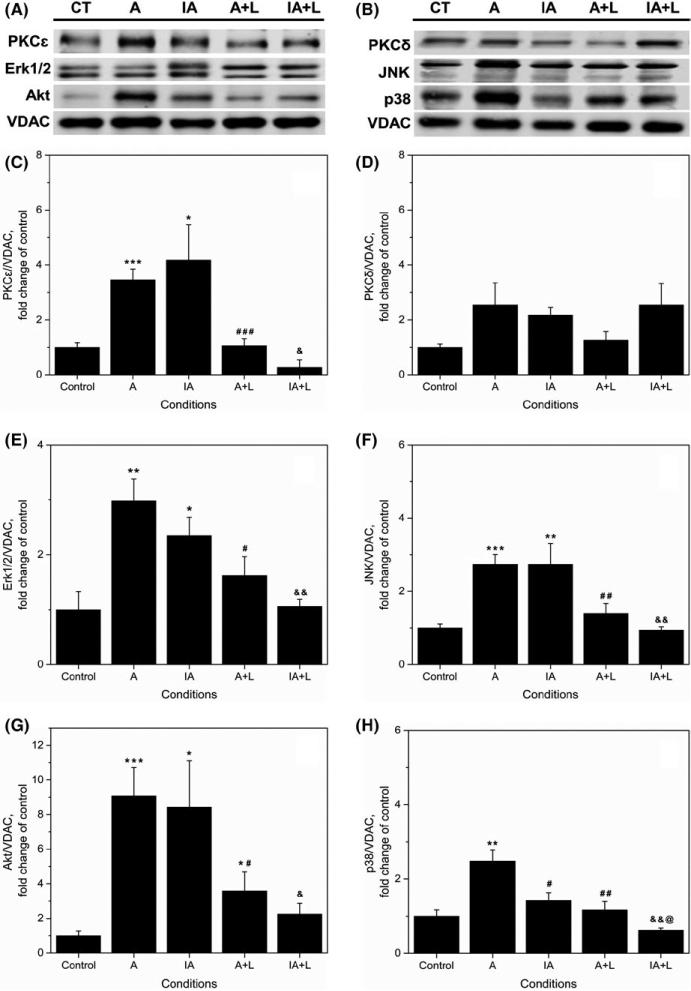
2.6 | Effect of losartan on PKCε, PKCδ, Erk1/2, Akt, JNK, and p38 MAPK in mitochondrial fractions
Figure 7A-H depicts the effect of 10 μmol/L losartan on protein levels of PKCε, PKCδ, Erk1/2, Akt, JNK, and p38 MAPK in mitochondria. Losartan completely prevented APC- and IPC/APC-induced increase of PKCε (Figure 7C), Erk1/2 (Figure 7E) and Akt (Figure 7G) in mitochondrial fractions. PKCε decreased 70% (P <.001) and 76% (P <.05) by losartan in APC and IPC/APC, respectively, while Erk1/2 was reduced 46% (P <.05) and 55% (P <.01), respectively. Similarly, Akt decreased by losartan 60% (P <.05) and 74% (P <.05) in APC and IPC/APC, respectively. PKCδ protein levels (Figure 7D) were not significantly affected by losartan although there was a tendency of the drug to inhibit protein levels in APC. JNK (Figure 7F) protein in APC and IPC/APC, however, were reduced 77% (P <.01) and 100% (P <.05) by losartan, respectively. Likewise, the enhancement of p38 protein levels by APC (Figure 7H) was inhibited 89% (P <.01) by losartan. The drug also depressed p38 protein levels in IPC/APC (P <.01). These findings indicate that the increase of PKCε, Erk1/2, and Akt in mitochondria by APC and IPC/APC requires AT1-Rs.
FIGURE 7.
Effects of losartan on protein levels of PKCε, PKCδ, Erk1/2, JNK, Akt, and p38 MAPK in isolated mitochondria. (A, B) Representative Western blots and quantitative data of total (C) PKCε; (D) PKCδ; (E) Erk1/2; (F) JNK; (G) Akt; (H) p38. Proteins were calculated as the ratio of total protein to VDAC, and normalized to control for each kinase. The values are shown as means ± SEM of five experiments per group. *P <.05, **P <.01, ***P <.001 vs Control; #P <.05, ##P <.01, ###P <.001, vs A; &P <.05, &&P <.01 vs IA; @P <.05 vs A+L. CT, control; I, IPC; A, APC; IA, IPC/APC; A+L, APC+losartan; IA+L, IPC/APC+losartan
3 | DISCUSSION
In this work, we evaluated the signalling mechanisms involved in APC to define the role of PKC-dependent signalling pathways in chelerythrine- and losartan-treated hearts. We demonstrated that mitochondrial levels of PKCε, but not PKCδ, markedly increased by APC, IPC and their combination. These findings suggest that activation and translocation of PKCε to the mitochondria is part of the mechanism by which these preconditionings mediate cardioprotection. Indeed, the translocation of PKCε to mitochondria was abolished by chelerythrine, a drug that inhibits the activity of all PKC isoforms by interacting with its catalytic domain.14 The increased mitochondrial PKCε levels were associated with chelerythrine-sensitive Erk1/2 and Akt translocation, supporting an important role of the prosurvival kinase pathway in the cardioprotective effects of IPC, APC, and IPC/APC. The important role of the PKCε-dependent signalling in cardioprotection is evidenced by the fact that chelerythrine abrogated the reduction of infarct size by the APC or IPC/APC when added either at preconditioning or reperfusion. These findings also indicate that the reperfusion phase is required for preconditioning-induced cardioprotection as previously reported.35 Altogether, these studies are consistent with a central role of PKCε in IPC and pharmacological preconditioning.36-44
Although protein levels of PKCε in APC or IPC/APC in the presence of chelerythrine were not statistically different from control hearts, PKCδ levels were 2-fold (P <.05) higher in the preconditioning groups, suggesting a cross-talk by which inhibition of PKCε promotes PKCδ translocation to the mitochondria. These findings agree with a previous report45 indicating that inhibition of PKCε is associated with phosphorylation of PKCδ and its translocation to the mitochondria. The fact that chelerythrine inhibited the APC and IPC/APC-mediated translocation of PKCε but increased that of PKCδ points to a primary effect of chelerythrine on PKCε at preconditioning and increased translocation of PKCδ to the mitochondria at reperfusion.27,42 Therefore, these studies suggest that APC and IPC/APC increases the ratio of PKCε/PKCδ protein levels in mitochondria in a chelerythrine-sensitive manner.
It is of interest to note that while chelerythrine reversed the PKCε/PKCδ ratio, it did not affect the improvements of cardiac function (LVDP) by APC or IPC/APC as previously reported.7 These findings suggest the activation of alternate signalling pathways for cardioprotection when PKCε-dependent mechanisms are inhibited. They also indicate that the increased mitochondrial PKCδ observed here, is not necessarily detrimental to heart function as previously suggested.46 Indeed, studies in PKCδ knockout mice demonstrate increased injury after IPC.44 Also, beneficial effects of PKCδ on cardioprotection have also been found in adult rats exposed to an intermittent hypoxia47,48 and sevoflurane-induced preconditioning.49 Preconditioning by chronic hypoxia, however, appears to depend on PKCε activity.50 Therefore, the role of PKCε or PKCδ in cardioprotection is likely dependent on the preconditioning stimulus and the experimental conditions used. To our knowledge, this is the first study demonstrating that the cardioprotective effect of APC in chelerythrine-treated hearts is associated with reduced PKCε/PKCδ ratio in the mitochondria.
mPTP is an important target of the PKC-dependent signalling mechanisms involved in cardioprotection. Our data suggest that an AT1-R-mediated translocation of PKCε, Erk1/2, and Akt to mitochondria promote inhibition of mPTP.19,35 Indeed, we found that both APC and IPC/APC significantly reduced mPTP opening in a chelerythrine- and losartan-sensitive manner. Because inhibition of PKC-dependent signalling is associated with mPTP opening, the post-ischaemic cardiac recovery in the presence of chelerythrine apparently involves mPTP-independent pathways. This effect of chelerythrine is at variance with that of losartan that inhibited the post-ischaemic cardiac recovery, the translocation of PKCε, Akt, Erk1/2, JNK and p38, and the APC-mediated, mPTP inhibition. Therefore, our data suggest that APC and IPC/APC promote cardiac recovery after IR through an AT1-R-mediated PKCε-dependent inhibition of the mPTP. However, in the presence of chelerythrine other cardioprotective mechanisms appear to be recruited leading to dissociation of the PKCε-dependent signalling and cardiac function.
The mechanisms involved in dissociating the post-ischaemic cardiac recovery and PKCε-dependent signalling with chelerythrine are unknown. We have previously reported that chelerythrine inhibits cardiac function during preconditioning with APC7 and other investigators14 have shown that at 1 μmol/L it abolishes IPC when administered for 3 minutes before preconditioning protocols. However, studies in rat hearts51 employing a prolonged (40 minutes) chelerythrine treatment before global ischaemia and reperfusion, show an enhanced post-ischaemic LVDP compared with non-treated hearts. Similar results were found by other groups52 with 30 minutes treatment with chelerythrine (5 μmol/L) during IPC. The protective effect of chelerythrine appears to involve stimulation of Na+/K+ ATPase activity and reduced intracellular Na+ levels secondary to the inhibition of PKC activity.51 Consistent with these findings, the drug also reduced creatine kinase release on reperfusion.51 Therefore, it is possible that in our studies with prolonged exposure to 5 μmol/L chelerythrine, cellular Na+ and hence, the inward movements of Ca2+ through Na+/Ca2+ exchange are reduced, leading to a diminished Ca2+ loading during ischaemia and reperfusion. These effects could enhance the post-ischaemic cardiac recovery by reducing the damage associated with Ca2+ loading as suggested by Lundmark and collaborators.51 Indeed, the ischaemic contracture, a function of Ca2+ loading during ischaemia, was inhibited by chelerythrine in controls, APC and IPC/APC supporting the idea that the drug reduces the post-ischaemic damage. This finding is consistent with suppression of LDH release by chelerythrine at the start of reperfusion in all groups evaluated.
Of note, chelerythrine also improved the post-ischaemic recovery (LVDP) of control hearts, suggesting that the improvement of cardiac function by the drug is independent of preconditioning. As mentioned above, the enhancement of cardiac recovery by chelerythrine could involve a reduction of contracture-induced tissue damage. However, it is likely that activation of PKCδ also contributes to the post-ischaemic cardiac recovery by the drug. This kinase can be phosphorylated on treonine505 in a chelerythrine-insensitive manner.52 Moreover, PKCδ activity has also been related to alterations of Ca2+ influx via Na+/Ca2+ exchange activity and Na+/K+ ATPase activity53 and could be modulated by ROS-dependent mechanisms during reperfusion.49 A positive inotropic effect of PKCδ has been reported in adult ventricular myocytes when the kinase localises to intracellular sites.54 Therefore, both mechanisms (reduced contracture-induced damage and PKCδ activation) could contribute to the increased LVDP observed in the presence of chelerythrine. Additional experiments are required to settle this point.
It is important to note that while chelerythrine inhibited LDH activity at the start of reperfusion in both control and preconditioning groups, it reversed the reduction of infarct size and mPTP opening by APC and IPC/APC at the conclusion of reperfusion (90 minutes after global ischaemia). The latter findings strongly suggest that inhibition of PKCε-dependent signalling and increased mPTP opening are linked to myocardial tissue damage. Considering that LDH activity monitors the tissue injury wavefront, while infarct size is a measure of IR-induced tissue damage,55 the apparent discrepancy between these indicators in chelerythrine-treated hearts, could arise from the expression of different temporal events (early and late) associated with reperfusion damage. It is possible that at early reperfusion the drug reduces myocardial cell Ca2+, reperfusion damage, and LDH release, while as reperfusion injury proceeds, its effect on PKCε-dependent signalling and mPTP, reduce myocardial integrity and cell survival, and increases infarct size. It should be noted that although chelerythrine prevented the reduction of infarct size by preconditioning, it promoted the post-ischaemic cardiac recovery (LVDP). These findings suggest a compensatory increase in myocardial contractility when tissue damage develops. We can only speculate that this compensation to sustain cardiac function, is due at least in part, to a PKCδ-mediated, enhanced contractility, as previously mentioned. By contrast with chelerythrine, losartan diminished the PKCε-dependent signalling, the ischaemic contracture, LDH release and the post-ischaemic cardiac recovery. These findings agree with the role that PKC and intracellular Ca2+ play in the reported inotropic effect of Ang II in cardiac tissue.56
Accumulating data suggest that Akt is the upstream signalling of PKCε activation in IPC.8 This fact is consistent with the relative Akt insensitivity to chelerythrine observed in this study. Indeed, the inhibitor partially reduced the enhancement of Akt by APC (49%) and IPC/APC (29%). These results indicate that while Akt activation mediates cardioprotective effects through PKC in IPC, both PKC-dependent and PKC-independent mechanisms are involved in APC. Our findings are consistent with previous studies on the PKC-independent activation of Akt during ischaemia and PKC-dependent activation of Akt at reperfusion through GPCR sensitization as found for the adenosine receptor (A2bAR).8,44 In contrast, Erk1/2 was markedly inhibited by chelerythrine in both APC and IPC/APC, suggesting that its activation is PKC-dependent. The latter possibly involves the Raf-MEK1/2 pathway at reperfusion.35 Our results also show losartan-sensitive, upregulation of PKCε, Erk1/2, and Akt in APC and IPC/APC, indicating their involvement in AT1-R signalling pathways.9
Another kinase that appears to be affected by APC and IPC/APC in a chelerythrine- and losartan-sensitive manner is JNK. Both chelerythrine and losartan decreased translocation of JNK to mitochondria and prevented the inhibition of the PTP by preconditioning. These findings suggest a role for JNK in the PKCε-dependent signalling mechanisms of APC and IPC/APC. Indeed, we have previously reported that inhibition of JNK with SU3327 causes mitochondrial dysfunction and aggravates the recovery of the heart after IR injury.34 However, the stress-mediated activation of JNK in the heart is complex and depends on the spatial-temporal localisation and isoform activated.8,33 JNK promotes reactivation of Akt, and interacts with the apoptosome, delaying the activation of caspase 9.57,58 Whether its actions on the mitochondria, Akt or the apoptosome are involved in the post-ischaemic recovery observed in the present study remains to be determined. Two additional kinases that are involved in preconditioning are p38 and GSK-3β. In this study, mitochondrial p38 was increased by APC in a chelerythrine and losartan-sensitive manner. In IPC/APC, this effect was not observed although losartan significantly (56%, P <.01) reduced p38 levels in IPC/APC. These findings agree with previous studies on the role of Ang II in cardioprotection by PKC-dependent, ROS-mediated p38 MAPK activation.10,29 However, its role in cardioprotection is still controversial.8,44 GSK-3β is a kinase that is phosphorylated and inhibited during preconditioning. This action has been linked to mPTP inhibition and cardioprotection.59 GSK-3β appears to reside in the cytosol because its inhibition in isolated mitochondria has no effect on mPTP opening.60 We did not find significant changes in the mitochondrial protein level of GSK-3β by the preconditioning protocols.
In addition to the mechanisms mentioned above, cardioprotective effects of APC can be mediated through the mitochondrial angiotensin system. Abundant and functional Ang II type 2 receptors (AT2-R) colocalised with endogenous Ang II, were found in mitochondria of mouse kidney tubular cells. Stimulation of mitochondrial AT2-Rs increased nitric oxide production and modulated the respiratory function of mitochondria.61 Likewise, our group has reported the expression of Ang II receptors, predominantly, AT2-Rs in mitochondria of healthy rats.62 Therefore, these findings support the idea that the mitochondrial angiotensin system could also play a role in APC.
Some limitations must be considered in the analysis of the current work. In this study, we used chelerythrine, a pan-PKC inhibitor that limits the evaluation of isoform-specific PKC-signalling in cardioprotection. We also measured total protein levels of PKCε, Erk1/2, Akt, PKCδ, JNK, p38 MAPK and GSK-3β in mitochondrial fractions at the end of reperfusion, to assess whether the translocation of these kinases was associated with the preconditioning protocols. The strong pattern of change in total kinase levels observed here, and the inhibitory effect of chelerythrine on PKCε, Erk1/2, Akt, and JNK, but not PKCδ and GSK-3β, support their activation and translocation to the mitochondria. However, other studies failed to show changes in total protein levels by IPC. Indeed, phospho-Akt and phospho-Erk1/2, but not total protein levels of these kinases have been reported to be increased by IPC.35 The reason for these apparent controversies is unknown, but it is possible that the use of homogenates35 versus the mitochondrial fractions in this study contributes to the differences observed. Indeed, Churchill et al63 also reported increased total PKCε levels in mitochondrial fractions obtained from IPC-hearts, an effect that was inhibited by the εV1-2 peptide that blocks PKCε translocation. Therefore, we are confident that the changes in total protein levels observed here reflect the translocation of the kinases evaluated in this study, rather than the activation of discreet mitochondrial pools.
In summary, the findings of this study indicate that APC induces an AT1-R-dependent increase in the ratio of PKCε/PKCδ protein levels in the mitochondria. This effect together with the recruitment of Erk1/2, Akt and JNK-dependent signalling and mPTP inhibition, appear to be critical in the cardioprotection by APC and IPC/APC. Chelerythrine abrogated the reduction of infarct size induced by preconditioning supporting the role of PKCε -dependent signalling in cardioprotection. However, the drug reduced the mitochondrial PKCε/PKCδ ratio but preserved the post-ischaemic cardiac recovery. The latter could be secondary to the attenuation of contracture-induced damage at reperfusion and/or an enhanced contractility by PKCδ activation. The study of these mechanisms could lead to new paths of pharmacological cardioprotection and treatment of IR-induced damage.
4 | METHODS
Male Sprague-Dawley rats weighing 140-160 g were purchased from Charles River (Wilmington, MA, USA). All experiments were performed according to protocols approved by the University Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
4.1 | Langendorff-mode perfusion
Hearts were isolated and perfused in the Langendorff mode as described previously.7 Briefly, rats were anaesthetized intraperitoneally with pentobarbital sodium (35 mg/kg body weight). The hearts were rapidly excised and immediately arrested in ice-cold buffered Krebs-Henseleit solution (KHS) containing (in mmol/L): 118 NaCl, 4.8 KCl, 1.2 KH2PO4, 1.25 CaCl2, 1.2 MgSO4, 11 glucose, and 25 NaHCO3 equilibrated at pH 7.4 with 5% CO2/95% O2, and mounted on a Langendorff's perfusion apparatus. Isolated hearts were perfused at constant flow (10-12 mL/g heart weight per min) with KHS. A water-filled latex balloon was inserted into the left ventricle and connected to a pressure transducer for continuous monitoring of left ventricular pressure. Balloon volume was set to give an initial left ventricular end diastolic pressure (LVEDP) of 4-6 mmHg. Data acquisition to determine left ventricular developed pressure (LVDP) was performed with the labscribe2 Data Acquisition Software (iWorx 308T, Dover, NH, USA). Samples of perfusate were collected during the procedure to determine the LDH activity by an enzymatic method as previously described.64 A subset of hearts underwent the corresponding perfusion protocols for each group to determine infarct size at the end of reperfusion by TTC staining method, as described previously.7 Results were expressed as infarcted area/total area × 100.
4.2 | Animals groups
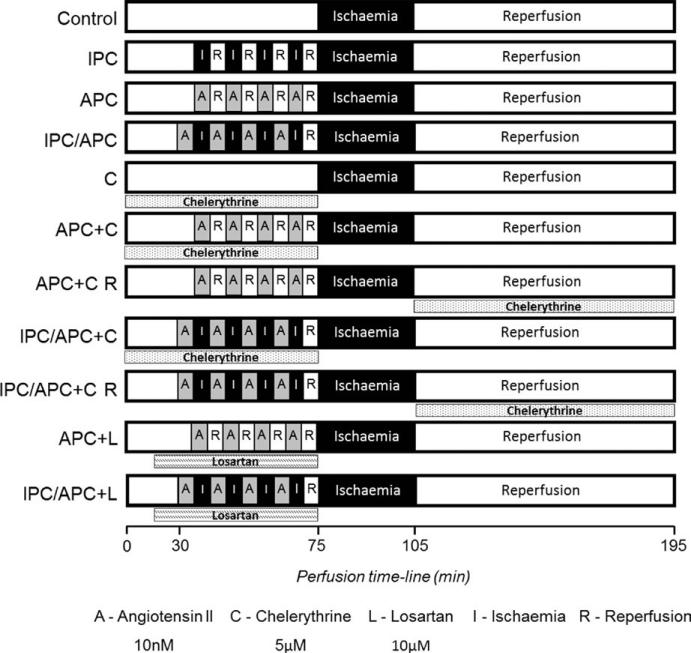
All hearts were equilibrated to the perfusion condition for 20 minutes and then, subjected to different perfusion protocols as described in the following groups: (i) IR (control) group, hearts were perfused for 75 minutes without any interventions followed by 30 minutes of global ischaemia and 90 minutes of reperfusion; (ii) IPC group, hearts underwent four cycles of 5 minutes of global ischaemia and 5 minutes of reperfusion for a total of 40 minutes prior to the 30 minutes global ischaemia and 90 minutes reperfusion; (iii) APC group, hearts underwent four cycles of 5 minutes perfusion with Ang II (10 nmol/L) and 5 minutes washout for a total of 40 minutes prior to the 30 minutes global ischaemia and 90 minutes reperfusion; (iv) IPC/APC group, hearts underwent for four cycles of 5 minutes of global ischaemia and 5 minutes of Ang II perfusion for a total of 40 minutes prior to the 30 minutes global ischaemia and 90 minutes reperfusion; (v) chelerythrine group, hearts were infused with chelerythrine (pan-PKC inhibitor, 5 μmol/L) during pre-ischaemia, followed by 30 minutes global ischaemia and 90 minutes reperfusion; (vi) APC+chelerythrine group, hearts were infused with chelerythrine for 30 minutes prior and during the APC protocol followed by 30 minutes global ischaemia and 90 minutes reperfusion; (vii) APC+chelerythrine reperfusion group, hearts were subjected to the APC protocol, followed by 30 minutes global ischaemia and infused with chelerythrine during the 90 minutes of reperfusion; (viii) IPC/APC+chelerythrine group, hearts were infused with chelerythrine for 30 minutes prior and during the IPC/APC protocol followed by 30 minutes global ischaemia and 90 minutes reperfusion; (ix) IPC/APC+chelerythrine reperfusion group, hearts were subjected to the IPC/APC protocol, followed by 30 minutes global ischaemia and infused with chelerythrine during the 90 minutes of reperfusion; (x) APC+losartan group, hearts were infused with losartan (AT1-R blocker, 10 μmol/L) for 15 minutes prior and during APC protocol followed by the 30 minutes global ischaemia and 90 minutes reperfusion; and (xi) IPC/APC+losartan group, hearts were infused with losartan for 15 minutes prior and during IPC/APC protocol followed by the 30 minutes global ischaemia and 90 minutes reperfusion. All groups of IPC/APC had a 5 minutes washout period prior to the 30 minutes ischaemia. Global ischaemia was induced by switching off the pump. During ischaemia, hearts were immersed in KHS buffer maintained at 37°C. A diagram of perfusion protocols is illustrated in Figure 1.
FIGURE 1.
Schematic representation of the perfusion protocols. See “Methods” for details
4.3 | Isolation of mitochondria
At the end of reperfusion, mitochondria were isolated as described previously.7 Briefly, the ventricles were cut, weighed, and homogenized with a Polytron homogenizer in 3 mL of ice-cold sucrose buffer containing 300 mmol/L sucrose, 10 mmol/L Tris-HCl, and 2 mmol/L EGTA; pH 7.4. Mitochondria were isolated from homogenate by centrifugation at 2000 g for 3 minutes, followed by centrifugation of the supernatant at 10 000 g for 6 minutes. The pellet was then resuspended and washed two times by centrifugation at 10 000 g for 6 minutes. The final pellet containing mitochondria was resuspended and used for determination of mPTP opening and analysis of protein expression.
4.4 | Determination of mPTP opening
Swelling of de-energized mitochondria as an indicator of mPTP opening in the presence and absence of Ca2+ was determined by monitoring the decrease in light scattering at 520 nm as described previously.7 Isolated mitochondria were incubated at 25°C in 1 mL of a buffer containing 150 mmol/L KSCN, 20 mmol/L MOPS, 10 mmol/L Tris, and 2 mmol/L nitrilotriacetic acid, supplemented with 0.5 μmol/L rotenone, 0.5 μmol/L antimycin and 2 μmol/L A23187. The reaction was started by the addition of 40 μL of the mitochondrial suspension containing approximately 220 μg of protein and swelling was initiated by addition of 200 μmol/L CaCl2. Sanglifherin A (0.2 μmol/L), an mPTP inhibitor, was used in vitro as a positive control of mPTP inhibition.
4.5 | Western blot analysis
Immunoblotting was performed as described previously.64 Protein concentration in mitochondria was determined using a Bradford assay kit (Bio-Rad, Hercules, CA, USA). Equal amounts of mitochondrial protein from the different experimental groups were resolved on 10% SDS-PAGE, and transferred onto Amersham Hybond ECL nitrocellulose membranes (GE Healthcare Bio-Sciences Corp., Pittsburgh, PA, USA). Membranes were blocked with 5% BSA in TBS. The antibodies used in this study were against total forms of PKCε (1:1000), PKCδ (1:2000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), Akt (1:1000), Erk1/2 (1:1000), JNK (1:1000), p38 (1:1000), GSK-3β (1:1000), and VDAC (1:1000) (Cell Signaling Technology, Danvers, MA, USA). The signals were visualized by Odyssey CLx Quantitative Fluorescent Imaging System with the secondary infrared antibody IRDye 800CW goat anti-rabbit (1:25 000). Results were analysed with image studio lite Software (LI-COR Biotechnology, Lincoln, NE, USA).
4.6 | Statistical analysis
Data are presented as mean ± SEM of 5-14 experiments per group. Statistical differences between groups were analyzed using 2-tailed unpaired Student's t test. Cardiac function data were compared using ANOVA followed by Tukey-Kramer post-hoc tests. Differences were considered significant when P ≤ .05.
ACKNOWLEDGEMENTS
The authors recognize the contribution of Ms. Miriam Castro who helped with LDH activity and mPTP opening determinations. This study was supported by the NIH RCMI Program grant No. G12M007600, NHLBI grant SC1HL118669 (SJ), and the University of Puerto Rico.
Footnotes
DISCLOSURE
The authors declare no conflict of interests.
REFERENCES
- 1.Liu Y, Tsuchida A, Cohen MV, Downey JM. Pretreatment with angiotensin II activates protein kinase C and limits myocardial infarction in isolated rabbit hearts. J Mol Cell Cardiol. 1995; 27:883-892. [DOI] [PubMed] [Google Scholar]
- 2.Diaz RJ, Wilson GJ. Selective blockade of AT1 angiotensin II receptors abolishes ischemic preconditioning in isolated rabbit hearts. J Mol Cell Cardiol. 1997; 29:129-139. [DOI] [PubMed] [Google Scholar]
- 3.Sharma A, Singh M. Role of angiotensin in cardioprotective effect of ischemic preconditioning. J Cardiovasc Pharmacol. 1999; 33:772-778. [DOI] [PubMed] [Google Scholar]
- 4.Sharma A, Singh M. Possible mechanism of cardioprotective effect of angiotensin preconditioning in isolated rat hearts. Eur J of Pharmacol. 2000; 406:85-92. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira AJ, Santos RA, Almeida AP. Angiotensin-(1-7): Cardioprotective effect in myocardial ischemia/reperfusion. Hypertension. 2001; 38:665-668. [DOI] [PubMed] [Google Scholar]
- 6.Das S, Engelman RM, Maulik N, Das DK. Angiotensin preconditioning of the heart: Evidence for redox signaling. Cell Biochem Biophys. 2006; 44:103-110. [DOI] [PubMed] [Google Scholar]
- 7.Nuñez RE, Castro M, Javadov S, Escobales N. Angiotensin II and ischemic preconditioning synergize to improve mitochondrial function while showing additive effects on ventricular postischemic recovery. J Cardiovasc Pharmacol. 2014; 64:172-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008; 88:581-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta PK, Griendling KK. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007; 292:C82-C97. [DOI] [PubMed] [Google Scholar]
- 10.Zhang GX, Lu XM, Kimura S, Nishiyama A. Role of mitochondria in angiotensin II-induced reactive oxygen species and mitogen-activated protein kinase activation. Cardiovasc Res. 2007; 76:204-212. [DOI] [PubMed] [Google Scholar]
- 11.Opie LH, Sack MN. Metabolic plasticity and the promotion of cardiac protection in ischemia and ischemic preconditioning. J Mol Cell Cardiol. 2002; 34:1077-1089. [DOI] [PubMed] [Google Scholar]
- 12.Lai CC, Tang CY, Chiang SC, Tseng KW, Huang CH. Ischemic preconditioning activates prosurvival kinases and reduces myocardial apoptosis. J Chin Med Assoc. 2015; 78:460-468. [DOI] [PubMed] [Google Scholar]
- 13.Krijnen PA, Nijmeijer R, Meijer CJ, Visser CA, Hack CE, Niessen HW. Apoptosis in myocardial ischaemia and infarction. J Clin Pathol. 2002; 55:801-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawamura S, Yoshida K, Miura T, Mizukami Y, Matsuzaki M. Ischemic preconditioning translocates PKC-delta and -epsilon, which mediate functional protection in isolated rat heart. Am J Physiol. 1998; 275:H2266-H2271. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell MB, Meng X, Ao L, Brown JM, Harken AH, Banerjee A. Preconditioning of isolated rat heart is mediated by protein kinase C. Circ Res. 1995; 76:73-81. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Ytrehus K, Downey JM. Evidence that translocation of protein kinase C is a key event during ischemic preconditioning of rabbit myocardium. J Mol Cell Cardiol. 1994; 26:661-668. [DOI] [PubMed] [Google Scholar]
- 17.Liang BT. Protein kinase C-dependent activation of KATP channel enhances adenosine-induced cardioprotection. Biochem J. 1998; 336:337-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dekker LR, Coronel R, VanBavel E, Spaan JA, Opthof T. Intracellular Ca2+ and delay of ischemia-induced electrical uncoupling in preconditioned rabbit ventricular myocardium. Cardiovasc Res. 1999; 44:101-112. [DOI] [PubMed] [Google Scholar]
- 19.Javadov S, Jang S, Agostini B. Crosstalk between mitogen-activated protein kinases and mitochondria in cardiac diseases: Therapeutic perspectives. Pharmacol Ther. 2014; 144:202-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seki T, Matsubayashi H, Amano T, Shirai Y, Satio N, Sakai N. Phosphorylation of PKC activation loop plays an important role in receptor-mediated translocation of PKC. Genes Cells. 2005; 10:225-239. [DOI] [PubMed] [Google Scholar]
- 21.Zatta AJ, Kin H, Lee G, et al. Infarct-sparing effect of myocardial post-conditioning is dependent on protein kinase C signalling. Cardiovasc Res. 2006; 70:315-324. [DOI] [PubMed] [Google Scholar]
- 22.Wang QJ, Bhattacharyya D, Garfield S, Nacro K, Marquez VE, Blumberg PM. Differential localization of protein kinase C delta by phorbol esters and related compounds using a fusion protein with green fluorescent protein. J Biol Chem. 1999; 274:37233-37239. [DOI] [PubMed] [Google Scholar]
- 23.Baines CP, Song CX, Zheng YT, et al. Protein kinase C epsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res. 2003; 92:873-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Churchill EN, Szweda LI. Translocation of δPKC to mitochondria during cardiac reperfusion enhances superoxide anion production and induces loss in mitochondrial function. Arch Biochem Biophys. 2005; 439:194-199. [DOI] [PubMed] [Google Scholar]
- 25.Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase C delta activation induces apoptosis in response to cardiac ischemia and reperfusion damage: A mechanism involving BAD and the mitochondria. J Biol Chem. 2004; 279:47985-47991. [DOI] [PubMed] [Google Scholar]
- 26.Gray MO, Karliner JS, Mochly-Rosen D. A selective epsilon-protein kinase C antagonist inhibits protection of cardiac myocytes from hypoxia-induced cell death. J Biol Chem. 1997; 272:30945-30951. [DOI] [PubMed] [Google Scholar]
- 27.Inagaki K, Hahn HS, Dorn GW II, Mochly-Rosen D. Additive protection of the ischemic heart ex vivo by combined treatment with delta-protein kinase C inhibitor and epsilon-protein kinase C activator. Circulation. 2003; 108:869-875. [DOI] [PubMed] [Google Scholar]
- 28.Kimura S, Zhang GX, Nishiyama S, et al. Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension. 2005; 45:860-866. [DOI] [PubMed] [Google Scholar]
- 29.Daiber A. Redox signaling (cross talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta. 2010; 1797:897-906. [DOI] [PubMed] [Google Scholar]
- 30.Fukai T. Mitochondrial thioredoxin: Novel regulator for NADPH oxidase and angiotensin II-induced hypertension. Hypertension. 2009; 54:224-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Giusti VC, Caldiz CI, Ennis IL, Pérez NG, Cingolani HE, Aiello EA. Mitochondrial reactive oxygen species (ROS) as signaling molecules of intracellular pathways triggered by the cardiac renin-angiotensin II-aldosterone system (RAAS). Front Physiol. 2013; 4:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heusch G. Molecular basis of cardioprotection: Signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. 2015; 116:674-699. [DOI] [PubMed] [Google Scholar]
- 33.Rose BA, Force T, Wang Y. Mitogen-activating protein kinase signaling in the heart: Angels versus demons in a heart-breaking tale. Physiol Rev. 2010; 90:1507-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jang S, Javadov S. Inhibition of JNK aggravates the recovery of rat hearts after global ischemia: The role of mitochondrial JNK. PLoS ONE. 2014; 9:e113526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005; 288:H971-H976. [DOI] [PubMed] [Google Scholar]
- 36.Liu GS, Cohen MV, Mochly-Rosen D, Downey JM. Protein kinase C-epsilon is responsible for the protection of preconditioning in rabbit cardiomyocytes. J Mol Cell Cardiol. 1999; 31:1937-1948. [DOI] [PubMed] [Google Scholar]
- 37.Ludwig LM, Weihrauch D, Kersten JR, Pagel PS, Warltier DC. Protein kinase C translocation and Src protein tyrosine kinase activation mediate isoflurane-induced preconditioning in vivo: Potential downstream targets of mitochondrial adenosine triphosphate-sensitive potassium channels and reactive oxygen species. Anesthesiology. 2004; 100:532-539. [DOI] [PubMed] [Google Scholar]
- 38.Novalija E, Kevin LG, Camara AK, Bosnjak ZJ, Kampine JP, Stowe DF. Reactive oxygen species precede the epsilon isoform of protein kinase C in the anesthetic preconditioning signaling cascade. Anesthesiology. 2003; 99:421-428. [DOI] [PubMed] [Google Scholar]
- 39.Qui Y, Ping P, Tang XL, et al. Direct evidence that protein kinase C plays an essential role in the development of late preconditioning against myocardial stunning in conscious rabbits and that epsilon is the isoform involved. J Clin Invest. 1998; 101:2182-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toma O, Weber NC, Wolter JI, Obal D, Preckel B, Schlack W. Desflurane preconditioning induces time-dependent activation of protein kinase C epsilon and extracellular signal-regulated kinase 1 and 2 in the rat heart in vivo. Anesthesiology. 2004; 101:1372-1380. [DOI] [PubMed] [Google Scholar]
- 41.Ping P, Zhang J, Qui Y, et al. Ischemic preconditioning induces selective translocation of protein kinase C isoforms epsilon and eta in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res. 1997; 81:404-414. [DOI] [PubMed] [Google Scholar]
- 42.Inagaki K, Chen L, Ikeno F, et al. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation. 2003; 108:2304-2307. [DOI] [PubMed] [Google Scholar]
- 43.Inagaki K, Churchill E, Mochly-Rosen D. Epsilon protein kinase C as a potential therapeutic target for the ischemic heart. Cardiovasc Res. 2006; 70:222-230. [DOI] [PubMed] [Google Scholar]
- 44.Yang X, Cohen MV, Downey JM. Mechanism of cardioprotection by early ischemic preconditioning. Cardiovasc Drugs Ther. 2010; 24:225-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayr M, Liem D, Zhang J, et al. Proteomic and metabolomic analysis of cardioprotection: Interplay between protein kinase C epsilon and delta in regulating glucose metabolism of murine hearts. J Mol Cell Cardiol. 2009; 46:268-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sivaraman V, Hausenloy DJ, Kolvekar S, et al. The divergent roles of protein kinase C epsilon and delta in simulated ischaemia-reperfusion injury in human myocardium. J Mol Cell Cardiol. 2009; 46:758-764. [DOI] [PubMed] [Google Scholar]
- 47.Neckár J, Marková I, Novák F, Nováková O, Szárszoi O, Ost’ádal B, Kolár F. Increased expression and altered subcellular distribution of PKC-δ in chronic hypoxic rat myocardium: Involvement in cardioprotection. Am J Physiol Heart Circ Physiol. 2005; 288:H1566-H1572. [DOI] [PubMed] [Google Scholar]
- 48.Hlavácková M, Neckár J, Jezková J, et al. Dietary polyunsaturated fatty acids alter myocardial protein kinase C expression and affect cardioprotection induced by chronic hypoxia. Exp Biol Med (Maywood). 2007; 232:823-832. [PubMed] [Google Scholar]
- 49.Bouwman RA, Musters RJ, van Beek-Harmsen BJ, de Lange JJ, Boer C. Reactive oxygen species precede protein kinase C-delta activation independent of adenosine triphosphate-sensitive mitochondrial channel opening in sevoflurane-induced cardioprotection. Anesthesiology. 2004; 100:506-514. [DOI] [PubMed] [Google Scholar]
- 50.Holzerová K, Hlavácková M, Zurmanová J, et al. Involvement of PKCε in cardioprotection induced by adaptation to chronic continuous hypoxia. Physiol Res. 2015; 64:191-201. [DOI] [PubMed] [Google Scholar]
- 51.Lundmark JL, Ramasamy R, Vulliet PR, Schaefer S. Chelerythrine increases Na-K-ATPase activity and limits ischemic injury in isolated rat hearts. Am J Physiol. 1999; 277:H999-H1006. [DOI] [PubMed] [Google Scholar]
- 52.Uecker M, Da Silva R, Grampp T, Pasch T, Schaub MC, Zaugg M. Translocation of protein kinase C isoforms to subcellular targets in ischemic and anesthetic preconditioning. Anesthesiology. 2003; 99:138-147. [DOI] [PubMed] [Google Scholar]
- 53.Bouwman RA, Salic K, Padding FG, et al. Cardioprotection via activation of protein kinase C-δ depends on modulation of the reverse mode of the Na+/Ca2+ exchanger. Circulation. 2006; 114:I226-I232. [DOI] [PubMed] [Google Scholar]
- 54.Kang M, Walker JW. Protein kinase C delta and epsilon mediate positive inotropy in adult ventricular myocytes. J Mol Cardiol. 2005; 38:753-764. [DOI] [PubMed] [Google Scholar]
- 55.Rossello X, Hall AR, Bell RM, Yellon DM. Characterization of the Langendorff perfused isolated heart model of global ischemia-reperfusion injury: Impact of ischemia and reperfusion length on infarct size and LDH release. J Cardiovasc Pharmacol Ther. 2016; 21:286-295. [DOI] [PubMed] [Google Scholar]
- 56.Salas MA, Vila-Petroff MG, Palomeque J, Aiello A, Mattiazzi A. Positive inotropic and negative lusitropic effect of angiotensin II: Intracellular mechanisms and second messengers. J Mol Cell Cardiol. 2001; 33:1957-1971. [DOI] [PubMed] [Google Scholar]
- 57.Tran TH, Andreka P, Rodrigues CO, Webster KA, Bishopric NH. Jun kinase delays caspase-9 activation by interaction with the apopto-some. J Biol Chem. 2007; 282:20340-20350. [DOI] [PubMed] [Google Scholar]
- 58.Shao Z, Bhattacharya K, Hsich E, et al. c-Jun N-terminal kinases mediate reactivation of Akt and cardiomyocyte survival after hypoxic injury in vitro and in vivo. Circ Res. 2006; 98:111-118. [DOI] [PubMed] [Google Scholar]
- 59.Murphy E, Steenbergen C. Inhibition of GSK-3beta as a target for cardioprotection: The importance of timing location, duration and degree of inhibition. Expert Opin Ther Targets. 2005; 9:447-456. [DOI] [PubMed] [Google Scholar]
- 60.Garlid KD, Costa AD, Quinlan CL, Pierre SV, Dos Santos P. Cardioprotective signaling to mitochondria. J Mol Cell Cardiol. 2009; 46:858-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abadir PM, Foster DB, Crow M, et al. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci USA. 2011; 108:14849-14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parodi-Rullan R, Barreto-Torres G, Ruiz L, Casasnovas J, Javadov S. Direct renin inhibition exerts an anti-hypertrophic effect associated with improved mitochondrial function in post-infarction heart failure in diabetic rats. Cell Physiol Biochem. 2012; 29:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Churchill EN, Ferreira JC, Brum PC, Szweda LI, Mochly-Rosen D. Ischaemic preconditioning improves proteasomal activity and increases the degradation of δPKC during reperfusion. Cardiovasc Res. 2010; 85:385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barreto-Torres G, Parodi-Rullán R, Javadov S. The role of PPARα in metformin-induced attenuation of mitochondrial dysfunction in acute cardiac ischemia/reperfusion in rats. Int J Mol Sci. 2012; 13:7694-7709. [DOI] [PMC free article] [PubMed] [Google Scholar]