Abstract
Noroviruses (NoV) have enhanced tropism for the gastrointestinal (GI) tract and are the major cause of nonbacterial gastroenteritis in humans. Titanium dioxide (TiO2) nanoparticles (NPs) used as food additives, dietary supplements, and cosmetics accumulate in the GI tract. We investigated the effect anatase TiO2 NPs on NoV replication and host response during virus infection, using murine norovirus (MNV-1) infection of RAW 264.7 macrophages. Pretreatment with 20 μg/ml anatase NPs significantly reduced the viability of macrophages alone or during virus infection, but did not alter virus replication. In contrast, pre-incubation with 2 μg/ml anatase NPs reduced virus replication fivefold at 48 h. The presence of anatase NPs during MNV-1 infection evoked a pro-inflammatory response, as measured by a significant increase in expression of cytokines, including IL-6, IFN-γ, TNFα and the TGFβ1. No genotoxic insults due to anatase TiO2 NPs alone or to their presence during MNV-1 infection were detected. This study highlights important safety considerations related to NP exposure of the GI tract in individuals infected with noroviruses or other foodborne viruses.
Keywords: Murine Norovirus, Titanium, Pro-inflammatory response, Genotoxicity, Nanoparticles, Biomarkers
INTRODUCTION
Nanomedicine has evolved into a discipline with high impact and potential in the fields of infectious disease therapeutics, vaccine delivery systems, and cancer therapeutics, among others (1-5). A variety of nanomaterials, including polymeric NPs, polymeric micelles, graphite, silver, gold, silica, carbon nanotubes and polylactide-coglycolides (PLGA) are being used as drug delivery vehicles or as adjuvants for vaccines (6). Several features of NPs, including size, shape, and their absorptive, catalytic, conductive and magnetic properties, make them attractive candidates for targeted delivery of drugs and biologics to humans (7). Although the foreign nature of NPs can elicit immune system stimulation, the convenient scaffold for engineering proteins and other biomaterials on the surface of the nanomaterial makes them attractive for use as vaccine platforms and as adjuvants to stimulate the immune response (8). In addition, there has been increased use of a variety of NPs in consumer products (9, 10), with over 1500 products currently used in several sectors, including foods, beverages, cosmetics and home appliances (11, 12).
TiO2 NPs are widely used in pharmaceuticals, cosmetics and food products (13, 14). They are employed as a pigment to provide whiteness and opacity to paints, coatings, plastics, papers, inks, food products, pills, tablets and toothpastes (15). The use of TiO2 as food additive results in an estimated dietary consumption of 5.4 mg/person/day, and approximately 36% of TiO2 used in food products is nanosized (10, 16). The use of TiO2 NPs in cosmetics, including lip balm and spray, allows for increased oral uptake, leading to increased exposure and accumulation in the GI tract (17, 18). The levels of human exposure of TiO2 NPs warrant safety assessment on the risk potential in the GI tract and systemically. There have been mixed reports regarding the ability of TiO2 NPs to cross the intestinal wall and redistribute to various organs (19, 20). However, Cho et al. (21) demonstrated that administration of high doses (1041.5 mg/kg) of TiO2 NPs for 13 weeks resulted in no free Ti in the bloodstream, suggesting a lack of intestinal permeability and localized accumulation of these NPs in the GI tract. Furthermore, several studies have reported toxicity of TiO2 NPs to in vitro human intestinal cells, including Caco-2, suggesting that accumulation of TiO2 NPs in the GI tract might result in toxicity (22, 23).
Accumulation of TiO2 NPs in the GI tract can also result in interaction with foodborne viruses within the GI tract (24). While these interactions could affect the virus life cycle, they may also result in the exacerbation of viral replication, pathogenesis and host response. Among the enteric viruses, noroviruses (NoVs) are highly infectious and pathogenic, causing acute gastroenteritis in humans. The highly infectious nature of NoVs stems from their low infectious doses (as few as 10 viral particles can infect a host), their resistance to environmental stressors, and the absence of mechanisms in humans for long-term immunity to NoVs (25-27). NoVs cause roughly 80% of all human nonbacterial gastroenteritis infections worldwide (28, 29). Within the US alone, NoVs cause ~19–21 million acute cases each year, resulting in 800 deaths and 71,000 hospitalizations, accounting for the majority of US foodborne illness episodes. In healthy individuals, NoV infection is generally mild and of short duration, but NoV infection can prove fatal in immunocompromised individuals. The World Health Organization (WHO) estimates that NoV infections in developing countries may result in up to 1.1 million hospitalizations and 218,000 deaths for immunocompromised children less than 5 years of age, with an attack rate of 56% (30-33). NoV contamination of food sources, including seafood, poses serious potential threats to human health and challenges to the food industry.
Due to the fecal-oral transmission of NoVs and their tropism to the GI tract, it is probable that TiO2 NPs interact with these enteric pathogens. The effect of NoV-TiO2 NP interaction on the virus cycle and host cell biology has not been reported previously. Given the lack of an established cell culture model for human NoV, we utilized murine NoV (MNV-1) infection of RAW 264.7 murine macrophages as the model system for these studies. We tested the effect of anatase TiO2 NPs, on MNV infection, host response, and imparting toxicity to the macrophages. We present evidence that while pre-incubation with TiO2 NPs has inert effects on virus replication, they do not lead to genotoxic damage, but do, however, promote differential modulation of the host response and toxicity on the host cells.
MATERIALS AND METHODS
Characterization of Nanoparticles
Nanoparticle diameters were measured through imaging using a JEOL JEM-2100F, Transmission Electron Microscope (TEM). The nanoparticles were dispersed in ethanol by sonication in an ice bath, before a few drops of the suspension were deposited on a holey carbon coated copper grid. After drying for ~10 minutes, the grid was inserted into TEM for observation at 80kV. The particle diameters were obtained from measuring over 130 particles in random fields of view. EMAN1 and ImageJ software were utilized to determine the major and minor diameters of each particle, and the geometric mean of the two is defined as the average diameter. Bruker D8 Discover XRD (X-ray Diffraction) system with Histar two dimensional detector and GADDS software was utilized to obtain the XRD spectrum of the anatase nanoparticles. X-ray source used in the analysis was Cu Kα line (λ=0.154nm) at 1600 Watts output power (40kV at 40mA). Bruker’s Eva software wasused to analyze the spectrum.
Cell lines, TiO2 NP incubation and MNV infection
RAW 264.7 cells were obtained from American Type Culture Collection (ATCC), grown in DMEM supplemented with 10% fetal bovine serum and 1% Penicillin/Streptomycin (GIBCO). Murine Norovirus genotype 1 (MNV-1, ATCC PTA-5935) was propagated in RAW 264.7 cells. The cells were treated with anatase TiO2 NPs, at concentrations of 20 μg/ml and 2 μg/ml for 3 h (where indicated) and then infected with MNV-1 at a multiplicity of infection (MOI) of 5 and 0.5 particles per cell (as indicated). Two different MOI (5 and 0.5 MOI), were chosen for the viral infection, as they are reflective of the range of differential amounts of virus exposure in the gut. After 24 and 48h post incubation, infectious virus was determined by plaque assay and genome copy numbers of MNV-1 were using Real Time RT-PCR. TNF-α, IL-6, IL-12, INF-γ and TGFβ1 levels were measured in the cellular supernatants collected as described below.
Viral RNA extraction and analysis of genome copy number
Viral RNA was extracted from 115μl of cell culture supernatant using a MagMAXTM viral RNA isolation kit and the MagMAX™ Express Magnetic Particle Processor (Applied Biosystems/Ambion, Austin, TX). Extracted RNA samples were either analyzed immediately by RT-PCR for genome copy number or stored at −80°C until use. RNA samples were analyzed for norovirus by a real-time one-step RT-PCR, using the Rotor-Gene Multiplex RT-PCR kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The reactions were carried out in duplicate, using primers and a Cy5-labeled fluorescent probe (Integrated DNA Technology, Coralville, IA). MNV RNA was used to generate a standard curve and 18S RNA (Applied Biosystems, Foster City, CA) was used as an internal control (16). RNA from mock-infected (no viral infection) RAW cell supernatant was used as a negative control and RNAse-free water was used as a non-template control. The detection limit of the assay was determined to be 2 RNA copies. For the assay, 5 μl of RNA was transferred to a Qiagen Rotor-Gene strip tube containing 10 μl of 2 × Rotor-Gene Multiplex RT-PCR master mix, 800 nM of each norovirus primer, MNVF (5′-TGCAAGCTCTACAACGAAGG-3′) and MNVR (5′-CACAGAGGCCAATTGGTAAA-3′), 200 nM of probe MNVP (5′-CCT TCC CGA CCG ATG GCA TC-3′-Cy5), and 0.2 μl of Rotor-Gene RT mix. The thermal cycling conditions consisted of reverse transcription at 50°C for 30 min, 95°C for 10 min, and then 50 cycles of 94°C for 30 sec and 48°C for 60 sec. Amplification was detected using a Rotor Gene Q6-plex machine from Qiagen.
1.2.4 Plaque assay for MNV
Viral titers were determined by plaque assay using RAW cells (34). Briefly, virus supernatants were serially diluted in MEM, and the dilutions were inoculated on a confluent monolayer of RAW macrophages and incubated at room temperature for 1 h. Viral inoculum was removed and cells were washed twice with PBS. A 1.5% agarose overlay in DMEM was added to the cells and incubated at 37°C. Plaques were counted at 48-72 h post incubation. Virus titers were estimated by multiplying the mean of plaque numbers in at least two wells times the reciprocal of the lowest virus dilution with visible plaques and adjusted to 1 mL volume.
1.2.5 Enzyme linked immunosorbent assay (ELISA)
Cell culture supernatants collected at 24 h post infection were dispensed into 200 μl volumes and frozen at −80°C. An ELISA test was conducted for IFN-γ, IL-6, TNF-α, IL-12p70 and TGF-β1 according to the manufacturer’s instructions (R&D Biosystems, Minneapolis). Briefly, Nunc Maxisorp 96-well plates were coated with anti-mouse IL-6 (2 μg/ml), anti-mouse IFN-γ (4 μg/ml), anti-mouse TNFα (0.8 μg/ml), anti-mouse IL-12 p70 (4 μg/ml) or TGFβ1 (4 μg/ml) (R&D BioSystems) overnight at room temperature. Before use, the plates were blocked with PBS containing 1% bovine serum albumin fraction V (BSA, Sigma) for 2h at Room Temperature. Cell culture supernatant samples (100 μl) were added to the wells and the control samples were diluted in PBS + 1% BSA. After 2 h incubation at RT, wells were washed 4 times with PBS containing 0.05% Tween 20. The addition of biotinylated monoclonal antibodies for each of the cytokines (IL-6 150 ng/ml; IFN-γ 300 ng/ml; TNFα 50 ng/ml; IL-12 p70 400ng/ml; TGFβ1 75 ng/ml) were added and incubated for 2 h at room temperature. Horseradish peroxidase-conjugated streptavidin (R&D Biosystems) was added according to the manufacturer’s recommendations and incubated for 30 min at room temperature. Standard curves were generated using purified recombinant IL-6, IFN-γ, TNFα, IL-12p70 and TGFβ1 according to the manufacturer’s recommendations (R&D BioSystems) using a MaxPro-generated four-parameter curve-fit for each cytokine.
1.2.6 Trypan Blue Exclusion Assay
Macrophages treated under indicated experimental conditions were harvested by scraping, and single suspensions were prepared in cold phosphate buffered saline (1x, pH 7.4, GIBCO). Trypan Blue (Ambion) 0.2% was diluted 1:2 in 1x PBS, and a 1:10 dilution of cell suspension was made using the diluted trypan blue solution. Viability was measured using Cellometer Auto T4 Manual (EMD Millipore, Germany).
1.2.7 Alkaline single-cell gel electrophoresis (Comet) assay
Here, we analyzed DNA damage and cell viability in RAW cells following MNV-1 infection (at 5 MOI) alone, or in combination with exposure to 20 μg/ml of anatase TiO2 NPs. Mock infected RAW macrophages treated with a single 3h exposure to a dose of 20 μg/ml methyl methanesulfonate (MMS) were used as a positive control for the Comet assay. To determine if MNV-1 infection alone, and/or NP exposure alone, or in combination with viral infection cause DNA damage consistent with genotoxicity in single cells, we infected or mock-treated RAW cells with high MOI viral infection, and exposed all combinations of cells to either high dosing of NP exposure or control treatment, and subsequently performed alkaline Comet assays and analysis. After dissociating RAW cells into single cell suspensions, global cell viabilities were determined using Trypan Blue Exclusion Tests. For the alkaline comet assay (35, 36), 50 μl of single-cell suspensions, in phosphate buffered saline (PBS), derived from each treatment condition were mixed with 450 μl 0.8% (diluted in PBS) low melting-point (LMP) agarose at 37°C, and 100μl of this suspension was applied to microscope slides (Fisher Scientific, St. Louis, MO) previously thin-coated with 1% agarose. After solidification of the LMP agarose/embedded cell mixture at 4°C, the slides were placed in freshly prepared lysis buffer (2.5 M NaCl, 0.1 M ethylenediaminetetraacetic acid (EDTA), 10 mM Tris, 10% dimethyl sulfoxide (DMSO) and 1% Triton X-100, pH 10.0) at 4°C in the dark for 3 h. The slides then were washed once in neutralization buffer (0.4 M Tris, pH 7.5) in the dark for 5 min at 4°C and then transferred into chilled alkaline unwinding/electrophoresis solution (300 mM NaOH, 1 mM EDTA, pH > 13) in the dark for 30 min to unwind DNA. Immediately following unwinding, electrophoresis was performed in the same solution at 4°C in the dark for 30 min at 0.8 V/cm and ∼300 mA. After the slides were removed from the electrophoresis chamber, they were washed three times (5 min each) with neutralization buffer, fixed with ice-cold ethanol (100%) and dried for 30 min. To visualize nucleic acids and facilitate scoring, the slides were stained with SYBR Gold (Invitrogen, Carlsbad, CA) (1:10,000 dilutions in Tris Buffered EDTA buffer). Three slides were scored from each treatment; at least 100 representative cells were selected randomly from each slide and scored via a system comprised of a Nikon 501 fluorescence microscope and Comet IV digital imaging software (Perceptive Instruments, Wiltshire, UK). Percent (%) DNA in the tail, defined as the fraction of DNA in the tail divided by the total amount of DNA associated with a cell multiplied by 100, was used as the parameter for DNA damage analysis.
1.2.8 Statistical analyses
All data were plotted and analyzed; statistical analysis was performed using Graph Pad Prism software, Microsoft Excel (Microsoft Corporation, Redmond, WA) and/or SigmaPlot (Systat Software, Inc., San Jose, CA). Two tailed T-tests were used to analyze statistical significance between the experimental groups.
1.3 RESULTS
1.3.1 Anatase TiO2 NPs decrease cell viability of murine macrophages at higher doses
Prior to analyzing the effects of TiO2 toxicity and modulation of host response in murine macrophages in the context of MNV infection, we first examined whether the Anatase TiO2NPs alone exerted any effects on cell morphology and cell viability to the murine macrophages. We dosed the macrophages at two concentrations, 20 μg/ml and 2 μg/ml of Anatase NPs, and analyzed viability and morphology of RAW cells. Importantly, our dose range was based on our extrapolation of data indicative of the amount of human daily NP consumption. When RAW cells were incubated with 20 μg/ml of anatase NPs and monitored for 48 h, we did not observe any cytopathic effects (cell rounding, detachment and floating) compared to the mock-infected controls (Fig. 1A). Although there were no visible cytopathic effects, pre-incubation with 20 μg/ml anatase caused a 30% reduction in cell viability, (Fig. 1B), as measured by a Trypan Blue exclusion assay. Pre-incubation with 2 μg/ml anatase did not alter morphology or viability of RAW macrophages as compared to mock controls (data not shown).
Fig. 1.
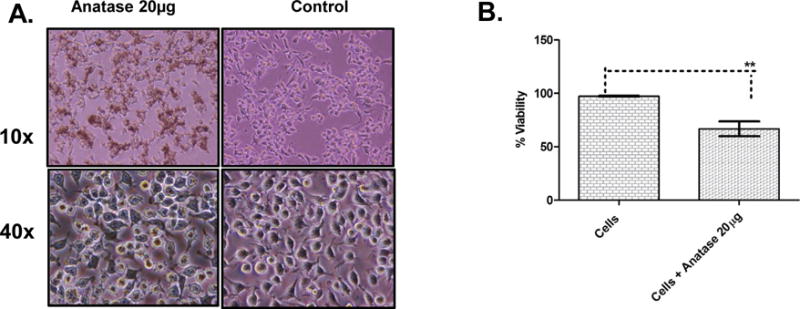
Effect of TiO2 nanoparticles on cell morphology and viability of RAW 264.7 cells.
A. Bright-field images (10X and 40X) of RAW 264.7 macrophages taken at 24 h post incubation with 20 μg/ml (indicated as 20 μg in the figure) of anatase TiO2 NPs, shown in comparison to mock-infected cells. B. Percentage cell viability calculated using a Trypan Blue exclusion assay with cells under indicated experimental conditions harvested at 24 h post incubation. Error bars represent SEM.
1.3.2 MNV Replication is altered in cells exposed to TiO2 NPs
We next analyzed whether pre incubation of RAW cells with high (20 μg/ml) or low dose (2 μg/ml) anatase NPs altered MNV replication and/or the production of infectious virus particles. The MNV-induced cytopathic effect observed at 48 h post infection was used a surrogate marker for viral replication, as MNV has been shown to cause cell rounding, shrinking and cell death following virus replication (37). At 24 h post infection, MNV-1 replicated to reach peak viral titers of 1×107 pfu/ml, when infected with 5 MOI, and ~6×106 pfu/ml, when infected with 0.5 MOI (Fig. 2A, left and right panels, MNV control). Replication of MNV-1 at 5 MOI was accompanied by rounding, shrinking and floating of cells in the monolayer, demonstrating the cytopathic effect due to MNV infection (Fig. 3A). These cytopathic effects resulted in a significant reduction in cell viability by 50% at 24 h and 75% at 48 h post infection, respectively, as measured by a Trypan Blue exclusion assay (Fig. 3C). Infection with 0.5 MOI MNV-1 had similar cytopathic effects (data not shown).
Fig. 2.
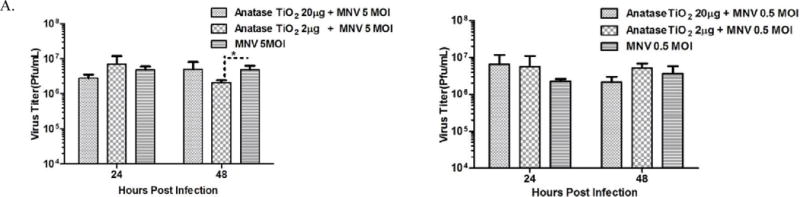
Pre-incubation with TiO2 nanoparticles alters production of infectious virus particles.
A. Infectious virus titers determined by plaque assay from cell culture supernatants harvested at 24 h and 48 h post infection, from cells incubated with 20 μg/mL and 2 μg/mL(indicated as 20 μg and 2 μg in the figure) anatase for 3 h, and infected with 5 MOI (left panel) and 0.5 MOI (right panel).
Fig. 3.
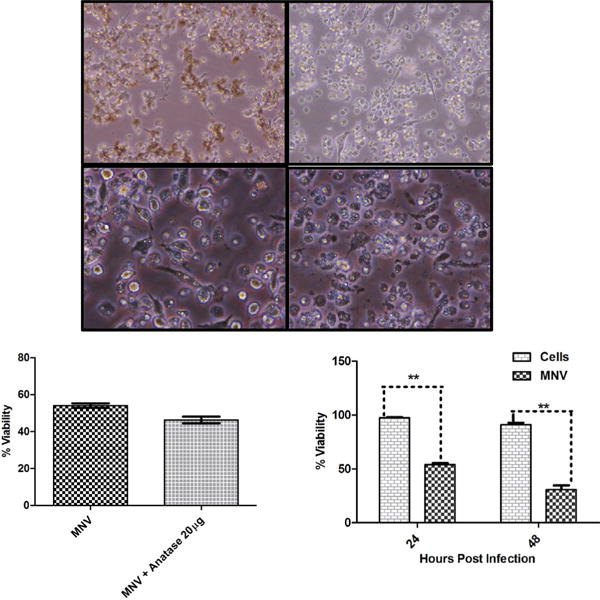
Titanium Di-oxide Nanoparticles alter the production of Infectious Virus Particles.
A. Bright-field images (10X and 40X) of RAW 264.7 macrophages pre-incubated with 20 μg/mL (indicated as 20 μg in the figure) of TiO2 NPs for 3 h followed by 5 MOI MNV infection, shown in comparison with MNV infection alone. B. Percentage cell viability calculated using a Trypan Blue exclusion assay with cells under indicated experimental conditions harvested at 24 h post incubation. C. Percentage cell viability determined at 24 h and 48 h from cells infected with MNV alone. Error bars represent SEM.
While both doses of anatase had no effect on infectious virus titers at 24 h post infection (for 5 MOI infection), pre-incubation of cells with 2 μg/ml anatase caused a statistically significant (~3 fold) reduction in infectious virus titers at 48 h post infection for 5 MOI Infection (Fig. 2A, left panel). Furthermore, cytopathic effects were clearly evident in cells incubated with both 20 μg/ml (Fig. 3A) and 2 μg/ml (data not shown) of anatase NPs under conditions of 5 MOI. Pre-incubation of RAW cells with 20 μg/ml anatase, followed by 5 MOI MNV-1 infection, resulted in significant reduction of cell viability (Fig. 3B, ~15%) as measured by a Trypan Blue exclusion assay at 24 h post infection. No significant difference in virus titer was observed at 24 h and 48 h after pre-incubation with both the doses of anatase NPs followed by infection with 0.5 MOI (Fig. 2A, right panel).
1.3.3 Viral copy numbers in the supernatant are altered upon exposure to different doses of NPs
We next analyzed whether MNV-1 genome copy numbers in the cell culture supernatant are altered due to exposure of the RAW cells to NPs followed by MNV infection. While pre-incubation of cells with 20 μg/ml anatase followed by 5 MOI did not result in alterations of viral genome copy numbers (as compared to controls), pre-incubation with 2 μg/ml resulted in a non-significant reduction (non-significant P>0.05), in genome copy number at 24 h and 48 h post infection, respectively (Fig. S1A, left panel). Similarly, pre-incubation of cells with either 20 μg/ml or 2 μg/ml of anatase followed by infection with 0.5 MOI did not affect viral genome copy numbers when compared to controls alone (Fig. S1A, right panel).
1.3.4 Secretion of cytokines by RAW macrophages is modulated by TiO2 NPs
Macrophages secrete several pro-inflammatory cytokines including Interleukin 6 (IL-6), Interferon Gamma (IFN γ), Tumor Necrosis Factor Alpha (TNFα), Interleukin 12 (IL-12), and Transforming Growth Factor Beta 1 (TGFβ1) in response to infection by microorganisms or antigenic stimulation. Since TiO2 NPs stimulate the immune system due to their foreign nature, we hypothesized that their presence during MNV-1 infection would result in a synergistic increase in host immune response and increased production of pro inflammatory cytokines. We therefore analyzed the secretion of pro-inflammatory cytokines during high and low MOI virus infection, in the presence of high and low doses of TiO2 NPs. Results from cytokine analysis are grouped in Fig. 4 (anatase 20 μg/mL and 2 μg/mL) under conditions of low and high MOI MNV-1 infection.
Fig. 4.
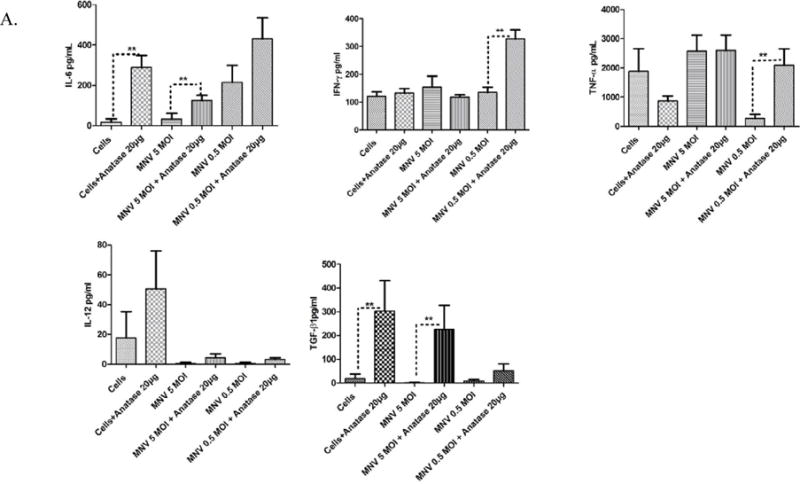
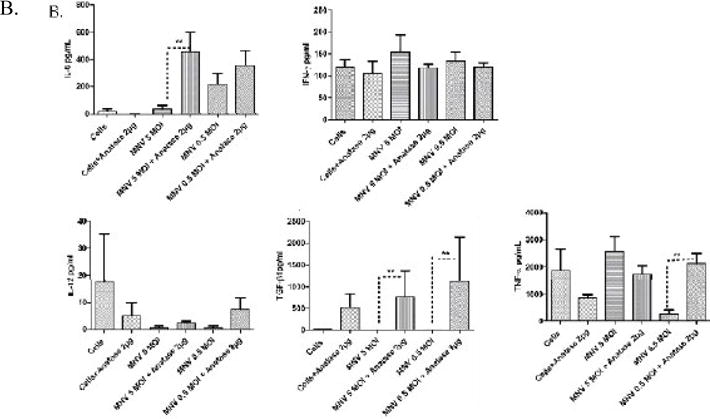
Production of pro-inflammatory cytokines in RAW 264.7 cells incubated with anatase
NPs and infected with 5 and 0.5 MOI MNV. A. Levels of IL-6, IFN-γ, TNFα, IL-12, and TGFβ as measured by an ELISA from culture supernatants harvested at 24 h from cells incubated with 20 μg/mL anatase alone (indicated as 20 μg in the figure) or infected with 5 or 0.5 MOI of viruses. B. Levels of IL-6, IFN-γ, TNFα, IL-12, and TGFβ as measured by ELISA from culture supernatants harvested at 24 h from cells incubated with 2 μg/mL (indicated as 2 μg in the figure) anatase alone or infected with 5 or 0.5 MOI of viruses.
Pre-incubation with 20 μg/mL of anatase resulted in a statistically significant increase (~3-4 fold) in secretion of IL-6 by macrophages, both alone and in the context of 5 MOI MNV infection (Fig. 4A). A similar trend (~2 fold increase) was found during 0.5 MOI MNV infection. In contrast, IFN-γ and TNF-α were elevated during low MOI MNV infection (~4 fold increase, p<0.05), while there was no significant increase during 5 MOI MNV infection (Fig. 4A). Whereas IL-12 showed no significant change during high or low MOI infection, pre-incubation with 20 μg/mL of anatase resulted in a statistically significant increase (~4 fold, P<0.05) of TGFβ1 during infection with 5 MOI MNV-1. Pre-incubation with 2 μg/ml anatase further caused a significant increase (~6 fold P<0.05) of IL-6 during 5 MOI MNV infection, whereas the apparent surge in IL-6 during low MOI infection was not statistically significant (Fig. 4B). The levels of IFN-γ showed no significant alterations during 5 and 0.5 MOI MNV-1 infection in macrophages pre-incubated with 2μg/ml anatase, TNFα showed a significant increase (~6 fold) during 0.5 MOI MNV-1 infection under the same conditions (Fig. 4B). Interestingly, TGFβ-1 was significantly elevated (~5 to 7 fold, P<0.05), under conditions of 5 and 0.5 MOI infection when pre-incubated with 2 μg/ml anatase (Fig. 4B). Overall, anatase NPs during MNV-1 infection evoked a pro-inflammatory response, as shown by significant increases in IL-6, IFN-γ, TNFα and TGF-β1 expression.
1.3.5 Assessment of DNA Damage
Literature studies have shown that NPs, including silica NPs, are capable of inducing single cell DNA damage consistent with genotoxicity that can be deciphered via the alkaline Comet assay (38). While viabilities ranged among cell sample preparations, there was a general reduction in viability as a result of either MNV-1 infection or NP exposure, consistent with known cytotoxic effects of these treatments. In contrast to cells exposed to Methyl Methane Sulfonate MMS (positive control), no statistically significant differences in DNA damage were observed for anatase TiO2 NP in the presence or absence of virus infection, when compared to the responses in the vehicle control group (Fig. 5). The RAW macrophages were susceptible to single cell DNA damage, consistent with genotoxicity, after exposure to MMS, a known genotoxicant.
Fig. 5.
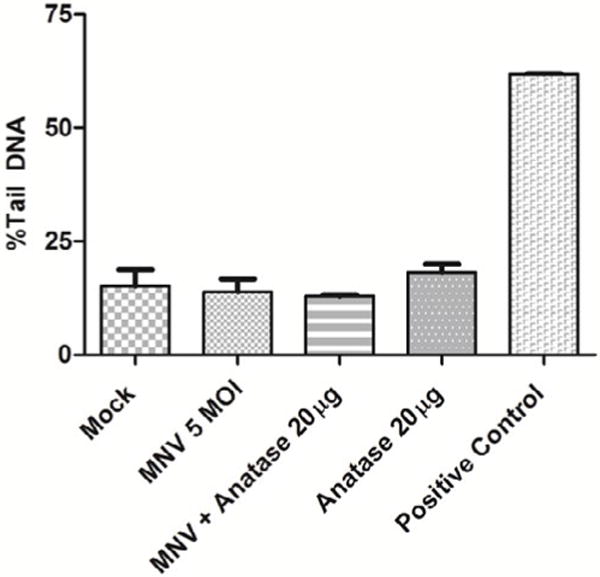
Effect of TiO2 NPs on genotoxic insults to cells. Genotoxic insult, shown as % tail DNA under indicated experimental conditions, as measured by the Comet assay. Note that the positive control (MMS) showed a significant increase in genotoxic insult, while no significant differences were observed in other conditions tested.
1.4 DISCUSSION
The use of TiO2 NPs in food additives and cosmetics results in accumulation of these NPs in the GI tract, which may allow interaction with enteroviruses. While several studies (16, 19, 20) have investigated the safety and toxicity of TiO2 NPs on intestinal cell lines, the direct effects of TiO2 NP accumulation on enteric virus replication, immune response of the host and genotoxic insult have not been studied before. Since noroviruses (NoVs) infect the GI tract by fecal-oral transmission and represent the leading cause of viral gastroenteritis (39, 40), we investigated the effect of TiO2 NPs accumulation on NoV replication and immune response of the host. Because MNV is the only NoV cultivable in vitro, we utilized MNV-1 and RAW macrophages as the host model system to analyze the safety hazard impact of TiO2 NPs in the context of MNV-1 infection. Although previous studies (41) have analyzed the effects of TiO2 NPs on MNV replication, those studies involved pre-incubation of MNV with TiO2 NPs followed by analysis of virus infectivity in RAW macrophages. In contrast, our study is the first to analyze the effects of pre-incubation of RAW macrophages with different doses of anatase TiO2 NPs on MNV replication, host immune response and genotoxicity.
Before viral infection, we analyzed the effects of anatase TiO2 NPs on morphology and cell viability in RAW macrophages. Although there were no distinct visual changes in cell viability, pre-incubation with 20 μg/ml of anatase resulted in ~30% decrease in viability. This effect of anatase was pronounced with high MOI MNV-1 infection, where there was a significant reduction in cell viability. Alkaline Comet assay analysis indicated there was no significant genotoxic insult imparted by 20 μg/ml of anatase alone or in combination with 5 MOI MNV, indicating that the observed cell death likely was mediated by mechanisms other than subtle DNA damage. Cytokine analysis showed an increase in pro-inflammatory cytokine IL-6 upon anatase incubation alone or in combination with MNV-1 infection. This elevation in IL-6 may have contributed to the reported decreases in cell viability, as cell death due to enhanced interleukin production stimulated by NPs in soil dust has been reported previously (42).
Pre-incubation of RAW cells with 20 μg/ml of the anatase form of TiO2 NPs had no effect on infectious virus production at 5 and 0.5 MOI of MNV-1 infection, whereas a tenfold reduction in dose (2 μg/ml) caused a significant reduction in infectious virus titers at 5 MOI but not 0.5 MOI (Fig. 2A). This was accompanied by a large increase (~6 fold) in IL-6 levels, further indicating that the increased expression of the pro-inflammatory cytokine could have contributed to the observed reduction in virus replication. Reduction of influenza virus and hepatitis B virus replication by increased IL-6 levels has been previously documented (43, 44).
The effects of TiO2 NPs and viral infection on cytokine secretion, analyzed by ELISA, yielded several interesting observations. Pro-inflammatory cytokines, including IL-6, IFN-γ and TNFα, were increased by both doses of anatase during virus infection. Anatase NPs have been shown to induce the inflammatory response by upregulating cytokines, in many human cells (48, 49).
Overall, anatase NPs evoked a pro-inflammatory response in the presence of MNV infection, as shown by the increase in indicated cytokines. Previous studies have documented the induction of pro-inflammatory response in macrophages by TiO2 NPs (48, 50), but our study is the first to report the effect of NPs during MNV infection in a macrophage model. Pre-incubation of RAW macrophages with titanium NPs resulted in enhanced inflammation, which was exacerbated by virus infection. The increase in pro-inflammatory cytokines did not have a high impact on virus replication, although several of the cytokines, including IL6, IFN-γ, and TNFα, have an antiviral effect in many systems tested (51, 52). Rather, this increase could facilitate the establishment of persistent or chronic virus infection, resulting in enhanced damage to the intestinal system. A recent report have shown an increase in inflammation, resulting in enhanced pneumonia, in mice inoculated with TiO2 NPs followed by infection with RSV virus (53).
Our rationale for using MNV-1 in this study was to confirm our in vitro findings using a mouse model, because MNV causes disease in mice. Studies investigating morbidity, virus replication and enhancement of disease upon pretreatment with TiO2 NPs are underway in mouse models to confirm our in vitro findings. Nevertheless, our study is the first to indicate that such phenomena could also occur in the GI tract, resulting in enhanced inflammation and damage due to interaction of NPs and enteric pathogens.
We used the alkaline Comet assay to investigate the genotoxicity of TiO2 NPs and of MNV infection. Although we used doses that were consistent with decreases in overall global viability suggestive of cytotoxic effects, even at the highest viral MOI or TiO2 NP exposure conditions, none of these groups induced DNA damage. These negative responses contrasted with the strong positive responses produced by MMS, consistent with previous reports using this chemical (38). The absence of a detectable response in the alkaline Comet assay in the presence of known global cytotoxic damage on the corresponding cellular suspensions was unexpected. The DNA damage may have occurred in a way that did not generate strand breaks detectable by the alkaline Comet assay, or perhaps DNA damage and repair took place outside of the time frame of our observations. To examine the likelihood of the latter possibility, we examined cells at a later time point after MNV infection (48 h). We again saw no difference in DNA damage between mock and MNV infection by the alkaline Comet assay. Our viability and global DNA damage data potentially suggest that large-scale DNA damage and apoptosis occur on such a scale that does not allow for retention of embeddable single cells and resulting genotoxic events. Although we did not detect genotoxicity in our current study, the effects of TiO2 NPs and MNV infection on cell viability, and thus likely on cytotoxicity, warrant further investigation of the safety hazards of TiO2 NPs.
Supplementary Material
Acknowledgments
We thank Dr. John Sutherland and Dr. Saeed Khan for critical review and editing of the manuscript.
Footnotes
Disclaimer
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the US FDA.
Declaration of Interest
The authors declare no potential conflict of interest.
Author Contributions
SA, TT, and MA designed experiments, performed analysis and wrote the manuscript. LM performed the experiments. FW, TM and AB prepared and characterized NPs, while MM originally developed and optimized the Comet assay described in this study.
References
- 1.Torchilin VP. Application of nanomedical approaches in experimental and clinical oncology. Anticancer Agents Med Chem. 2006;6:501. doi: 10.2174/187152006778699103. [DOI] [PubMed] [Google Scholar]
- 2.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 3.Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov. 2014;13:813–827. doi: 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrari M. Nanotechnology-enabled medicine. Discov Med. 2005;5:363–366. [PubMed] [Google Scholar]
- 5.Ferrari M, Downing G. Medical nanotechnology: shortening clinical trials and regulatory pathways? BioDrugs. 2005;19:203–210. doi: 10.2165/00063030-200519040-00001. [DOI] [PubMed] [Google Scholar]
- 6.Geng Y, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58:1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal JA, Chen L, Baker JL, Putnam D, DeLisa MP. Pathogen-like particles: biomimetic vaccine carriers engineered at the nanoscale. Curr Opin Biotechnol. 2014;28:51–58. doi: 10.1016/j.copbio.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhry Q, et al. Applications and implications of nanotechnologies for the food sector. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008;25:241–258. doi: 10.1080/02652030701744538. [DOI] [PubMed] [Google Scholar]
- 10.Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol. 2012;46:2242–2250. doi: 10.1021/es204168d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dekkers S, et al. Presence and risks of nanosilica in food products. Nanotoxicology. 2011;5:393–405. doi: 10.3109/17435390.2010.519836. [DOI] [PubMed] [Google Scholar]
- 12.Kessler R. Engineered nanoparticles in consumer products: understanding a new ingredient. Environ Health Perspect. 2011;119:a120–125. doi: 10.1289/ehp.119-a120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buettner KM, Valentine AM. Bioinorganic chemistry of titanium. Chem Rev. 2012;112:1863–1881. doi: 10.1021/cr1002886. [DOI] [PubMed] [Google Scholar]
- 14.Buettner KM, Snoeberger RC, Batista VS, Valentine AM. Pharmaceutical formulation affects titanocene transferrin interactions. Dalton Trans. 2011;40:9580–9588. doi: 10.1039/c1dt10805k. [DOI] [PubMed] [Google Scholar]
- 15.Contado C. Nanomaterials in consumer products: a challenging analytical problem. Front Chem. 2015;3:48. doi: 10.3389/fchem.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song ZM, et al. Biological effect of food additive titanium dioxide nanoparticles on intestine: an in vitro study. J Appl Toxicol. 2015;35:1169–1178. doi: 10.1002/jat.3171. [DOI] [PubMed] [Google Scholar]
- 17.Chen XX, et al. Characterization and preliminary toxicity assay of nano-titanium dioxide additive in sugar-coated chewing gum. Small. 2013;9:1765–1774. doi: 10.1002/smll.201201506. [DOI] [PubMed] [Google Scholar]
- 18.Chen BT, et al. Nanoparticles-containing spray can aerosol: characterization, exposure assessment, and generator design. Inhal Toxicol. 2010;22:1072–1082. doi: 10.3109/08958378.2010.518323. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Du LJ, Song ZM, Chen XX. Progress in the characterization and safety evaluation of engineered inorganic nanomaterials in food. Nanomedicine (Lond) 2013;8:2007–2025. doi: 10.2217/nnm.13.176. [DOI] [PubMed] [Google Scholar]
- 20.Skocaj M, Filipic M, Petkovic J, Novak S. Titanium dioxide in our everyday life; is it safe? Radiol Oncol. 2011;45:227–247. doi: 10.2478/v10019-011-0037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho WS, et al. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part Fibre Toxicol. 2013;10:9. doi: 10.1186/1743-8977-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brun E, et al. Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia. Part Fibre Toxicol. 2014;11:13. doi: 10.1186/1743-8977-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Angelis I, et al. Comparative study of ZnO and TiO2 nanoparticles: physicochemical characterisation and toxicological effects on human colon carcinoma cells. Nanotoxicology. 2013;7:1361–1372. doi: 10.3109/17435390.2012.741724. [DOI] [PubMed] [Google Scholar]
- 24.Bergin IL, Witzmann FA. Nanoparticle toxicity by the gastrointestinal route: evidence and knowledge gaps. Int J Biomed Nanosci Nanotechnol. 2013;3 doi: 10.1504/IJBNN.2013.054515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Roda Husman AM, et al. Calicivirus inactivation by nonionizing (253.7-nanometer-wavelength [UV]) and ionizing (gamma) radiation. Appl Environ Microbiol. 2004;70:5089–5093. doi: 10.1128/AEM.70.9.5089-5093.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheesbrough JS, Green J, Gallimore CI, Wright PA, Brown DW. Widespread environmental contamination with Norwalk-like viruses (NLV) detected in a prolonged hotel outbreak of gastroenteritis. Epidemiol Infect. 2000;125:93–98. doi: 10.1017/s095026889900432x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Souza DH, et al. Persistence of caliciviruses on environmental surfaces and their transfer to food. Int J Food Microbiol. 2006;108:84–91. doi: 10.1016/j.ijfoodmicro.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 28.Berg DE, Kohn MA, Farley TA, McFarland LM. Multistate outbreaks of acute gastroenteritis traced to fecal-contaminated oysters harvested in Louisiana. J Infect Dis. 2000;181(Suppl 2):S381–386. doi: 10.1086/315581. [DOI] [PubMed] [Google Scholar]
- 29.Fankhauser RL, et al. Epidemiologic and molecular trends of “Norwalk-like viruses” associated with outbreaks of gastroenteritis in the United States. J Infect Dis. 2002;186:1–7. doi: 10.1086/341085. [DOI] [PubMed] [Google Scholar]
- 30.Debbink K, Donaldson EF, Lindesmith LC, Baric RS. Genetic mapping of a highly variable norovirus GII.4 blockade epitope: potential role in escape from human herd immunity. J Virol. 2012;86:1214–1226. doi: 10.1128/JVI.06189-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall AJ, et al. Norovirus disease in the United States. Emerg Infect Dis. 2013;19:1198–1205. doi: 10.3201/eid1908.130465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopman B, et al. Environmental transmission of norovirus gastroenteritis. Curr Opin Virol. 2012;2:96–102. doi: 10.1016/j.coviro.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Payne DC, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med. 2013;368:1121–1130. doi: 10.1056/NEJMsa1206589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez-Hernandez MB, Bragazzi Cunha J, Wobus CE. Plaque assay for murine norovirus. J Vis Exp. 2012:e4297. doi: 10.3791/4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 36.Ding W, et al. Methyleugenol genotoxicity in the Fischer 344 rat using the comet assay and pathway-focused gene expression profiling. Toxicological sciences an official journal of the Society of Toxicology. 2011;123:103–112. doi: 10.1093/toxsci/kfr153. [DOI] [PubMed] [Google Scholar]
- 37.Bok K, Prikhodko VG, Green KY, Sosnovtsev SV. Apoptosis in murine norovirus-infected RAW264.7 cells is associated with downregulation of survivin. J Virol. 2009;83:3647–3656. doi: 10.1128/JVI.02028-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manjanatha MG, et al. Genotoxicity of doxorubicin in F344 rats by combining the comet assay, flow-cytometric peripheral blood micronucleus test, and pathway-focused gene expression profiling. Environmental and molecular mutagenesis. 2014;55:24–34. doi: 10.1002/em.21822. [DOI] [PubMed] [Google Scholar]
- 39.Karst SM, Wobus CE, Goodfellow IG, Green KY, Virgin HW. Advances in norovirus biology. Cell Host Microbe. 2014;15:668–680. doi: 10.1016/j.chom.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green SM, et al. Human enteric Caliciviridae: a new prevalent small round-structured virus group defined by RNA-dependent RNA polymerase and capsid diversity. J Gen Virol. 1994;75(Pt 8):1883–1888. doi: 10.1099/0022-1317-75-8-1883. [DOI] [PubMed] [Google Scholar]
- 41.Lee J, Zoh K, Ko G. Inactivation and UV disinfection of murine norovirus with TiO2 under various environmental conditions. Appl Environ Microbiol. 2008;74:2111–2117. doi: 10.1128/AEM.02442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veranth JM, et al. Inflammatory cytokines and cell death in BEAS-2B lung cells treated with soil dust, lipopolysaccharide, and surface-modified particles. Toxicol Sci. 2004;82:88–96. doi: 10.1093/toxsci/kfh248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuo TM, et al. HBV replication is significantly reduced by IL-6. J Biomed Sci. 2009;16:41. doi: 10.1186/1423-0127-16-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dienz O, et al. Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol. 2012;5:258–266. doi: 10.1038/mi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biermer M, Puro R, Schneider RJ. Tumor necrosis factor alpha inhibition of hepatitis B virus replication involves disruption of capsid Integrity through activation of NF-kappaB. J Virol. 2003;77:4033–4042. doi: 10.1128/JVI.77.7.4033-4042.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seo SH, Webster RG. Tumor necrosis factor alpha exerts powerful anti-influenza virus effects in lung epithelial cells. J Virol. 2002;76:1071–1076. doi: 10.1128/JVI.76.3.1071-1076.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Wimmer E, Paul AV. Cis-acting RNA elements in human and animal plus-strand RNA viruses. Biochim Biophys Acta. 2009;1789:495–517. doi: 10.1016/j.bbagrm.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Refai AK, Textor M, Brunette DM, Waterfield JD. Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines. J Biomed Mater Res A. 2004;70:194–205. doi: 10.1002/jbm.a.30075. [DOI] [PubMed] [Google Scholar]
- 49.Giudiceandrea F, et al. Mechanisms of bone resorption: analysis of proinflammatory cytokines in peritoneal macrophages from titanium implant–an experimental design. J Craniofac Surg. 1998;9:254–259. [PubMed] [Google Scholar]
- 50.Schanen BC, et al. Exposure to titanium dioxide nanomaterials provokes inflammation of an in vitro human immune construct. ACS Nano. 2009;3:2523–2532. doi: 10.1021/nn900403h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheeran MC, et al. CD4(+) lymphocyte-mediated suppression of cytomegalovirus expression in human astrocytes. Clin Diagn Lab Immunol. 2000;7:710–713. doi: 10.1128/cdli.7.4.710-713.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheeran MC, Hu S, Gekker G, Lokensgard JR. Decreased cytomegalovirus expression following proinflammatory cytokine treatment of primary human astrocytes. J Immunol. 2000;164:926–933. doi: 10.4049/jimmunol.164.2.926. [DOI] [PubMed] [Google Scholar]
- 53.Hashiguchi S, et al. Titanium dioxide nanoparticles exacerbate pneumonia in respiratory syncytial virus (RSV)-infected mice. Environ Toxicol Pharmacol. 2015;39:879–886. doi: 10.1016/j.etap.2015.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


