Abstract
Sand flies, similar to most vectors, take multiple blood meals during their lifetime1-4. The effect of subsequent blood meals on pathogens developing in the vector, and their impact on disease transmission have never been examined. Here, we show that ingestion of a second uninfected blood meal by Leishmania-infected sand flies triggers dedifferentiation of metacyclic promastigotes, considered a terminally differentiated stage inside the vector5, to a leptomonad-like stage, the retroleptomonad promastigote. Reverse metacyclogenesis occurs after every subsequent blood meal where retroleptomonad promastigotes rapidly multiply and differentiate to metacyclic promastigotes enhancing sand fly infectiousness. Importantly, a subsequent blood meal amplifies the few Leishmania parasites acquired by feeding on infected hosts by 125 folds, and increases lesion frequency by 4 folds, in twice-fed compared to single-fed flies. These findings place readily available blood sources as a critical element in transmission and propagation of vector-borne pathogens.
Human leishmaniasis, a neglected disease afflicting an estimated one million people worldwide, is transmitted by phlebotomine sand flies6. Multiple blood meals (BM)s increase the capacity of vectors, including sand flies, to transmit disease by promoting contact with susceptible hosts 2,3,7. The effect of a second uninfected blood meal in Leishmania development inside the sand fly gut and its consequence in parasite transmission have not been studied.
Here, we show that ingestion of a second uninfected BM by Leishmania-infected sand flies triggers parasite dedifferentiation and amplification that greatly enhance disease transmission. Experimentally, Leishmania parasites develop transmissible infections, characterized by terminally differentiated metacyclic promastigotes, 8-12 days post infection (PI) 8-10. Sand flies were membrane-fed on blood containing 2×106 Leishmania parasites per ml, and half were provided a second uninfected BM by feeding on a healthy mouse 12 days PI (Fig. 1a). Eighteen days PI and 6 days post second BM, the midgut of twice engorged (E2) sand flies is dense and distended showing an infection enhancement compared to once engorged (E1) sand flies (Fig. 1a). For E1 sand flies, L. infantum followed the expected developmental cycle in Lutzomyia longipalpis, developing mature infections with a median of 9.6×104 parasites (Fig. 1b, blue symbols, Supplementary Table 1) and a median of 79% metacyclic promastigotes by day 12 (Fig. 1c, blue symbols, Supplementary Table 1). In contrast to E1 sand flies where the infection remained stable, 24 hours after E2 sand flies had a second BM a rapid and abrupt drop in the proportion of metacyclic promastigotes from 79% to 5.6% was observed despite an unchanged total number of parasites per midgut (Fig. 1c, orange symbols, Supplementary Table 1). Instead of highly motile metacyclic promastigotes (Fig. 1d, Supplementary Video 1a and b), “leptomonad-like” parasites with a large cell body, a shorter flagella and low motility were observed (Fig. 1e, Supplementary Video 1c and d). Since these forms resulted from dedifferentiation of metacyclic promastigotes, they were termed “retroleptomonad promastigotes”. The multiplication of retroleptomonad promastigotes in E2 sand flies significantly increased the number of parasites per midgut from a median of 1.08 ×105 on day 13 to a median of 5.19 ×105 on day 18 (Fig. 1b, orange symbols, Supplementary Table 1). This resulted in a 4.5-fold increase in the number of metacyclic promastigotes per midgut on day 18 PI in E2 sand flies (Fig. 1f). We compared parasite viability in E1 and E2 sand flies every 6 hours during the first 24 hours after E2 sand flies were provided the uninfected BM. Parasite viability was comparable in both groups and remained above 90% at all times (Fig. S1). The appearance of retroleptomonads coincided with the disappearance of metacyclic promastigotes 24 hours after a subsequent BM (Fig. 1g, purple symbol). Conversely, the disappearance of retroleptomonad promastigotes coincided with the appearance of metacyclic promastigotes at day 18 post infection (Fig. 1g, orange symbols). This phenomenon was not observed in E1 sand flies (Fig. 1h). The reverse metacyclogenesis phenomenon, the transformation of metacyclic promastigotes into a proliferative stage in response to sequential blood feeding, appears to be ubiquitous as it was also observed in L. major-infected Phlebotomus papatasi (Supplementary Figure 2a and c) and L. donovani-infected Lu. longipalpis (Supplementary Figure 2b and d).
Fig. 1. Leishmania metacyclics differentiate into replicative retroleptomonads after a subsequent blood meal enhancing sand fly infectiousness.
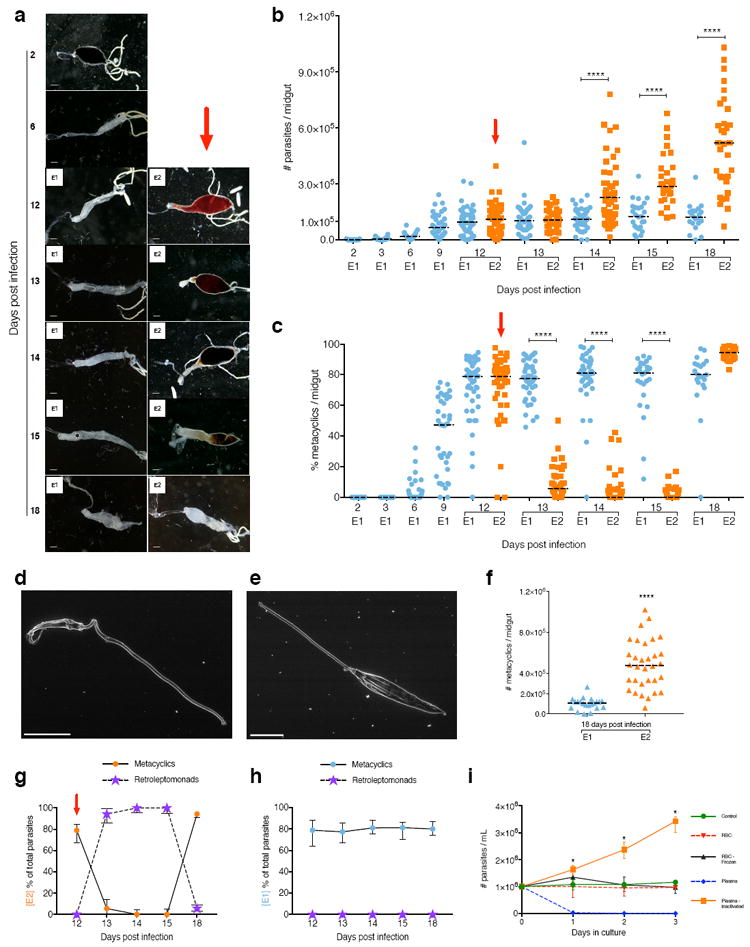
(a to h) Leishmania infantum-infected Lutzomyia longipalpis sand flies. (a) Midgut images. Bars, 150 μm. (b) Midgut parasite number. (c) Percent metacyclics. (d and e) Metacyclic (d) and retroleptomonad (e) EM images. Bars, 5 μm. (f) Number of metacyclics. (g and h) Proportion of metacyclics to retroleptomonads in E2 (g) or E1 (h) sand flies. E1, sand flies engorged on an infected blood meal (BM). E2, infected sand flies engorged on a subsequent uninfected BM. Red arrow, subsequent BM. (i) Metacyclics cultured with blood components. Bar, Median (shown ± interquartile range for g to i). (b to c and f to h) Cumulative data shown from four independent experiments; n for each condition is specified in Supplementary Table 1; (a) and (d and e) images are representative of four or two independent experiments, respectively. *P<0.05, ****P≤0.0001 determined by Mann-Whitney’s U-test for parasite number and by N1-Chi-squared test for percent metacyclics.
Reverse metacyclogenesis also occurs in vitro. Addition of inactivated plasma (to inactivate the complement cascade) triggered dedifferentiation of metacyclics into proliferative promastigotes (Fig. 1i). As previously reported11, fresh normal plasma killed metacyclics promastigotes after a few hours in culture (Fig, 1i). Medium supplemented with either red blood cells, disrupted red blood cells or medium alone had no effect on metacyclics (Fig. 1i). These data suggest that a component from plasma triggers reverse metacyclogenesis. We hypothesize that metacyclic promastigotes sense a nutrient from blood causing them to dedifferentiate and proliferate. As the blood is digested and excreted, the retroleptomonads differentiate again into metacyclic promastigotes. To visualize this phenomenon, we imaged metacyclic promastigotes every minute for 18 hours after the addition of inactivated serum. We captured the transformation of metacyclic promastigotes into retroleptomonad promastigotes (Supplementary Video 2 a,b,c). During this transition, the flagellum of a metacyclic promastigote shortens significantly, its body increases more than twice in length and the emergent promastigote starts to divide (Supplementary Video 2 a,b,c). We named this parasite stage retroleptomonad due of its similarity to a leptomonad-like promastigote but more importantly due to its origin, the metacyclic promastigote and its temporal placement in the life cycle within the midgut. Transcriptomics, proteomics, and glycomics analysis will determine whether this stage can be also classified as a leptomonad or has distinct molecular and biochemical characteristics. Future studies are also needed to identify the molecule (s) in blood that trigger reverse metacyclogenesis and how it is sensed by the metacyclic parasite12.
Sand flies take a BM every 5-6 days throughout their life span 1,13 and full parasite development to metacyclic promastigotes inside the sand fly gut occurs later, 9-12 days PI14. We tested whether a subsequent BM taken six days after infection with Leishmania would have consequences for the developing early infection. For both L. infantum-infected Lu. longipalpis (Fig. 2a, Supplementary Table 2) and L. major-infected P. papatasi (Fig. 2b, Supplementary Table 2), E2 sand flies that were provided a second BM six days post infection showed a significant increase in the median parasite burden per midgut compared to E1 sand flies. A significantly lower percent of metacyclics was observed for E2 compared to E1 sand flies on day 9 for both L. infantum-infected Lu. longipalpis (Fig. 2c) and L. major-infected P. papatasi (Fig. 2d), indicative of a transient delay in the appearance of metacyclics in E2 sand flies. This delay resulted in a significant increase in both parasite number and percent of metacyclics three days later (Fig. 2c, d, Supplementary Table 2). Similar to mature infections, this amplification resulted in a significant enhancement of the number of metacyclics per midgut from a median of 8.4×104 in E1 to 4.08×105 in E2 L. infantum-infected Lu. longipalpis (Fig. 2e), and a median of 1.5×103 in E1 to 9.75×104 in E2 L. major-infected P. papatasi (Fig. 2f), on day 12 PI. Thus, a subsequent BM by a Leishmania-infected sand flies results in parasite amplification regardless of the stage of infection. Of relevance, this phenomenon was also observed when the subsequent BM was provided by feeding on a chicken (Supplementary Figure 3, Supplementary Table 2), a favored peridomestic blood source for sand flies 15 that supports Leishmania development in infected sand flies 16. This implicates chickens, that are refractory to Leishmania infection, in parasite amplification in the sand fly and, together with other readily available animal blood sources17, in disease propagation in nature.
Fig. 2. A subsequent uninfected blood meal enhances early Leishmania infection in the sand fly.
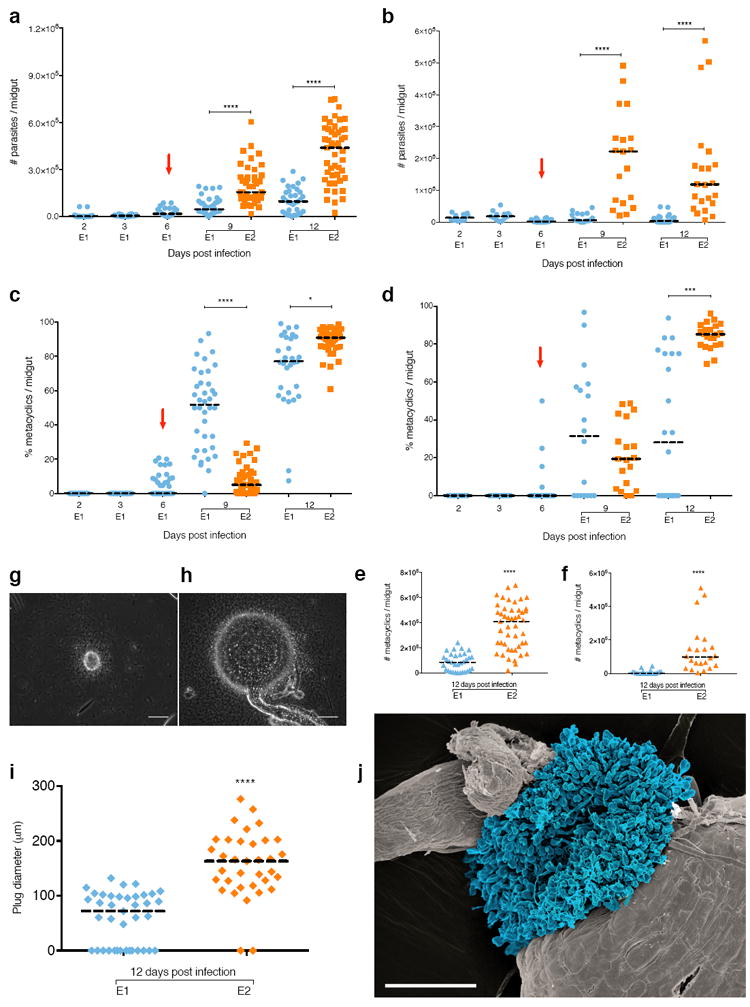
(a to f) Parasite number (a and b), and percent (c and d) and number (e and f) of metacyclics in Leishmania infantum-infected Lutzomyia longipalpis (a, c and r) or L. major-infected Phlebotomus papatasi (b, d and f) sand flies. (g to j), Haptomonads parasite sphere (HPS) in Lu. longipalpis. (g and h) HPS image in E1 (g) or E2 (h) sand flies. Bar, 50 μm. (i) HPS diameter. (j) In situ SEM of HPS. Blue, Haptomonads. Bar, 25 μm. E1, sand flies engorged on an infected blood meal (BM). E2, infected sand flies engorged on a subsequent uninfected BM. Red arrow, subsequent BM. Bar, Median. Cumulative data shown from four (a, c and e) or two (b, d, f and i) independent experiments; (a to f) n for each condition is specified in Supplementary Table 2. (g and h) images are representative of two independent experiments. P<0.05, ***P≤0.001, ****P≤0.0001 determined by Mann-Whitney’s U-test for parasite number and by N1-Chi-squared test for percent metacyclics.
Another significant finding related to parasite transmission was observed in infected sand flies after a second uninfected BM. The haptomonad stage of an E2 sand fly enlarges to form an extensive spherical structure, termed the haptomonad parasite sphere (HPS), that occludes the stomodeal valve (Fig. 2g, h, Supplementary Video 3). Haptomonads adhere to the lining of the stomodeal valve and have been associated to chitinase-mediated destruction of its structure, thereby facilitating transmission of metacyclics 8. E2 sand flies developed a significantly larger (HPS) with a median diameter of 163μm compared to 72μm for E1 sand flies (Fig. 2i). Fig. 2j shows part of the HPS in situ as it protrudes from the stomodeal valve of a sand fly at 12 days PI. Of note, the HPS encompasses a massive physical structure at the stomodeal valve. Formation of this large spherical structure after a second blood meal implicates it as a key component, in addition to the promastigote secretory gel18, in the blockage of parasites at the anterior part of the sand fly midgut, thereby promoting regurgitation and enhancing parasite transmission during feeding. These findings support the previously hypothesized importance of haptomonad promastigotes in parasite transmission5, and suggest they may be more relevant to its success than previously considered5.
The number of parasites acquired by a sand fly feeding on an infected host remains unknown. Importantly, the effect of a second blood meal on parasites acquired from infected animals has not been previously studied. To assess the effect of a subsequent BM on sand flies that fed on a Leishmania-infected host, we first established that Lu. longipalpis sand flies ingest a median of 52 parasites after feeding on a sick L. infantum-infected hamster, while P. papatasi takes in a median of 80 parasites after feeding on a L. major footpad lesion (Fig. 3 a, b). This establishes that the number of parasites naturally acquired by sand flies is smaller than that provided through experimental infections. Under this natural setting, E1 sand flies that picked-up Leishmania parasites from infected animals and did not take a second BM developed poor infections in Lu. longipalpis (Fig. 3c, Supplementary Table 3) and P. papatasi (Fig. 3d, Supplementary Table 3), and produced a median of 4.1% metacyclics for Lu. longipalpis (Fig. 3e, Supplementary Table 3) and zero for P. papatasi (Fig. 3f, Supplementary Table 3) by day 12 PI. In contrast, the number of parasites in sand flies that had a subsequent uninfected BM (E2) increased 69-fold for L. infantum-infected Lu. longipalpis (Fig. 3c, Supplementary Table 3) and 125-fold for L. major-infected P. papatasi (Fig. 3d, Supplementary Table 3) by day 12 PI. Additionally, E2 L. infantum-infected Lu. longipalpis (Fig. 3e, Supplementary Table 3) and L. major-infected P. papatasi (Fig. 3f, Supplementary Table 3) developed a median of 68.3% and 86% metacyclics, respectively, on day 12 PI, a significantly higher percent of metacyclics compared to E1 sand flies. This translates to an increase in the number of metacyclics per midgut from a median of zero in E1 sand flies to a median of 5.85×104 and 6×104 for L. infantum-infected Lu. longipalpis (Fig. 3g) and L. major-infected P. papatasi (Fig. 3h) E2 sand flies, respectively. These data indicate that a second BM amplifies the small number of parasites acquired by feeding on infected hosts facilitating their establishment in the sand fly. This led us to hypothesize that infected sand flies that take a second BM would be more efficient at transmitting parasites to a mammalian host. The transmission success after the bite of a single L. major-infected P. papatasi E1 sand fly, that fed once on footpad lesions, was compared to an E2 sand fly provided a second uninfected BM at day 6 PI. Leishmania transmission by an E2 sand fly bite was 4-fold higher than an E1 sand fly, assessed by the frequency of cutaneous leishmaniasis lesions in mice at week 4 post-challenge with a single infected sand fly (Fig. 3i). Interestingly, we did not observe an increase in pathology, strongly suggesting that infected flies that took a second blood meal will produce “more cases” of disease and not necessarily “more severe” disease. Lesions resulting from E1 sand flies are likely caused by infrequent high-dose transmitters19. In contrast, the higher frequency of lesions after bites of E2 sand flies suggests that a second blood meal likely produces high-dose transmitters in the majority of E2 sand flies, a hypothesis that remains to be verified in future studies. The enhanced frequency of transmission in E2 sand flies may have epidemiological implications for leishmaniasis. They reveal that a second blood meal is vital for vector competence of infected sand flies, and should be accounted for when considering their vectorial capacity. Additionally, our findings have consequences for xenodiagnosis, a technique used to determine if an animal or a person is infectious to the vector. To date, these studies lack sensitivity, and most investigators assess ‘pick-up’ of parasites rather than the epidemiologically relevant status of mature infections. The relatively small number of parasites acquired by sand flies after feeding on infected reservoirs will most likely survive and expand in the gut of the insect only if a second blood meal is taken. We propose that a second uninfected blood meal should be implemented for xenodiagnosis to better reflect the true infectiousness of a target species.
Fig. 3. A subsequent uninfected blood meal rescues parasites in sand flies fed on Leishmania-infected animals.
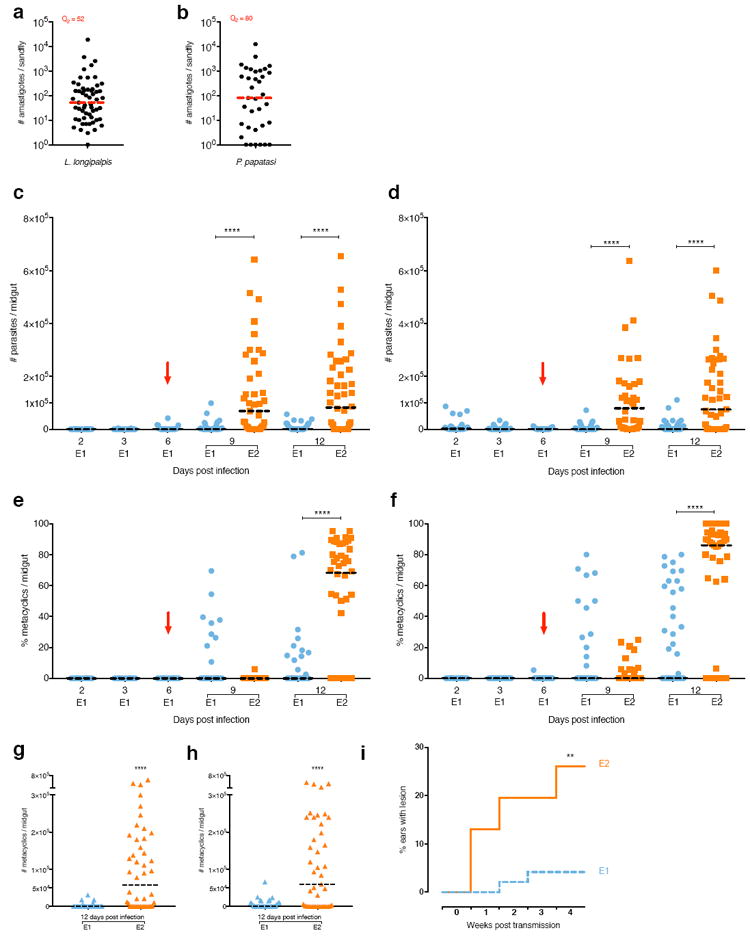
(a to i) Lutzomyia longipalpis and Phlebotomus papatasi were infected by feeding on a sick Leishmania infantum-infected hamster and a L. major-footpad lesion, respectively. (a and b) Number of parasites acquired by a single Lu. longipalpis (a) or P. papatasi (b). Q2 = median. (c to h) Parasite number (c and d) and percent (e and f) and number (g and h) of metacyclics in L. infantum-infected Lu. longipalpis (c, e and g) or L. major-infected P. papatasi (d, f and h) sand flies. (i) Developing lesions in mice ears exposed to a single L. major-infected P. papatasi sand fly. E1, sand flies engorged on an infected blood meal (BM). E2, infected sand flies engorged on a subsequent uninfected BM. Red arrow, subsequent BM. Bar, Median. Cumulative data shown from three independent experiments; (a to h) n for each condition is specified in Supplementary Table 3; (i) n= 48 for E1 and E2. **P≤0.01 and ****P<0.0001 determined by Mann-Whitney’s U-test for parasite number, by N1-Chi-squared test for percent metacyclics and by log-rank (Mantel-Cox) test for percent ears with lesions.
The life cycle of Leishmania parasites in the midgut of a sand fly was thought to conclude with terminally differentiated infective metacyclic promastigotes 8,18,20 (Fig. 4a). It was accepted that the complex developmental cycle of Leishmania parasites in the sand fly includes two multiplicative forms, procyclic and leptomonad promastigotes 8,18,20,21 (Fig. 4a). Here, we provide direct experimental evidence that metacyclic promastigotes are more plastic than previously thought and can respond to environmental cues, dedifferentiating in vivo into a leptomonad-like replicative stage, the retroleptomonad, upon the ingestion of additional uninfected BMs (Fig. 4b). These retroleptomonads multiply before redifferentiating into metacyclics, amplifying the number of infectious parasites in the sand fly prior to the next transmission event (Fig. 4b).
Fig. 4. Revising natural transmission of Leishmania by vector sand flies.
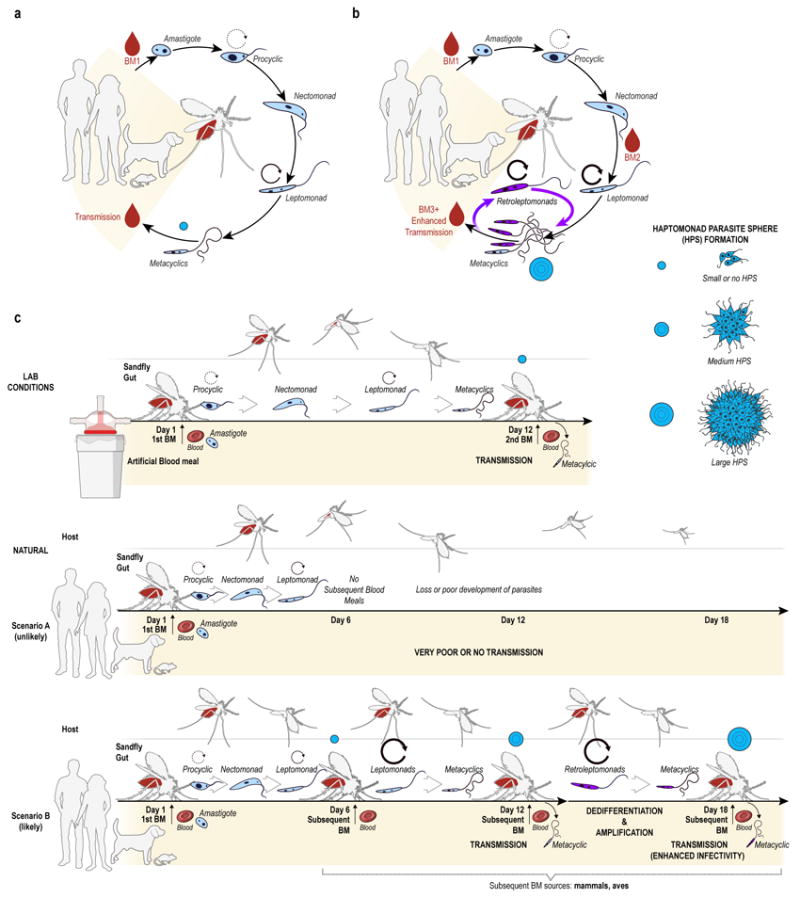
(a) Classical midgut developmental cycle of Leishmania parasites. (b) Subsequent blood meals promote Leishmania establishment by triggering metacyclic dedifferentiation into multiplicative retroleptomonads amplifying their numbers. (c) Illustrating experimental versus natural Leishmania transmission by sand flies. Infection is either initiated under artificial experimental conditions or either naturally by a sand fly taking only one infected blood meal (Scenario A, unlikely) or following it by successive blood meals (Scenario B, likely). Circular arrows depict a multiplicative stage. Blue circles represent the HPS formation and development in each scenario.
Classically, the life cycle of Leishmania parasites within the sand fly midgut has been based on observations done with experimental infections that require administration of millions of parasites/ml, typically in a single artificial BM (Fig. 4c, Experimental conditions). In this work, we show that for sand flies that pick-up <100 Leishmania amastigotes from infected animals, a subsequent blood meal is a major determinant of sand fly infectiousness. Sand flies that do not take a subsequent BM upon laying their eggs, a rare and unlikely scenario, will produce poor infections (Fig. 4c, Scenario A). Comparatively, sand flies that take multiple blood meals, the most likely scenario in nature, driven by an evolutionary need to lay as many batches of eggs as possible throughout their lifespan, establish a healthy infection and augment their infectivity by continuously amplifying the infection by expanding their metacyclic promastigote population (Fig. 4c, Scenario B). These laboratory observations are relevant to field conditions since previous studies have established that sand flies take multiple blood meals every 5-6 days throughout their life span 1,13, including while they are infected22, and that certain species take multiple blood meals before they lay their eggs1. Our findings reveal a fundamental role for multiple blood meals in establishing Leishmania infection, and in perpetually enhancing the infectiousness of sand fly vectors. As most vectors of disease take blood meals after becoming infected, their pathogens may have evolved similar mechanisms to promote their survival and transmission redefining the role of uninfected blood meals in the epidemiology of vector-borne diseases.
Methods
Ethics statement
All animal experimental procedures were reviewed and approved by the National Institute of Allergy and Infectious Diseases (NIAID) Animal Care and Use Committee under animal protocol LMVR4E. The NIAID DIR Animal Care and Use Program complies with the Guide for the Care and Use of Laboratory Animals and with the NIH Office of Animal Care and Use and Animal Research Advisory Committee guidelines. Detailed NIH Animal Research Guidelines can be accessed at https://oma1.od.nih.gov/manualchapters/intramural/3040-2/.
Animals
Six to eight weeks old female BALB/c mice and four weeks old White Leghorn chickens were obtained from Charles River laboratories. Three to six weeks old male Golden Syrian hamsters (Hsd Han TM- AURA strains) were purchased from Harlan Laboratories. Animals were housed under pathogen-free conditions at the NIAID Twinbrook animal facility, Rockville, MD. Lutzomyia longipalpis and Phlebotomus papatasi sand flies were mass reared at the Laboratory of Malaria and Vector Research insectary according to the protocols described by Lawyer et al, 201623. Adult females were maintained on a 30% sucrose diet (commercial sugar) and were starved for 12 hours before feeding. The number of flies dissected per time point was similar for all conditions and was determined empirically based on previous studies. For parasite transmission to mice, animals were randomly assigned to different experimental groups, and were randomly selected at each time point. From historical data, 66% of mice develop lesions after sand fly transmission with Phlebotomus papatasi infected with Leishmania major when 10 infected sand flies were used as the infectious challenge. Here, we used a single fly transmission comparing E1 and E2 thus reducing the likelihood of lesions development in E1 to an estimated 7 %. Taking into considerations that E2 sand flies have ~125 times more parasites in their gut we theorized that E2 transmission would occur at least 3 times more often (21%). With these numbers in mind, we calculate that to find a statistically significance difference of P<0.05 with a power of 95% probability comparing E1 to E2 flies, we will need at least n=16 per group on each experiment on lesion size prevalence. Samples from the different experimental groups were processed and assayed simultaneously.
Parasites
Parasite strains used in this study: Leishmania infantum (MCAN/BR/09/52) isolated from a dog spleen in Natal, Brazil9; Leishmania major (WR 2885) isolated from a soldier deployed to Iraq24; and Leishmania donovani (MHOM/SD/62/1S) maintained by serial passages in Golden Syrian hamsters as described before9. L. major was grown at 26°C in Schneider’s insect medium (Lonza Biowhittaker, 04-351Q) supplemented with 20% heat inactivated fetal bovine serum (Gibco, 16140071) for purification of metacyclic promastigotes by PNA (Vector Laboratories, L-1070) agglutination25. Purified metacyclics were used to infect mouse footpads and for differentiation experiments in vitro. For in vitro dedifferentiation experiments, L. major PNA double-purified metacyclics were seeded in Grace’s insect medium (Lonza BioWhittaker, 04-457F). Experiments were performed with pure medium or with medium supplemented with one of the following: 20% fresh rabbit plasma, 20% inactivated rabbit plasma, PBS-washed red blood cells (RBC) or RBC disrupted by 3 freeze/thaw cycles. L. major PNA double-purified metacyclics were also used to record the dedifferentiation event. Metacyclics promastigotes were suspended in PBS and transferred to poly-L-lysine (Sigma – P8920) coated bottom glass base dish (Thermo Scientific – 150682). Dishes were coated for 5 min with sterile poly-L-lysine, excess solution was removed and let dry for 1h. Dishes glass area were washed 25 times with 1 ml of PBS. After dry, metacyclic promastigotes suspension was added to dish and let settle for 10 min. Dish was extensively washed with medium (Schneider’s + 20% FBS) to remove unattached and poorly attached parasites. Three mL of medium was added and attached metacyclic promastigotes were imaged every minute for 18h.
Animal infection
For L. infantum, thirty to fifty 12 day-infected Lu. longipalpis were allowed to feed on an anesthetized hamster for one hour. Six to eight months later, symptomatic animals 9 were exposed to sand flies or used to harvest Leishmania amastigotes. For L. major, metacyclic promastigotes were harvested from stationary phase cultures and purified using PNA agglutination as previously described 26. Metacyclics (1×105) were injected into a BALB/c mouse footpad. After four to six weeks, swollen non-ulcerated footpads (<5 mm thickness) were offered to sand flies or were used to harvest amastigotes.
Sand fly infections
Sand flies were infected either artificially, by mixing animal blood with tissue-harvested amastigotes in a custom-made glass feeder (Chemglass Life Sciences, CG183570) capped with chick skin, or naturally on a Leishmania-infected animal. In the former, sand flies were allowed to feed on heparinized blood containing 2×106 Leishmania amastigotes per mL. The feeding apparatus was kept at 37°C with circulating heated water. Lu. longipalpis was artificially infected using naïve dog blood seeded with L. infantum amastigotes harvested from infected hamster spleens as described elsewhere 27. P. papatasi was artificially infected with L. major amastigotes harvested from infected BALB/c mice footpads 26. Flies were allowed to feed for 3 hours in the dark. For natural infections, Lu. longipalpis and P. papatasi were fed on symptomatic hamsters exposed seven months earlier to L. infantum-infected sand flies or on L. major footpad lesions of BALB/c mice, respectively. Sand flies were allowed to feed on an anesthetized hamster placed in a custom made plexiglas cage (L14cm × W14cm × H14cm), whereas the infected footpad of an anesthetized mouse was inserted through a hole made in a mesh covering a cardboard pint containing the sand flies. Feeding was carried out for one hour in the dark. After artificial and natural sand fly infections, blood fed females were sorted for further experimentation.
Sand fly subsequent blood meals
After the sand flies had taken an infected blood meal, either by feeding on a glass feeder or an infected animal, they were kept on a 30% sucrose diet for either 6 or 12 days. The sand flies were then allowed to blood engorge on either an anesthetized naïve mouse or a restrained young chicken for one hour.
Midgut parasite load assessment by direct counting
The midguts of infected sand flies were dissected in PBS on microscope slides using tweezers and fine needles. Dissected midguts were then transferred to 1.7mL microtubes (Denville Scientific, C2172) filled with 30μL of PBS and ground with disposable pellet mixers and a cordless motor (Kimble, 7495400000). Dilutions were made as necessary, and 10 μL of each sample was loaded onto Neubauer improved chambers (Incyto, DNC-NO1). Leishmania parasites were counted following the manufacture’s recommendations. To provide accurate counts of the rapidly swimming metacyclic promastigotes, they were slowed down by the addition of formalin to PBS at a final concentration of 0.005%. Parasites were counted under a phase contrast Axiostar plus microscope (Zeiss) at 400X magnification.
Midgut parasite load assessment by Quantitative PCR
Immediately after feeding on infected animals, the whole body of fully fed females was lysed individually for DNA purification using QIAamp DNA Micro kits (Qiagen, 56304), following the manufacture’s recommendations (tissue protocol – plus grind with disposable pellet mixers at the lysis buffer). Twenty nanograms of sample DNA was used as template in a Taqman-based quantitative PCR (qPCR) to amplify a fragment of the Leishmania kinetoplast minicircle DNA as described elsewhere 28. In order to obtain a standard curve and assess the parasite concentration at a given cycle threshold (CT), cultured Leishmania parasites were serially diluted from 106 to 101 and individually mixed with one uninfected female sand fly for DNA extraction. qPCR was carried out for all the standards, and the CT values were plotted against parasite concentrations (log10 scale). Standard curves were performed separately for L. infantum-infected Lu. longipalpis and L. major-infected P. papatasi. Water only as well as DNA from uninfected sand fly females were used as negative controls. As the amplification of Lu. longipalpis uninfected sand fly DNA displayed CT values similar to the 101 L. infantum-spiked DNA equivalent, such a dilution was excluded from the standard curve. Primer-probe amplification efficiencies were calculated using the equation: E = 10(-1/slope), where the slope was obtained from the linear regression analysis.
Transmission of Leishmania parasites via sand fly bites
A single P. papatasi female was placed into a cylindrical custom made plastic vial covered with a fine mesh as previous described 29. A small hole was made in the mesh to insert the ear of an anesthetized mouse inside the vial. The sand flies were kept in direct contact with the mouse ear for 3 hours in the dark at 26°C and 75% room humidity. Afterwards, the sand flies were checked for blood under a stereoscope. Lesions developing on mice ears were measured weekly for 4 weeks using a Vernier caliper (Mitutoyo, 500-195).
Haptomonad sphere dissection and measurement
The midguts of sand flies at late stage infections (12 days after the 1st blood meal for once or twice engorged sand flies) were dissected as described above. The haptomonads parasite spheres (HPS) were obtained by pulling the crop and the midgut apart, which sometimes resulted in the removal of intact HPS from the cardiac valve. Whenever the HPS stayed behind connected to the cardia, direct dissection of the cardiac valve with fine needles was performed. As the HPS was isolated, tissue debris were removed from the surroundings, and a coverslip was placed onto the HPS for posterior measurements. Pictures of HPS were obtained, and voxel sizes were determined by image acquisition using a stage micrometer calibration slide (AmScope, MR400). The HPS’ diameters were measured using the Image J software 30.
Microscopy, stereomicroscopic imaging and video recording
Whole midgut images were taken using an iphone 6s camera connected to the Stemi 508 stereomicroscope (Zeiss) ocular by a microscope mount (iDu Optics, iDu Professional iPhone 6/6S microscope adapter with built-in 30mm 10X WF lens) and voxel sizes were determined by image acquisition using a stage micrometer calibration slide (Omax, A36CLAM1). Phase contrast micrographs were taken using the Axiocam mRm camera coupled to Axiovert 200 microscope (Zeiss). Midguts videos were recorded using a DFC345 FX camera coupled to a DMI6000 B microscope (Leica). Raw files were opened using Image J software and exported at 10 frames per second (Supplementary video 1a,b,c) and 25 frames per second (Supplementary video 2a,b,c). For Supplementary videos 2, images were cropped to focus on single parasite and compiled until 18h (Using Image J software). Video files were loaded to Movavi video editor software (Movavi video suite – v16.5) to add arrow on initial frames.
Scanning electron microscopy
Parasite samples were fixed in 25 uL of 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer and allowed to settle on silicon chips for twenty minutes. After a brief buffer wash, samples were post-fixed with 1.0% osmium tetroxide in 0.1 M sodium cacodylate buffer. Specimens were dehydrated with a graded ethanol series, critical point dried under CO2 in a Bal-Tec model cpd 030 Drier (Balzers), mounted on aluminum studs, and sputter coated with 50 A of iridium in a model IBSe ion beam sputter coater (South Bay Technologies) and viewed at 5 kV in a Hitachi SU-8000 field emission scanning electron microscope (Hitachi). Scanning Electron Micrographs were converted to RGB color in Adobe Photoshop CS6 from Grayscale. The embedded SEM image details were masked using the Fill- Content Aware function. The midgut was selected and a Levels adjustment layer was added to adjust for improved contrast. The midgut mask was modified with a Hue/Saturation layer (Hue: 28, Saturation:13, Lightness: -34). The background was selected as a mask and the contrast was modified with the Levels adjustment layer. The background mask was colored a deep ombre (#130f05) with a Color Fill adjustment layer set at an opacity of 81% and fill of 100% using an Overlay transfer mode. The parasites were selected as a mask and adjusted for contract using the Levels adjustment mode. The parasite mask was then colored cyan (#0eb7f6) with a Color Fill adjustment layer using an opacity of 97% and fill of 99%. The transfer mode was set to Multiply. The highlights within the parasites were selected and colored yellow (#c5c722) using a Color Fill adjustment layer with an opacity and fill of 77% for each and an Overlay transfer mode. A new layer was added and using the gradient tool, shadowing was added to the top edge of the image.
Flow cytometry
Flow cytometry analysis was used to determine viability of gut-residing sand fly promastigotes during the first 24 hours after the 2nd blood meal using propidium iodide incorporation 12. At day 12 post infection, once or twice engorged sand fly midguts were dissected in PBS and the thoracic portion isolated. The dissected portion of ten midguts was transferred to 0.5 mL of PBS in a pyrex 9 depression glass spot plate well (Corning, 7220-85), opened longitudinally or “unzipped” to flush the contents out into the supernatant. After 10 min, the contents of the thoracic midguts were collected and the concentration adjusted to 2×106 parasites/ml in PBS. At the moment of acquisition, 5μl/ml of a propidium iodide staining solution (BD pharmingen, 556463) was added to the samples. Data were collected in MacsQuant flow cytometer (Miltenyi Biotec). Data analysis was performed using FlowJo v.10 software (Tree Star Inc). At least twenty thousand events were collected for each sample.
Statistical analysis
Data were first analyzed by the D'Agostino & Pearson normality test. Due to the non-normal distribution for most of the parasite counts, statistical comparisons were performed with the Mann Whitney-U test. To calculate the differences between groups for the proportions of metacyclics, we used “N-1” Chi-squared test. Lesion appearance curves were analyzed by the log-rank (Mantel-Cox) test. Graphs depict the individual sample values and their median; or the median +/- interquartile range. Graphs and analyses were made using GraphPad Prism 7.0c software. For comparison of proportions between two samples, we used the Medicalc free web calculator comparison of proportions (https://www.medcalc.org/calc/comparison_of_proportions.php).
Data Availability
The data that support the findings of this study are available from the corresponding authors upon request.
Supplementary Material
Acknowledgments
We would like to thank Elizabeth Fischer and Stacy Ricklefs from the Research Technology Branch (RTB), NIAID, for electron microscopy support; Ryan Kissinger from RTB, NIAID, for illustration support; Alec Perkins and Waldione de Castro from VMBS, NIAID, for technical support; Venansa Vernyuy, Timothy R. Wilson and Brian G. Bonilla from LMVR, NIAID for sand fly insectary support; Drs. Ranadhir Dey and Hira Nakhasi from CBER, FDA, for help with qPCR; Dr. Aline M. A. Souza for help with statistical analysis and Drs. Carolina Barillas-Mury and Jose M.C. Ribeiro from LMVR, NIAID, for critical reading of the manuscript. This research was supported by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases.
Footnotes
Author contributions:
T.D.S. and I.V.C.A. designed and performed the experiments. T.D.S. analyzed the data. I.V.C.A analyzed qPCR data. C.M. performed sand fly insectary work. J.G.V., S.K., and F.O. were involved in the design, interpretation and supervision of this study. All authors wrote the manuscript.
References
- 1.Guzman H, Walters LL, Tesh RB. Histologic detection of multiple blood meals in Phlebotomus duboscqi (Diptera: Psychodidae) J Med Entomol. 1994;31:890–897. doi: 10.1093/jmedent/31.6.890. [DOI] [PubMed] [Google Scholar]
- 2.Norris LC, Fornadel CM, Hung WC, Pineda FJ, Norris DE. Frequency of multiple blood meals taken in a single gonotrophic cycle by Anopheles arabiensis mosquitoes in Macha, Zambia. Am J Trop Med Hyg. 2010;83:33–37. doi: 10.4269/ajtmh.2010.09-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kramer LD, Ebel GD. Dynamics of flavivirus infection in mosquitoes. Adv Virus Res. 2003;60:187–232. doi: 10.1016/s0065-3527(03)60006-0. [DOI] [PubMed] [Google Scholar]
- 4.Abbasi I, Cunio R, Warburg A. Identification of blood meals imbibed by phlebotomine sand flies using cytochrome b PCR and reverse line blotting. Vector Borne Zoonotic Dis. 2009;9:79–86. doi: 10.1089/vbz.2008.0064. [DOI] [PubMed] [Google Scholar]
- 5.Bates PA. Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies. Int J Parasitol. 2007;37:1097–1106. doi: 10.1016/j.ijpara.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.W H O vector-borne diseases. 2014:1–30. http://apps.who.int/iris/handle/10665/206531?mode=full.
- 7.Das S, Muleba M, Stevenson JC, Pringle JC, Norris DE. Beyond the entomological inoculation rate: characterizing multiple blood feeding behavior and Plasmodium falciparum multiplicity of infection in Anopheles mosquitoes in northern Zambia. Parasit Vectors. 2017;10:45. doi: 10.1186/s13071-017-1993-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dostalova A, Volf P. Leishmania development in sand flies: parasite-vector interactions overview. Parasit Vectors. 2012;5:276. doi: 10.1186/1756-3305-5-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aslan H, et al. A new model of progressive visceral leishmaniasis in hamsters by natural transmission via bites of vector sand flies. J Infect Dis. 2013;207:1328–1338. doi: 10.1093/infdis/jis932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collin N, et al. Sand fly salivary proteins induce strong cellular immunity in a natural reservoir of visceral leishmaniasis with adverse consequences for Leishmania. PLoS Pathog. 2009;5:e1000441. doi: 10.1371/journal.ppat.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard MK, Sayers G, Miles MA. Leishmania donovani metacyclic promastigotes: transformation in vitro, lectin agglutination, complement resistance, and infectivity. Exp Parasitol. 1987;64:147–156. doi: 10.1016/0014-4894(87)90138-x. [DOI] [PubMed] [Google Scholar]
- 12.Serafim TD, et al. Leishmania metacyclogenesis is promoted in the absence of purines. PLoS Negl Trop Dis. 2012;6:e1833. doi: 10.1371/journal.pntd.0001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ready PD. Biology of phlebotomine sand flies as vectors of disease agents. Annu Rev Entomol. 2013;58:227–250. doi: 10.1146/annurev-ento-120811-153557. [DOI] [PubMed] [Google Scholar]
- 14.Kamhawi S. Phlebotomine sand flies and Leishmania parasites: friends or foes? Trends Parasitol. 2006;22:439–445. doi: 10.1016/j.pt.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Alexander B, de Carvalho RL, McCallum H, Pereira MH. Role of the domestic chicken (Gallus gallus) in the epidemiology of urban visceral leishmaniasis in Brazil. Emerg Infect Dis. 2002;8:1480–1485. doi: 10.3201/eid0812.010485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sant'anna MR, et al. Chicken blood provides a suitable meal for the sand fly Lutzomyia longipalpis and does not inhibit Leishmania development in the gut. Parasit Vectors. 2010;3:3. doi: 10.1186/1756-3305-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guimaraes ESAS, et al. Leishmania infection and blood food sources of phlebotomines in an area of Brazil endemic for visceral and tegumentary leishmaniasis. PLoS One. 2017;12:e0179052. doi: 10.1371/journal.pone.0179052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers ME. The role of leishmania proteophosphoglycans in sand fly transmission and infection of the Mammalian host. Front Microbiol. 2012;3:223. doi: 10.3389/fmicb.2012.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimblin N, et al. Quantification of the infectious dose of Leishmania major transmitted to the skin by single sand flies. Proc Natl Acad Sci U S A. 2008;105:10125–10130. doi: 10.1073/pnas.0802331105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bates PA. Leishmania sand fly interaction: progress and challenges. Curr Opin Microbiol. 2008;11:340–344. doi: 10.1016/j.mib.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gossage SM, Rogers ME, Bates PA. Two separate growth phases during the development of Leishmania in sand flies: implications for understanding the life cycle. Int J Parasitol. 2003;33:1027–1034. doi: 10.1016/s0020-7519(03)00142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Killick-Kendrick R, Rioux JA. Mark-release-recapture of sand flies fed on leishmanial dogs: the natural life-cycle of Leishmania infantum in Phlebotomus ariasi. Parassitologia. 2002;44:67–71. [PubMed] [Google Scholar]
- 23.Lawyer PMC, Rowland T, Rowton E. Care and Maintenance of Phlebotomine Sand Flies. Vector Biology Research Resources (BEI Resources) 2016 [Google Scholar]
- 24.Oliveira F, et al. A sand fly salivary protein vaccine shows efficacy against vector-transmitted cutaneous leishmaniasis in nonhuman primates. Sci Transl Med. 2015;7:290ra290. doi: 10.1126/scitranslmed.aaa3043. [DOI] [PubMed] [Google Scholar]
- 25.Sacks DL, Melby PC. Animal models for the analysis of immune responses to leishmaniasis. Curr Protoc Immunol. 2015;108:19 12 11–24. doi: 10.1002/0471142735.im1902s108. [DOI] [PubMed] [Google Scholar]
- 26.Sacks DL, Perkins PV. Identification of an infective stage of Leishmania promastigotes. Science. 1984;223:1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- 27.Gomes R, et al. Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proc Natl Acad Sci U S A. 2008;105:7845–7850. doi: 10.1073/pnas.0712153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selvapandiyan A, et al. Intracellular replication-deficient Leishmania donovani induces long lasting protective immunity against visceral leishmaniasis. J Immunol. 2009;183:1813–1820. doi: 10.4049/jimmunol.0900276. [DOI] [PubMed] [Google Scholar]
- 29.Kamhawi S, Belkaid Y, Modi G, Rowton E, Sacks D. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science. 2000;290:1351–1354. doi: 10.1126/science.290.5495.1351. [DOI] [PubMed] [Google Scholar]
- 30.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon request.


